The epidemic caused by the Zika virus (ZIKV), beginning in 2015, is a major public health shock for Brazil, confronting reproductive-age women with an array of potentially dire risks for pregnancy and for the health of their children. As these risks have become more widely known, we would expect to see responses on the part of vulnerable women in changed childbearing intentions and in sexual and contraceptive behavior. In this study we investigate these responses. In particular, we examine the interplay of women’s desires, behaviors, and healthcare access and use during the first 18 months of the epidemic, drawing on rich new qualitative data.
The ZIKV epidemic has several defining features. First, the virus is transmissible via a mosquito (the Aedes Aegypti) that is already familiar to most Brazilians because of its long history transmitting other viruses, including dengue fever and chikungunya (Petersen et al. 2016). This familiarity may affect the ways in which people understand and respond to the vector. Second, ZIKV is transmissible not only via mosquitoes, but also via sexual intercourse, blood transfusions, and amniotic fluid (ibid.).1 The fact that it is transmissible via amniotic fluid means that pregnant women can transmit the virus to their fetuses (Brasil et al. 2016; Brito 2015; Petersen et al. 2016). Moreover, infection at any point during pregnancy can have deleterious effects on fetal development (Brasil et al. 2016). Third, the symptoms of ZIKV infection can range in severity from rashes and fevers to temporary paralysis (Guillain-Barré Syndrome; Petersen et al. 2016). Among fetuses, ZIKV infection can lead to congenital Zika syndrome, of which microcephaly is a possible outcome (França et al. 2016; Johansson et al. 2016; Miranda-Filho et al. 2016; Petersen et al. 2016).2 Fourth, despite its wide range of potential symptoms, ZIKV infection can often be asymptomatic (Johansson et al. 2016), allowing it to go unnoticed and/or to be unknowingly transmitted. Finally, there is as yet no vaccine or treatment for the virus (Marston et al. 2016; Thomas et al. 2016). Together, these features of the epidemic imply that the only way to guarantee against Zika-related birth defects until a vaccine is developed, the epidemic subsides, and/or an effective treatment becomes available is to avoid becoming pregnant or to terminate a pregnancy.
In this article, we provide an overview of the Brazilian context and describe how and why the ZIKV epidemic may affect reproductive processes. We then use new focus group data collected in two regions of Brazil with different onsets of the ZIKV epidemic and ZIKV and microcephaly rates to explore how the epidemic is affecting women’s fertility intentions and contraceptive use. We pay special attention to how women’s socioeconomic status and geographic location are related to their responses to the epidemic. Focus groups are especially well suited for capturing the complexity of individual motivations and behaviors because exchanges between participants often provoke the detailed expression and/or qualification of different perspectives (Morgan and Krueger 1993). To conclude, we highlight the implications of our observations for researchers and policymakers seeking to better understand and address how the ZIKV epidemic is affecting reproductive decision-making and disparities in reproductive health.
The Brazilian context
In the decades before the ZIKV epidemic, Brazil’s fertility fell dramatically, from 5.8 births per woman in the 1970s (Berquó and Cavenaghi 2004) to 1.9 by 2010 (Cavanaghi and Berquó 2014) . Because fertility was already below replacement level, many women may have wanted to prevent pregnancy even before the epidemic began. If so, then the epidemic could exert little influence on women’s already predominantly low fertility desires.
However, unintended fertility remains high in Brazil, where approximately 50 percent of all births are deemed unintended (Le et al. 2014). This large unintended birth rate reveals an important disconnect between the fertility desires and behaviors of many women. Thus, even if women wish to prevent pregnancy during the epidemic, it is unlikely that all of them will be able to do so successfully. Some women who do become pregnant may seek an abortion, despite the fact that abortion is highly restricted in Brazil (Aiken et al. 2016). These abortions may pose additional health risks because many are unsafe (Fusco and Andreoni 2012; Grimes et al. 2006), and they may be especially risky if performed in the second or third trimester. Because unintended pregnancy disproportionately occurs among women of lower socioeconomic status (Prietsch et al. 2011; Theme-Filha et al. 2016), women with greater economic resources may be more successful in preventing unwanted pregnancy during the epidemic and beyond.
Appendix Table 1 highlights fertility patterns by socioeconomic status and geographic region in Brazil.3 Overall fertility and adolescent fertility levels are highest among women with low socioeconomic status (Cavenaghi and Berquó 2014). Moreover, women with lower status have a greater number of children and tend to have them earlier in their reproductive years than women with higher status (Camarano et al. 2014; Cavenaghi and Berquó 2014). For example, in 2010, the highest age-specific fertility rates among women in households with less than 1 minimum wage4 occurred when women were aged 20–24; among their counterparts in households with more than 2 minimum wages, the highest age-specific fertility rates occurred at ages 30–34. This pattern suggests that a greater share of younger women with lower status were likely to view their childbearing as completed when the ZIKV epidemic began than women of the same age with higher socioeconomic status. At the same time, evidence also suggests that women with higher socioeconomic status tend to delay pregnancy (Camarano et al. 2014; Cavenaghi and Berquó 2014), which may mean that more women with higher status had not yet completed their childbearing at the epidemic’s onset.
Moreover, because economic development has disproportionately occurred in the Southern regions of Brazil (Diniz 2002) and poverty levels are higher in the Northern regions (IPEA 2010), reproductive health disparities persist across space. For instance, adolescent fertility rates are significantly higher in the lesser developed North and Northeast than in the South and Southeast (Appendix Table 1). Likewise, higher fertility rates are observed in the North than in the South. For example, in 2010 the total fertility rates in the North and Northeast were 2.47 and 2.06, respectively, while they were below replacement levels in the South and Southeast (1.78 and 1.70, respectively, Appendix Table 1).
Underlying these socioeconomic and geographic disparities in fertility are parallel disparities in contraceptive use. Our calculations show that among non-pregnant women in unions with 9 years of schooling or more in 2006, 89.3 percent used modern contraceptive methods at ages 15–19 and 87.3 percent at ages 20–24. In contrast, among women with less than 3 years of schooling, only 61.1 percent and 76.7 percent used modern contraception at these respective ages. Rates of modern contraceptive use were lowest in the North and Northeast in the 1990s, but gaps declined by the mid-2000s.5 Higher rates of contraceptive failure, discontinuation, and switching are also found in the Northeast (Leite and Gupta 2007).
Despite disparities in reproductive health, Brazil’s unified health system (Sistema Único de Saúde-SUS) is designed to provide universal and equitable health care to all citizens (Costa 2016a; Macinko and Harris 2015). Brazil’s SUS system6 provides most health procedures and prescribed medications free of charge, including contraception. A branch of the health care offered by SUS is formed by community-based primary care called Estratégia Saúde da Família (Family Health Strategy), which provides comprehensive primary health care7 for a large proportion of Brazil’s population (62 percent in 2014) (Macinko and Harris 2015).
Importantly for this research, ZIKV was first detected in Northeast Brazil (Diniz 2016; Pan American Health Organization 2015; Zanluca et al. 2015), and the region was initially hit hardest by the epidemic (Brasil 2017a; Oliveira et al. 2017). For instance, in November and December 2016 (epidemiological weeks 45/2015 to 52/2016), 1,049 municipalities had reported ZIKV cases in the Northeast compared to only 381 in the Southeast (Brasil 2017b). Figure 1 shows that in the state of Pernambuco alone, 106 municipalities had confirmed ZIKV cases in this period; in Minas Gerais, only 16 municipalities had confirmed ZIKV cases. Moreover, the Northeast presents the highest number of confirmed microcephaly cases8 in the country (1,730 cases out of the total of 2,347). A combination of lower economic development, high temperatures, stagnant water, and sanitation problems are possible explanations for why Brazil’s Northeastern region was affected first by the ZIKV epidemic (Ali et al. 2017; Diniz 2016; Zanluca et al. 2015).
FIGURE 1.
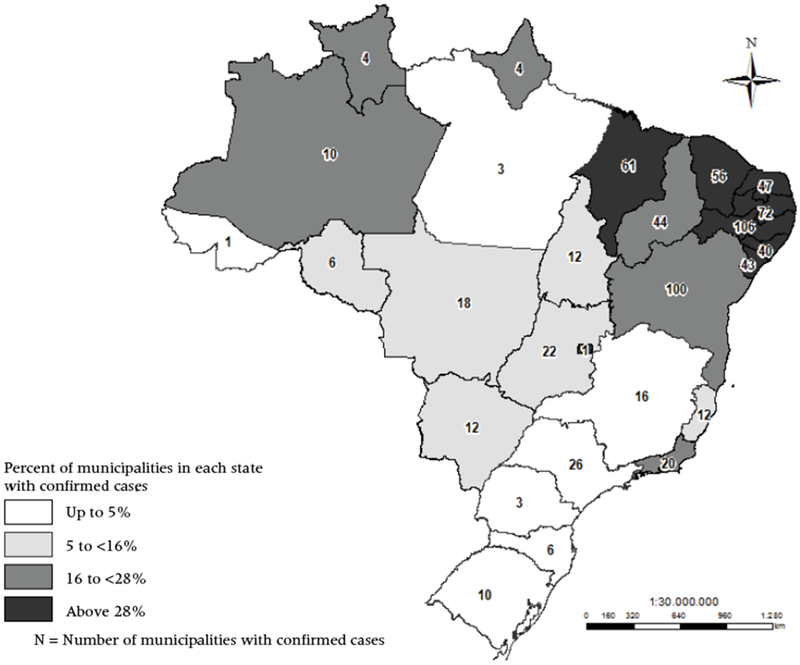
Relative and absolute number of municipalities with confirmed cases of ZIKV by state in the period between epidemiological weeks 45/2015 and 52/2016: Brazil, 2015–2016
SOURCE: Brasil 2017b.
Risk perceptions and reproductive intentions
The presence of Zika and the threats it poses to developing fetuses may lead some women to want to prevent or delay9 pregnancy and childbearing. In fact, early in the epidemic, some officials in the Brazilian Ministry of Health encouraged women to postpone pregnancy (Romero 2015) because doing so was the only legal way to avoid Zika-related birth defects until more was understood about the virus and the prevention of intrauterine transmission.
