Abstract
Can we create robots with the behavioral flexibility and robustness of animals? Engineers often use bio-inspiration to mimic animals. Recent advances in tissue engineering now allow the use of components from animals. By integrating organic and synthetic components, researchers are moving towards the development of engineered organisms whose structural framework, actuation, sensing, and control are partially or completely organic. This review discusses recent exciting work demonstrating how organic components can be used for all facets of robot development. Based on this analysis, we propose a Robotic Taxonomic Key to guide the field towards a unified lexicon for device description.
1. Introduction
In robotics, animals have long served as inspiration for device development, providing both abstract structural inspiration (1), and inspiration for detailed systems design (2, 3). While such bio-inspiration has contributed greatly to the development of advanced robots, many capabilities seen in animals have yet to be achieved. Standard materials for robotic fabrication do not exhibit self-healing or have the ability to autonomously generate energy as is seen in biological systems. Robotic actuation is also somewhat limited. For example, many existing small-scale traditional robotic actuators lack the compliance, energy efficiency, and power-to-weight ratio observed in musculoskeletal systems. Furthermore, robotic sensing is still limited. For example, there are no affordable, responsive, and reliable chemical sensors that can replace dogs for drug or explosive detection. Finally, the behavioral flexibility, complex control, and advanced learning capabilities observed in animals have not been fully captured by current robotic controllers. As a consequence, organic components provide highly promising alternatives to traditionally engineered systems for the development of complex autonomous robotic systems.
Current efforts to integrate organic components with robotic systems require expertise from many different disciplines. Researchers in tissue engineering have sought to develop devices for biomedical applications and pharmacology, whereas those in control engineering have looked to natural nervous systems for insight into neural network algorithms and artificial intelligence development. In robotics, engineers have been drawn to the possibility of using living muscle as a lightweight, compliant actuator for robotic systems.
Complete robotic devices require structural frameworks, actuators, sensors, and control. In the field of biohybrid and completely organic robotics, researchers are striving to create each, or even all, of these components with organic materials. As a consequence, current devices are made up of three fundamental materials: organic components, hybrid components (combining both organic and synthetic material), and fully synthetic components. Despite the recent momentum in the field (Figure 1), there is no comprehensive review of robotic systems using organic components. Several previous reviews on this subject exist, ranging from discussions of biohybrid devices within the context of biofabrication and biologically inspired design (4), to focused reviews of particular systems, such as the use of biomolecular motors in the development of nanoscale systems (5). These reviews have tended to focus on fabrication strategies (6, 7), or the use of organic material for a particular component or subset of components in robotic design. The primary focus is typically on organic actuators. Several reviews have discussed the use of muscle cells (8–14) or biomolecular motors (5, 11) as organic actuators. Biohybrid actuators are even being included in textbooks on microrobots (15). Other reviews have instead focused on the use of microorganisms and flagellated cells to provide both actuation and basic sensing capabilities for biohybrid devices (12, 13, 16). Discussion of the use of organic components as control systems has been limited, although the need for neuron-based controllers and neuromuscular junctions for muscle-actuated robots has been addressed (14). A unifying review that analyzes the existing literature for organic structural frameworks, actuators, sensors, and controllers would provide insight to the community that is developing microsystems and next generation robots. To this end, our review discusses the use of these organic components in robotic devices, highlighting the material combinations that make up existing devices. We quantitatively compare current mobile robots incorporating organic components with the performance of animals. Whereas our focus is on devices and applications to enable the development of mobile robotic platforms, it should be noted that the development of biohybrid systems incorporating plants (Flora robotica (17)) is also a growing area of research. Interested readers should refer to Skrzypczak et al. for additional insights on plant-based biohybrid systems (18). We address the need to formulate a clear and descriptive lexicon for device description that does not currently exist. Finally, we conclude by discussing future avenues of device development.
Fig. 1.
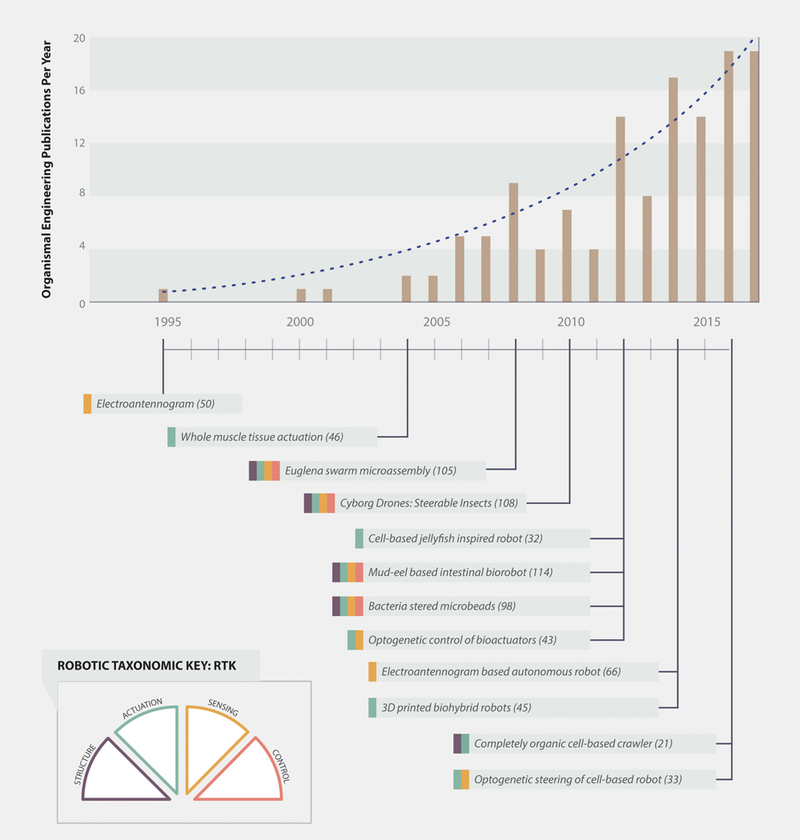
While in its infancy, the field of biohybrid and organic robots is growing quickly, and many papers on organobots, biohybrid robots, and cyborg drones have been published in the past few years (Top). During the past two decades, many exciting devices have been developed, as indicated by the small selection provided here (Bottom). These devices are color coded to indicate the organic components as outlined by the Robotic Taxonomic Key (RTK; Bottom left; see Figure 2 for RTK details).
2. Robotic Taxonomic Key for Device Characterization
A complete, autonomous robotic system requires four elements: structural framework (henceforth “structure”), actuation, sensors, and control. Each of these elements can consist of organic components, hybrid components, or synthetic components. To better categorize devices that have been previously reported in the literature, we have developed a simple robotic taxonomic key (RTK) as shown in Figure 2.A. This key provides a quick and convenient guide for device classification, allowing devices to be easily categorized by more accurate keywords in the future. Each robotic element is represented by one of the RTK’s four wedges. For each wedge, there is a simple question: Is the component organic (colored fill), hybrid (striped fill), or synthetic (white fill)? If the device lacks a particular component, the corresponding wedge can simply be greyed out. This classification scheme allows quick visualization of the organic make-up of a device, whether that device has organic components (Figure 2.B), or is fully synthetic (Figure 2.C). By asking these questions, one can categorize all existing devices, as well as devices yet to come.
Fig. 2.
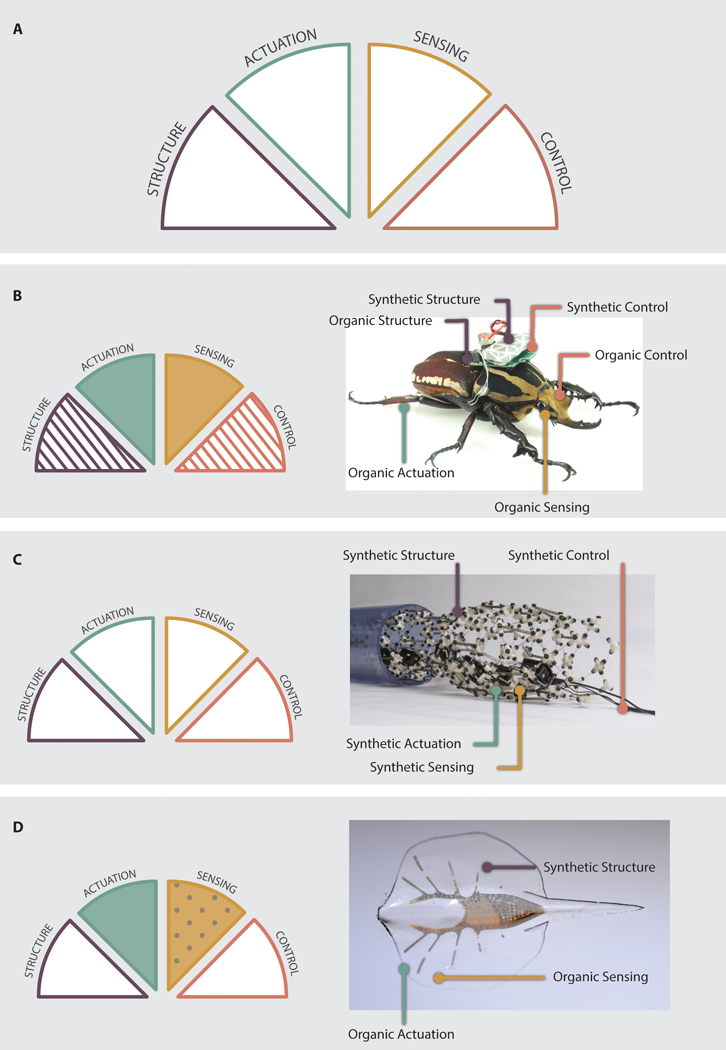
The Robotic Taxonomic Key and examples of its usage. A: The RTK consists of four wedges: Structure, Actuation, Sensing, and Control. Each wedge can be shaded or patterned to visually describe robotic devices. B: Application of the RTK to an organism based robot (146, 147). The device uses a beetle as a base that provides organic structure, actuation, sensing and control. It is, however, augmented with synthetic structures and control. As a result, sensing and actuation have solid colored fills and control and structure have striped fills. C: Application of the RTK to a synthetic robot (148). Since no components are organic and the robot has all four components, each branch has a solid white fill. D: Application of the RTK to a biohybrid device with genetically engineered components (33). The cells have been modified to respond to light; as a consequence, the sensing wedge has a dot pattern.
