Abstract
Background
Practice guidelines recommend that chronic insomnia be treated first with cognitive behavioural therapy for insomnia (CBT-I), and that hypnotic medication be considered only when CBT-I is unsuccessful. Although there is evidence of CBT-I’s efficacy in research studies, systematic reviews of its effects in primary care are lacking.
Aim
To review the effects on sleep outcomes of CBT-I delivered in primary care.
Design and setting
Systematic review of articles published worldwide.
Method
Medline, PsycINFO, EMBASE, and CINAHL were searched for articles published from January 1987 until August 2018 that reported sleep results and on the use of CBT-I in general primary care settings. Two researchers independently assessed and then reached agreement on the included studies and the extracted data. Cohen’s d was used to measure effects on sleep diary outcomes and the Insomnia Severity Index.
Results
In total, 13 studies were included. Medium-to-large positive effects on self-reported sleep were found for CBT-I provided over 4–6 sessions. Improvements were generally well maintained for 3–12 months post-treatment. Studies of interventions in which the format or content veered substantially from conventional CBT-I were less conclusive. In only three studies was CBT-I delivered by a GP; usually, it was provided by nurses, psychologists, nurse practitioners, social workers, or counsellors. Six studies included advice on withdrawal from hypnotics.
Conclusion
The findings support the effectiveness of multicomponent CBT-I in general primary care. Future studies should use standard sleep measures, examine daytime symptoms, and investigate the impact of hypnotic tapering interventions delivered in conjunction with CBT-I.
Keywords: chronic insomnia, cognitive behavioural therapy, family practice, primary health care, sleep disorders, sleep initiation and maintenance disorders
INTRODUCTION
Insomnia is the most prevalent sleep disorder, affecting 10–15% of the adult population1 and 19–44% of primary care patients worldwide.2–4 Chronic insomnia disorder is defined as early-morning awakening or difficulty initiating or maintaining sleep that occurs at least 3 nights a week for at least 3 months and impairs functioning.5 It is associated with numerous health problems6,7 and a number of psychiatric illnesses, most notably depression.8 As well as the demand this creates on health care, insomnia also burdens the economy through work absences and is associated with absenteeism and reduced productivity.9,10
For patients seeking professional help for insomnia, GPs are, by far, the health professionals most likely to be consulted.11 Practice guidelines recommend cognitive behavioural therapy for insomnia (CBT-I) as the first-line treatment, with hypnotic medication to be considered only if CBT-I does not work.12,13 Compared with pharmacotherapy, CBT-I has been shown to be superior in reducing symptoms of insomnia14 and in maintaining sleep improvements for years.15,16 Furthermore, non-pharmacological approaches are often preferred by patients, as they are considered to be better at improving daytime functioning and less likely to produce adverse side effects.17 The components of CBT-I are typically:
stimulus control — pairing the bed with sleep, leaving the bed when awake;
sleep restriction — reducing time in bed to increase sleep pressure;
cognitive therapy — challenging alerting thoughts about sleep loss; and
relaxation training.
Although these components can be delivered individually, the therapy is most effective when all components are delivered together as full CBT-I.18
Despite abundant evidence for the efficacy of CBT-I, the approach is under-utilised.19 GPs are often aware of the need to reduce hypnotic prescribing but have limited knowledge about, and access to, CBT-I.20 In order to refer patients for CBT-I or provide it in their practice, GPs first need to know whether, and how well, it works in primary care specifically. Although much literature exists about the efficacy of CBT-I, systematic reviews focused on the effectiveness of its use in primary care are lacking. As such, this study was undertaken to review the effects of CBT-I on sleep outcomes in general primary care settings and provide a pragmatic overview of the results for general practice.
METHOD
Database searches
A systematic review was conducted with searches of the Medline (Ovid), PsycINFO (Ovid), EMBASE, and CINAHL databases. Search strategies combining indexing keywords relevant to CBT-I in primary care (Box 1) were used with each database. The initial search included articles published from January 1987 until 16 November 2017. Studies published in English or French were included. The results of the searches were combined and duplicates removed. Articles were reviewed by two authors to identify studies possibly suitable for inclusion based on their title and abstract. These articles were then reviewed in depth by the same authors to determine their relevance. The database searches and procedures used to identify relevant articles were repeated on 8 August 2018 using the exact terms as before, but with a date range of January 2017 to August 2018, to identify any new relevant articles.
Box 1.