There are several reasons why the epidemic may lead some women to want to avoid a pregnancy. First is the possibility of becoming infected during pregnancy and transmitting the virus to the developing fetus. The health belief model (Rosenstock 1974) suggests that the motivation to adapt one’s behaviors to mitigate health-related risks is often contingent on simultaneously believing that one is at risk of infection, that infection poses grave health consequences, that specific actions will reduce the risk of infection, and that the benefits of preventing infection outweigh the costs (Rosenstock 1974; Rosenstock, Strecher, and Becker 1994). Applied to the ZIKV epidemic, this model would suggest that in order for women to want to postpone pregnancy they must believe they are at risk of ZIKV infection, understand the potential in utero consequences of infection, believe that abstinence and/or contraception will successfully prevent pregnancy, and believe that not becoming pregnant is worth any monetary, physical, or psychological costs incurred from abstinence and/or contraception. This last belief may be less common among women who desired more children before the epidemic began, especially if these women are toward the end of their reproductive years. For these women, the potential cost of delaying pregnancy may mean an inability to conceive at a later date.
A second and related reason is that the epidemic may be affecting social norms and values. Both the theory of planned behavior (Ajzen 1991) and the theory of reasoned action (Fishbein and Ajzen 2011) suggest that individuals’ intentions are related to their attitudes, beliefs, and perceived norms, which are derived from their social surroundings. Thus, if public officials’ discouragement of pregnancy during the early stages of the epidemic has increased stigma against pregnant women and especially against infected pregnant women, then this stigma may heighten women’s desire to avoid pregnancy. Likewise, increased attention to congenital Zika syndrome, particularly microcephaly, both among the media and among women’s peers, may also affect women’s perceptions of risk and attitudes toward becoming pregnant during the epidemic, which may in turn affect their pregnancy intentions.
Assuming that at least some women want to postpone pregnancy, the theory of planned behavior and the theory of reasoned action also suggest that this desire should result in deliberate actions to prevent becoming pregnant, such as using contraception more frequently or switching to a more effective form of contraception (Moreau et al. 2013; Reinecke, Schmidt, and Ajzen 1996; Sutton, McVey, and Glanz 1999). Similarly, the cognitive social model of fertility intentions (Bachrach and Morgan 2013) suggests that the desire to avoid pregnancy may lead to both deliberate and automatic reactions that increase efforts to prevent pregnancy.
Preliminary evidence indicates a slight decline in live births, starting in the second half of 2016, underscoring the possibility that at least some Brazilian women are changing their fertility behavior during the period of the ZIKV epidemic.10 Figure 2 shows the absolute numbers of vaginal and cesarean-section deliveries occurring in hospitals. These numbers declined in December 2016 after remaining stable for the previous two years.11 Correspondingly, Figure 3 shows the total number of live births in Pernambuco12 (a state with high ZIKV incidence). In this state, the number of live births declined by 10 percent between 2015 and 2016, starting in August 2016, a little more than a year after the epidemic began.
FIGURE 2.
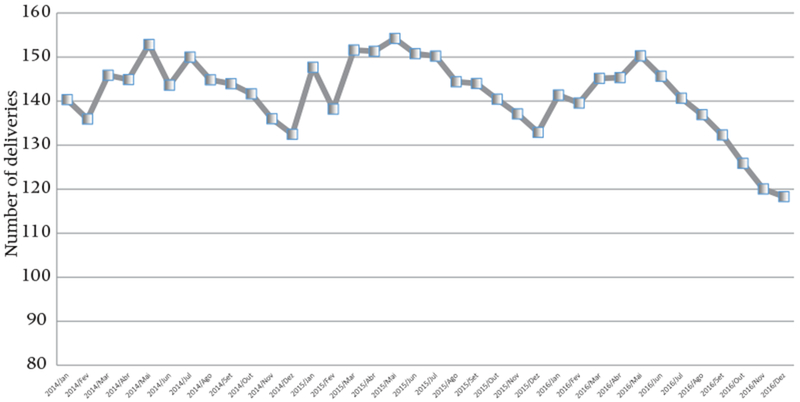
Absolute number of hospitalizations due to deliveries (all types) by month and year, Brazil 2014–2016
SOURCE: Ministério da Saúde 2017. Data available for tabulation at www.datasus.gov.br.
FIGURE 3.
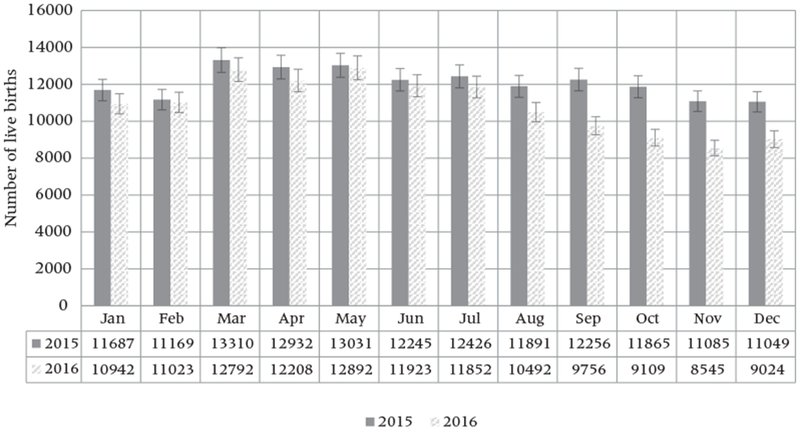
Number of live births by month and year, 2015–2016: Pernambuco, Brazil
SOURCE: Ministério da Saúde 2017. Data available through the Microcephaly Epidemic Research Group.
Distal determinants of reproductive responses to the ZIKV epidemic
To prevent or delay pregnancy, women must either limit their sexual activity or use effective forms of contraception consistently (Bongaarts 1978). However, women’s consistent use of contraception often varies with their social context. For instance, socioeconomic status shapes women’s ability to obtain and afford reproductive services (Campbell, Sahin-Hodoglugil, and Potts 2006; Dennis and Grossman 2012) and also influences which individuals women interact with on a regular basis, which over time can affect their attitudes toward contraception (Bachrach and Morgan 2013).
Socioeconomic status may also shape women’s use of contraception by determining the type of reproductive health services to which they have access (Potter et al. 2003). Recent research in Recife, for example, suggests that nurses intentionally omit information about emergency contraception to women in order to prevent the method from gaining popularity (Spinelli et al. 2014). If women believe that the quality of care at public clinics is low or that clinics are crowded (Barnes-Josiah, Myntti, and Augustin 1998), they may avoid using public health services even when these are the only services they can afford. Likewise, women may fear a lack of privacy at public rather than private clinics (Hatzenbuehler, Phelan, and Link 2013). This lack of privacy may contribute to a fear of stigmatization when women are seeking services for unwanted or unplanned pregnancies or for STI (sexually transmitted infections) treatment.
Analytic strategy
Data
We conducted eight focus groups in Belo Horizonte, Minas Gerais (Southeast), and eight focus groups in Recife, Pernambuco (Northeast) approximately 18 months after the epidemic began in Brazil. Each focus group consisted of six to eight women between the ages of 18 and 49 years (N=114). This large age range was intended to capture women both toward the beginning and the end of their reproductive years. We stratified focus groups by socioeconomic status,13 conducting half with women of low status and half with women of high status, which we proxied with the neighborhood of recruitment (described below). We hypothesized that the manner in which the ZIKV epidemic affects the reproductive intentions of women differs by socioeconomic status. Stratifying focus groups by socioeconomic status was also intended to allow participants to identify more readily with one another’s experiences and therefore to express themselves more freely (Knodel 1993). Participants included both pregnant and non-pregnant women, with the majority not pregnant at the time of participation.
Belo Horizonte and Recife represent distinct demographic, economic, and epidemiological settings in Brazil, yet both are urban state capitals with great influence on their regional economies. Recife, the capital of Pernambuco, is one of the locations where the first cases of ZIKV and microcephaly were diagnosed in Brazil. In 2016, microcephaly cases in Pernambuco constituted 21.9 percent of all cases nationally (Brasil 2016). In contrast, microcephaly cases in Minas Gerais represented only 1.9 percent of cases nationally in the same year (ibid.). The early onset of the ZIKV epidemic and the high microcephaly rate in Pernambuco suggest differences in both the likelihood of infection and the likelihood of contact with infected individuals according to geographic location. While not a perfect proxy, geographic location suggests variation in the social environment related to Aedes Aegypti mosquitoes, ZIKV, microcephaly, and congenital Zika syndrome.
These disparities in the onset of the ZIKV epidemic also reflect broader disparities between the two states. The Municipal Human Development Index (IDHM) for 2010, for example, was 0.772 in Recife and 0.810 in Belo Horizonte (Brasil 2017d). The adolescent childbearing rate in Recife (11.1 percent) is almost twice that of Belo Horizonte (6.5 percent). A larger proportion of households lack infrastructure in Recife, with increased exposure to mosquitoes; in 2010, 11 percent of Recife households had open sewage, compared to only 1.3 percent in Belo Horizonte households (Brasil 2012). In contrast to Belo Horizonte, Recife has an ideal environment for the reproduction of the Aedes Aegypti, owing to its hot temperatures and high humidity (at least 70 percent) throughout the year. Recife also has a chronic problem of water supply and distribution, which is worsened in poor neighborhoods, where people have difficulty saving water inside their houses to drink, cook, clean, and use for personal hygiene.