Emerging technologies and applications can be added to the RTK by using additional pattern options. For example, genetic engineering of cells to induce responses to optical stimuli has been used to create optical sensors for robotic devices. Therefore, we can add the question, “Is the organic component genetically engineered?” to the RTK classification process. If the answer is ‘yes’, the wedge can be filled with a dot pattern (Figure 2.D). Similarly, the RTK can be updated by future researchers as new, unforeseen technologies are developed. A list of selected publications demonstrating the use of the key for classification of the devices is presented in Table 1, along with measures of device performance.
Table 1.
Selected papers detailing the synthetic (S) or organic (O) components of devices incorporating biological components in the previously described categories: Structure (Str.), Actuation (Act.), Sensing (Sens.), Control (Con.). Relevant metrics for the devices are reported as available: velocity in body lengths (BL)/s for locomoting device and success rate (S.R.). Where success rate is the percentage of total trials in which the devices successfully accomplished the locomotion task tested (e.g. following a line of a certain length). Asterisks indicate inclusion of the reported device in allometric scaling analysis (See Section 4).
| Paper | Year | Str. | Act. | Sens. | Con. | Body Length (BL) | Vel. (BL/s) | S.R. % |
|---|---|---|---|---|---|---|---|---|
| Kuwana (50) | 1995 | S | S | O | S | |||
| Beer (149) | 1998 | S | S | S | S | |||
| Webb (150) | 2001 | S | S | S | S | |||
| Herr (46)* | 2004 | S | O | - | S | 120 cm | 0.33 | |
| Xi (24) * | 2005 | S | O | - | - | 138 μm | 0.27 | |
| Kim (151) | 2006 | S | O | - | - | |||
| Feinberg (27) * | 2007 | S | O | - | - | 3–6 mm | 0.008–0.045 | |
| Kim (25) * | 2007 | S | O | - | - | 2.5 mm | 0.034 | |
| Sato (108, 109) | 2010 | S/O | O | O | S/O | 56 | ||
| Takemura (19) | 2010 | O | O | - | - | 2 mm | 0.003 | |
| Warwick (152) | 2010 | S | S | S | S/O | 46 | ||
| Hayashi (80) | 2011 | S | S | S | S/O | 80 | ||
| Chan (26) * | 2012 | S | O | - | - | 7 mm | 0.033 | |
| Kim (98) * | 2012 | S/O | S | O | O | 10–20 μm | 0.13–0.329 | |
| Latif (112) | 2012 | S/O | O | O | S/O | 10 | ||
| Nawroth (32) * | 2012 | S | O | - | - | 2 mm | 0.4–0.7 | |
| Zhu (114) * | 2012 | S/O | O | O | S/O | 380 ± 20 mm | 0.046 | |
| Magdanz (107) * | 2013 | S/O | S/O | - | S | 300 μm | 0.016–0.033 | |
| Cvetkovic (45) * | 2014 | S | O | - | - | 6 mm | 0.026 | |
| Ijspeert (153) | 2014 | S | S | S | S | |||
| Martinez (66) | 2014 | S | S | O | S | 96 | ||
| Park (99) | 2014 | S/O | O | O | O | 80 | ||
| Williams (31) * | 2014 | S | O | - | - | 2 mm | 0.005 | |
| Barroso (100) * | 2015 | S/O | O | O | O | 2 um | 3.5 | |
| Huang (63) | 2015 | S | S | O | S/O | 42 | ||
| Sanchez (111) | 2015 | S/O | O | O | S/O | 70 | ||
| Holley (28) * | 2016 | S/O | O | - | - | 9.2 mm | 0.015 | |
| Li (97) | 2016 | S/O | O | O | S/O | 89.5 | ||
| Park (33) * | 2016 | S | O | O | S | 14 mm | 0.23 | |
| Webster (21) * | 2016 | O | O | - | - | 4 mm | 0.003 | |
| Webster (48) * | 2016 | S/O | O | - | - | 4 cm | 0.003 | |
3. Organic Components in Robotics
Although devices may have multiple categories of organic components, we systematically discuss each category separately: structures, actuators, sensors, and control. We begin by discussing the use of organic structures in robotic platforms. To date, the development of such structures has been limited to the use of collagen or through building robotic systems based on existing organisms (organism-based). More research has been focused on the use of organic actuators, sensors, and controllers wherein the organic components can be cell-based or make use of whole tissues or organs. Furthermore, cyborg drone systems have been developed in which existing organisms are used as a platform on which the robotic control system is built. These devices naturally employ organic structures, actuators, sensors, and controllers that are subsequently augmented by synthetic components. To reduce repetition, such organism-based devices are discussed separately (Section 3.5).
3.1. Organic Structures
Structural components provide the groundwork upon which robotic devices are built, as the kinematics and material properties of the structure affect the modes of actuation, sensors, and control architectures that will be incorporated into the device. However, as the field of biohybrid and organic robots grows, most devices continue to implement hybrid systems in which the organic actuators and sensors interface with inorganic structural components. In such systems, the structures used have been primarily synthetic, biocompatible polymers. Some exceptions exist, however, through the use of biological polymers or existing organisms (organism-based devices are discussed in detail in Section 3.5).
As a biological polymer, collagen provides an intriguing material for soft robotic structures. Collagen can be readily isolated from a variety of tissues and natively promotes cellular attachment. Takemura et al. used collagen gels, with a stiffness of 270 Pa, as a structure for a cell-powered jellyfish-inspired device (19). Additionally, commercially available collagen sheets have been used in a proof-of-concept system for establishing a cardiomyocyte driven joint for potential use in legged robots (20). Cells seeded on one side of the collagen were able to bend the joint.
Organic structures can also be used to not only promote cellular attachment, but also cause cellular alignment. By using electrocompacted and aligned collagen (ELAC) sheets as a structure for both cardiomyocyte and skeletal muscle cell-seeded robots, Webster et al. fabricated self-assembling devices capable of crawling (21). The use of ELAC as a structure for completely organic robots has many advantages over synthetic polymers. ELAC is biocompatible, does not require micro-patterning to promote cellular attachment or alignment, and the stiffness of the material can be controlled by crosslinking and/or by varying compaction time. The devices reported by Webster et al. had an indentation stiffness of ~640 N/m2; the stiffness of ELAC can be varied several orders of magnitude with chemical crosslinking. In addition, the collagen causes cellular alignment without micro-patterning. Additional devices without synthetic structure components have been developed using bacteria and protists as will be discussed in Section 3.5.
3.2. Organic Actuators
Organic actuators fall into two categories: cell-powered and tissue-powered. Cell-powered devices are developed by isolating individual cells and culturing them in such a way as to produce coordinated locomotion. For such devices, research has focused primarily on cardiomyocytes and skeletal muscle cells. Tissue-powered devices are fabricated by isolating intact tissue, primarily skeletal muscle tissue, from a living organism.
3.2.1. Cell-based Devices
Cardiomyocyte-based:
Cardiomyocytes are a natural choice as actuating cells, as isolated cardiomyocytes spontaneously contract in culture and can be paced with an external electrical field, eliminating the need for complicated on-board circuitry or microfluidics. Early efforts were often inspired by the results of organ-on-a-chip experiments using cantilevers, such as those reported by Park et al. (22, 23), where one side of a micro-cantilever was seeded with cells. The contraction of the cell layer resulted in bending of the cantilever. By using one or more cantilevers in device body geometries, a wide range of cardiomyocyte-powered devices has been created. For example, in 2005, Xi et al. used this technique to fabricate a two-legged crawling device with a micro-patterned silicon backbone to induce attachment and alignment of seeded cardiomyocytes (24). Contraction of the cardiomyocyte layer resulted in locomotion with a maximum speed of 38 μm/s. Furthermore, Kim et al. demonstrated the possibility of fabricating cell-powered robots that functioned over long periods of time by combining micro-patterned PDMS with cardiomyocytes (25). The resulting asymmetric six-legged robots (Figure 3.A) were capable of functioning for up to 11 days at an estimated speed of 86.8 μm/s. Similarly, Chan et al. have developed a single-legged cantilever-based cell-powered robot with a 3D printed polyethylene (glycol) diacrylate (PEGDA) substrate (26). Using a stereolithographic printer, a body was fabricated that consisted of a thick base block with asymmetric cantilever arms on the top. Cells were subsequently seeded on either the top or the bottom of the cantilevers to produce different locomotion gaits.