Search terms
| Search terms used for all four databases. Words in each section combined with ‘or’; each section combined with ‘and’ in order to search each combination of terms. |
|---|
| insomnia* Sleep initiation sleep maintenance sleep disorder |
| psychotherap* cognitive behavio* therap* behavio* therapy CBT CBT-I CBTI cognitive therapy sleep hygiene stimulus control sleep restriction relaxation imagery |
| primary care general practice family practice family medicine |
How this fits in
| The primary care physician is the health professional most likely to be consulted by a patient with insomnia. Although cognitive behavioural therapy for insomnia (CBT-I) is the recommended first-line treatment, reviews of its effectiveness in the primary care setting are lacking. This review provides evidence that multicomponent CBT-I (group or individual) is effective at improving sleep outcomes for primary care patients with chronic insomnia. |
To be included in the review, studies had to:
report results of original research (not reviews, protocols, recommendations, conference abstracts, or clinical advice papers) on the effects of CBT-I;
be based in a general primary care population;
have at least 10 adult (≥18 years) patients; and
have quantitative measures of sleep outcomes.
Participants could have medical or psychological comorbidity and could use hypnotic medication, and online or telephone therapy was included if the participants had been recruited in primary care. Randomised controlled trials (RCTs) and pre-post clinical case series were included; the latter’s design — an important step between RCTs and direct clinical application of CBT-I — provides data on within-subject changes experienced by patients in their primary care setting.
Studies had to report Insomnia Severity Index (ISI) scores or at least one of the following outcomes derived from sleep diaries:
sleep onset latency (SOL);
wake after sleep onset (WASO);
total sleep time (TST); or
sleep efficiency (SE).
The ISI is a brief self-report measure of the severity of insomnia symptoms and their impact on functioning; it has been validated in primary care.21 Higher scores reflect greater insomnia severity. As insomnia is defined by a complaint of sleep difficulty, sleep diaries22 and the ISI are standard tools to assess insomnia and monitor response to treatment.
For the results to be maximally applicable to physicians in general practice settings, the focus was on general primary care; studies conducted in special settings (for example, universities, active military or veterans’ treatment centres, community pharmacies) were excluded.
Study quality and risk of bias
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations on the reporting of systematic reviews23 were followed. For each of the 13 studies, all three authors independently assessed the extent of sleep disorder screening and the risk of bias for common validity indicators.23 The three then met and reached consensus on the values in bias tables (information available from the authors on request).
Data extraction
Information about participants, interventions, and results of the included studies were extracted for the evidence tables. Two researchers independently extracted data from the studies; when there was discrepancy they met to reach agreement on the final entries.
If not provided in the article, effect sizes were calculated based on reported sleep data, then checked by two researchers. Cohen’s d was used as the standard measure of effect size and, in line with Cohen’s suggestion, interpreted as small (0.20), medium (0.50), or large (0.80).24 Although study designs varied and authors used various formulas for d, this review reported classical Cohen’s d for all between-group studies in order to make effect sizes comparable, as outlined by Morris and DeShon.25
The formula was:
In the formula, M1 and M2 are the post-treatment means of the two groups, SDpooled = √[(n1 − 1)s12 + (n2 − 1)s22]/(n1 + n2) and n and s are the sample size and standard deviation (SD) of each group. This gives an estimate of the effect of CBT-I relative to a control or comparator condition.
For clinical case series, within-subject effect sizes were reported; the following formula was used:
In this formula, z = difference score, and Mz and SDz are the mean and SD of the difference scores. Within-subject ds are useful here because they estimate the pre-post effect that would be expected for patients in regular practice.
For the description of studies, conventional CBT-I was defined as multicomponent treatment consisting of sleep restriction, stimulus control therapy, cognitive therapy, and relaxation delivered in 4–8 sessions. Low-impact control was defined as a control condition that was assumed to be relatively inactive compared with CBT-I and included wait list, self-monitoring, and/or sleep hygiene.26 If prescription or withdrawal of hypnotic medication was combined with the CBT-I treatment, this information was noted as part of the intervention.