In each city, we recruited women from two adjacent neighborhoods selected on the basis of four criteria. First, we ranked all neighborhoods and regions in each municipality by their LIRAa index, that is, the Aedes Aegypti larval infestation index (LIRAa—Levantamento de Índice Rápido do Aedes Aegypti). The LIRAa index maps localities according to Aedes Aegypti infestation. Not surprisingly, Recife has overall higher LIRAa indices than Belo Horizonte (LIRAa 2015, 2016). Of the eight focus groups we conducted in each municipality, four were conducted in areas with low to medium relative levels of Aedes Aegypti larvae infestation and four in areas with medium to high relative levels of larvae infestation (ibid.). The second criterion was differing proximities to bodies of water. In Belo Horizonte we selected only areas with close proximity to bodies of water so that these areas are comparable to Recife, which is a city characterized by mangroves. The third selection criterion was that all areas we selected presented contrasting physical infrastructures, that is, high-rise apartments and single-story homes. The fourth criterion was the presence of segregated but adjacent high and low socioeconomic status residences. Within each area, two focus groups were conducted in neighborhoods with high socioeconomic status and two others in neighborhoods with low socioeconomic status. The fourth criterion should result in the participation of women whose ability to prevent unintended pregnancies differs substantially.
To generate an overall sample that was diverse in terms of race/ethnicity, age, and socioeconomic status, we recruited participants from a central location—outside of grocery and convenience stores—by passing out flyers containing basic information about the study. Participants were remunerated US$15 (R$50) for their participation—a sum large enough to pay for their transportation costs and one meal on the day of their participation. Focus groups were conducted in rented, safe spaces within the neighborhoods from which women were recruited. All focus groups were led by the same interviewer and were audio recorded with participants’ oral consent. Immediately following each focus group, the interviewer recorded field notes that conveyed her perceptions of the group’s dynamic, body language, and anything else that could not be audio-recorded. Once all focus groups were completed, the audio-recordings (including field notes) were transcribed.
Methods
The same interview protocol was followed for all focus groups, consisting of open-ended questions about participants’ pregnancy intentions and birth histories; perceptions of ZIKV symptoms; ZIKV transmission; where they receive information about ZIKV; demand for and access to contraception; and what kind of advice they would offer to women who became pregnant during the epidemic. Questions about the effects of ZIKV on women’s reproductive plans and behaviors, including abortion, were asked in the latter half of the interview to minimize the possibility of biasing responses.
Focus group transcripts were coded and analyzed by two researchers. Coding of the data and thematic analysis were performed manually; software was used to organize overarching themes, highlight codes and excerpts, and quantify codes and their combinations. A literature review on risk perception and reproductive health informed the initial codebook, which included such codes as ”sources of information about ZIKV,” “strategies of protection against infection,” “use and type of contraceptives,” and “ZIKV affecting pregnancy by postponement.” Development of the codebook was an iterative process; as new themes emerged (e.g., belief in the severity of ZIKV), a corresponding code was created and added to the codebook and then applied to all previous respondents, an approach widely used in qualitative research (Weiss 1994; Coffey and Atkinson 1996). Many of the codes were accompanied by sub-codes that represented distinct responses within a given code category. In the case of sources of information, for example, sub-codes included “TV,” “Townhall,” “Flyer/folder (cartilha),” “Health agents who visit their homes,” “Children’s school,” “Hospital/Posto (waiting room banner),” “On the bus,” “Don’t trust word of mouth,” “Internet,” “Radio,” “Newspaper,” “Scientific literature,” “Magazine,” and “Had a class about the topic at school.” Finally, the program combined like-coded passages into an “overview grid” (Knodel 1993), allowing us to relate different data points to one another across comparative contexts. A key strength of this approach is that it highlighted systematic points of convergence and divergence among women from different socioeconomic and geographic backgrounds.
Results
Reproductive intentions during the ZIKV epidemic
We begin by examining whether the ZIKV epidemic has affected the reproductive intentions of Brazilian women. In all groups, regardless of socioeconomic status (SES) or geographic location, women expressed fear of contracting ZIKV. When asked whether they or their friends who want to have (more) children intend to postpone pregnancy because of ZIKV, the majority of both low- and high-SES women mentioned their intentions to postpone pregnancy, regardless of whether they lived in cities with low (Belo Horizonte) or high (Recife) ZIKV prevalence. Women who reported not wanting more children mentioned they would postpone pregnancy if hypothetically they (still) wanted (more) children:
Respondent: If I wanted to have children now, I certainly wouldn’t do it. Exactly because I don’t want to risk being bitten by a mosquito, right?
Moderator: But has it crossed your mind to have children at this moment and you postponed because of Zika?
Respondent: Yes. That was in the beginning of the year. I said “now, no way.” Running the risk, no. (BH, High SES)
Respondent: So, I thought about getting pregnant and now I don’t think as much, so I will wait until everything is solved. This is a tragedy and could happen with anyone and I wouldn’t like it to happen to me and to my family, so this is something that changed in my mind. I avoid getting pregnant now much more than before. (BH, Low SES)
Respondent: I think about fear and about not wanting to get pregnant now. If I had a plan of having a child in one year, it wouldn’t be one year anymore, it would be at least 4 years just because of Zika. (Recife, High SES)
Moderator: Do you know anyone who does not want to have children because of Zika?
Respondent: I do, my niece. Because she is afraid, right?
Moderator: And how long will she wait to get pregnant?
Respondent: She said three more years because she wants the outbreak to pass. (Recife, Low SES)
When discussing how long women intended to postpone pregnancy because of ZIKV, responses varied from specific periods, like 2 or 3 years, to more abstract answers, such as: “when they find a cure,” “when they create a vaccine,” or “until doctors learn more about the epidemic and the mechanisms by which it affects the baby.” Women also reported their own and their friends’ intentions to postpone pregnancy until the winter months, reasoning that the lower mosquito proliferation during the winter made it temporarily safer for them to become pregnant. Seasonality might have also affected women’s pregnancy intentions, because once the number of ZIKV cases declined during the winter and the media stopped constantly providing the public with ZIKV information, women reported feeling safe about pregnancy again.
Intentions regarding pregnancy postponement differed by women’s socioeconomic status, reflecting that high-SES women in Brazil have a first pregnancy later than low-SES women and therefore have a shorter amount of time in which to achieve their desired family size. The median age at first pregnancy14 in Northeast Brazil is 19.9 for women with less than high school education and 26.7 for women with at least a high school diploma. In Southeast Brazil the corresponding median ages at first pregnancy are 20.8 and 27.6. In our focus groups, we found that age was a key factor that explained a short period of postponement or no postponement during the ZIKV epidemic for several high-SES women, with high-SES women discussing at length how the “age factor” was a determinant for their own or their friends’ decision whether or not to postpone pregnancy. Notably, these discussions about age were not as frequent among low-SES women, who tended to have children at a much younger age:
Respondent: My sister is 36 and got pregnant at the end of last year. She couldn’t wait longer. She was already trying for a long time. (BH, High SES)
Respondent: I have a niece who is trying to get pregnant. And with the age factor, she is crazy to become a mother. (BH, High SES)
Moderator: Why do you think [she got pregnant with the risk of getting Zika]?
Respondent: Because of her age. She said “I am 35 and I want to have my daughter. With or without a Zika outbreak.” (Recife, High SES)
While all women reported a fear of contracting ZIKV, concerns were stronger among women trying to get pregnant or desiring (more) children than among women who had achieved their desired family size and had no intention to become pregnant. Because most low-SES women have children early in Brazil, concerns over ZIKV were lower among low-SES groups. Specifically, low-SES women stated that they would be more concerned about ZIKV if they were “trying to get pregnant” or if they “wanted more children.” At the same time, in discussions about their own and their friends’ birth histories, low-SES women reported that most of their (and their friends’) pregnancies, particularly the first ones, occurred at a young age and were unplanned:
Respondent: I’m going to tell you something. I was very sad. I took my pre-natal classes with 15 women, and I was the only one who wanted to get pregnant. All of them didn’t want to get pregnant. It’s very sad. (BH, Low SES)
Respondent: I was afraid to death to get pregnant. My first [pregnancy] was unexpected, right? We weren’t ready to become parents.… We had no financial conditions for that, but when I saw it, it was done. (Recife, Low SES)
Contrary to this sense of uncontrolled reproductive intentions found in the discourse of low-SES women, high-SES women appear to have a sense of control over their reproductive intentions. Young high-SES women reported significant control over their reproductive intentions, describing precise life plans where college, graduate school, and a career came before childbearing. This sense of control was reflected in how high-SES women approached their fertility during the ZIKV epidemic. Despite reporting fear of being infected, high-SES women also reported feeling equipped to prevent or time a birth as a result of their superior access to both contraception and safe abortion. High-SES women often mentioned their control over pregnancy intentions and ZIKV, stating that by living in “better areas of the city” or in “tall buildings” common to the wealthy neighborhoods of both Belo Horizonte and Recife, they were at lower risk of infection. These women also reported that their health care providers suggested that if they took precautions such as using insect repellant15 and avoiding areas of the city with high mosquito infestation, open sewage, poor sanitary conditions, and large bodies of water, then their babies would likely be fine:
Respondent: Even when Zika started, she continued to try to get pregnant, you know, but she took really good care of herself, and we helped, we gave her information: use repellant, use pants, don’t go to high-incidence areas…and she did it. She took really good care and all went well. The baby was born last month. (BH, High SES)
Respondent: I would ask God, use repellant and that’s it…. (BH, High SES)
Moderator: What strategies do you think rich women can use that poor women cannot?
Respondent: Living in tall buildings. The mosquito doesn’t go to the 14th floor at all. (BH, High SES)
Respondent: I asked my doctor [whether I should wait to get pregnant] and he said, “look, in terms of Brazil, the Aedes Aegypti will never end, so if you want to have a baby you have to try now…” (BH, High SES)
An additional aspect of women’s reproductive intentions present in all focus groups was whether women would seek an abortion if they contracted ZIKV. Participants discussed whether they believed that the proportion of women intending to have an abortion would increase as a consequence of the epidemic. In all focus groups, regardless of SES or geographic location, women felt that the threat of ZIKV would result in an increase in women’s intentions to have an abortion if infected with ZIKV. Women often mentioned knowing friends who had an abortion when they contracted ZIKV, citing the difficulties of raising a child with microcephaly to justify their intentions:
Moderator: Do you think Zika will increase this search [for abortion]?
Respondent: I think it has already. (Recife, High SES)
Respondent: When she heard she was pregnant, and that she had Zika, she had it [an abortion]. If you see this TV show, and you know what Zika is…and I know a whole bunch of women who had dengue, Zika, and they had children with microcephaly. Then, you fear it. (Recife, Low SES)
Moderator: Do you think that because you could be pregnant and get Zika, an abortion would become an option?