Fig. 3.
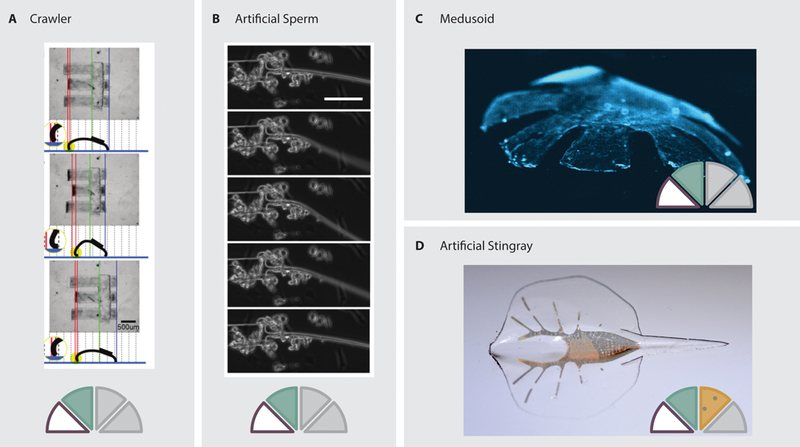
Examples of cardiomyocyte-powered devices. A: A three-legged crawler driven by cardiomyocyte actuation of long cantilever-based legs (34). B: A spermatozoa inspired biohybrid device that swims due to cardiomyocytes driving the actuation of a long filamentous tail (31). C: A jellyfish-inspired device in which mircopatterning is used to direct the growth of cardiomyocytes seeded on the radial arms (32). D: A stingray inspired device powered by optogenetically modified cardiomyocytes to facilitate steering (33).
Micro-patterning can be used to orient cells at any angle to the base substrate. Feinberg et al. have demonstrated this concept by using angled patterning to fabricate a wide variety of actuators, including radial coiled actuators, cylindrical actuators with displacement along the long axis, and micro-grippers (27). In the same study, the effect of anisotropic vs. isotropic cell orientations was demonstrated through the fabrication of triangular-shaped swimming cell-powered robots.
More complex swimming devices have also been developed, including a PDMS-based self-stabilizing swimming robot powered by cardiomyocytes (28–30). The device consisted of three layers: a PDMS-Nickel composite layer, a PDMS cantilever layer, and a PDMS-microballoon composite layer. The cantilever layer served as an actuating tail sandwiched between the nickel-composite ballast and the microballoon-composite top. By varying the ratios of nickel to microballoon PDMS, the stability of the device could be tuned. Furthermore, the nickel layer was magnetic, allowing for control of the device’s position during cell seeding.
Animals also provide a source for body morphology inspiration, leading to biologically-inspired cell-powered devices. Taking bio-inspiration from spermatozoa, Williams et al. developed a synthetic flagellar swimmer (31). Cardiomyocytes were localized at the junction between a broad head and filament tail (Figure 3.B). Contraction of the cells subsequently deflected the tail, propelling the robot forward. In 2010, Takemura et al. developed a hemispherical jellyfish-inspired, cell-powered swimming robot using gel-encapsulated cardiomyocytes (19). Cells were seeded in a thin cylindrical collagen gel. Due to the thickness of the gel, cells within the bulk of the material were starved of nutrients and died off, leaving only an external layer of living cells. The passive and active stresses of this layer caused the cylinder to deform, resulting in a dome with living cardiomyocytes on the concave bottom surface. Active contraction of the gel resulted in pulsating displacement that served to propel the device at a speed of 6.25 μm/s. Using inspiration from another species of jellyfish, Nawroth et al. reverse-engineered a body and musculature pattern mimicking that of the animal (32). By patterning an approximate musculature on a PDMS film with radially oriented arms, the device was capable of locomotion between 0.8 and 1.4 mm/s (Figure 3.C).
Optogenetically modified actuating cells have also been used to control biohybrid devices. In 2016, Park et al., used optogenetics to create a steerable cell-powered robot inspired by the sting-ray (33). Cells were seeded on an engineered body with spatially varying geometric and mechanical properties (Figure 3.D). The cells on each side of the body were genetically modified to respond to light at different wavelengths. When the matching wavelength is focused on the front of the body, the cells contract and signal proximal cells via electrical gap junctions. This results in a contractile wave propagating along the body of the device, moving it forward. By adjusting the strength of light on the two sides of the device, the contraction force on opposite sides of the body can be varied, thereby steering the robot.
Many other interesting cardiomyocyte-powered devices have been developed. For example, cardiomyocytes have been used to actuate micro-pumps (34). Additionally, cantilevers can be used for ‘heart-on-a-chip’ applications, which may allow the effects of pharmaceuticals on cellular contraction to be tested (22, 35, 36).
Skeletal muscle-based:
Skeletal muscle has also been used for fabricating cell-powered devices. Skeletal muscle cells have been shown to produce faster locomotion as compared to cardiomyocytes when both are seeded on the same type of device (21). Development of skeletal muscle cell-powered devices has, in many ways, mirrored that of cardiomyocyte-powered devices, with early efforts making use of skeletal myotubes cultured on cantilevers (37–40). Although most research efforts have focused on mammalian or avian cell sources, contractile cells have also been isolated from insects (41, 42). Similar to cardiomyocytes, skeletal muscle cells can be optogenetically modified to contract in response to light stimuli. Using such techniques, Sakar et al. developed optically controllable skeletal muscle actuators on tissue gauges (Figure 4.A) (43). Similarly, Neal et al. have demonstrated the ability to use optogenetically modified cells to control the position and rotation of suspended PDMS blocks (Figure 4.B) (44).
Fig. 4.
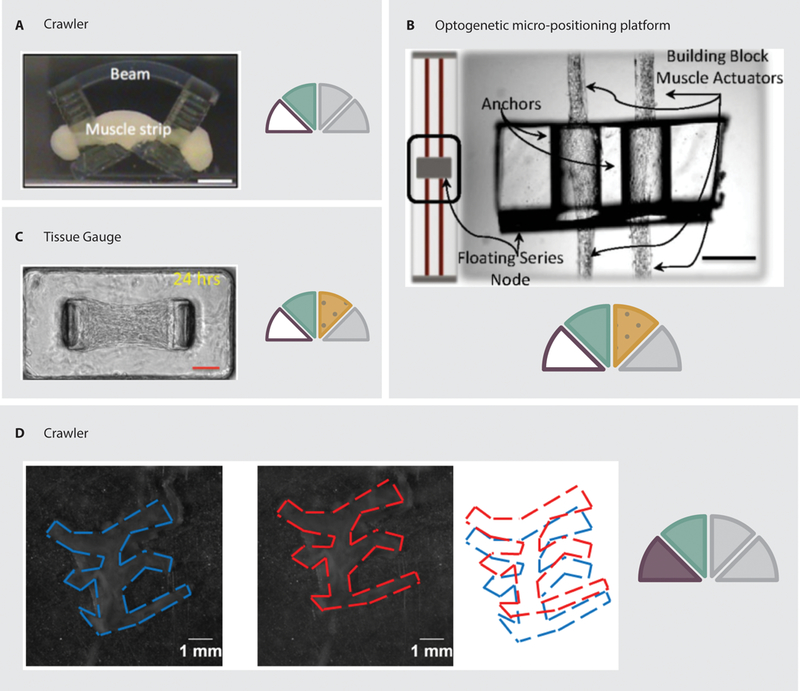
Examples of skeletal muscle powered devices. A: A crawling device driven by contraction of a cell/gel tissue construct supported by a 3D printed structure (45). B: A micro-positioning platform suspended by optogenetically modified cell/gel muscle actuators, which allow the position and rotation of the platform to be controlled via light stimuli (44). C: A stationary culture system in which cells are seeded in a gel mixture around flexible pillars. Deflection of the pillars allows the contraction force to be calculated (43). D: A completely organic device using electrocompacted and aligned collagen as a substrate (21).
Mobile devices powered by skeletal muscle cells have also been developed. In 2014, Cvetkovic et al. developed a 3D-printed device powered by skeletal muscle cells (45). The device was printed with a long body and two T-shaped legs. A cell/gel mixture was then seeded around the legs while the device was upside down. During culture, the passive stresses of the cells tightened the gel around the legs, forming a tissue construct. When the device was flipped over, slight asymmetries in the structure allowed the cell-powered robot to move forward (Figure 4.C). Furthermore, Webster et al. created completely organic multi-legged devices powered by skeletal muscle cells using electrocompacted collagen structures (21). During fabrication, the collagen molecules were compacted into a robust sheet and aligned along the leg axis of the device. This method aligns the cells so that their contraction is coordinated. The devices were capable of crawling in response to an external electrical field (Figure 4.D).