RESULTS
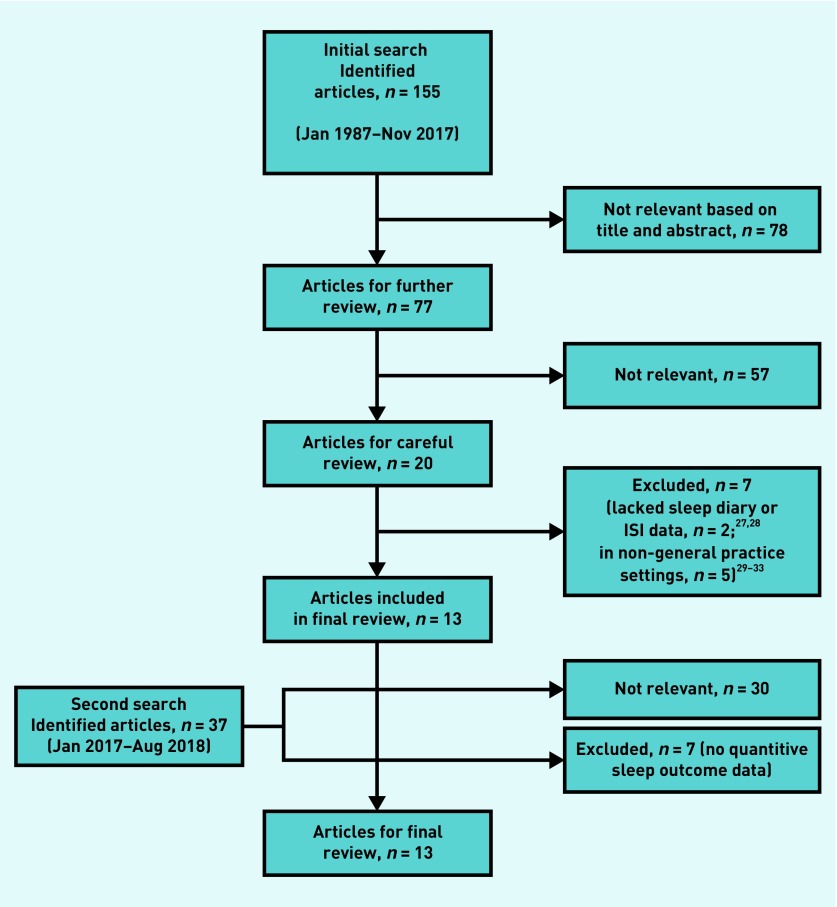
Figure 1 shows the steps in the evaluation and selection of studies. In total, 13 studies were included in the final review.34–46
Figure 1.
Flow diagram of article selection process. ISI = Insomnia Severity Index.
Study quality and bias
Ten studies used a RCT design. There was one cluster randomised trial in which 31 physicians were randomly assigned to provide one of two treatments.40 Two studies were case series.45,46 The examination of sources of bias across studies raised questions about the impact on reported outcomes of the following factors: level of screening for sleep disorders; type of health professional; the provider’s amount of CBT-I training; treatment fidelity; lack of blinding of patients, health providers, and analysts; and high rates of loss to follow-up in some studies (information available from the authors on request).
RCTs with mixed-age samples
Seven studies used an RCT design with mixed-age adult samples in primary care (Table 1). The samples were largely composed of females; the mean age was in the low–mid-50s. Four studies34–37 used conventional CBT-I in group format provided by nurses or social workers; the other three38–40 provided variations of CBT-I (Table 1).
Table 1.
Randomised controlled trials of CBT-I in general primary care with mixed-age adult samples
| Study | Sample size, n | Sex, % female | Mean age, years | Country/region | Intervention | Sessions, n | Session duration | Control condition | Intervention delivered by | Effect sizes, Cohen’s d | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SOL | WASO | TST | SE | ISI | ||||||||||
| Bothelius et al (2013)34 | 66 | 86.4 | 50.7 | Sweden | CBT-I group, HT | 5 | 60–90 mins | Wait list | Nurses, social workers | 0.57 | 0.55 | – | – | 0.90 |
| Espie et al (2001)35 | 139 | 68.3 | 51.4 | UK | CBT-I group, HT | 6 | 50 mins | Monitoring | Nurses | 0.97 | 0.48 | 0.32 | – | – |
| Espie et al (2007)36 | 201 | 68.2 | 54.3 | UK | CBT-I group | 5 | 60 mins | Treatment as usual | Nurses | 0.58 | 0.35 | 0.13 | 0.68 | – |
| Sandlund et al (2017)37 | 165 | 72.7 | 54.5 | Sweden | CBT-I group, HT | 7 (6 + 1-month follow-up) | 120 mins | Treatment as usual | Nurses | 0.51 | 0.39 | 0.40 | 0.62 | 1.23 |
| Falloon et al (2015)38 | 94 | 77.3 | 53.5 | New Zealand | Sleep restriction (brief), individual | 2 | Not specified, GP appointment | Sleep hygiene guidelines | GP | 0.13 | 0.36 | 0.01 | 0.21 | 0.55 |
| Wong et al (2017)39 | 216 | 78.2 | 56.1 | Hong Kong | MBCT-I + sleep restriction + sleep-specific cognitive therapy, group | 8 | 150 mins | Psychoeducationa + stretching | MBCT instructors/physiotherapists | 0.02 | 0.50 | 0.14 | 0.14 | 0.36 |
| Katofsky et al (2012)40 | 80 | 77.5 | 51.5 | Germany | Self-help CBT-I manual + 4-week lormetazepam start and taper, individual | 6 | Not specified, GP appointment | Short term lormetazepam | GPs | 0.41 | 0.18 | 0.10 | – | – |
Included stimulus control therapy and sleep hygiene. Dashes signify not reported. CBT-I = cognitive behavioural therapy for insomnia. HT = hypnotic medication tapering. ISI = Insomnia Severity Index. MBCT = mindfulness-based cognitive therapy. SE = sleep efficiency. SOL = sleep onset latency. TST = total sleep time. WASO = wake after sleep onset.