Respondent 3: For me, yes, I don’t know for the others.
Respondent 1: Yes, because this girl is from the favela and they want to have kids, but they won’t take the consequences [of microcephaly]. (BH, Low SES)
Respondent 1: I even think that abortion should be legal in case of microcephaly.
Respondent 2: I totally agree.
Respondent 3: Me too.
Respondent 1: People should have the right to choose [an abortion] because if the government is unable to prevent Zika, then it needs to deal with the consequences.
Respondent 2: That’s what I think. (BH, High SES)
To summarize, most women in our focus groups, regardless of socioeconomic status or geographic location, either did not want any more children regardless of the epidemic or, if they did want more children, intended to postpone pregnancy during the epidemic. Relatedly, many women suggested that they would consider abortion if they were pregnant and contracted ZIKV (in either order). There were nonetheless two key differences related to socioeconomic status. First, most high-SES women considered age when determining whether or for how long they intended to postpone pregnancy, whereas virtually all low-SES women reported not desiring more children independently of the epidemic. Second, high-SES women expressed a sense of control over their reproductive intentions, while low-SES women did not. Low-SES women’s lack of perceived control is confirmed by their higher rate of unwanted childbearing, suggesting that women with low SES may indeed have been more vulnerable to unwanted pregnancies and/or unwanted infected pregnancies during the first 18 months of the epidemic.
Reproductive behaviors during the Zika epidemic
We now report on how women described their reproductive behaviors in relation to the ZIKV epidemic. The majority of women in our low-SES focus groups who believed they were at risk of getting pregnant reported that they were reinforcing their contraceptive behaviors because of the ZIKV epidemic. These actions included more concerted efforts to use contraception consistently and simply going from no method to starting a method. Reports of contraceptive change were especially common in the Recife focus groups, where exposure to the risk of contracting ZIKV is higher than in Belo Horizonte:
Respondent: I didn’t want to get [pregnant] and after this outbreak, I am always preventing. I do not delay my injection, I do not delay anything. (Recife, Low SES)
Respondent: People who had Zika are afraid of getting pregnant.… If one gets pregnant, you know, there is the risk of being born with a birth defect. Do you think I will risk it? No!
Moderator: Are you taking care?
Respondent: Of course I am now. From every side. (Recife, Low SES)
Most high-SES women had previously experienced nearly perfect contraceptive use (few or no unintended pregnancies), which gave them a sense of control over their fertility. Accordingly, these women reported continuing their use of contraceptives to avoid unwanted pregnancy:
Respondent: Yes, I am using oral contraceptives and I had Zika. In none of those moments I wanted to be a mother, so I didn’t even bother to worry about having a child with microcephaly. I have been taking contraceptives for years, doing everything right, so I wasn’t even concerned with the possibility. (Recife, High SES)
Moderator: Do you know anyone who is trying to prevent pregnancy right now?
Respondent: Me. I am on the pill, but it has nothing to do with Zika. (Belo Horizonte, High SES)
Among the few women who had been actively planning to become pregnant before the ZIKV epidemic, some reported switching their method (looking for more efficient alternatives), ensuring consistent use of the method they were using, or simply going from no method to starting a method in order to postpone childbearing and avoid an infected pregnancy:
Respondent: Yes, I was going to have [the IUD] removed in the beginning of the year. Then, it started to rain and it rained all month in February, when there were these major campaigns against Zika, and there was a case in Rio de Janeiro, and everything, and then I talked to her [the doctor] and she said: “let’s wait because we don’t know how it is going to be.” (BH, High SES)
Moderator: Do you think women are more cautious now?
Respondent: Yes.
Moderator: Every woman?
Respondent: I think everybody who left the health clinic with a diagnosis of Zika, left the clinic thinking about having this kind of care [enforcing contraceptives], of not getting pregnant right now because if you have Zika, you can transmit it to your baby (Recife, High SES)
While both low- and high-SES women discussed adjustments in contraceptive use, women from the two groups indicated different barriers to these adjustments in both Belo Horizonte and Recife. First, low-SES women reported less effective and fewer choices of contraception than did high-SES women. For instance, low-SES women frequently reported using the pill, injections, or condoms—all of which are available at public clinics—while high-SES women reported going to private clinics and being able to afford a larger variety of contraceptive methods, including IUDs and vaginal rings. Moreover, low-SES women frequently offered lengthy accounts of inconsistent use, failure, or absence of methods despite their fear of contracting ZIKV and their desire not to have (more) children.
Respondent: I wanted my second daughter, but not at that moment, but then I got pregnant unexpectedly. (BH, Low SES)
Respondent: I continue to forget about the pill, but when I forget, I get worried [about a pregnancy during the Zika epidemic] and then I take two [at once]. (Recife, Low SES)
When discussing contraceptive failure, low-SES women often mentioned that sterilization was the only way in which they were certain they could avoid pregnancy, even though some also reported dealing with the failure of sterilization surgery.16 This is particularly important because, in times of ZIKV, unmet need for contraception could expose low-SES women to ZIKV and, in turn, increase the risk of microcephaly:
Respondent: We don’t think about having [more children], but we are not ligada [sterilized], so we are risking.
Moderator: You think you are risking [a pregnancy] because you didn’t do the tubal ligation?
Respondent: Because I didn’t do the tubal ligation. So, I just intend not to get pregnant [respondent’s emphasis on intend]. (Recife, Low SES)
Respondent: I have two, one is seventeen and the other is five.
Moderator: And another one in your belly?
Respondent: And I was sterilized.
Moderator: Oh, you were sterilized?
Respondent: It was horrible. I didn’t want [the pregnancy]. (Recife, Low SES)
A second barrier to more effective contraceptive use that low-SES women commonly reported was low bargaining power with their partners. Low-SES women frequently mentioned that they were the to prevent or delaypregnancy and childbearing. When asked whether ZIKV had affected their partner’s reproductive behavior, women reported that their partners were not concerned about getting infected with ZIKV. Some even suggested that their partner’s apathy stemmed from the fact that ZIKV had largely been portrayed by the Brazilian Ministry of Health as an illness that is transmitted by mosquitoes and affects pregnancy, with little attention to the sexual transmission of ZIKV or to its potential consequences for men’s health:
Moderator: Do you think men are worried about Zika, or about anything else?
Respondent: Men don’t worry! Men only want sex. And the silly [girls] are giving in. (BH, Low SES)
Moderator: Do you think Zika changed the behavior of the men you know?
All respondents together: No!
Moderator: Have you heard men saying they were worried about Zika?
All respondents together: No!
Respondent 1: I think they don’t even know [about it]. (BH, Low SES)
Respondent 1: I talked to my partner [about Zika being sexually transmitted]. [I told him] “I have Zika. You will get it.”
Moderator: What happened?
Respondent 1: He got it.
Other respondents: laughter
Moderator: Do you think he got Zika through sex?
Respondent 1: Yes, he got it through sex.
Moderator: Even with Zika he refused to use a condom?
Respondent: He doesn’t use it. My husband says… “that plastic…that plastic.” (Recife, Low SES)
Respondent 3: I didn’t see anything about that; that fathers and husbands who intend to have children should be careful with Zika not to transmit it to their partners through sex. At no point was there talk about that.
Respondent 2: The image I remember, the posters I saw show two images: the mosquito and the woman. You don’t see males associated with that. It’s the mosquito or the woman. (Recife, High SES)
Respondent: I think that it is a matter of information, because [men] hear at length that it [Zika] causes microcephaly, then men create a barrier in their minds that they don’t need to protect themselves because it’s all about the baby. (BH, Low SES)
While high-SES women told similar stories regarding their partner’s reluctance to use contraception during the ZIKV epidemic, they more frequently reported demanding protection and were able to successfully negotiate for it, particularly because their partners also feared unintended pregnancies, which the partners of low-SES women did not:
Moderator: Are your boyfriends worried about Zika?
Respondent: [Says no with the head]. Men are very absentminded for that…women are more worried.
Moderator: But do they tell you, let’s not get pregnant now because of Zika?
Respondent: Not because of Zika, but because of pregnancy. (BH, High SES)
Moderator: Do you think they [their partners] are worried [about the epidemics]?
All of them: No. Not even a bit.
Respondent: I think they might worry if the wife is pregnant. So, they are worried about their wives and their babies. But the man who doesn’t have [a wife]…no. (Recife, High SES)
Respondent: If you aren’t firm and don’t say that it will have to be with a condom, then it’ll be without a condom.
Moderator: And can your friends be firm about it?
All respondents: Yes, of course. (Recife, High-SES)
Another factor contributing to differences in low- and high-SES women’s contraceptive changes during the ZIKV epidemic was access to contraception. Low-SES women reported feeling stigmatized when they went to public clinics for contraceptives, oftentimes because of a violation of privacy by staff in health clinics.17 For example, low-SES women in both cities described instances in which the staff joked and gossiped about other women in front of them, or revealed pregnancy and test results out loud in the waiting room. This fear of stigma and violation of privacy was exacerbated by the fact that clinic staff commonly lived in the same community as their patients:
Respondent: We go to the public clinic and everybody will know about your life. Why didn’t I go there when I lost the baby? If I went there, then the whole place would know “oh, that little girl is already a woman [lost her virginity].” (Recife, Low SES)
Respondent: Full of gossipy women. Like for your pregnancy test. You are sitting there. Instead of calling you inside the room, they call you out loud and say: here it is, go there and pee here [showing the recipient]. Then, they give you a huge paper and say out loud: go to the reception and schedule your prenatal care. (BH, Low SES)
Respondent: I was there [public clinic] for an appointment and I was listening to this talk in a room, they were telling women not to get pregnant and to be careful, to use repellant, but I wasn’t participating, it was in a room and I was outside and I could still hear everything. (Recife, Low SES)
In addition to accounts of stigma and violations of privacy, a few low-SES women reported not knowing that they could obtain certain types of contraception in public clinics, despite the fact that contraceptive methods are available free of charge in these clinics. With a required prescription, contraceptives can also be purchased at a subsidized price in pharmacies participating in the governmental program Farmácia Popular (Brasil 2015). However, going to a clinic each month for contraception is time consuming. Another barrier is that in order to pay the subsidized price, women need to present a valid prescription and a picture ID, requirements that are not necessary when purchasing at full price. Women also reported that the public clinics were crowded during the summer months, when ZIKV reached its peak in Brazil.
