Abstract
Purpose
We used parcellation based on 264 putative functional areas to explore the difference of amplitude of low-frequency fluctuation (ALFF) between refractory depression and non-refractory depression patients.
Patients and methods
Sixty first episode drug-naive patients with major depressive disorder (MDD) and 20 healthy controls (HCs) were recruited in this study; the MDD group was divided into a refractory depression (TRD) group (n=15) and a non-refractory depression (non-TRD) group (n=18) according to the treatment effect following up for 2 years. All the subjects underwent magnetic resonance imaging scanning and performed the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) and all the patients with MDD finished the 17-item Hamilton Depression Rating Scale (HAMD17). We used a parcellation based on 264 putative functional areas to explore the difference of ALFF measures in the three groups. The correlation between the abnormal ALFF value and characteristics of MDD was examined.
Results
RBANS total scores and index scores in the HCs were significantly different from that of the MDD group. HAMD-17 in the TRD group was significantly higher than that of non-TRD group. Relative to HCs, MDD groups showed significantly lower ALFF within the right default mode network, which was positively correlated with the immediate memory and language in the MDD group. Compared with the non-TRD group, the TRD group showed higher ALFF in the right sensory/somatomotor hand, right auditory and left default mode network.
Conclusion
Dysfunction of the somatosensory areas, right auditory and left default mode network may be a marker for specific psychopathology symptoms of TRD.
Keywords: amplitude of low frequency fluctuation, refractory depression, 264 putative functional area
Introduction
Major depressive disorder (MDD) is highly prevalent psychiatric disorders and is a leading cause of disability worldwide.1 About 30% of patients with MDD do not respond to standard pharmacotherapy or psychotherapy.2 They are classified as treatment-resistant depression (TRD).3 Although MDD has been extensively studied by neuroimaging studies in recent years,4-6 the pathomechanism of TRD at neural level is still under debate.
Neuroimaging studies demonstrate that TRD is accompanied by functional and structural abnormalities in many brain regions. Change of grey matter volume has also been observed in patients with TRD in frontal and temporal regions, including the parahippocampus and amygdala.7 Furthermore, research showed hyperperfusion in the left subcortical regions (amygdala, putamen and pallidum) and the left dorsomedial prefrontal, left anterior cingulate cortex in the TRD and chronically depressed patients by arterial spin labeling perfusion magnetic resonance imaging.8 Abnormal functional connectivity with thalamus and medial prefrontal regions has also been discovered to be associated with refractoriness.9 Also, comparison between TRD and non-treatment-resistant depression (non-TRD) showed reduced functional connectivity in the left amygdala-anterior cingulate cortex-right insula-precuneus in non-TRD group.10 The involvement of specific brain regions in refractoriness was furthermore confirmed by the improving clinical symptoms through theta burst stimulation in the left dorsolateral prefrontal cortex (LDLPFC).11 Repetitive transcranial magnetic stimulation (rTMS) has too been reported to be a promising technique for cognitive enhancement by acting on brain areas in TRD.12 However, fewer magnetic resonance studies have investigated the neural mechanisms between TRD and non-TRD by differential functional integration within neural networks.
Resting state MRI (rs-MRI) that measures brain baseline fluctuations in the BOLD signal when a subject with open or closed eyes is not doing anything in the scanner.13 rs-fMRI actually might be of great significance approach for investigating the abnormal neural mechanisms of depression.Two reviews detail a good deal of studies that have utilized rs-fMRI to study various neurological and psychiatric conditions.14,15 Rs-fMRI provides good signal-to-noise, requires minimal subject compliance, and is well suited for translation into the clinical realm.16 In rs-fMRI, regional characteristics of low-frequency oscillations are used commonly to examine with amplitude of low-frequency fluctuation (ALFF), which was developed as a power spectrum of low-frequency (0.01–0.08 Hz) fluctuations in the blood oxygen level-dependent signal with higher sensitivity and specificity. In ALFF analysis, the baseline intensity or the amplitude of low-frequency oscillations is quantified as a regional characteristic of resting state natureneural activity. Alterations in ALFF values in MDD have been one of the topics in a few recent rs-fMRI studies. Overwhelming majority of studies mainly have used structure atlases such as the automated anatomical labeling (AAL) atlas, which focused on the structural units of ALFF changes in depression.17,18 There is growing evidence that the neural networks of MDD are damaged.19,20 Nevertheless, the AAL that consists of 90 regions within the cerebral cortex provides a coarse parcellation of the brain based on then on-overlapping structural units. The 264 putative functional areas atlas that parceled the whole brain into 264 distinct regions were derived in task-free data using methods with no prior information about node identity.19 Many properties of this functional areas atlas should be fairly direct reflections of functional brain organization. Using the 264 putative functional areas atlas obtained superior results compared to the AAL atlas.20
The current study is the first study to examine the differences in resting state activity as measured by ALFF between non-TRD and TRD based on 264 putative functional areas atlas. We hypothesized that TRD and non-TRD are characterized by distinct functional deficits in distributed brain system,and the functional deficits of patients are associated with symptoms and cognitive function. First, we recruited 60 MDD with first episode and drug-naive, MDD was divided into 15 TRD and 18 non-TRD according to the therapeutic effect after followed up for 2 years. In addition, we use a parcellation based on 264 putative functional areas to explore functional abnormality using ALFF measures in refractory depression, non-refractory depression and comparison subjects. Furthermore, we conducted correlation analysis between abnormal ALFF value and characteristics of MDD.