CBT-I group studies
For the four CBT-I group studies,34–37 the effect sizes for SOL were medium to large and those for WASO were small to medium. CBT-I was associated with a mean reduction of 9–30 minutes for SOL, and a mean reduction of 22–36 minutes for WASO; for controls, the mean reductions were 1–4 minutes and 6–8 minutes for SOL and WASO respectively. Effect sizes for TST were absent or small, while medium effects were found for SE, where reported.36,37 Large effects were found for the ISI in the two studies that reported it;34,37 CBT-I was associated with a mean ISI reduction of 6–8 points versus the 0–1 points for controls.
CBT-I variants
Falloon et al 38 studied the effects of a sleep restriction protocol, carried out within two GP appointments, compared with sleep hygiene. Post-treatment measures were reported 6 months after the second appointment. There were small effects for SOL and SE, a negligible effect for TST, and a medium effect for the ISI.
Wong et al39 compared mindfulness-based cognitive therapy for insomnia (MBCT-I) with eight sessions of sleep psychoeducation — which included sleep hygiene and stimulus control therapy — plus stretching and strengthening exercises. MBCT-I was associated with a negligible effect for SOL, a medium effect for WASO, and small effects for TST, SE, and the ISI.
Katofsky et al 40 compared self-help CBT-I provided in 6 weekly chapters (of the self-help manual referred to in Table 1), plus short-term (introduced and tapered within 4 weeks) lormetazepam with short-term lormetazepam alone. GPs, rather than patients, were randomised to provide one of the two treatments. Small effect sizes were found for SOL, WASO, and TST.
RCTs with older primary care patients
Table 2 summarises the four studies that included RCTs of CBT-I in older patients in primary care. Approximately two-thirds to three-quarters of the samples were female, and participants’ mean age was late 60s–late 70s. In the study by Vitiello et al,44 patients also had osteoarthritis. All four studies used education or sleep hygiene as the control, and variations (both in content and format) of conventional CBT-I: two studies provided four treatment sessions, two of which were face-to-face and two of which were conducted over the telephone;41,42 one study used mailed-out booklets;43 and one used group CBT-I combined with cognitive behavioural therapy (CBT) for pain management.44
Table 2.
Randomised controlled trials of CBT-I with older patients in primary care
| Study | Sample size, n | Sex, % female | Mean age, years | Country/region | Intervention | Sessions, n | Session duration | Control condition | Intervention delivered by | Effect sizes, Cohen’s d | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SOL | WASO | TST | SE | ISI | ||||||||||
| Buysse et al (2011)41 | 79 | 68.3 | 71.6 | US | Sleep restriction, SCT, sleep education; individual | 4 | 45–60 mins face to face; 20 mins telephone; 30 mins face to face; 20 mins telephone |
Education (articles), 10-mins telephone call | Mental health nurse practitioner | 3.38 | 3.91 | 1.83 | 3.43 | – |
| McCrae et al (2007)42 | 20 | 65.0 | 77.2 | US | Sleep restriction, SCT relaxation; individual | 4 | 2 × 50-min face to face; 2 × 30-mins telephone |
Sleep hygiene education | Mental health workers or social workers | 0.82 | 1.06 | 0.99 | 1.14 | – |
| Morgan et al (2012) 43a | 193 | 66.3 | 66.7 | UK | Self-help CBT-I booklets, HT info | 6 booklets | Weekly booklet | Sleep hygiene sheet | Booklet | – | – | – | b | 0.74 |
| Vitiello et al (2013) 44c (two of three study arms) | 245 | 77.6 | 73.1 | US | CBT for pain and insomnia; group | 6 | 90 mins | Education on pain + sleep management | Counsellor, psychologist | – | – | – | b | 0.40 |
Patients had long-term conditions.
Reported but not based on sleep diaries.
Patients had osteoarthritis. Dashes signify not reported. CBT-I = cognitive behavioural therapy for insomnia. HT = hypnotic medication tapering. ISI = Insomnia Severity Index. SCT = stimulus control therapy. SE = sleep efficiency. SOL = sleep onset latency. TST = total sleep time. WASO = wake after sleep onset.