3.2.2. Tissue or Organ-based Devices
Several devices have used muscle tissue to directly power devices. Because the structure of the muscle is maintained, overall contractile force is improved. Removal of the muscle from the supporting vasculature, however, results in rapid deterioration. As a consequence, devices have limited lifetimes. In 2004, Herr et al. developed a swimming robot, actuated by tissue from the semitendinosus muscle of a frog (46). In this device, the muscle was sutured to the synthetic body consisting of a rigid fore-body and compliant tail. The muscle was subsequently stimulated, using on-board circuitry, via electrodes wrapped around the tissue. The device swam for 4 hours over a 42 hour lifetime when stimulated with a 10 % duty cycle. This lifetime is much shorter than that of devices using cultured cell layers, likely due to the inability of nutrients to diffuse throughout the thick muscle tissue.
Recently, invertebrate muscle has found use in biohybrid systems. Many invertebrates are capable of surviving in a wide range of environmental conditions and, as a consequence, have developed robust tissues. Insect dorsal vessel has been demonstrated to be a suitable actuator for microrobots. Akiyama et al. combined excised dorsal vessel tissue with a molded PDMS frame to create a stepping robot capable of locomotion at 66 μm/s with a locomotion efficiency of 17% (47). Furthermore, the velocity of the device can be increased 8-fold by addition of crustacean cardioactive peptide to the surrounding medium. Additionally, the marine mollusk Aplysia californica has been investigated as a source of muscle tissue (48). Aplysia’s open circulatory system allows thin muscles to function without specific vasculature, which may simplify the design of larger robots. Using the thin I2 muscle from the animal’s feeding apparatus, Webster et al. fabricated a crawling robot with a 3D printed body capable of locomotion speeds of approximately 0.08 mm/s. Muscle tissue from earthworms has also been used to drive a bio-micropump (49). In this pump, a section of earthworm muscle was isolated and flattened to drive the displacement of a diaphragm.
3.3. Organic Sensors
Although many recent research efforts in the biohybrid and organic robotics field have focused on developing organic actuators, the use of organic materials as robotic sensors is not new. In fact, it might be said that the first “Biorobot” was a device using organ-based sensors (50) (See below). Organic sensors have been developed for a wide variety of sensory modes using both cell-based and tissue or organ-based systems.
3.3.1. Cell-based Devices
Mammalian cell-based systems have been used for olfactory, visual, and tactile sensing. In 2013, Du et al. developed a cell-based olfactory sensor using genetically engineered olfactory sensory neurons (51). To target a specific olfactory ligand, diacetyl, the cells were modified by transfection with the C. elegans gene for the ODR-10 olfactory receptor. The sensor demonstrated the ability to distinguish between odorants with and without diacetyl. Furthermore, the position of olfactory cells relative to the recording array can be modified with DNA-directed immobilization (52). This enables precise placement of cells on electrode pads, thereby improving recording sensitivity. Organic components have also been used as optical and visual sensors. As mentioned above, muscle cells have been optogenetically modified to contract in response to specific light cues, allowing local position control of small PDMS blocks (43, 44), and steering of a sting-ray inspired robot (33). Furthermore, the walking robot initially presented by Cvetkovic et al. (45) has been further developed to include optogenetically modified cells for stimulation (53), demonstrating similar locomotion capabilities between electrically stimulated and optogenetically stimulated devices. The slime mold Physarum polycephalum can also be used as an optical sensor (54). Physarum exhibits phototaxis behavior, and regular oscillations in the cell’s surface potential are measurable via external electrodes. Adamatzky has demonstrated that a slime mold cultured across two electrodes displays unique changes in surface potential in response to red, blue, and green light, but did not exhibit discrimination between green and white light (54). This phenomenon could be used for the future development of slime mold circuits and robots.
Biohybrid systems can also be used to create tactile sensors. In such platforms, mechanical stimulation of the biological components results in changes in membrane potentials of the cells that are monitored via electrodes. This technique has been previously used with fibroblasts, and even with slime mold, serving as the biological components. In 2011, Buselli et al. developed a stretchable skin-like sensor in which a cell monolayer was seeded on a PDMS substrate in a 100 μm gap between two Au/Ti electrodes (55). Stretching the PDMS substrate applied mechanical loads to the cells, resulting in the activation of ion channels and subsequent changes in intracellular ion concentrations. Using similar concepts, Cheneler et al. proposed a fibroblast-based sensor in which the cells are encapsulated in alginate between a PDMS cover and a conductivity sensor (56, 57). Microfluidic channels were incorporated into the base of the device to provide nutrients to the cells. Rather than sensing the lateral stretch of the cell culture substrate, the sensor responds to pressure to the PDMS cover above the cell-laden alginate. Such pressure sensors can be developed with a variety of cells. Using Madin Darby Canine Kidney cells, Salgarella et al. developed a biohybrid tactile sensor and investigated the effect of including a hydrogel layer over the cells as compared to only growth media, finding a significant improvement in sensor performance in the growth media alone (58).
As the fundamental principle behind using living cells in biohybrid tactile sensors is the mechanochemical properties of cell membranes and ion channels, the concept is not limited to mammalian cells. In fact, Adamatzky has previously presented a tactile sensor using Physarum polycephalum as the living component in a biohybrid sensor (59). Physarum exhibits natural periodic oscillation in surface electrical potential with amplitudes ranging from 0.1–5 mV. To use Physarum in a biohybrid tactile sensor, Adamatzky cultivated a single Physarum cell between two electrode pads. Pressure to the protoplasmic tube that formed between the pads resulted in measurable spikes in membrane potential and alterations in the overall oscillation. Similarly, bristles can be attached to the electrodes, and the Physarum will grow around the base of the bristles (60). Deflection of the bristle then resulted in a measurable voltage change across the electrodes. A more detailed review of biohybrid and bio-inspired tactile sensors can be found in Lucarotti et al. (61).
3.3.2. Tissue or Organ-based Devices
Whole organ sensors in robotic systems have primarily used insect tissues as visual or olfactory sensors. The visual system of the blowfly, specifically the H1-cells, has been used to provide visual input to a robotic system (62, 63). The H1-cells are excited by back-to-front visual motion. The blowfly was mounted on a wheeled robotic platform and extracellular electrodes were used to record from the cells of interest (Figure 5.B). The robot was preprogrammed to move in a sinusoidal pattern to provide back-to-front visual motion with a front-facing blowfly. Sensory input then served to modify the trajectory of the robot so that it could follow a wall down a corridor.
Fig. 5.
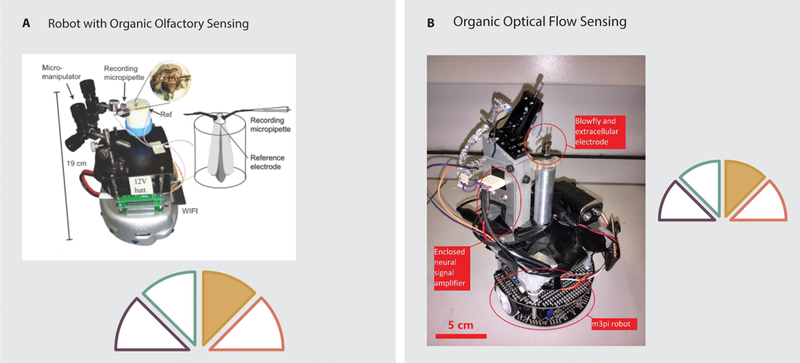
Examples of organic sensing in robotics. A: A robot capable of olfactory sensing via electroantennograms. Electrical signals are recorded from the antennae of an intact moth and used to control the heading of a wheeled robotic platform in order to track odor plumes (66). B: A robot capable of wall following due to the visual sensory input from H1-cells of a blowfly mounted on a wheeled robotic platform (62, 63).
Early biohybrid olfactory sensors used insect antennae to provide sensing capabilities. In 1995, Kuwana et al. built a robotic platform which read sensory input from the isolated antennae of silk moths (50). This robot consisted of a simple synthetic motor-driven platform. Two isolated antennae were attached to an amplifier that provided input to a microprocessor. When pheromones were sprayed on the right sensor, the robot turned to the right, and when sprayed on the left sensor, the robot turned to the left. Using insect-based electroantennograms, Myrick et al. demonstrated the ability to discriminate odors in real time using 4–8 antennae to provide electrical inputs to a classifier (64). However, the longevity of the isolated antennae was low (30–90 minutes). Myrick et al. therefore modified their four channel electroantennogram to hold four live moths, thus reducing the problems of longevity. With this platform, they were able to locate a distant odor source, although the system was not mounted on a robotic platform (65). More recently, Martinez et al. have developed a mobile platform using a synthetic base and controller with organic sensors (66). As with Myrick et al.’s live moth electroantennogram, Martinez et al. have developed a device in which an intact moth is mounted on a robotic platform (Figure 5.A). Electroantennograms are then recorded from the animal. This resulted in a significantly longer operating time for the sensors than those observed for Kuwana et al.’s mobile platform. Whereas isolated antennae showed a significant decrease in output over a few hours, the use of the intact animals showed no such decrease over an 8 hour trial period.