Materials and methods
Participants
We recruited 60 outpatient clinics with MDD from First Hospital of Shanxi Medical University between December 2015 and July 2017. All the patients fulfilled DSM-IV criteria for major depression as diagnosed by the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV)and completed the 17-item Hamilton Depression Rating Scale (HAMD-17). The MDD was divided into 15 TRD and 18 non-TRD according to the therapeutic effect after followed up for 2 years, and 27 patients fell off. Inclusion criteria for depressed subjects were as follows: 1) aged between 18 and 50 years; 2) HAM-D-17>17; 3) patients with first episode and drug-naive; 4) no comorbid mental disorders. Exclusion criteria for depressed patients were as follows: 1) those having mental disorders caused by organic changes; 2) those meeting criteria for substance dependence within the past year; 3) those who had experienced positive urinary toxicology screening at recruitment ;4) continuous drinking for more than a week ; 5) those who were strong suicidal ideation or serious suicidal behavior,according to suicide score ≥3 of the HAMD-17; 6) those with serious medical or neurological illness; 7) those who were pregnant or breastfeeding at the time of the study.
TRD was defined as the lack of response to at least two kinds of antidepressants for at least 4–6 weeks in adequate dosages, and with continuing moderate to severe psychopathology.21 Non-TRD patients were in remittance from a major depressive episode. We further recruited a group of 20 healthy controls matched with the MDD patients for age, gender and educational years. There was no history of psychiatric illness in the participants and their first-degree relatives, and having no substance dependence; having no serious medical or neurological illness. All participants performed the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS).
This study’s protocol was approved by the Ethical Committee for Medicine of the First Hospital of Shanxi Medical University, Taiyuan, People's Republic of China. All the participants or their guardians provided written informed consent and approved this study. This study was conducted in accordance with the Declaration of Helsinki.
Clinical questionnaire
HAMD-17 was developed by Hamilton in 1960,22 that was administered to assess the severity of depressive symptoms. The κ score of the HAM-D-17 was 0.91, and the Cronbach’s α score was 0.714.23 RBANS24 was consisted by 12 subtests that including a total score and index scores across five domains: Immediate Memory, Visuospatial-Constructional, Language, Attention and Delayed Memory. Index scores are converted to age-based standard scores (M=100, SD=15).
MRI scanning
MRI data were acquired from all the participants using a MAGNETOM Trio Tim 3.0 T system (Siemens Medical Solutions, Germany) at the Shanxi Provincial People’s Hospital, Taiyuan, People's Republic of China. Foam pads and earplugs were used to limit head motion and reduce scanner noise. Participants were positioned with their heads in a 32 channel head coil. All subjects were asked to rest quietly with their eyes closed, and confirmed that they did not sleep during the scan. We acquired 212 three-dimensional image volumes with the following parameters: TR=2,000 ms; TE=30 ms; section thickness=3 mm sagittal slices=32;FA=90º;FOV=240×240 mm2; and matrix=64×64 mm2.
Data processing and analysis
Resting-state fMRI data preprocessing was conducted using SPM8 (www.fil.ion.ucl.ac.uk/spm/software/spm8) and the Data Processing Assistant for Resting-State fMRI toolbox (DPARSF V2.0).25 Standard pre-processing steps were performed on the rsfMRI data sets, including the following steps: remove the first 10 time points, slice timing, realign, normalize (using an EPI standard template from the Montreal Neurological Institute ), spatial smoothing (6 mm full-width at half-maximumGaussian filter), linear detrending. Linear regression of global mean signal, head motion parameters, cerebrospinal fluid signal and white matter signal was executed to remove the effects of nuisance covariates. Data were excluded if their head motion was >2 mm or an angular rotation of 2° in any direction.
The 264 putative functional areas atlas partitioned the whole brain into 264 distinct regions 15. These areas were identified using two sets of regions of interests (ROIs) resulted from two independent methods: meta-analytic and functional connectivity (fc)-mapping. The first method of identifying assumed functional areas utilized multiple datasets (n>300) for brain regions that showed remarkable activity when specific tasks were performed. This method consolidated to 151 final ROIs by spatial averaging. The second method was applied to the rs-fMRI data from healthy young adults (n=40).The (fc)-mapping method generated representative set of 193 non-overlapping ROIs. Technical details and full information about the fc-mapping are referred to the reporter.26,27 The combination of meta-analytic and (fc)-mapping yielded 264 putative areas across the cerebral cortex, subcortical structures and the cerebellum. We extracted ALFF values of all subjects with the identified 264 putative functional areas. The 264 regions of ALFF values between the patient groups and controls were examined using one-way ANOVA analysis followed by post hoc two-sample t-tests utilizing the SPSS 23.0. For all analyses, the threshold is p<0.05 with false discovery rate (FDR) correction for multiple spatial comparisons at the cluster level across the whole brain.