The studies by Buysse et al41 and McCrae et al42 found large effects for SOL, WASO, TST, and SE. Together, these studies found reductions in SOL and WASO of 23–25 minutes and 24–37 minutes respectively, versus the control reductions of 0–1 minutes and 3–18 minutes. Based on the ISI data, Morgan et al43 found a medium-to-large effect for self-help booklets, while Vitiello et al44 found a small effect for CBT-I combined with CBT for pain.
Clinical case series
Two studies45,46 reported sleep outcomes in clinical case series studies. In both, patients received advice on tapering hypnotic medication, if relevant. In Baillargeon et al’s study,45 GPs provided stimulus control therapy to 15 patients and recorded a large reduction in mean SOL (44 minutes; large effect size). Of note, the treatment was provided until SOL reached ≤30 minutes, or for 10 sessions, thereby potentially inflating the effect. In the study by Davidson et al,46 81 consecutive primary care patients were studied before and after group CBT-I was provided by a psychologist and a nurse practitioner or graduate student. Effect sizes were large for SOL, WASO, SE, and the ISI, and small for TST. The mean reductions in SOL, WASO, and the ISI were 31 minutes, 37 minutes, and nine points, respectively.
Adjunctive hypnotic medication interventions
In six of the 13 studies, the treatment protocols included advice on step-by-step reduction of hypnotic medication,34,35,37,45 provided general information on sleep medication in booklet format,43 or offered an adjunct programme of hypnotic withdrawal.46 In an additional study, hypnotic medication was both started and then tapered within 4 weeks.40 The remaining six studies did not mention interventions aimed at hypnotic medication use.
Follow-up data
Two studies found effects were somewhat reduced at 6 months36 and 18 months34 post-treatment, although follow-up outcomes were still superior to baseline. Otherwise, follow-up data (where available), showed good maintenance of therapeutic gains at 3 months,39,43,45 6 months,40,41,43,45 9 months,44 and 12 months.35,37
DISCUSSION
Summary
The sleep outcomes of 13 studies of CBT-I set in general primary care were examined. Those studies that provided either full CBT-I, or an intervention that included stimulus control and sleep restriction in at least four sessions, showed the greatest effects on SOL, WASO, and the ISI. This was true for mixed-age samples and for older patients. Variations on conventional CBT-I were not as successful, for example, limiting the intervention to two brief appointments for sleep restriction38 led to smaller effect sizes; adding self-help CBT-I booklets to medication led to only modest improvements over medication alone.40 Methodological issues within the studies — including the use of a CBT-I component in the control condition,39 the absence of sleep diary data,43,44 and delayed collection of post-treatment data38 — rendered the results of non-conventional CBT-I less conclusive.
Strengths and limitations
Although CBT-I is recommended as the first-line treatment of chronic insomnia, to the authors’ knowledge, this was the first systematic review of its use in primary care (since the current paper went to press, another review has appeared47).
This review also used methods in accordance with PRISMA standards. Limitations should be noted, however. First, the study focused on subjective sleep outcomes and not daytime symptoms (fatigue, functioning, mood), which are also important insomnia treatment outcomes. Second, the requirement of sleep diary or ISI data led to the exclusion of two large pragmatic studies.27,28 Both found positive effects of CBT-I on non-standard sleep estimates and, as such, their exclusion does not appear to alter the main findings of this review. Third, the application of these results to clinical practice rests on the assumption that patients are carefully screened for other sleep disorders before doing CBT-I (based on the quality and bias analyses). Fourth, publication bias in the form of negative, unpublished trials cannot be ruled out. Finally, this review was limited to studies in general practice settings — the results do not necessarily represent special populations, such as university students or the military.
Comparison with existing literature
The results presented here converge with recent meta-analytic reviews of CBT-I in adults in general,48–50 older adults,51 and in patients with medical or mental comorbidity.52–54 For example, the effect sizes for SOL, WASO, SE, and the ISI for conventional CBT-I found in this study were similar to those found by Trauer et al,50 Koffel et al,49 and Geiger-Brown et al.52 They are somewhat lower than the large effect sizes for CBT-I at behavioural sleep medicine clinics.55–57 Consistent with other studies,49,52,55 TST did not increase substantially. Also consistent with the literature,15,16 sleep improvements were generally well maintained at follow-up.
Implications for research and practice
For the primary care provider, the results of this review provide evidence that CBT-I (group or individual) is effective at improving sleep onset and maintenance in primary care patients with chronic insomnia. SOL can be expected to decrease by 9–31 minutes and WASO by 22–37 minutes. Sleep improvements are sustained 3–12 months after treatment. The effectiveness of interventions that deviate markedly in terms of content or number of sessions from the 4–6 session conventional CBT-I format are, as yet, unproven in this setting.