Olfactory sensors have also been developed using intact mammalian tissues. In 2010, Liu et al. developed a ‘bioelectronic nose’ by recording extracellular potentials from intact olfactory epithelium isolated from rats (67). By isolating intact tissue, Liu et al. maintained the functional structure of the organ, including the support cells as well as the receptor neurons, along with any spatial structure associated with each neuron’s unique odorant response. Using power spectrum analysis, this sensor was able to discriminate between two different odorants. Using this platform, Liu et al. subsequently developed a system capable of distinguishing between four odors presented in real-time (68). As with the intact moth electroantennograms previously discussed, mammalian tissues do not necessarily need to be isolated to be used as olfactory sensors. In 2013, Dong et al. used in vivo recording from a single site within the olfactory bulb of rats via a brain-computer-interface to achieve odor sensing accuracies of up to 95% (69). Although the system demonstrated high sensitivity to odor stimuli, the neural activity was not analyzed to distinguish between different odors.
3.4. Organic Controllers
The vast majority of research into onboard organic controllers for mobile robots to date has focused on the development of cell-based devices. These systems used dissociated neurons with electrodes or multi-electrode arrays to record from and stimulate cultured neural circuits. Furthermore, recent research has begun to investigate the use of whole ganglia, intact clusters of neurons, as simple onboard controllers for muscle-actuated robots. Additionally, off-board organic control can be achieved using brain-computer-interfaces (BCIs). Two overarching categories of BCIs exist, those in which the interface is used to externally control an organism (i.e., cyborg drones), and those in which the organism controls mobile elements or external devices via the interface (e.g., functional electrical stimulation of leg muscles to bypass spinal cord injuries). This distinction is based on the locus of the goal setting for the biohybrid device. In the case of cyborg drones, an external user dictates the goals of the organism, whereas in most rehabilitation applications, the patient’s goals dictate changes in their body or external systems (internal goal BCI). For the purposes of our review, we classify cyborg drones as organism-based devices (see Section 3.5), whereas we will discuss internal goal BCIs in the context of organ-based control. As BCIs are a prevalent tool in neurorehabilitation research, and have been extensively reviewed elsewhere, we will only briefly discuss such systems in the context of mobile robots. Interested readers are encouraged to refer to the recent BCI reviews by Lebedev et al. (70) and Pohlmeyer et al. (71).
3.4.1. Cell-based Devices
To develop organic controllers, researchers have often used neurons cultured on electrode arrays, so that they may record from and stimulate the neurons. In early efforts to use living neurons to control an artificial system, DeMarse et al. demonstrated the ability of cortical neurons cultured on a multi-electrode array to control a simulated animal (72). While the simulation received input from the living culture and, in turn, provided sensory feedback in the form of stimulation to the cells, the simulation was not trained to perform any particular task. Similar neural cultures have been used to control external mobile robots for more specific goals (73). For example, using a Khepera robot, Novellino et al. developed a bidirectional neural interface that allowed the small wheeled robot to perform obstacle avoidance in an arena while under the control of cultured neurons (74). In 2010, De Santos et al. developed a system using neuroblastoma cells, in which cell activity was recorded and processed (Figure 6.A). Control signals were subsequently transmitted to a remote mobile robot system (75). Similarly, Warwick et al. developed a robot using a synthetic wheeled platform. To control the robot, approximately 100,000 cortical neurons were cultured on a multi-electrode array. The neural activity served as input to the platform’s actuators and the cultured cells were stimulated based on signals from onboard ultrasonic sensors to provide feedback (76, 77). While the device would turn in response to the presence of a wall, demonstrating closed loop capabilities using neural control, it also generated spontaneous turns, in which no stimuli were present, at a higher frequency than meaningful turns. In fact, 54% of turns were spontaneous.
Fig. 6.
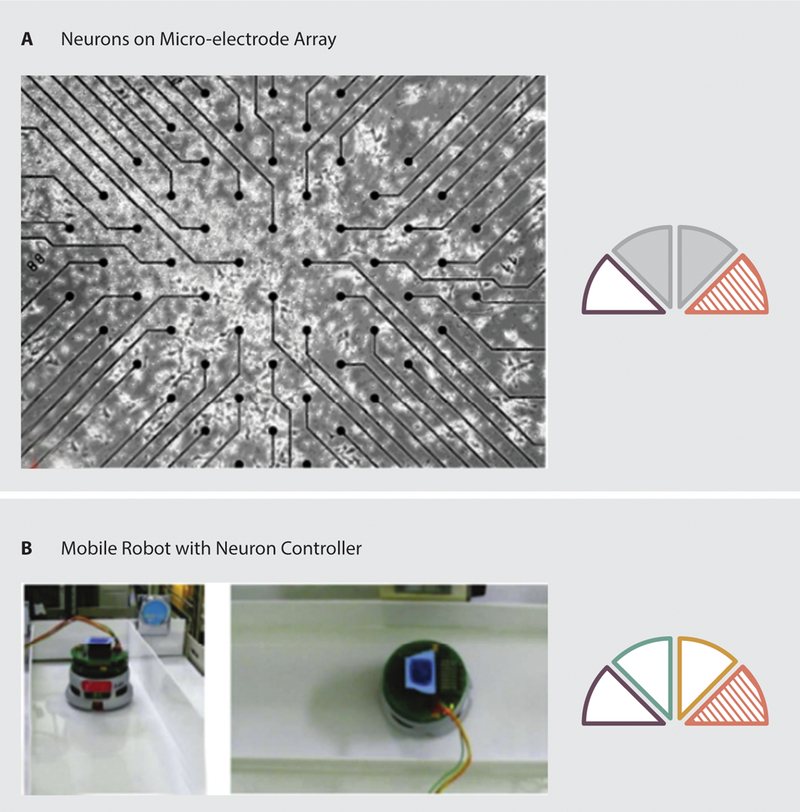
Examples of organic control in robotics. A: neurons cultured on a multi-electrode array (75). B: A wheeled mobile platform with organic control resulting in wall following (80).
Neural cultures have also been used to control robotic drawing arms, bringing together biology, art, and robotics (78, 79). As with neuron controlled mobile robots, this system used multi-electrode arrays to record from and stimulate living cells. The neural culture was used to control two robotic arms, each grasping a pen. The activity of the neurons was transmitted remotely to the arms which subsequently drew using the pens. A camera mounted above the drawing then transmitted sensory information back to be input to the neural culture. In this way, the platform could be installed in art galleries around the world (78).
Cultured neurons are often combined with artificial intelligence techniques to serve as controllers. For example, in 2011, Hayashi et al. presented a neuron-controlled mobile robot, in which they used fuzzy logic to have the device follow a line (Figure 6.B). Signals from the neuron culture were represented as fuzzy If-Then rules and activity in the culture controlled motor speed (80). Using this system on a commercially available Khepera robot platform, the device was able to follow a line while avoiding wall collisions with an 80% success rate.
Furthermore, the potential learning capabilities of cultured neuron controllers have also been investigated. Building on the Khepera platform developed by Novellino et al. discussed earlier, Novellino et al. subsequently incorporated a learning phase to enforce obstacle avoidance as the robot navigated an arena (81). Conditional stimuli were provided as a pairing of tetanic bursts with low-frequency stimuli when the robot struck an obstacle (82). Following this training protocol, the robots exhibited a 30% decrease in the number of obstacles struck. In 2016, Li et al. developed a robotic system in which cultured neurons controlled the robot’s heading by stimulating turns based on received inputs such that the robot would move towards an object. Li et al. showed that providing high frequency training stimuli when the robot turned the wrong way significantly improved the robot’s ability to reach the object (83). Human neurons differentiated from human fetal neural stem cells (obtained from donated miscarried fetuses) have also been used to investigate learning and operate a small mobile robot. Pizzi et al. developed a control architecture using these human neurons cultured on a micro-electrode array as a pattern classifier (84). Patterned stimuli were provided to the culture and subsequent responses were recorded using the electrode array. An artificial neural network (ANN) was then used to process the output and provide information to the robotic platform.
3.4.2. Tissue or Organ-based Devices
Incorporating the natural neural architectures can enhance the effectiveness of organic controllers. Researchers have primarily used cultured cells rather than intact isolated tissues as controllers for mobile robots in vitro. However, in 2017, Webster et al. developed a mobile biohybrid robot using the natural neural circuitry and neuromuscular junctions from Aplysia californica (85). This robot used an intact muscle (the I2 protractor muscle (86)), from the animal’s feeding apparatus as an actuator. Native innervation from the buccal ganglia (motor control) and cerebral ganglion (higher level control) was kept intact. The muscle and associated ganglia were then mounted in a 3D printed, inchworm-inspired body that isolated the cerebral ganglion from the rest of the circuit, allowing chemical stimulation to be applied to it, without directly stimulating the muscle. The application of carbachol, which is known to induce biting patterns in semi-intact in vivo preparations (87), resulted in cyclic contraction of the muscle, without the use of external electrical stimulation. In this way, the ganglia acted as a motor controller for the muscle, thereby producing locomotion.