Differences of age and years of education between the MDD (including TRD and non-TRD)and controls were examined using ANOVA. Gender was compared using Chi-square test. The illness duration and HAMD total scores between TRD and non-TRD were estimated utilizing two-sample t-tests. One-way ANOVA was conducted to compare RBANS total scores and index scores (Immediate Memory, Visuospatial-Constructional, Language, Attention and Delayed Memory) between the three groups, and post-hoct-tests were performed to identify variations across groups with least-significant difference (LSD). The partial correlation is examined between abnormal ALFF and HAMD total scores with age, years of education and illness duration as covariates in patients. The partial correlation between the ALFF value and the RBANS total scores,Immediate Memory scores, Visuospatial-Constructional scores, Language scores, Attention scores and Delayed Memory scores was examined with age, years of education and illness duration as covariates.
Results
Demographic and clinical characteristics
Age, sex and years of education were not significantly different between TRD, non-TRD and HC group. Illness duration of MDD has not significantly different between TRD and non-TRD. Depression severity (HAMD17 score) with TRD was significantly higher than that of non-TRD (p=0.016; Table 1). RBANS total scores, Immediate Memory scores, Language scores, Attention scores and Delayed Memory scores with MDD (including TRD and non-TRD) were significantly different from that of the HC group (p<0.05). No significant differences were observed for Visuospatial-Constructional scores among the three groups (p>0.05). Post-hoc t-tests displayed that compared with HC, TRD and non-TRD showed significantly lower scores in RBANS total scores (TRD vs HC, p=0.001; non-TRD vs HC, p<0.001), Immediate Memory scores (TRD vs HC, p=0.001; non-TRD vs HC, p<0.001), Language scores (TRD vs HC, p=0.022; non-TRD vs HC, p=0.001), Attention scores (TRD vs HC, p=0.012; non-TRD vs HC, p<0.001) and Delayed Memory scores (TRD vs HC, p=0.003; non-TRD vs HC, p<0.001). However, RBANS total scores and index scores were not significantly different between TRD and non-TRD(p>0.05; Table 2).
Table 1.
Demographic and clinical characteristics of the patients with MDD and HCs
| Characteristic | TRD (N=15) mean±SD | Non-TRD (N=18) mean±SD | HC (N=20) mean±SD | F/t | p-value |
|---|---|---|---|---|---|
| Age (years) | 36.87±8.98 | 36.06±9.48 | 31.90±9.04 | 1.55 | 0.223 |
| Gender (male/female) | 6/9 | 7/11 | 9/11 | 0.17 | 0.921 |
| Education (years) | 11.07.±2.82 | 12.22±3.34 | 12.85±2.21 | 1.75 | 0.163 |
| Illness duration (months) | 14.53±19.32 | 10.17±14.93 | NA | 0.73 | 0.469 |
| HAMD (Hamilton Depression Scale) | 23.07±2.76 | 20.17±3.59 | NA | 2.56 | 0.016* |
Note: *p<0.05.
Abbreviations: TRD, treatment-resistant depression; HC, healthy controls ; non-TRD, non-treatment-resistant depression; MDD, major depressive disorder.
Table 2.
ANOVA and post-hoc t-tests analysis for RBANS with TRD, non-TRD and HCs
| Characteristic | TRD (N=15) mean±SD | Non-TRD (N=18) mean±SD | HC (N=20) mean±SD | p-value |
|---|---|---|---|---|
| Immediate Memory | 71.8±11.47 | 66.06±11.8 | 90.25±22.76 | <0.001* |
| TRD vs non-TRD | - | - | - | 0.460 |
| TRD vs HC | - | - | - | 0.002* |
| Non-TRD vs HC | - | - | - | <0.001* |
| Visuospatial-Constructional | 82.00±15.55 | 81.67±17.03 | 90.90±20.16 | 0.211 |
| Language | 84.67±9.25 | 79.00±16.60 | 95.32±12.07 | 0.001* |
| TRD vs non-TRD | - | - | - | 0.224 |
| TRD vs HC | - | - | - | 0.022* |
| Non-TRD vs HC | - | - | - | 0.001* |
| Attention | 103.40±13.06 | 96.72±18.28 | 117.20±14.51 | 0.001* |
| TRD vs non-TRD | - | - | - | 0.225 |
| TRD vs HC | - | - | - | 0.012* |
| Non-TRD vs HC | - | - | - | <0.001* |
| Delayed Memory | 81.93±11.92 | 79.78±11.76 | 96.00±15.20 | 0.001* |
| TRD vs non-TRD | - | - | - | 0.643 |
| TRD vs HC | - | - | - | 0.003* |
| Non-TRD vs HC | - | - | - | <0.001* |
| Total score | 79.67±10.06 | 76.11±13.69 | 98.05±17.95 | <0.001* |
| TRD vs non-TRD | - | - | - | 0.490 |
| TRD vs HC | - | - | - | 0.001* |
| Non-TRD vs HC | - | - | - | <0.001* |
Note: Post-hoc t-tests with least-significant difference (LSD), *p<0.05.