Respondent 1: If you ask a gynecologist at the public clinic [for contraception], you get it, I think. I did research, you have to choose among 5 to 8 methods of prevention. There are Mirena, IUD, pill, condoms, injectables, they have them all.
Respondent 2: And through the family planning program you can get your sterilization in the clinic.
Moderator: And why do you think people don’t make use of it?
Respondent 3: I think that for some of these methods, especially IUD and Mirena, it’s a lack of knowledge that the clinics can offer all of that. (BH, Low SES)
Respondent: The epidemics here started in December or January, right? She got pregnant during carnival [February]. The epidemic was really strong during December and January, when I had Zika, when you would go to a hospital and everything was crowded. I got there at 7 in the morning and left at 8 at night. There were no UPA [Public Urgent Care Unit] that could assist you because everywhere was crowded.
All respondents: Crowded, crowded. (Recife, Low SES)
High-SES women, on the other hand, visited private clinics or bought their contraceptives at pharmacies without a prescription. The combination of stigma, crowded clinics, the requirement to go to the clinic for a prescription, lack of knowledge, and lower bargaining power suggests that, at least for some low-SES women, access to contraception has been a barrier to making adjustments to reproductive behavior during the ZIKV epidemic.
While our focus groups suggest that SES has influenced how Brazilian women altered their contraceptive behavior during the first 18 months of the ZIKV epidemic, they also suggest that geographic location, a proxy for ZIKV social environment, has been influential. Women in Recife often reported, for example, that the risk of ZIKV became much more concrete for them when they saw mothers carrying babies with microcephaly on the city bus:
Respondent 1: I would give the kid to the government [if he had microcephaly]. That is why I prevent [pregnancy]. Because if I had a sick child, I wouldn’t even look [at the baby].
Respondent 8: That is why I do everything I can to prevent [transmission], because I am sure I wouldn’t give my child away. It is your fruit. You made him. The only difference is that he was born sick. (Recife, Low SES)
Respondent 1: I have a friend who had a child with microcephaly.
Moderator: And then what?
Respondent 1: I know a father who has [such a child].
Respondent 2: When you see it, you think, “now I really do not want to get pregnant at all,” got it? (Recife, High SES)
Moderator: Do you think about having more [children]?
Respondent: I do. But I am seriously considering [not to]. Because I see my friend’s child [with microcephaly], and he is almost the size of my baby and I am almost giving up. And staying with only one. I cried so much before having my child. The father would say “don’t cry now because the baby was not born with the little head yet. Save your cries for when the baby is born, because you will have the rest of your life to cry.” It was on TV all the time. Thank God the baby wasn’t born [with microcephaly]. (Recife, Low SES)
Women in Recife clearly recognized that they had a high risk of exposure to ZIKV and were extremely vulnerable to contracting the virus. This was in part because women in Recife tended to know of babies with microcephaly, which they claimed motivated them to prevent pregnancy. Thus, the experiences of women in Recife with knowing people infected with ZIKV and their babies who had been infected in utero compelled them more than their Belo Horizonte counterparts to become proactive about their use of contraception. Low-SES women in Belo Horizonte offered more fatalistic views about becoming pregnant during the ZIKV epidemic. Their high-SES counterparts in Belo Horizonte, on the other hand, felt overwhelmed with information about ZIKV. Thus, even though both low- and high-SES women faced lower ZIKV prevalence in Belo Horizonte than in Recife, high-SES women in Belo Horizonte acted as if their risk was very high, reporting high levels of concern. Finally, regardless of socioeconomic status or geographic location, women reported that they themselves or other women they knew resorted to abortion because of ZIKV:
Respondent: My doctor told me: I don’t do it. But my “insemination” doctor told me that he understands that the person has the right to have a healthy baby, so if the person wants one, he can refer them… (BH, High SES)
Respondent 1: Me, as a mother, I do everything I can to eliminate the concentration [of mosquitoes] in my house. But if I got pregnant, I wouldn’t think twice before doing an abortion.
Respondent 6: My friend got pregnant right at the top of the Zika epidemic. She said she was going to do the ultrasound and if it was positive, she would take the baby out. I said: oh, don’t do that! And she said: Do you know what it feels like to have a child like that for the rest of your life? Then, I gained courage [to admit I’m pro-abortion]. (BH, Low SES)
Moderator: Your friend got an abortion? But did she have Zika?
Respondent 1: When she heard she was pregnant, and it was a time of Zika, she took the baby out.
Respondent 5: If you watch these programs every day and you know what Zika is… I know a lot of women who had Dengue, Zika, and had a child with microcephaly. Then, you fear it. She was already suffering with so many problems, the love, the concern is bigger so she did it [an abortion]. (Recife, Low SES )
Among low-SES women, references to abortion included knowing of friends or family members who bought abortion pills on the black market or used herbs and teas believed to induce abortion. Low-SES women also mentioned that they or women they knew often had to suffer the consequences of an unsafe abortion such as hemorrhage, secondary infertility, and even death. High-SES women reported that they and their friends could rely on their private doctors to perform surgical abortions or to refer them to other trusted private doctors who would perform safe surgical abortions:
Respondent: I know a couple, a woman who got pregnant, and she said: I simply don’t see myself taking care of a child and I won’t be able to give him what I wish. One day I want to get pregnant, but not now. Then, this person looked for a doctor, explained to her and the doctor was super…
Moderator: Her own doctor?
Respondent: Yes, she was super open minded. She accepted it, did the right procedure, she got the abortion and didn’t have any problems. (Recife, High SES)
Respondent: They [friends] were doing it [abortion], I learned from them.
Moderator: You say you don’t know where to go or who to look for…
Respondent: I needed the Pau Brasil [Brazilian native tree], you know, I got the Pau Brasil around here, cut down the bark, made the tea and smoke….
Moderator: So you did it in your house, relaxed, no sequels?
Respondent: No, I went to the hospital.
Moderator: You felt bad?
Respondent: I went into cardiac arrest, stayed in the ER for 15 days. After that, I had another [pregnancy].
Moderator: Wait, after that you got pregnant again and [attempted another abortion]?
Respondent: Again, I did it again.
Moderator: The same way, with the Pau Brasil…
Respondent: Same way. (Recife, Low SES)
Moderator: So you think that if a woman wanted to end her pregnancy, she could?
Respondent 2: Yes. But she needs money. It costs R$500. Like this girl who took the medicine at night and had to go to the hospital. When she got there, the baby got out and stayed in her panties. The doctor said: “don’t do this anymore! I don’t report you because you have two children to raise.”
Respondent 3: But there are other more natural ways.
Respondent 4: There are teas.
Respondent 3: Many of my relatives took the tea. Pinga [local alcoholic drink] with that stink flower, arruda [common rue]. Or they stick in mamona [ricinus].
Respondent 2: Yeah, but then the baby might not come out and is born with trouble. (BH, Low SES)
Thus, women with high SES were able to wait longer before deciding whether to have a safe abortion, granting them more time to detect microcephaly or other types of congenital Zika syndrome. Low-SES women, on the other hand, had to decide either to abort in the first trimester, when the status of fetal development was often unclear, or to wait until the second trimester but risk having an unsafe surgical abortion if they decided to terminate the pregnancy after learning of fetal malformation.
Many women in our focus groups reported changes in their contraceptive use and/or knowing of women seeking abortion in response to ZIKV. Yet a large proportion of low-SES women reported barriers to effective contraception, such as low bargaining power with their partners, contraceptive failure, inconsistent use, and supply-side factors such as stigma and crowded clinics. High-SES women, meanwhile, reported having greater bargaining power, going to private clinics, and discreetly buying contraceptives. Comparing across regions, low-SES women in Recife reported feeling more motivated to make contraceptive changes than their Belo Horizonte counterparts, who had a more fatalistic view of ZIKV, thus suggesting that exposure to ZIKV and regionally targeted public health campaigns have activated behavioral changes.
Discussion and conclusions
This study has offered a rich description of women’s reproductive responses during the first 18 months of the ZIKV epidemic in Brazil, based on new focus group data collected from a socioeconomically diverse sample of women in two distinct regions of the country with different degrees of ZIKV and microcephaly incidence. Analysis of these data has brought to light the interplay of women’s fertility desires, behaviors, and healthcare access and use across differing geographic and social structural contexts.
Most women in our focus groups were aware of the intrauterine consequences of ZIKV infection, and many did not want to become pregnant during the epidemic, with the exception of older women who had not yet achieved their desired family size—most of whom were of high socioeconomic status. These women reported that they and their friends were trying to conceive in the dry winter months when mosquito prevalence is lowest. They also mentioned taking precautions such as using mosquito repellant and wearing long-sleeve shirts, and they rationalized a pregnancy during the ZIKV epidemic by living in tall buildings or in neighborhoods where the prevalence of Aedes Aegypti is low. By contrast, women of low socioeconomic status often reported not wanting any more children irrespective of the epidemic because they had already completed their desired fertility at a younger age. Nevertheless, low-SES women reported numerous obstacles to using contraception and expressed little confidence in their ability to prevent unwanted pregnancies during the epidemic or otherwise. Despite these obstacles and low perceived self-efficacy, these women still suggested that they were attempting to use contraception (primarily the pill and condoms) more consistently. Meanwhile, high-SES women reported greater confidence that they would successfully avoid pregnancy during the epidemic, sought more effective forms of contraception to do so, and more commonly negotiated with their partners to prevent the sexual transmission of ZIKV.