Another way to incorporate natural neural architectures is to use brain-computer-interfaces (BCIs). Most BCI research is geared towards functional restoration following stroke or spinal cord injury through the use of implanted electrodes. Using implanted electrodes enables direct mapping of neural signals for controlling robotic arms and prosthetics, as well as driving wheelchairs. In 2000, Wessberg et al. demonstrated the ability to train owl monkeys to control a robotic arm in real-time via implanted micro-wire arrays in the cortical areas of the frontal and parietal lobes (88). The ability of primates to control not only robotic arms, but also provide whole body movement via a BCI controlled wheelchair has also been demonstrated using implanted electrodes (89). Monkeys were placed in a chair mounted on a wheelchair base and were trained to control the wheelchair to move towards a food reward station. Initially, the wheelchair was driven externally so that the monkeys observed that motion to the feeding station resulted in a reward. Subsequently, both monkeys learned to navigate to the reward station using the interface. Over the 3–6 week experimental period, both monkeys achieved significant reductions in travel time, indicating improved control abilities (89). Implanted electrodes have also been used in humans with tetraplegia to control reaching and grasping with a robotic arm (90). Using 96-channel micro-electrode arrays implanted in the motor cortex of each participant, Hochberg et al. decoded spiking signals to use as control inputs for a robotic arm. Success rates in reaching and grasping trials ranged from 47.9 – 95.6 % (90).
The natural neural architecture may generate non-invasive signals that can also be used for control. Although many patients with severe motor disabilities would be willing to accept implanted electrodes (91), non-invasive solutions are needed for many applications, particularly remote control of robotic systems by healthy individuals. Non-invasive BCI systems have been developed that use external sensors to monitor brain activity and translate the signals to control commands. Examples of such systems include EEG control of wheelchairs (92–94), humanoid robots (95), and telepresence platforms (96). With EEG control, users are instructed to execute mental tasks that correspond to higher level control concepts. For example, the wheelchair control system developed by Galán et al. required users to execute three mental tasks, including imagined left hand movement, word association, or mental rest, that corresponded to the wheelchair turning left, right, or going forward, respectively (93). To control a humanoid robot, the system developed by Bell et al. used EEG signals corresponding to patient focus on higher level concepts, such as objects and locations in the testing arena, to provide goals and behaviors to the robot, which then autonomously completed the commanded task (95). EEG based BCIs have even been used to control cyborg drone cockroaches (97) as described in Section 3.5.
3.5. Organism Based Robots
More traditional engineered components have also been used to ‘hijack’ living systems. Such ‘hijacked’ systems have been developed with bacteria, spermatozoa, protists, and insects. Several devices have used bacteria or protists to provide actuation, sensing, and control by attaching single-celled-organisms to a synthetic structure. In 2012, Kim et al. demonstrated that bacteria could be attached to the surface of polystyrene microbeads. The hybrid system would subsequently locomote towards a chemoattractant without outside input (98). In this case, the attached bacteria were sensing the chemoattractant and actuating their flagella to propel the microbeads. In 2014, Park et al. demonstrated similar chemical gradient navigation with bacteria. In contrast to the device developed by Kim et al., Park et al. used selectively patterned microbeads that resulted in bacteria attaching to a specific hemisphere of the bead (99). This served to coordinate the actuation of the bacteria such that the flagella pointed in a more uniform direction. Similarly, in 2015, Barroso et al. developed an optical assembly technique that allowed a bacterium to be attached to individual zeolite L crystals (100). The assembled devices were able to locomote in response to a chemical gradient.
Rather than using chemical gradients to direct bacteria, some devices make use of magnetism. A single bacterium can be captured in a magnetic microtube. The swimming bacterium becomes a bioengine to drive the microtube, which can be steered externally via a magnetic field (Figure 7.A) (101). Additionally, by using MC-1 magnetotactic bacteria, the swimming direction of the bacteria can be influenced directly by application of a magnetic field that applies a torque to a single chain of membrane-bound magnetic crystals (102). Using this mechanism, swarms of bacteria can be formed, which could be used to manipulate small blocks. When using nonpathogenic, magnetotactic bacteria, such as MC-1 or MSR-1, such devices have demonstrated applicability in targeted drug delivery (103, 104).
Fig. 7.
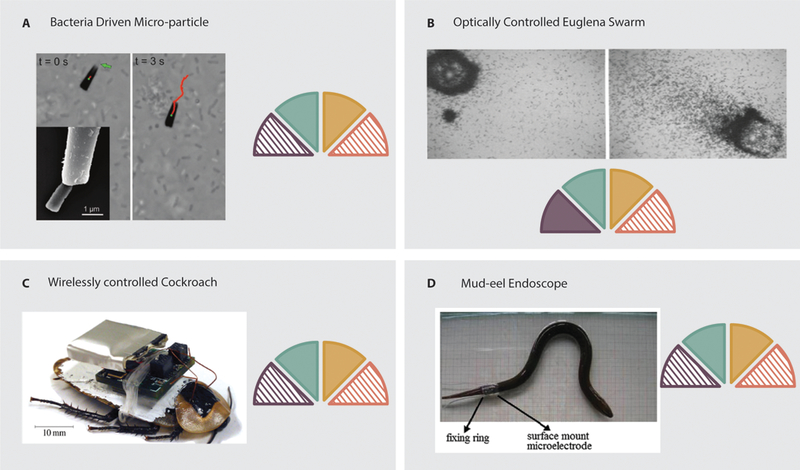
Examples of organism based systems. A: A bacteria driven microtube. The bacteria is trapped in the tube and serves as an actuator (101). B: A swarm of Euglena that can be steered via light stimuli. The swarm can be used to move micro particles (105). C: A wirelessly controlled cockroach that can be steered remotely via neural implants (111). D: A mud-eel driven endoscope. The locomotion of the eel is controlled via surface-mount electrodes on the tail, allowing controlled locomotion (114).
Similarly, swarms of protists have been used to manipulate objects. In 2008, Itoh et al. used the phototaxis capabilities of Euglena to form swarms of organisms (Figure 7.B) capable of transporting particles requiring up to 70 nN to move (105). Swarms could be guided at speeds of 1–5 μm/s. Negative inhibition of actuating bacteria can be used to control object manipulation. In 2013, Wong et al. developed micro-robots by attaching bacteria to the surface of polymer (SU8) objects (106). By applying UV light to certain regions of the object, bacteria at those locations could be immobilized, allowing control over rotational motion.
Whereas bacteria and protists have been used to steer microparticles, flagellated cells have also been used to provide actuation. Rather than using an organism to drive a synthetic structure, Magdanz et al., developed a technique for steering spermatozoa. Spermatozoa were confined in magnetic microtubes (107), and the orientation of the microtubes was controlled by application of an external electric field. Therefore, the flagellar action of the spermatozoa provided forward thrust, while the microtubes provided heading control.
Engineered control systems have also been used to ‘hijack’ much more complicated organic systems. Since the goals of such devices are set externally, we refer to these as ‘cyborg drones’. Insects have been the primary focus for development of autonomous cyborg drones as has been reviewed previously (108). We will provide a brief overview of previous work and discuss more recent devices. In such devices, the sensori-neuro system of the insect is stimulated in response to external input to control the locomotion of the insect. In 2009, Sato et al. demonstrated the ability to remotely control insects in free flight via an implantable neural stimulation system (109, 110). Flying beetles were equipped with on-board stimulation systems that allowed initiation and cessation of flight, as well as elevation control, to be elicited via neural stimulation of the brain, while turning was controlled via direct stimulation of the basilar muscles. More recently, Sanchez et al. demonstrated locomotion control of a cockroach via stimulation of the prothoracic ganglion (Figure 7.C) (111). They found that the turning radius could be controlled by varying either the frequency or voltage of the stimulation. Such cyborg drone insects can also be controlled via sensory organs. In 2012, Latif et al. modified a cockroach so that it was capable of line following when the appropriate antenna was stimulated (112). Using such techniques, cockroaches have been modified so that they can be controlled by human subjects via a brain-computer-interface (97). While the success rates were low (20%), this demonstrates the ability of human operators to potentially control other organisms. Furthermore, insects provide a possible power source for the onboard electronics needed for control. Schwefel et al. have demonstrated that by using a bio-fuel cell implanted in a cockroach’s thorax, energy available in the animal’s hemolymph can power onboard electronics for wireless communication (113).
The use of organisms for biorobot fabrication is not limited to insects. In 2012, Zhu et al. developed an intestinal biorobot using the mud eel as a source of locomotion for a potential endoscopy system (114). The mud eel was encapsulated in an outer shell, and locomotion was controlled by stimulating the eel via a surface-mount microelectrode (Figure 7.D). The encapsulated eel was capable of navigating a simulated intestinal tract, as well as sections of isolated intestine, at rates of 12–24 mm/s.
4. Allometric scaling in robots with organic components
Can general principles be derived for robots that incorporate organic elements? To achieve this goal, there should be more consistency in robotic metrics reported for each device, making device-to-device comparisons, and the development of design guidelines, possible. In our efforts to investigate scaling laws in such devices, we assessed 28 research articles that reported mobile devices and performance metrics (indicated by an asterisk in Table 1), excluding cyborg drones. These articles provide a sample of the field including a range of size-scales, actuators, and materials. Cyborg drones were not included in this analysis, as they are fundamentally animals upon which an electronic control system is built. Most cyborg drones use the animal’s natural locomotion abilities and only apply turning or locomotion initiation cues. As a consequence, their allometric length-velocity scaling would coincide with the animal systems on which they are constructed.