Abbreviations: RBANS, the Repeatable Battery for the Assessment of Neuropsychological Status; TRD, treatment-resistant depression; HC, healthy controls; non-TRD, non-treatment-resistant depression.
Group differences in ALFF
Significant group differences of ALFF were found in 10 regions between TRD, non-TRD and Healthy Subjects by ANCOVA, followed FDR correction for multiple spatial comparisons (p<0.05). Relative to the healthy group, both TRD groups and non-TRD groups showed significantly lower ALFF within the right default mode network (posterior cingulate cortex [PCC]). Compared with non-TRD and healthy group, TRD showed higher ALFF in right sensory/somatomotor hand. Compared with non-TRD, TRD groups showed significantly higher in right auditory (temporal lobe) and left default mode network (LDLPFC). Compared with healthy group, non-TRD group showed significantly higher in left sensory/somatomotor hand, right auditory (insula), right fronto-parietal task control and right subcortical, and significantly lower in left fronto-parietal task control and right fronto-parietal task control (Table 3 and Figure 1, Table 4 and Figure 2).
Table 3.
ALFF value differences in the TRD, non-TRD and HCs
| Number | ROI area | RSN | Side | MNI coordinate | F | p | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| 1 | 19 | Sensory/somatomotor hand | R | 13 | 33 | 75 | 6.271 | 0.004* |
| 2 | 24 | Sensory/somatomotor hand | L | −40 | −19 | 54 | 3.339 | 0.044* |
| 3 | 61 | Auditory | R | 32 | −26 | 13 | 3.667 | 0.033* |
| 4 | 62 | Auditory | R | 65 | −33 | 20 | 3.694 | 0.032* |
| 5 | 89 | Default mode | R | 6 | −59 | 35 | 5.456 | 0.007* |
| 6 | 99 | Default mode | L | −16 | 29 | 53 | 4.992 | 0.011* |
| 7 | 177 | Fronto-parietal task control | L | −53 | −49 | 43 | 3.636 | 0.034* |
| 8 | 179 | Fronto-parietal task control | R | 53 | −53 | −14 | 3.816 | 0.029* |
| 9 | 196 | Fronto-parietal task control | R | 40 | 18 | 40 | 3.530 | 0.037* |
| 10 | 234 | Subcortical | R | 9 | −4 | 6 | 4.001 | 0.024* |
Note: *p<0.05.
Abbreviations: RSN, resting state network, MNI, Montreal Neurological Institute space; ROI, regions of interests; TRD, treatment-resistant depression; HC, healthy controls; non-TRD, non-treatment-resistant depression; ALFF, amplitude of low-frequency fluctuations.
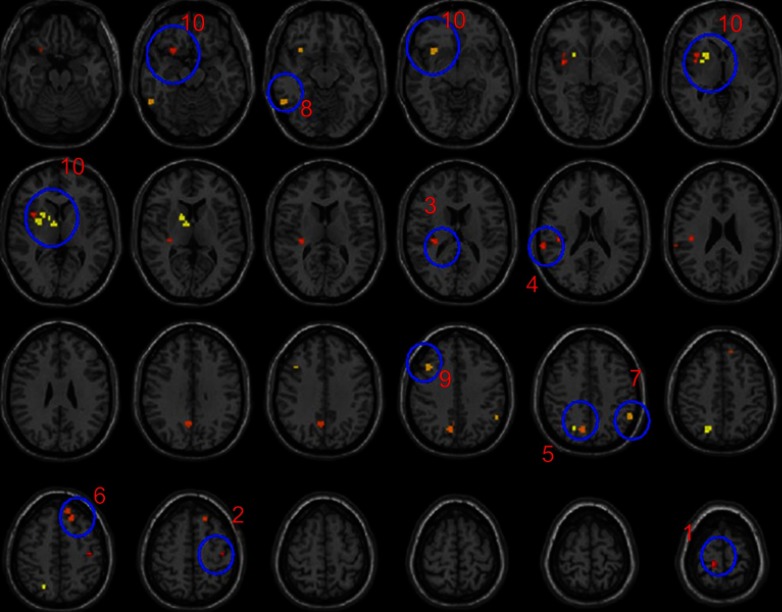
Figure 1.
F-statistical maps showing ALFF differences among TRD, non-TRD and HCs.
Notes: The blue circles represents significant clusters of ALFF differences among TRD, non-TRD and HCs.Significant clusters were shown after FDR correction for multiple spatial comparisons (p<0.05). 1: ROI:19, sensory/somatomotor hand; 2: ROI:24, sensory/somatomotor hand; 3: ROI:61, auditory (insula); 4: ROI:62, auditory (temporal); 5: ROI:89, default mode (PCC); 6: ROI:99, default mode (LDLPFC); 7: ROI:177, fronto-parietal task control; 8: ROI:179, fronto-parietal task control; 9: ROI:196, fronto-parietal task control; 10: ROI:234, subcortical.
Abbreviations: ROI, regions of interests; TRD, treatment-resistant depression; HC, healthy controls ; non-TRD, non-treatment-resistant depression; ALFF, amplitude of low-frequency fluctuations; FDR, false discovery rate; PCC, posterior cingulate cortex; LDLPFC, left dorsolateral prefrontal cortex.