Although abortion is illegal under most circumstances in Brazil (Aiken et al. 2016; Grimes et al. 2006) and the Brazilian government has shown no concrete sign of improving legal access to abortion in light of the ZIKV epidemic (Sandy 2016), women across different socioeconomic strata reported a willingness to have an abortion if they became infected with the virus. However, the types of abortion to which women reported having access varied with SES. While women with lower status primarily reported obtaining homeopathic and medical abortions (teas and pills, respectively), women with higher status reported a combination of medical and surgical abortions, the latter of which they often accessed through referrals from their primary care doctors.
Taken together, these findings suggest that socioeconomic status and ZIKV prevalence have shaped women’s reproductive responses to the epidemic. More specifically, they indicate that inequalities in reproductive health care access are likely to determine which women face the greatest risk of unwanted pregnancy during the epidemic, especially in Recife where ZIKV incidence has been highest. Moreover, because women with lower socioeconomic status not only have higher rates of unintended pregnancy (Theme-Filha et al. 2016), but also higher rates of mosquito-borne illnesses (Almeida, Medronho, and Valencia 2009; Siqueira et al. 2004) and STIs (Szwarcwald et al. 2005), they may also perceive a greater risk of unwanted and ZIKV-infected pregnancies. This risk, compounded by the fact that infection can occur and have fetal consequences at any point during pregnancy (Brasil et al. 2016), and that low-SES women lack access to safe surgical abortions, further points to the possibility that poorer, lower status women may be more likely to carry infected pregnancies to term and thus to bear children with brain abnormalities and cognitive deficits.
Although reporting concrete concerns about a pregnancy during the ZIKV epidemic, low-SES women also reported barriers to effective contraception. Because high-SES women constitute a relatively small group with an older fertility schedule in Brazil, a potential effect on overall fertility driven by this group is likely to be difficult to discern. Nonetheless, our focus groups also shed light on women’s responses to the epidemic and suggest that the declining number of births in Brazil (Figure 2) and in Pernambuco (Figure 3), although slight and preliminary, may not be aberrations. For instance, women reported becoming warier of pregnancy toward the start of the rainy season (which varies in the Northeast and Southeast regions), which suggests the potential for a seasonal effect in fertility, especially among high-SES women when not under the pressure of the “age effect.”
As the epidemic continues to unfold and WHO’s global risk assessment remains high, policymakers must take concrete actions to address barriers to contraception among low-SES women, in order to prevent unwanted pregnancies that run the risk of becoming infected with ZIKV. These actions may include increasing education and guidance related to effective contraceptive use; increasing the availability of long-acting reversible forms of contraception, such as IUDs, in public clinics; and offering women vouchers to buy a wider variety of contraceptives in pharmacies and without the requirement to present a picture ID or a prescription. Improving the affordability of contraceptives in all pharmacies may be especially useful for women of low socioeconomic status, not only because it increases the number of locations where they can obtain contraception, some of which may be closer to their homes, but also because women with low socioeconomic status report fearing violation of privacy and stigmatization when they seek contraception (and the required prescription) at public health clinics. Related to this last point, the Ministry of Health could increase the extent to which women seek free contraception at public health clinics by implementing policies that punish confidentiality breaches, such as developing an accountability system for patients to report such breaches or monitoring clinics that violate patient privacy. ZIKV health campaigns should also include men, not only because the virus can be sexually transmitted but also because many women with low-SES reported difficulties negotiating sex and contraceptive use with their male partners.
While our findings identify patterns in women’s reproductive responses to the ZIKV epidemic in Brazil, there are limitations to this study. First, because our data come from a limited sample, we cannot generalize our findings to all Brazilian women of reproductive age. Further, we interviewed women in two cities only, and it is possible that women in other parts of the country, such as in rural areas, perceive the risk of ZIKV infection and its consequences differently from women in the urban centers we examined. Finally, we interviewed women only and therefore cannot offer direct insights into men’s reproductive responses to ZIKV. While some of these limitations are inherent to qualitative research (Maxwell 2012), qualitative analysis presents a unique opportunity to map the ground of a new health threat such as the ZIKV epidemic and to understand patterns, mechanisms, and interpretations. Here, qualitative data were used to indicate how women are navigating a new epidemic, providing new insights into an emerging social phenomenon.
From this study, one can deduce that while many women who feel vulnerable to ZIKV may want to avoid pregnancy, some women—especially those of low socioeconomic status—may have a more difficult time doing so than others. These differences in women’s ability to prevent unwanted pregnancy and/or births reflect longstanding disparities in reproductive health but present new public health implications amidst the ZIKV epidemic. When women are unable to prevent unwanted pregnancies, or are unable to safely terminate those pregnancies, they must additionally contend with the risk of an intrauterine infection that may lead to severe fetal abnormalities. Improving equality in women’s access to a range of reproductive health services is thus essential to reducing or eliminating the longer-term consequences of health inequalities in Brazil.
Supplementary Material
Acknowledgments
Funds for this research were provided by a seed grant from the Population Research Center and the Population Research Initiative at the University of Texas at Austin. The authors are grateful to Kathleen Gerson for generous feedback on the design of the focus group protocol; to Abigail Aiken, Jennifer Barber, Suzana Cavenaghi, Rob Crosnoe, Gilvan Guedes, Deb Umberson, and Ana Paula Verona for feedback on this study’s overall design; to Constância Ayres, André Monteiro Costa, and Márcia Castro for generous exchanges on the ZIKV epidemic; and to Breno Bittencourt, Gabriela Bonifácio, and Bruno Firmino for excellent research assistance.
Footnotes
Infective Zika viral particles have been found in breast milk, but there are no documented cases of ZIKV being transmitted via breast milk (Petersen et al. 2016).
However, only 1 to 13 percent of fetuses infected in the first trimester are estimated to develop microcephaly (Cauchemez et al. 2016; Johansson et al. 2016). Congenital Zika syndrome encompasses a variety of illnesses of which microcephaly is one form (Chang et al. 2016; França et al. 2016). A significant increase in babies with microcephaly has been identified in Brazil (Araújo et al. 2016; Brasil 2017a). The Brazilian Ministry of Health has consistently released figures on microcephaly prevalence and incidence (e.g., Brasil 2017a, Tables 8 and 9), and the majority of Brazil’s public health campaigns related to ZIKV consistently used the term microcephaly to describe the possible consequences of ZIKV for fetuses. Microcephaly is also the term Brazilian women in our focus groups used to describe the possible consequences of ZIKV for babies. For these reasons, we use the term microcephaly when discussing women’s discourses about the consequences of ZIKV.
Appendix Table 1 is available at the supporting information tab at wileyonlinelibrary.com/journal/pdr.
The monthly minimum wage in Brazil is currently 970 reais, approximately US$320.
Authors’ calculations using data from the 1996 Demographic and Health Survey (BEMFAM, 1997) and the 2006 Pesquisa Nacional de Demografia e Saúde (CEBRAP 2008). The DHS and the PNDS have similar methodologies and are widely comparable, unlike the 2013 PNS (Pesquisa Nacional de Saúde), which presents limited measures of sexual and reproductive health and excluded women aged 17 and younger in the sample. The 2006 PNDS is the most current nationally representative dataset on reproductive health available for Brazil (CEBRAP 2008).
For detailed information on Brazil’s SUS response to the ZIKV epidemic, see Brasil 2017c.
The home visit teams include physicians, nurses, and community health agents. As part of its monthly visit to households, the team evaluates household members’ health status and results of preventive tests, discusses health information, and evaluates individuals for risk factors. If necessary, the home visit team distributes medication and suggests further measures and interventions (Macinko and Harris 2015; Malagutti 2012). Interestingly, some argue that fertility declined as a consequence of the FHS program because of improved access to health services (Bhalotra, Rocha, and Soares 2016).
Brasil 2017a, Table 8, referring to the period between March and October 2016. We used the term microcephaly here to correspond to the report from Brazil’s Ministry of Health.
Because delaying pregnancy requires preventing it at least in the short term, we refer to both as pregnancy prevention.
At the same time, it is also important to note that the economic and political crisis in Brazil since 2014 may account for some of the decline (Vrachnis et al. 2014).
Data from Ministério da Saúde 2017. Although hospital deliveries are only a proxy for births, 98.4 percent of births in Brazil occur in hospitals (MS/SVS/DASIS/SINASC-Sistema de Informações sobre Nascidos Vivos). Until April 2017, national vital statistics data had not been publicly released for the years 2015–2016. The authors have access to vital statistics data for the state of Pernambuco as part of an agreement between the State Health Secretariat and the Microcephaly Epidemic Research Group (MERG).
Administrative data on live births in developing countries face data quality issues, including delay in registration and misreporting, though data from SINASC in the state of Pernambuco are considered accurate (Pereira et al. 2013). While corrections to vital statistics are still expected throughout the year and can certainly account for some of the difference in live births (Szwarcwald et al. 2014), it is unlikely that corrections will account for the entire decline shown in Figure 2. According to a recent IBGE report, while there were delays in registering almost 15 percent of births in Pernambuco in 2003, the figure declined to 3 percent in 2012 (IBGE 2015).
Demographers have long been interested in the connections between socioeconomic status and fertility. In the case of Brazil, researchers have shown that both adolescent mothers (Marteleto and Villanueva 2016) and their children (Marteleto and Dondero 2013) are educationally disadvantaged compared to women who give birth later (or remain childless) and their children. Research has also shown that Brazilian women with high levels of education tend to have fewer children than women with low levels (Cavenaghi and Berquó 2014).
Authors’ calculations using data from the Pesquisa Nacional de Saúde (PNS 2013), collected by the Brazilian Census Bureau (IBGE). Here we opted to use the 2013 PNS (instead of the PNDS 2006) because this dataset has information that allows for this calculation, though the questionnaire is limited in scope and, for example, has information on age at first pregnancy rather than age at first birth.
A recent study reports that ZIKV-negative women were more likely to have used insect repellent than ZIKV-positive women (Brasil et al. 2016, p. 2323).
In the decades prior to the expansion of women’s health services in Brazil, which gained momentum after the Cairo Conference in 1994, sterilization was the most widely available and used contraceptive method; in 1996, 40.1 percent of all women aged 15–49 were sterilized (Caetano 2014). Many researchers attribute the decline in Brazilian fertility to sterilization, which helped women restrict fertility at higher orders (Curtis and Diamond 1995; Potter 1999; Potter, Schmertmann, and Cavenaghi 2002; Amaral and Potter 2015). Researchers also found that 75 percent of women who were sterilized had the procedure before age 25 (Miranda-Ribeiro, Rios-Neto, and Carvalho 2013).