Typical robotic metrics were reported as follows: mass (6/28), speed (21/28), dimensions (23/28), actuator force (7/28), device success rate (10/28), cost-of-transport (0/28). Some of these metrics, such as cost-of-transport, may be difficult to determine due to the nature of the robotic systems. However, metabolic assays may serve to help quantify such information. As most of the assessed research articles did include device length and velocity information, we chose to investigate the allometric scaling of velocity with respect to body length (115). Without mass information, we are unable to calculate energy estimates, though energy can be expected to scale with .
For biohybrid robots and organobots, we compared the length-velocity scaling to the scaling laws observed in similar sized animals with similar modes of locomotion and environments (i.e. bacteria (116, 117), worms (118–122), and fish (123–133)). The length-velocity scaling of biohybrid robots and organobots was found to scale as , compared to for their animal counterparts. Using an analysis of variance, we found that while the intercept of the fits was significantly different (p = 9.9×10−12), the slope was not (p = 0.395). It is clear that the velocity of these engineered devices is significantly lower than that of similarly sized animals (Figure 8.A).
Fig. 8.
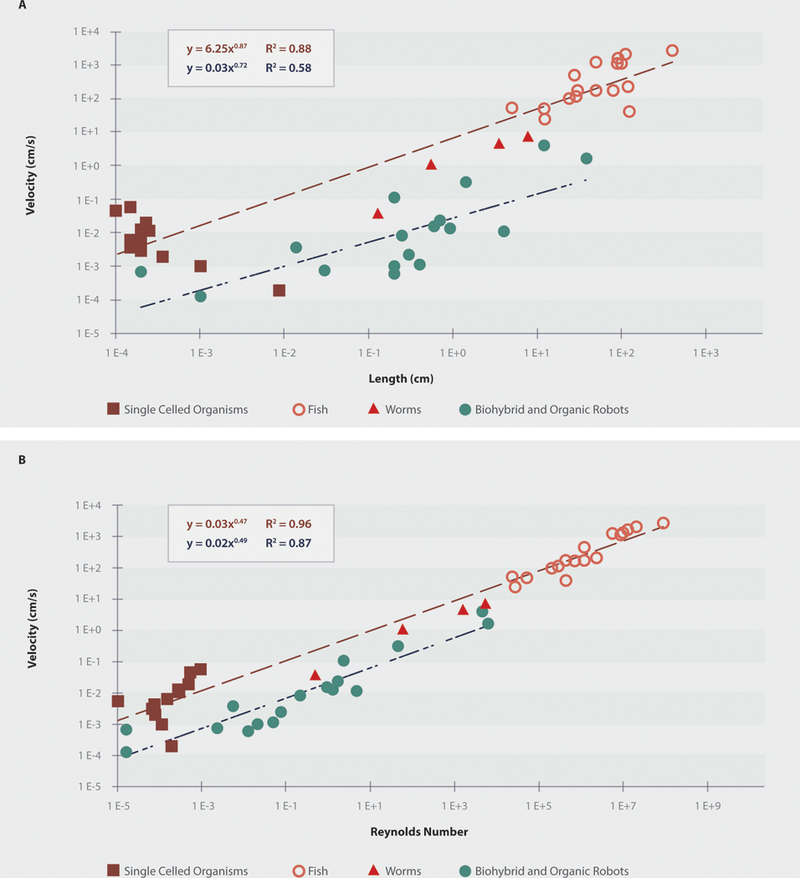
A: The length-velocity scaling of biohybrid and organic robots as compared to animals with similar locomotion types and environments on a log-log scale. Overall, the speed of robotic devices is about two orders of magnitude lower than that of similarly sized animals. B: The Reynolds number-velocity relationship of biohybrid and organic robots compared to animals with similar locomotion types and environments on a log-log scale. Overall, the robotic devices function in a lower Reynolds number regime, indicating the need for improvements in velocity, or attention to appropriate modes of locomotion.
Similarly, we calculated the Reynolds numbers for each device and animal using the length as the characteristic dimension due to lack of reported frontal areas for the robotic devices (Figure 8.B). As expected, after performing linear regression on the log of the values for the Reynolds number vs. speed data, we found that the intercept of the fit was significantly different (p = 4.9×10−11), but that the slope was not (p = 0.439). When comparing only the Reynolds numbers for the fish or worms to robots, neither the intercept (p=0.439 and p = 0.180, respectively) nor slope (p = 0.193 and p = 0.584, respectively) were significantly different. However, the majority of the robots operated in a much lower Reynolds number regime than did the fish.
This analysis suggests several conclusions. First, there is currently an inconsistency in how researchers are reporting findings with biohybrid and organic robots. As the field continues to grow and creates robots with improved capabilities, it will be important to consistently report robot parameters and metrics, as is common in traditional robotics. Such metrics include mass, speed, actuator metrics, device success rate, and cost-of-transport. Additionally, current biohybrid and robotic devices exhibit significantly lower speeds than similarly sized animals moving in similar environments. Interestingly, the robots with the highest Reynolds numbers made use of intact or native muscle, rather than dissociated cells. Future research should either seek to maintain, or better mimic the natural structure of muscle, or focus on locomotion modes appropriate for the Reynolds number regimes in which the robots are expected to function.
5. A nomenclature for describing robots with organic components
As with the performance metrics currently reported for robots with organic components, there is inconsistency in the language used to describe such devices. It is important to understand how the language of the field is developing and to ensure uniformity in linguistic designations across research groups. Robots with only organic components, robots with both synthetic and organic components, and robots using traditional materials (i.e., ‘nuts-and-bolts’ robots) whose designs are inspired by biology all incorporate biology and robotics. As a consequence, the terms describing such devices have overlapped, so that literature searches inevitably turn up results for all three kinds of devices.
To better understand how organic, hybrid, and synthetic bio-inspired robotic systems are described, we have analyzed 51 articles, including 3 reviews on biologically-inspired robots. From each article, we have identified key terms used to describe the devices. Such an analysis reveals that a wide range of terms is used to describe these devices, ranging from highly specific terms such as Flora robotica (17), to vague terms which fail to convey the presence of organic components (e.g., robot). Furthermore, “biohybrid”, “robot”, and “machine” are used most commonly to describe the analyzed systems with organic components (used in 12, 11, and 8 papers, respectively). However, with the most commonly used word appearing in less than 25% of the papers analyzed, the inconsistency in keywords is apparent. A consistent vocabulary is needed to enable researchers to readily locate research of interest. To propose a unifying lexicon, we first turn to the Robotic Taxonomic Key presented earlier. Fundamentally, the key first asks a simple question: Is the device completely synthetic? If the answer is ‘yes’, the language is simple. The device is a synthetic robot. As organic components are introduced, the vocabulary is no longer so straightforward. While the term “biohybrid” is commonly used in existing literature, and accurately describes devices combining organic and synthetic components, it fails to fully describe all devices in the field, as there is growing interest in completely organic devices. We propose the term “biohybrid” be used categorically for devices with both synthetic and organic components. The RTK can also guide the choice of additional keywords to provide further clarification about the device, such as specifying which components are organic (e.g., cardiomyocyte actuation). In cases where the device relies on an existing living organism, details about the organism should be clarified, such as using the term “cyborg drone” for devices that rely on steering a living multicellular animal, and organism-specific terms, such as “Bacteriobot”, for devices using single celled organisms. Additionally, we propose a novel term, “Organobot”, to describe robotic devices consisting of solely organic components. To aid in choosing consistent keywords and terminology, we have developed a dichotomous key (Supplementary Material 1), and taxonomy (Supplementary Material 2). Both of these tools can grow to include future technologies. Additionally, a table demonstrating how these terms would apply to devices previously reported in the literature is provided in Supplementary Material 3.
6. Conclusions
Structure, actuation, sensing, and control make up the fundamental building blocks of robots. To date, devices have been developed with organic structures, organic actuators, organic sensors, and organic controllers separately, but a true complete organobot has yet to be produced with all four components. While this review has presented a range of exciting devices, considerable work remains to be done. Current biohybrid devices and preliminary organobots are very simple when compared to more traditional robotic systems. They are often only able to perform one function, and lack the sensorimotor control system needed to perform complex tasks. Recent developments, however, in both tissue engineering and organic controllers suggest exciting possibilities for such devices in the near future.
Developing a consistent nomenclature is critical for unifying any field. For example, the use of genetic sequencing in the identification of ion channel genes has led to consistent naming conventions which better allow study and classification of new excitable tissues (134). A consistent naming scheme enables classification, concise communication of key concepts, and reveals gaps in the field that can lead to the development of novel devices. The Robotic Taxonomic Key presented in this review will be of value for all of these reasons. Similarly, developing consistent metrics for these robots will make comparisons possible and lead to general design principles for such robots.