Table 4.
Post-hoc t-tests of ALFF value analysis for TRD, non-TRD from HCs
| RSN | ROI area | MD | SE | p-value | 95% CI |
|---|---|---|---|---|---|
| HC vs TRD | |||||
| Sensory/somatomotor hand | 19 | −0.207 | 0.062 | 0.002* | −0.33 to −0.08 |
| Default mode (PCC) | 89 | 0.254 | 0.123 | 0.040* | 0.006 to 0.50 |
| HC vs non-TRD | |||||
| Sensory/somatomotor hand | 24 | −0.152 | 0.059 | 0.013* | −0.27 to −0.03 |
| Auditory (insula) | 61 | −0.050 | 0.018 | 0.009* | −0.09 to −0.01 |
| Default mode (PCC) | 89 | 0.378 | 0.117 | 0.002* | 0.14 to 0.61 |
| Fronto-parietal task control | 177 | 0.178 | 0.069 | 0.014* | 0.04 to 0.32 |
| Fronto-parietal task control | 179 | −0.198 | 0.076 | 0.017* | −0.34 to 0.04 |
| Fronto-parietal task control | 196 | 0.156 | 0.059 | 0.012* | 0.04 to 0.28 |
| Subcortical | 234 | −0.063 | 0.022 | 0.007* | −0.11 to −0.02 |
| TRD vs non-TRD | |||||
| Sensory/somatomotor hand | 19 | 0.177 | 0.63 | 0.007* | 0.05 to 0.30 |
| Auditory (temporal) | 62 | 0.252 | 0.097 | 0.012* | 0.06 to 0.45 |
| Default mode (LDLPFC) | 99 | 0.162 | 0.054 | 0.004* | 0.05 to 0.27 |
Note: Post-hoc t-tests with least-significant difference (LSD), *p<0.05.
Abbreviations: RSN, resting state network; ROI, regions of interests; MD, mean difference; SE, standard error; TRD, treatment-resistant depression; HCs, healthy controls; non-TRD, non-treatment-resistant depression; PCC, posterior cingulate cortex; LDLPFC, left dorsolateral prefrontal cortex; ALFF, amplitude of low-frequency fluctuations.
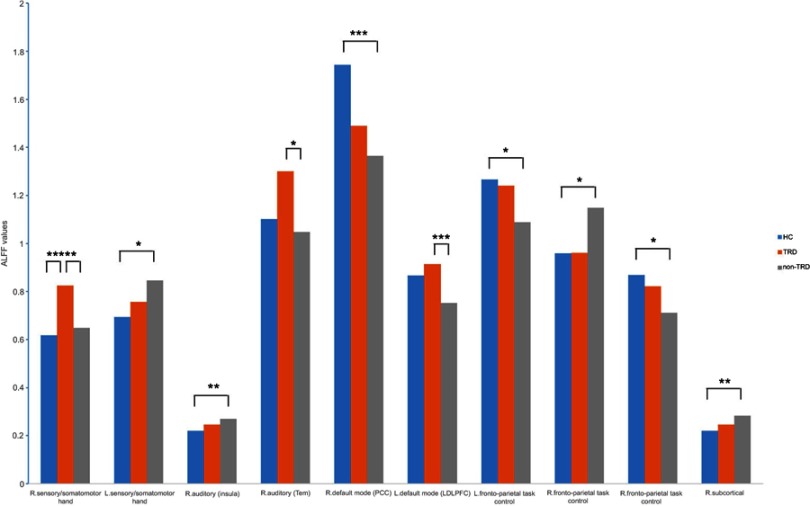
Figure 2.
Bar graph of post-hoc t-tests of ALFF value for TRD, non-TRD from HCs.
Note: *the threshold at p<0.05 ; ** the threshold at p<0.01; *** the threshold at p<0.005.
Abbreviations: TRD, treatment-resistant depression; HCs, healthy controls; non-TRD, non-treatment-resistant depression; PCC, posterior cingulate cortex; LDLPFC, left dorsolateral prefrontal cortex; ALFF, amplitude of low-frequency fluctuations.
Correlations between abnormal ALFF values and HAMD scores in patients with MDD
The partial correlation between abnormal ALFF (right Sensory/somatomotor hand,right auditory, left default mode network) and HAMD total scores in the patients were examined with age, years of education and illness duration as covariates, although age, years of education and ill duration were not significantly different between the groups. No correlations were observed.
Correlations between abnormal ALFF values and RBANS total scores, index scores
The partial correlation between abnormal ALFF (right default mode network) and RBANS total scores, index scores were performed with age, years of education, illness duration and HAMD total scores as covariates. The ALFF value of right default mode network (PCC) was positively and statistically significantly correlated with the immediate memory scores (r=0.426, p=0.021) and language scores (r=0.383,p=0.04). No correlations were observed between ALFF value of right default mode network (PCC) and RBANS total scores, attention scores and delayed memory scores.