The best methods of integrating CBT-I into primary care services need to be identified. In only three of the 13 studies reviewed was the GP directly involved in administering the CBT-I intervention;38,40,45 in the remaining 10 studies, other health professionals provided CBT-I, including nurses, nurse practitioners, social workers, mental health workers, and psychologists. In some cases, CBT-I involved support for the discontinuation of hypnotic medication, a component especially relevant to primary care settings. In two studies, the tapering schedule was designed by pharmacists.35,46 Overall CBT-I appears to be finding a place within primary health systems that support interdisciplinary teamwork.
This review revealed some variation in the intervention components, sleep disorders screening, and in the health discipline and CBT-I training of the providers — all of these are potential moderator variables that warrant further research. Future studies should use standard measures of sleep,22 measure daytime symptoms,58 and examine the impact of hypnotic tapering interventions delivered as part of CBT-I programmes for primary care patients.
Acknowledgments
The authors would like to thank Ms Sandra Halliday for her expert advice on the database searches and Dr Kate Harkness, Dr Helen Driver, and Dr Richard Beninger for their helpful comments on this manuscript.
Funding
None.
Ethical approval
Not applicable.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Morin CM, Benca R. Chronic insomnia. Lancet. 2012;379(9821):1129–1141. doi: 10.1016/S0140-6736(11)60750-2. [DOI] [PubMed] [Google Scholar]
- 2.Bhaskar S, Hemavathy D, Prasad S. Prevalence of chronic insomnia in adult patients and its correlation with medical comorbidities. J Family Med Prim Care. 2016;5(4):780–784. doi: 10.4103/2249-4863.201153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shochat T, Umphress J, Israel AG, Ancoli-Israel S. Insomnia in primary care patients. Sleep. 1999;22(Suppl 2):S359–S365. [PubMed] [Google Scholar]
- 4.Terzano MG, Parrino L, Cirignotta F, et al. Studio Morfeo: insomnia in primary care, a survey conducted on the Italian population. Sleep Med. 2004;5(1):67–75. doi: 10.1016/j.sleep.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Sleep Medicine . International classification of sleep disorders. 3rd edn. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 6.Budhiraja R, Roth T, Hudgel DW, et al. Prevalence and polysomnographic correlates of insomnia comorbid with medical disorders. Sleep. 2011;34(7):859–867. doi: 10.5665/SLEEP.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz DA, McHorney CA. The relationship between insomnia and health-related quality of life in patients with chronic illness. J Fam Pract. 2002;51(3):229–235. [PubMed] [Google Scholar]
- 8.Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1–3):10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Daley M, Morin CM, LeBlanc M, et al. The economic burden of insomnia: direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep. 2009;32(1):55–64. [PMC free article] [PubMed] [Google Scholar]
- 10.Hajak G, Petukhova M, Lakoma MD, et al. Days-out-of-role associated with insomnia and comorbid conditions in the America Insomnia Survey. Biol Psychiatry. 2011;70(11):1063–1073. doi: 10.1016/j.biopsych.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123–130. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125–133. doi: 10.7326/M15-2175. [DOI] [PubMed] [Google Scholar]
- 13.Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700. doi: 10.1111/jsr.12594. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs GD, Pace-Schott EF, Stickgold R, Otto MW. Cognitive behavior therapy and pharmacotherapy for insomnia: a randomized controlled trial and direct comparison. Arch Intern Med. 2004;164(17):1888–1896. doi: 10.1001/archinte.164.17.1888. [DOI] [PubMed] [Google Scholar]
- 15.Castronovo V, Galbiati A, Sforza M, et al. Long-term clinical effect of group cognitive behavioral therapy for insomnia: a case series study. Sleep Med. 2018;47:54–59. doi: 10.1016/j.sleep.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Morin CM, Colecchi C, Stone J, et al. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA. 1999;281(11):991–999. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 17.Vincent N, Lionberg C. Treatment preference and patient satisfaction in chronic insomnia. Sleep. 2001;24(4):411–417. doi: 10.1093/sleep/24.4.411. [DOI] [PubMed] [Google Scholar]