The violation of privacy in health clinics and hospitals in Brazil’s public health system has been documented (Diniz et al. 2012). Violation of privacy in public clinics is often linked to stigmatization and discrimination, particularly related to sexually transmitted infections (Malta et al. 2007) and adolescent pregnancy (Maranhão, Monteiro and Lago 2012). These situations limit access to care, becoming a barrier to the delivery of quality health.
Contributor Information
LETÍCIA J. MARTELETO, Department of Sociology and Population Research Center, University of Texas at Austin, Austin, TX 78712-1699
ABIGAIL WEITZMAN, University of Michigan, Population Studies Center, 426 Thompson Ave.
RAQUEL ZANATTA COUTINHO, Centro de Desenvolvimento e Planejamento Regional de Minas Gerais Belo Horizonte.
SANDRA VALONGUEIRO ALVES, Federal University of Pernambuco, Recife, Pernambuco, Brazil.
References
- Aiken ARA et al. 2016. “Requests for abortion in Latin America related to concern about Zika virus exposure,” New England Journal of Medicine 375(4): 396–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajzen I 1991. “The theory of planned behavior,” Organizational Behavior and Human Decision Processes 50(2): 179–211. [Google Scholar]
- Ali GO, Harber S, Harrison A, Houle L, and Ivory J. 2017. “Environmental and social change drive the explosive emergence of Zika virus in the Americas,” PLoS Neglected Tropical Diseases 11(2): e0005135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida AS, Medronho RA, and Valencia LIO. 2009. “Spatial analysis of dengue and the socioeconomic context of the city of Rio de Janeiro (Southeastern Brazil),” Revista de Saúde Pública 43(4): 666–673. [DOI] [PubMed] [Google Scholar]
- Amaral EFL and Potter JE. 2015. “Determinants of female sterilization in Brazil, 2001–2007,” RAND Working Paper Series WR 1093. [Google Scholar]
- Araújo TVB et al. 2016. “Association between Zika virus infection and microcephaly in Brazil, January to May, 2016,” The Lancet Infectious Diseases 16(12): 1356–1363. [DOI] [PubMed] [Google Scholar]
- Bachrach CA and Morgan SP. 2013. “A cognitive–social model of fertility intentions,” Population and Development Review 39(3): 459–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes-Josiah D, Myntti C, and Augustin A. 1998. “The ‘three delays’ as a framework for examining maternal mortality in Haiti,” Social Science and Medicine 46(8): 981–993. [DOI] [PubMed] [Google Scholar]
- Behrman JA, and Weitzman A. 2016. “Effects of the 2010 Haiti earthquake on women’s reproductive health,” Studies in Family Planning 47(1): 3–17. [DOI] [PubMed] [Google Scholar]
- BEMFAM. 1997. “Sociedade Civil Bem-Estar Familiar no Brasil,” DHS Final Reports. Brazil DHS, 1996. Rio de Janeiro, Brasil and Macro International Inc. [Google Scholar]
- Berquó E and Cavenaghi S. 2004. “Mapeamento sócio-econômico e demográfico dos regimes de fecundidade no Brasil e sua variação entre 1991 e 2000,” in Annals of the XIV National Meeting of the Brazilian Population Association (ABEP) Campinas, São Paulo. [Google Scholar]
- Bhalotra S, Rocha R, and Soares R. 2016. “Does universalization of health work? Evidence from health systems restructuring and maternal and child health in Brazil,” Working Paper No. 2016-16, Institute for Social and Economic Research. [Google Scholar]
- Bongaarts J 1978. “A framework for analyzing the proximate determinants of fertility,” Population and Development Review 4(1): 105–132. [Google Scholar]
- Brasil. 2012. Censo Demográfico 2010: nupcialidade, fecundidade e migração. Rio de Janeiro: IBGE. Instituto Brasileiro de Geografia e Estatística. [Google Scholar]
- Brasil. 2015. Brazil Popular Pharmacy Program. Brasilia: Portal da Saúde; http://portalsaude.saude.gov.br/index.php/o-ministerio/principal/secretarias/sctie/farmacia-popular Accessed 03/23/2017. [Google Scholar]
- Brasil. 2016. Boletim Epidemiológico Secretaria de Vigilância em Saúde. Volume 32, no. 11 Brasilia: Ministério da Saúde. [Google Scholar]
- Brasil. 2017a. Boletim Epidemiológico Secretaria de Vigilância em Saúde. Brasília: Ministério da Saúde, Volume 48, no. 7. [Google Scholar]
- Brasil. 2017b. Boletim Epidemiológico Secretaria de Vigilância em Saúde. Brasília: Ministério da Saúde, Volume 48, no. 6. [Google Scholar]
- Brasil. 2017c. Vírus Zika no Brasil: a resposta do SUS Ministério da Saúde, Secretaria de Vigilância em Saúde. Brasília: Ministério da Saúde [Google Scholar]
- Brasil. 2017d. IBGE Cidades, http://cidades.ibge.gov.br/xtras/home.php?lang= Accessed 23/03/2017.
- Brasil P et al. 2016. “Zika virus infection in pregnant women in Rio de Janeiro,” New England Journal of Medicine 375(24): 2321–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito C 2015. “Zika virus: A new chapter in the history of medicine,” Acta Médica Portuguesa 28: 679–680. [DOI] [PubMed] [Google Scholar]
- Caetano A 2014. “Esterilização cirúrgica feminina no Brasil, 2000 a 2006: Aderência à lei de planejamento familiar e demanda frustrada,” Revista Brasileira de Estudos de População 31(2): 309–331. [Google Scholar]
- Camarano AA, Kanso S, Barbosa P, and Alcântara VS. 2014. “Desigualdades na dinâmica demográfica e as duas implicações na distribuição de renda no Brasil,” in Novo Regime Demográfico: uma nova relação entre população e desenvolvimento? Rio de Janeiro: IPEA. [Google Scholar]
- Campbell M, Sahin-Hodoglugil NN, and Potts M. 2006. “Barriers to fertility regulation: A review of the literature,” Studies in Family Planning 37(2): 87–98. [DOI] [PubMed] [Google Scholar]
- Cauchemez S et al. 2016. “Association between Zika virus and microcephaly in French Polynesia, 2013–2015: A retrospective study,” Lancet 387(10033): 2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavenaghi SM and Berquó E. 2014. “Perfil socioeconômico e demográfico da fecundidade no Brasil de 2000 a 2010,” in Cavenaghi S and Cabella W (eds.), Comportamiento reproductivo y fecundidad en América Latina: una agenda inconclusa. Rio de Janeiro: ALAP, pp. 220–240. [Google Scholar]
- CEBRAP. 2008. Centro Brasileiro de Análise e Planejamento and DECIT, Departamento de Ciência e Tecnologia / MS. 2008. Pesquisa Nacional de Demografia e Saúde da Criança e da Mulher – PNDS 2006. [Google Scholar]
- Chang C, Ortiz K, Ansari A, and Gershwin ME. 2016. “The Zika outbreak of the 21st century,” Journal of Autoimmunity April 68: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey A and Atkinson P. 1996. “Narratives and stories,” in Making Sense of Qualitative Data: Complementary Research Strategies. Thousand Oaks. CA: Sage Publications, pp. 54–82. [Google Scholar]
- Costa NR 2016a. “The Family Health Strategy: Primary health care and the challenge of Brazilian metropolises,” Ciênc. saúde coletiva [online] 21(5): 1389–1398. [DOI] [PubMed] [Google Scholar]
- Costa AM 2016b. “A determinação social da microcefalia/Zika,” Desafios do Desenvolvimento IPEA 87. Brasília: IPEA. [Google Scholar]
- Creswell JW 2009. Research Design: Qualitative & Quantitative Approaches. Thousand Oaks. CA: Sage Publications. [Google Scholar]
- Curtis SL and Diamond I. 1995. “When fertility seems too high for contraceptive prevalence: An analysis of northeast Brazil,” International Family Planning Perspectives 21(2): 58–63. [Google Scholar]
- Dennis A and Grossman D. 2012. “Barriers to contraception and interest in over‐the‐counter access among low‐income women: A qualitative study,” Perspectives on Sexual and Reproductive Health 44(2): 84–91. [DOI] [PubMed] [Google Scholar]
- Diniz C 2002. “Repensando a questão regional brasileira: tendências, desafios e caminhos,” in Castro AC (ed.), Desenvolvimento em Debate – Painéis sobre o Desenvolvimento Brasileiro II. Rio de Janeiro: BNDES. [Google Scholar]
- Diniz D 2016. Do Sertão Nordestino à Ameaça Global. Rio de Janeiro: Civilização Brasileira. [Google Scholar]
- Diniz S, d’Oliveira G., Lucas AFP, and Sonia L. 2012. “Equity and women’s health services for contraception, abortion and childbirth in Brazil,” Reproductive Health Matters 20(40): 94–101. [DOI] [PubMed] [Google Scholar]
- Finlay J 2009. Fertility Response to Natural Disasters: The Case of Three High Mortality Earthquakes. Cambridge, MA: Harvard School of Public Health. [Google Scholar]
- Fishbein M and Ajzen I. 2011. Predicting and Changing Behavior: The Reasoned Action Approach. Taylor & Francis. [Google Scholar]
- França GVA et al. 2016. “Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation,” The Lancet 388(10047): 891–897. [DOI] [PubMed] [Google Scholar]
- Fusco CLB, and Andreoni S. 2012. “Unsafe abortion: Social determinants and health inequities in a vulnerable population in São Paulo, Brazil,” Cadernos de Saúde Pública 28(4): 709–719. [DOI] [PubMed] [Google Scholar]
- Goldberg RE 2012. “Family instability and early initiation of sexual activity in Western Kenya,” Demography 49(4): 725–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes DA et al. 2006. “Unsafe abortion: The preventable pandemic,” The Lancet 368(9550): 1908–1919. [DOI] [PubMed] [Google Scholar]
- Hatzenbuehler M, Phelan J, and Link B. 2013. “Stigma as a fundamental cause of population health inequalities,” American Journal of Public Health 103(5): 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPEA. 2010. Dimensão, evolução e projeção da pobreza por região e por estado no Brasil. Comunicados do Ipea No. 58. Instituto de Pesquisas Econômicas Aplicadas. Secretaria de Assuntos Estratégicos da Presidência da República. [Google Scholar]