Discussion
To our best knowledge, no prior study has used 264 putative functional areas atlas to examine the neural mechanisms between the TRD and non-TRD by differential functional integration within neural networks. Three main findings emerge from this study. First, the baseline depression severity (HAMD score) was significantly difference between TRD and non-TRD. Second, a significantly lower ALFF of right default mode network (PCC) between the depressed patient groups and healthy controls, moreover, right default mode network was correlated with the immediate memory and language in patients. Furthermore, abnormal ALFF values in right sensory/somatomotor hand, right auditory (temporal lobe) and left default mode network (LDLPFC) were observed between the TRD and non-TRD.
Cognitive deficits have now been a well-established feature of depressive disorder. The meta-analysis studies have shown that cognitive deficits in MDD mainly appeared in attention, memory, executive functioning, processing speed and selective cognitive control.28–30 In support of our studies suggesting cognitive functions in multiple dimensions with depressive patients were impaired.
The role of baseline severity as effect modifier in multifarious psychiatric disorders is one topic of controversy and of clinical import. In this study, baseline depression severity with TRD was significantly higher than that of non-TRD, does this indicate that the relationship between baseline depression severity and treatment response? A previous study reported late-life depression patients with high baseline depression severity are unlikely to respond after 12 weeks of treatment with venlafaxine XR.31 Another study showed lower baseline depression severity predicted a higher likelihood of improvement with placebo.32 These are all similar to our current result. However, the study of individual participant data meta-analysis revealed that the association between baseline severity and treatment was not statistically significant.33 Interestingly, there are studies that show that the depression spectrum (very severe, mild) increases the risk of poor response to treatment. Severe depression is associated with complex biological disorders, and for mild depression, drugs often fail to perform significantly better than placebos.34,35 The reason for the inconsistent results may be due to multifactorial disorder with clinically heterogeneous features in MDD.
The default mode network (DMN) is mainly composed of medial prefrontal cortex and PCC, both located along the brain’s midline, together with inferior parietal and medial temporal regions, which is comprised of regions that are most active during rest, and is suggested to be involved in self-referential processes.36 The DMN has been shown to be abnormal in depression patients.37 We found decreased ALFF value in PCC within DMN with MDD (including TRD and non-TRD) compared to HCs, which are correlated positively with the immediate memory and language in depression. Some previous studies have reported similar results: one study found significantly reduced correlation between the precuneus/posterior cingulate cortex (P/PCC) and the bilateral caudate in depression compared with controls,38 another research39 demonstrated dissociation between posterior and anterior functional connectivity within DMNs in first-episode, treatment-naive young adults with MDD, which suggest that increased functional connectivity in anterior medial cortex and decreased functional connectivity in posterior medial regions. However, a large proportion of MDD studies have reported increased DMN in MDD: using seed-based correlation, functional connectivity between PCC and subgenual anterior cingulate cortexhas been found to be increased.40,41 Discrepancy in results may be ascribed differences in sample size, scanning and analysis methods, clinical variations in medication status.
In the present study, compared with non-TRD subjects, TRD patients showed greater ALFF value in right sensory/somatomotor hand, temporal lobe and left default mode network (LDLPFC), the MDD patients’ HAMD score is uncorrelated with abnormal ALFF value. Convergent evidence from functional brain imaging and therapeutics suggests that depression is associated with dysfunction in several functionally integrated pathways, including somatosensory areas, temporal lobe and LDLPFC. The ENIGMA Major Depressive Disorder Working Group research shows MDD had lower total surface area in somatosensory areas by 20 cohorts worldwide.42 Reza Tadayonnejad’s results revealed abnormal alterations in ALFF in affective networks, corticostriatal circuits and motor/somatosensory networks, the significant positive correlation was found between higher frequency fALFF values in left somatosensory network and depression severity.43 A review44 summarized that the overall function of the anterior temporal lobe may be for semantic processing that is personally, socially or emotionally relevant. Another research showed MDD patients had significantly increased connectivity between medial thalamus and temporal areas, furthermore thalamo-temporal connectivity and severity of symptoms was positive correlation.45 The involvement of the LDLPFC specific in TRD was furthermore confirmed by the improving clinical symptoms through theta burst stimulation.11 Another study shows10 TRD is associated with disrupted functional connectivity mainly in thalamo-cortical circuits, while non-TRD is associated with the limbic system. Animal studies46 show that stress, through corticosteroid secretion, negatively modulates 5-HT1A receptors in the limbic system and is associated with alterations of corticoid receptor balance. Possible explanation is that the limbic system is also the target of standard antidepressants. Dysfunctional of somatosensory areas, right auditory (temporal) and left default mode network (LDLPFC) may be a marker for specific psychopathologies symptoms of TRD, that may be the reason that pharmacological treatment is effective in only one clinical group even though both groups show changed brain functioning.
We found that non-TRD but not TRD showed altered ALFF in left sensory/somatomotor hand, right auditory (insula), right fronto-parietal task control, right subcortical, in left fronto-parietal task control and right fronto-parietal task control. One possibility is that diversity of symptoms in non-TRD, and these areas are also the target of standard antidepressants. For example, the insula is believed mediate interpretation of sensory information from the body that contributes to emotional states.47 Therefore, abnormal activity of the insula might underlie such depressive symptoms as somatic complaints and negative bias in interpreting bodily feedback.