- 18.Harvey AG, Bélanger L, Talbot L, et al. Comparative efficacy of behavior therapy, cognitive therapy, and cognitive behavior therapy for chronic insomnia: a randomized controlled trial. J Consult Clin Psychol. 2014;82(4):670–683. doi: 10.1037/a0036606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ancoli-Israel S, Lieberman JA., 3rd Insomnia in primary care: overcoming diagnostic and treatment barriers. Introduction. Postgrad Med. 2004;116(6 Suppl Insomnia):4–6. doi: 10.3810/pgm.12.2004.suppl38.256. [DOI] [PubMed] [Google Scholar]
- 20.Siriwardena AN, Apekey T, Tilling M, et al. General practitioners’ preferences for managing insomnia and opportunities for reducing hypnotic prescribing. J Eval Clin Pract. 2010;16(4):731–737. doi: 10.1111/j.1365-2753.2009.01186.x. [DOI] [PubMed] [Google Scholar]
- 21.Gagnon C, Bélanger L, Ivers H, Morin CM. Validation of the Insomnia Severity Index in primary care. J Am Board Fam Med. 2013;26(6):701–710. doi: 10.3122/jabfm.2013.06.130064. [DOI] [PubMed] [Google Scholar]
- 22.Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen J. Statistical power analysis for the behavioral sciences. 2nd edn. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 25.Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods. 2002;7(1):105–125. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- 26.Chung KF, Lee CT, Yeung WF, et al. Sleep hygiene education as a treatment of insomnia: a systematic review and meta-analysis. Fam Pract. 2018;35(4):365–375. doi: 10.1093/fampra/cmx122. [DOI] [PubMed] [Google Scholar]
- 27.Cape J, Leibowitz J, Whittington C, et al. Group cognitive behavioural treatment for insomnia in primary care: a randomized controlled trial. Psychol Med. 2016;46(5):1015–1025. doi: 10.1017/S0033291715002561. [DOI] [PubMed] [Google Scholar]
- 28.Morgan K, Dixon S, Mathers N, et al. Psychological treatment for insomnia in the management of long-term hypnotic drug use: a pragmatic randomised controlled trial. Br J Gen Pract. 2003;53(497):923–928. [PMC free article] [PubMed] [Google Scholar]
- 29.Edinger JD, Sampson WS. A primary care ‘friendly’ cognitive behavioral insomnia therapy. Sleep. 2003;26(2):177–182. doi: 10.1093/sleep/26.2.177. [DOI] [PubMed] [Google Scholar]
- 30.Funderburk JS, Shepardson RL, Krenek M. Brief behavioral interventions for symptoms of depression and insomnia in university primary care. J Am Coll Health. 2015;63(6):398–402. doi: 10.1080/07448481.2015.1015031. [DOI] [PubMed] [Google Scholar]
- 31.Goodie JL, Isler WC, Hunter C, Peterson AL. Using behavioral health consultants to treat insomnia in primary care: a clinical case series. J Clin Psychol. 2009;65(3):294–304. doi: 10.1002/jclp.20548. [DOI] [PubMed] [Google Scholar]
- 32.Hryshko-Mullen AS, Broeckl LS, Haddock CK, Peterson AL. Behavioral treatment of insomnia: the Wilford Hall Insomnia Program. Mil Med. 2000;165(3):200–207. [PubMed] [Google Scholar]
- 33.Pigeon WR, Funderburk J, Bishop TM, Crean HF. Brief cognitive behavioral therapy for insomnia delivered to depressed veterans receiving primary care services: a pilot study. J Affect Disord. 2017;217:105–111. doi: 10.1016/j.jad.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Bothelius K, Kyhle K, Espie CA, Broman JE. Manual-guided cognitive-behavioural therapy for insomnia delivered by ordinary primary care personnel in general medical practice: a randomized controlled effectiveness trial. J Sleep Res. 2013;22(6):688–696. doi: 10.1111/jsr.12067. [DOI] [PubMed] [Google Scholar]
- 35.Espie CA, Inglis SJ, Tessier S, Harvey L. The clinical effectiveness of cognitive behaviour therapy for chronic insomnia: implementation and evaluation of a sleep clinic in general medical practice. Behav Res Ther. 2001;39(1):45–60. doi: 10.1016/s0005-7967(99)00157-6. [DOI] [PubMed] [Google Scholar]
- 36.Espie CA, MacMahon KM, Kelly HL, et al. Randomized clinical effectiveness trial of nurse-administered small-group cognitive behavior therapy for persistent insomnia in general practice. Sleep. 2007;30(5):574–584. doi: 10.1093/sleep/30.5.574. [DOI] [PubMed] [Google Scholar]
- 37.Sandlund C, Hetta J, Nilsson GH, et al. Improving insomnia in primary care patients: a randomized controlled trial of nurse-led group treatment. Int J Nurs Stud. 2017;72:30–41. doi: 10.1016/j.ijnurstu.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Falloon K, Elley CR, Fernando A, 3rd, et al. Simplified sleep restriction for insomnia in general practice: a randomised controlled trial. Br J Gen Pract. 2015 doi: 10.3399/bjgp15X686137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong SY, Zhang DX, Li CC, et al. Comparing the effects of mindfulness-based cognitive therapy and sleep psycho-education with exercise on chronic insomnia: a randomised controlled trial. Psychother Psychosom. 2017;86(4):241–253. doi: 10.1159/000470847. [DOI] [PubMed] [Google Scholar]