- Johansson MA, Mier-y-Teran-Romero L, Reefhuis J, Gilboa S, and Hills SL. 2016. “Zika and the risk of microcephaly,” New England Journal of Medicine 375(1): 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodel J 1993. “The design and analysis of focus group studies: A practical approach,” in Morgan DL (ed.), Successful Focus Groups: Advancing the State of the Art. Thousand Oaks: Sage, pp. 35–53 [Google Scholar]
- Le HH et al. 2014. “The burden of unintended pregnancies in Brazil: A social and public health system cost analysis,” International Journal of Women’s Health 6: 663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite IC and Gupta N. 2007. “Assessing regional differences in contraceptive discontinuation, failure and switching in Brazil.” Reproductive Health 4(1): 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIRAa.2015. Levantamento do Índice Rápido do Aedes Aegypti – LIRAa. Prefeitura de Belo Horizonte; http://www.pbh.gov.br/smsa/dengue/pag.php?p=2 Accessed 05/15/2016. [Google Scholar]
- LIRAa 2016. Levantamento do Índice Rápido do Aedes Aegypti – LIRAa. Prefeitura de Recife; http://www2.recife.pe.gov.br/etiquetas/liraa Accessed 05/15/2016. [Google Scholar]
- Macinko J and Harris MJ. 2015. “Brazil’s Family Health Strategy—Delivering community-based primary care in a universal health system, New England Journal of Medicine 372(4): 2177–2181. [DOI] [PubMed] [Google Scholar]
- Malagutti W 2012. Assistência Domiciliar – Atualidades da Assistência de Enfermagem (org). Rio de Janeiro: Rubio, 336. [Google Scholar]
- Malta M et al. 2007. “Knowledge, perceived stigma, and care-seeking experiences for sexually transmitted infections: A qualitative study from the perspective of public clinic attendees in Rio de Janeiro, Brazil,” BMC Public Health 7: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranhão TA, Monteiro CFS, and Lago TA. 2012. Violência contra adolescentes grávidas: uma revisão. Revista Interdisciplinar UNINOVAFAPI, Teresina. 5(3): 58–63. [Google Scholar]
- Marston HD, Lurie N, Borio LL, and Fauci AS. 2016. “Considerations for developing a Zika virus vaccine,” New England Journal of Medicine 375(13): 1209–1212. [DOI] [PubMed] [Google Scholar]
- Marteleto LJ and Dondero M. 2013. “Maternal age at first birth and adolescent education in Brazil,” Demographic Research 28: 793–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteleto LJ and Villanueva A. 2016. “Adolescent childbearing and union: Consequences for education in Brazil,” paper presented at the Annual Meeting of the Population Association of America, Washington DC. [Google Scholar]
- Maxwell JA 2004. “Using qualitative methods for causal explanation,” Field Methods 16(3): 243–264 [Google Scholar]
- Maxwell JA. 2012. “The importance of qualitative research for causal explanation in education,” Qualitative Inquiry 8(8): 655–661. [Google Scholar]
- McCord GC, Conley D, and Sachs JD. 2017. “Malaria ecology, child mortality, and fertility,” Economics & Human Biology 24: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministério da Saúde. 2017. DATASUS. Data available for selected tabulations from: www.datasus.gov.br Accessed 03/29/2017.
- Miranda-Filho DB et al. 2016. “Initial description of the presumed congenital Zika syndrome,” American Journal of Public Health 106(4): 598–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Ribeiro A, Rios-Neto ELG, and Carvalho JAM. 2013. “Efeitos tempo, parturição e quantum no Brasil: indicadores de período e evidências empíricas,” Revista Brasileira de Estudos de População 30(1): 145–170. [Google Scholar]
- Moreau C et al. 2013. “Effect of prospectively measured pregnancy intentions on the consistency of contraceptive use among young women in Michigan,” Human Reproduction 28(3): 642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DL and Krueger RA. 1993. “When to use focus groups and why,” in Morgan DL, SAGE Focus Editions: Successful Focus Groups: Advancing the State of the Art. Thousand Oaks, CA: SAGE Publications; 3–19 [Google Scholar]
- Nobles J, Frankenberg F, and Thomas D. 2015. “The effects of mortality on fertility: Population dynamics after a natural disaster,” Demography 52(1): 15–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira WK et al. 2017. “Zika virus infection and associated neurologic disorders in Brazil,” The New England Journal of Medicine. Correspondence. Published on 03–29-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan American Health Organization. 2015. Epidemiological Alert: Zika Virus Infection. Geneva, Switzerland. [Google Scholar]
- Pereira CCB, Vidal SA, Carvalho PI, and Frias PG. 2013. Avaliação da implantação do Sistema de Informações sobre Nascidos Vivos (Sinasc) em Pernambuco. Revista Brasileira de Saúde Materno Infantil 13(1). [Google Scholar]
- Petersen LR, Jamieson DJ, Powers AM, and Honein MA. 2016. “Zika virus,” New England Journal of Medicine 374(16): 1552–1563. [DOI] [PubMed] [Google Scholar]
- PNDS. 2006. Pesquisa Nacional de Demografia e Saúde da Mulher e da Criança. Ministério da Saúde. [Google Scholar]
- PNS. 2013. Pesquisa Nacional de Saúde. Sistema IBGE de Recuperação Automática-SIDRA; https://sidra.ibge.gov.br/tabela/5540 Accessed 03/29/2017. [Google Scholar]
- Potter JE 1999. “The persistence of outmoded contraceptive regimes: The cases of Mexico and Brazil,” Population and Development Review 25(4): 703–739. [Google Scholar]
- Potter JE et al. 2003. “Frustrated demand for postpartum female sterilization in Brazil,” Contraception 67(5): 385–390. [DOI] [PubMed] [Google Scholar]
- Potter JE, Schmertmann CP, and Cavenaghi SM. 2002. “Fertility and development: Evidence from Brazil,” Demography 39(4): 739–761. [DOI] [PubMed] [Google Scholar]
- Prietsch SOM., González-Chica DA, Cesar JA, and Mendoza-Sassi RA. 2011. “Gravidez não planejada no extremo Sul do Brasil: prevalência e fatores associados,” Cadernos de Saúde Pública 27(10). [DOI] [PubMed] [Google Scholar]
- Reinecke J, Schmidt P, and Ajzen I. 1996. “Application of the theory of planned behavior to adolescents’ condom use: A panel study,” Journal of Applied Social Psychology 26(9): 749–772. [Google Scholar]
- Romero S 2015. “Alarm spreads in Brazil over a virus and a surge in malformed infants,” New York Times, December 30. [Google Scholar]
- Rosenstock IM 1974. “The health belief model and preventive health behavior,” Health Education & Behavior 2(4): 354–386. [Google Scholar]
- Rosenstock IM, Strecher VJ, and Becker MH. 1994. “The health belief model and HIV risk behavior change,” Preventing AIDS. Springer. [Google Scholar]
- Sandy M 2016. “Brazilian Legislators Look to Increase Abortion Penalties in the Wake of Zika Outbreak,” Time. Rio de Janeiro, February 22. [Google Scholar]
- Siqueira JB et al. 2004. “Household survey of dengue infection in central Brazil: Spatial point pattern analysis and risk factors assessment,” The American Journal of Tropical Medicine and Hygiene 71(5): 646–651. [PubMed] [Google Scholar]
- Spinelli MBAS, de Souza AI, de Moraes Vanderlei LC, and Vidal SA. 2014. “Características da oferta de contracepção de emergência na rede básica de saúde do Recife, Nordeste do Brasil,” Saúde e Sociedade 23(1): 227–237. [Google Scholar]
- Sutton S, McVey D, and Glanz A. 1999. “A comparative test of the theory of reasoned action and the theory of planned behavior in the prediction of condom use intentions in a national sample of English young people,” Health Psychology 18(1): 72–81. [DOI] [PubMed] [Google Scholar]
- Szwarcwald CL, Barbosa-Júnior A, Pascom AR, and Souza-Júnior PR. 2005. “Knowledge, practices and behaviours related to HIV transmission among the Brazilian population in the 15–54 years age group, 2004,” AIDS 19: S51–S58. [DOI] [PubMed] [Google Scholar]
- Szwarcwald CL et al. 2014. “Correction of vital statistics based on a proactive search of deaths and live births: evidence from a study of the North and Northeast regions of Brazil,” Population Health Metrics 12: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theme-Filha MM et al. 2016. “Factors associated with unintended pregnancy in Brazil: Cross-sectional results from the Birth in Brazil National Survey, 2011/2012,” Reproductive Health 13(3): 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SJ, L’Azou M, Barrett ADT, and Jackson NAC. 2016. “Fast-track Zika vaccine development—Is it possible?,” New England Journal of Medicine 375(13): 1212–1216. [DOI] [PubMed] [Google Scholar]
- Trinitapoli J and Yeatman S. 2011. “Uncertainty and fertility in a generalized AIDS epidemic,” American Sociological Review 76(6): 935–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrachnis N, Vlachadis N, Iliodromiti Z, Vlachadi M and Creatsas G. 2014. “Greece’s birth rates and the economic crisis,” The Lancet 383(9918): 692–693. [DOI] [PubMed] [Google Scholar]
- Weiss RS 1994. Learning from Strangers: The Art and Method of Qualitative Interview Studies. New York: Free Press. [Google Scholar]
- World Bank. 2016. GINI Index. Washington, DC. [Google Scholar]
- Zanluca C et al. 2015. “First report of autochthonous transmission of Zika virus in Brazil,” Memórias do Instituto Oswaldo Cruz, 110(4): 569–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