Several main limitations should be considered in interpreting these results. First, due to the heterogeneous of depression, other factors that were not studied in our study may yet influence findings, such as age of onset. Second, the fMRI data of post-treatment were lacking because some patients refused to undergo fMRI scans again.Most patients believe depression has nothing to do with brain, and a small number of patients because of lack of time.Therefore, these function change dynamically after therapy remains to be established in longitudinal studies. Lastly, because of the relatively small sample size in this research, there was not enough data to examine neural network differences between the no-TRD and TRD. A larger sample size could help to clarify the neuromechanisms of TRD.
Conclusion
In conclusion, based on 264 putative functional areas atlas, we used the ALFF measure to test the difference of the baseline amplitude of intrinsic brain activity in non-TRD relative to TRD. We detected that TRD patient exhibits greater ALFF value in the right sensory/somatomotor hand, temporal lobe and DLPFC,dysfunction of that may be a marker for specific psychopathologies symptoms of the TRD.
Acknowledgments
We thank all the subjects who participated in this study. This study was funded by the National Natural Science Foundation of China (81471379 and 81701345),and the National Key Research and Development Program of China (2016YFC1307103).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- 2.Rush AJ, Kraemer HC, Sackeim HA, et al. Report by the ACNP task force on response and remission in major depressive disorder. Neuropsychopharmacology. 2006;31:1841–1853. doi: 10.1038/sj.npp.1301131 [DOI] [PubMed] [Google Scholar]
- 3.Stimpson N, Agrawal N, Lewis G. Randomised controlled trials investigating pharmacological and psychological interventions for treatment-refractory depression. Systematic review. Br J Psychiatry. 2002;181:284–294. [DOI] [PubMed] [Google Scholar]
- 4.Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff PHE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719–3735. doi: 10.1002/hbm.20801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorenzetti V, Allen NB, Fornito A, Yücel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J Affect Disord. 2009;117:1–17. doi: 10.1016/j.jad.2008.11.021 [DOI] [PubMed] [Google Scholar]
- 6.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li CT, Lin CP, Chou KH, et al. Structural and cognitive deficits in remitting and non-remitting recurrent depression: a voxel-based morphometric study. Neuroimage. 2010;50:347–356. doi: 10.1016/j.neuroimage.2009.11.021 [DOI] [PubMed] [Google Scholar]
- 8.Duhameau B, Ferré JC, Jannin P, et al. Chronic and treatment-resistant depression: a study using arterial spin labeling perfusion MRI at 3Tesla. Psychiatry Res. 2010;182:111–116. doi: 10.1016/j.pscychresns.2010.01.009 [DOI] [PubMed] [Google Scholar]
- 9.Greicius MD, Flores BH, Menon V, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lui S, Wu Q, Qiu L, et al. Resting-state functional connectivity in treatment-resistant depression. Am J Psychiatry. 2011;168:642–648. doi: 10.1176/appi.ajp.2010.10101419 [DOI] [PubMed] [Google Scholar]
- 11.Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391:1683–1692. doi: 10.1016/S0140-6736(18)30295-2 [DOI] [PubMed] [Google Scholar]
- 12.Serafini G, Pompili M, Belvederi Murri M, et al. The effects of repetitive transcranial magnetic stimulation on cognitive performance in treatment-resistant depression. A systematic review. Neuropsychobiology. 2015;71(3):125–139. doi: 10.1159/000381351 [DOI] [PubMed] [Google Scholar]
- 13.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201 [DOI] [PubMed] [Google Scholar]
- 14.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21(4):424–430. doi: 10.1097/WCO.0b013e328306f2c5 [DOI] [PubMed] [Google Scholar]
- 15.Gong Q, He Y. Depression, neuroimaging and connectomics: a selective overview. Biol Psychiatry. 2015;77(3):223–235. doi: 10.1016/j.biopsych.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 16.Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Zhu X, Wang X, et al. First-episode medication-naive major depressive disorder is associated with altered resting brain function in the affective network. PLoS One. 2014;9:e85241. doi: 10.1371/journal.pone.0085241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Ren L, Womer FY, et al. Alterations in amplitude of low frequency fluctuation in treatment-naïve major depressive disorder measured with resting-state fMRI. Hum Brain Mapp. 2014;35:4979–4988. doi: 10.1002/hbm.22526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Power JD, Cohen AL, Nelson SM, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khazaee A, Ebrahimzadeh A, Babajani-Feremi A. Application of advanced machine learning methods on resting-state fMRI network for identification of mild cognitive impairment and Alzheimer’s disease. Brain Imaging Behav. 2016;10:799–817. doi: 10.1007/s11682-015-9448-7 [DOI] [PubMed] [Google Scholar]
- 21.Berlim MT, Turecki G. Definition, assessment, and staging of treatment-resistant refractory major depression: a review of current concepts and methods. Can J Psychiatry. 2007;52:46–54. doi: 10.1177/070674370705200108 [DOI] [PubMed] [Google Scholar]