- 40.Katofsky I, Backhaus J, Junghanns K, et al. Effectiveness of a cognitive behavioral self-help program for patients with primary insomnia in general practice: a pilot study. Sleep Med. 2012;13(5):463–468. doi: 10.1016/j.sleep.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Buysse D, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171(10):887–895. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCrae CS, McGovern R, Lukefahr R, Stripling AM. Research Evaluating Brief Behavioral Sleep Treatments for Rural Elderly (RESTORE): a preliminary examination of effectiveness. Am J Geriatr Psychiatry. 2007;15(11):979–982. doi: 10.1097/JGP.0b013e31813547e6. [DOI] [PubMed] [Google Scholar]
- 43.Morgan K, Gregory P, Tomeny M, et al. Self-help treatment for insomnia symptoms associated with chronic conditions in older adults: a randomized controlled trial. J Am Geriatr Soc. 2012;60(10):1803–1810. doi: 10.1111/j.1532-5415.2012.04175.x. [DOI] [PubMed] [Google Scholar]
- 44.Vitiello M, McCurry SM, Shortreed SM, et al. Cognitive-behavioral treatment for comorbid insomnia and osteoarthritis pain in primary care: the lifestyles randomized controlled trial. J Am Geriatr Soc. 2013;61(6):947–956. doi: 10.1111/jgs.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baillargeon L, Demers M, Ladouceur R. Stimulus-control: nonpharmacologic treatment for insomnia. Can Fam Physician. 1998;44:73–79. [PMC free article] [PubMed] [Google Scholar]
- 46.Davidson JR, Dawson S, Krsmanovic A. Effectiveness of group cognitive behavioral therapy for insomnia (CBT-I) in a primary care setting. Behav Sleep Med. 2017 May 2;:1–13. doi: 10.1080/15402002.2017.1318753. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 47.Cheung JMY, Jarrin DC, Ballot O, et al. A systematic review of cognitive behavioral therapy for insomnia implemented in primary care and community settings. Sleep Med Rev. 2019;44:23–36. doi: 10.1016/j.smrv.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Okajima I, Komada Y, Inoue Y. A meta-analysis on the treatment effectiveness of cognitive behavioral therapy for primary insomnia. Sleep and Biological Rhythms. 2011;9(1):24–34. [Google Scholar]
- 49.Koffel EA, Koffel JB, Gehrman PR. A meta-analysis of group cognitive behavioral therapy for insomnia. Sleep Med Rev. 2015;19:6–16. doi: 10.1016/j.smrv.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trauer JM, Qian MY, Doyle JS, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191–204. doi: 10.7326/M14-2841. [DOI] [PubMed] [Google Scholar]
- 51.Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006;25(1):3–14. doi: 10.1037/0278-6133.25.1.3. [DOI] [PubMed] [Google Scholar]
- 52.Geiger-Brown JM, Rogers VE, Liu W, et al. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54–67. doi: 10.1016/j.smrv.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 53.Wu JQ, Appleman ER, Salazar RD, Ong JC. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis. JAMA Intern Med. 2015;175(9):1461–1472. doi: 10.1001/jamainternmed.2015.3006. [DOI] [PubMed] [Google Scholar]
- 54.Johnson JA, Rash JA, Campbell TS, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer ssurvivors. Sleep Med Rev. 2016;27:20–28. doi: 10.1016/j.smrv.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 55.Dolan DC, Taylor DJ, Bramoweth AD, Rosenthal LD. Cognitive-behavioral therapy of insomnia: a clinical case series study of patients with co-morbid disorders and using hypnotic medications. Behav Res Ther. 2010;48(4):321–327. doi: 10.1016/j.brat.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 56.Perlis M, Aloia M, Millikan A, et al. Behavioral treatment of insomnia: a clinical case series study. J Behav Med. 2000;23(2):149–161. doi: 10.1023/a:1005413117932. [DOI] [PubMed] [Google Scholar]
- 57.Perlis ML, Sharpe M, Smith MT, et al. Behavioral treatment of insomnia: treatment outcome and the relevance of medical and psychiatric comorbidity. J Behav Med. 2001;24(3):281–296. doi: 10.1023/a:1010770807823. [DOI] [PubMed] [Google Scholar]
- 58.Sandlund C, Hetta J, Nilsson GH, et al. Impact of group treatment for insomnia on daytime symptomatology: analyses from a randomized controlled trial in primary care. Int J Nurs Stud. 2018;85:126–135. doi: 10.1016/j.ijnurstu.2018.05.002. [DOI] [PubMed] [Google Scholar]