- 22.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Addington D, Addington J, Maticka-Tyndale E, Joyce J. Reliability and validity of a depression rating scale for schizophrenics. Schizophr Res. 1992;6:201–208. [DOI] [PubMed] [Google Scholar]
- 24.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823 [DOI] [PubMed] [Google Scholar]
- 25.Chao-Gan Y, Yu-Feng Z. DPARSF: a MATLAB toolbox for “Pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen AL, Fair DA, Dosenbach NU, et al. Defining functional areas in individual human brains using resting functional connectivity MRI. Neuroimage. 2008;41:45–57. doi: 10.1016/j.neuroimage.2008.01.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson SM, Cohen AL, Power JD, et al. A parcellation scheme for human left lateral parietal cortex. Neuron. 2010;67:156–170. doi: 10.1016/j.neuron.2010.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. 2009;119:1–8. doi: 10.1016/j.jad.2009.04.022 [DOI] [PubMed] [Google Scholar]
- 29.Bolsover FE, Murphy E, Cipolotti L, Werring DJ, Lachmann RH. Cognitive dysfunction and depression in Fabry disease: a systematic review. J Inherit Metab Dis. 2014;37:177–187. doi: 10.1007/s10545-013-9643-x [DOI] [PubMed] [Google Scholar]
- 30.Ahern E, Semkovska M. Cognitive functioning in the first-episode of major depressive disorder: a systematic review and meta-analysis. Neuropsychology. 2017;31:52–72. doi: 10.1037/neu0000319 [DOI] [PubMed] [Google Scholar]
- 31.Smagula SF, Butters MA, Anderson SJ, et al. Antidepressant response trajectories and associated clinical prognostic factors among older adults. JAMA Psychiatry. 2015;72:1021–1028. doi: 10.1001/jamapsychiatry.2015.1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trivedi MH, South C, Jha MK, et al. A novel strategy to identify placebo responders: prediction index of clinical and biological markers in the EMBARC trial. Psychother Psychosom. 2018;87:285–295. doi: 10.1159/000491093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furukawa TA, Maruo K, Noma H, et al. Initial severity of major depression and efficacy of new generation antidepressants: individual participant data meta-analysis. Acta Psychiatr Scand. 2018;137:450–458. doi: 10.1111/acps.12886 [DOI] [PubMed] [Google Scholar]
- 34.Serretti A , Fabbri C. 6 signs your patient is at risk for treatment-resistant depression. Psychiatric Times. 2016. Available from: https://www.psychiatrictimes.com/major-depressive-disorder/6-signs-your-patient-risk-treatment-resistant-depression. Accessed June 21, 2019. [Google Scholar]
- 35.Serretti A, Fabbri C. Factors that predispose patients to treatment-resistant depression. Psychiatric Times. 2014;31(9). Available from: https://www.psychiatrictimes.com/factors-predispose-patients-to-treatment-resistant-depression/page/0/1. Accessed July 10, 2019. [Google Scholar]
- 36.Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Den Heuvel MP, Stam CJ, Boersma M, Hulshoff PHE. Small-world and scale-free organization of voxel-based resting-state functional connectivity in the human brain. Neuroimage. 2008;43:528–539. doi: 10.1016/j.neuroimage.2008.08.010 [DOI] [PubMed] [Google Scholar]
- 38.Bluhm R, Williamson P, Lanius R, et al. Resting state default-mode network connectivity in early depression using a seed region-of-interest analysis: decreased connectivity with caudate nucleus. Psychiatry Clin Neurosci. 2009;63:754–761. doi: 10.1111/j.1440-1819.2009.02030.x [DOI] [PubMed] [Google Scholar]
- 39.Zhu X, Wang X, Xiao J, et al. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry. 2012;71:611–617. doi: 10.1016/j.biopsych.2011.10.035 [DOI] [PubMed] [Google Scholar]
- 40.Zhou Y, Yu C, Zheng H, et al. Increased neural resources recruitment in the intrinsic organization in major depression. J Affect Disord. 2010;121:220–230. doi: 10.1016/j.jad.2009.05.029 [DOI] [PubMed] [Google Scholar]
- 41.Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Soc Cogn Affect Neurosci. 2011;6:548–555. doi: 10.1093/scan/nsq080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmaal L, Hibar DP, Sämann PG, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 2017;22:900–909. doi: 10.1038/mp.2016.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tadayonnejad R, Yang S, Kumar A, Ajilore O. Clinical, cognitive, and functional connectivity correlations of resting-state intrinsic brain activity alterations in unmedicated depression. J Affect Disord. 2015;172:241–250. doi: 10.1016/j.jad.2014.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong C, Gallate J. The function of the anterior temporal lobe: a review of the empirical evidence. Brain Res. 2012;1449:94–116. doi: 10.1016/j.brainres.2012.02.017 [DOI] [PubMed] [Google Scholar]
- 45.Brown EC, Clark DL, Hassel S, MacQueen G, Ramasubbu R. Thalamocortical connectivity in major depressive disorder. J Affect Disord. 2017;217:125–131. doi: 10.1016/j.jad.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 46.Drevets WC, Thase ME, Moses-Kolko EL, et al. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol. 2007;34(7):865–877. doi: 10.1016/j.nucmedbio.2007.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894 [DOI] [PubMed] [Google Scholar]