6.1. Current limitations in the use of organic components in robotics
Despite recent developments in the field of biohybrid and organic robotics, there are still many limits on the use of organic components as structures, actuators, sensors, and controllers that will need to be addressed in the future. Many of these limitations stem from the effect of the structure on actuators, sensors, and control, particularly when working with cell based components. For example, actuators made from cells seeded on an isotropic structure lack the organization of native muscle. To compensate for this lack of organization, micro-patterning or aligned structures can be used to induce cellular organization. However, such devices have largely involved 2D structures, or used a single extracellular matrix component. As a consequence, the cultured tissue lacked the full 3D-organization and robustness of native muscle. Similarly, maintenance systems will need to be built into the structure of these devices to support long-term performance. Existing devices lack the vasculature needed to support long-term maintenance of cultured cells, or to provide nutrients to muscle tissue-based actuators. In addition, current devices have been engineered without regard to the protective mechanisms (e.g., immune system and skin) needed to allow devices to function outside of research labs. Finally, in animals, muscles interact with complex supporting structures in many ways (e.g., musculoskeletal systems, muscular hydrostats, and hydrostatic skeletons) (135, 136). These organizational structures allow animals to perform complex behaviors and can provide inspiration for future organic robots (137). Advances in bioprinting and biofabrication are likely to allow future devices to better replicate the supporting structures needed for robust robotic systems.
However, current limitations are not restricted to the structural components. Biological systems are highly interdependent, and to consider any component alone is to eliminate some supporting mechanisms. For example, removal of the nervous system from actuating cells or tissue may eliminate the modulatory effects observed when muscle is natively stimulated by motor neurons. Similarly, isolating a nervous system from sensory and actuating units essentially eliminates the feedback system through which native nervous systems function. While organic controllers have been developed for synthetic mobile robotic platforms, such controllers have yet to be used for higher-level control of organic actuators. Additionally, in such cases, the neurons have been used to perform input-output transformations, rather than make complex decisions. Therefore, it is possible that the neurons could be replaced with more traditional computing techniques such as ANNs. In the future, it will be important to demonstrate that cultured neural controllers have advantages over ANNs - for example, in their robustness to damage or unexpected inputs - to convincingly justify their use. It may be possible to exploit natural neural architectures that are capable of performing complex processing and decision making (e.g., the cerebellum) to control future devices.
6.2. Challenges
Clearly, while many exciting robotic devices have been developed using organic components, many challenges exist before such devices are deployed in the field. Currently, there are no field-specific software tools to aid researchers in the design and prototyping of robots with organic components. As a consequence, in the immediate future, there is a need to create engineering tools and computational frameworks for the design and simulation of novel biohybrid and organic systems. This necessitates multidisciplinary collaboration to bring together simulation techniques from neurobiology, dynamics, and soft body modeling in order to build a toolbox akin to those that exist for traditional robotic simulation and development. Efforts towards this goal are beginning. For example, Raman et al. have developed a modular protocol for designing and fabricating legged muscle-powered biohybrid robots (138), which is an important step towards the development of future design platforms.
Additionally, as researchers move towards the use of neurons as organic controllers, they will be adding additional layers of complexity to the devices, with significant unknowns. To advance the field, there is a need to better understand animal nervous systems in order to either grow our own organized cell-based control architectures, or exploit and retrain existing nervous systems to obtain complex autonomous control. This will likely require new mathematical frameworks, such as the Dynome (139), allowing for growth patterns or training protocols to be tested computationally prior to biological implementation. Along with fundamental biological research to better understand the nervous system, further efforts are needed to ensure muscle activation is efficient in order to limit device fatigue.
Furthermore, the nature of the organic components means that a continuous source of nutrition or power must be integrated into biohybrid and organic robots, especially for long-term operation. For shorter missions, nutrients may be provided by a fluid bladder and perfusion system. However, for longer missions, systems will need to be developed to extract nutrients from the environment. For biohybrid devices with synthetic electronics, a power source will be needed. In the case of insect-based cyborg drones, researchers have demonstrated the potential for the insect’s own hemolymph to serve as a biofuel cell to power onboard electronics (113, 140, 141). In the future, power systems will be needed to support not only cyborg drones, but also cell-based devices. To this end, it may be possible to develop supporting media inspired by hemolymph to build in such fuel cells, or to incorporate external microbial fuel cells to provide power (142). Alternatively, contractile cells themselves can be used to generate power for synthetic components via deflection of piezoelectric microcantilevers (143). Larger biohybrid robots and organobots may use more traditional robotic power supplies such as batteries or solar panels. Additionally, the presence of organic material makes such devices targets for bacteria and other contaminants. This also presents a major challenge for researchers seeking to create devices for long-term operation.
Finally, in any research involving tissue or cells, there are many ethical concerns that must be considered. The development of biohybrid robots and organobots is subject to many of the same concerns facing more traditional tissue engineering, such as the ethical procurement of cells or tissue as well as proper animal treatment for research using primary cells or tissue explants. In general, the costs of using animals should be weighed against the benefits of the potential robots made from animal components if doing so will induce pain or take the life of the animal. Such device development also presents unique questions and challenges, especially as the field progresses. With the development of more autonomous devices with advanced peripheries, concerns begin to arise over the possibility that the device may experience pain or stress, or may even begin to exist as a living organism unto itself. Autonomy may also imply that the device pursues its own goals, rather than those of its original fabricator, and these goals may have problematic consequences. These concerns will need to be addressed as the field progresses.
6.3. Future Directions
Beyond the challenges facing the field, there are several additional promising avenues for the development of future organobots. To improve actuation, devices should attempt to replicate the 3D environment of muscle tissue to make use of structural cues from the extracellular matrix. 3D bioprinting offers one exciting avenue for such developments (144). For organic actuators in large-scale robots and bioprosthetics, a vascular system providing nutrient-rich fluid will be necessary for long term operation. Recent advances in tissue engineering point to many approaches that can be integrated into future organobots (145). With further development, biohybrid devices, organobots, and cyborg drones have a bright future in many fields: from microscale devices capable of navigating the human vascular system and performing maintenance and diagnostic tasks, to the development of vascularized mobile platforms for environmental monitoring, to bioprosthetics. The development of robots with organic components is a growing and exciting area of research.
Supplementary Material
Acknowledgments:
We thank Grace Gongaware for scientific illustrations.
Funding: Supported by grants from the National Science Foundation (DGE-0951783, DMR-1306665, IIS-1065489, and CAREER Award 1552782), the National Institute of Health (R01 AR063701 (OA), Ruth L. Kirschstein National Research Service Award T32 AR007505 from the NIH NIAMS) and a GAANN Fellowship (Grant No. P200A150316).
Footnotes
Data and materials availability: Contact R.Q. for data and materials.
References and Notes:
- 1.Saranli U, Buehler M, Koditschek DE, RHex: A Simple and Highly Mobile Hexapod Robot. Int. J. Rob. Res. 20, 616–631 (2001). [Google Scholar]
- 2.Ijspeert AJ, Crespi A, Cabelguen J, Simulation and robotics studies of salamander applying neurobiological principles to the control. Neuroinformatics. 3, 171–195 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Szczecinski NS, Martin JP, Bertsch DJ, Ritzmann RE, Quinn RD, Neuromechanical model of praying mantis explores the role of descending commands in pre-strike pivots. Bioinspir. Biomim. 10 (2015), doi: 10.1088/1748-3190/10/6/065005. [DOI] [PubMed] [Google Scholar]
- 4.Raman R, Bashir R, Biomimicry, Biofabrication, and Biohybrid Systems: The Emergence and Evolution of Biological Design. Adv. Healthc. Mater., 1700496 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Bachand GD, Bouxsein NF, Vandelinder V, Bachand M, Biomolecular motors in nanoscale materials, devices, and systems. WIREs Nanomed Nanobiotechnol. 6, 163–177 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Stanton MM, Trichet-Paredes C, Sanchez S, Applications of three-dimensional (3D) printing for microswimmers and bio-hybrid robotics. Lab Chip. 15, 1634–1637 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Ricotti L, Fujie T, Thin polymeric films for building biohybrid microrobots. Bioinspir. Biomim. 12, 021001 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Ricotti L, Menciassi A, Bio-hybrid muscle cell-based actuators. Biomed. Microdevices. 14, 987–98 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Feinberg AW, Biological Soft Robotics. Annu. Rev. Biomed. Eng. 17, 243–265 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Duffy RM, Feinberg AW, Engineered skeletal muscle tissue for soft robotics: fabrication strategies, current applications, and future challenges. WIREs Nanomed Nanobiotechnol. 6, 178–195 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Chan V, Asada HH, Bashir R, Utilization and control of bioactuators across multiple length scales. Lab Chip. 14, 653–670 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Schwarz L, Medina-Sánchez M, Schmidt OG, Hybrid BioMicromotors. Appl. Phys. Rev. 4 (2017), doi: 10.1063/1.4993441. [DOI] [Google Scholar]
- 13.Carlsen RW, Sitti M, Bio-hybrid cell-based actuators for microsystems. Small. 10, 3831–3851 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Kamm RD, Bashir R, Creating Living Machines. Ann Biomed Eng. 42, 445–459 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sitti M, Mobile Micro-Robotics (MIT Press, 2017). [Google Scholar]
- 16.Hosseinidoust Z et al. , Bioengineered and biohybrid bacteria-based systems for drug delivery. Adv. Drug Deliv. Rev. 106, 27–44 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Hamann H et al. , Flora robotica-mixed societies of symbiotic robot-plant bio-hybrids. Proc. - 2015 IEEE Symp. Ser. Comput. Intell. SSCI 2015, 1102–1109 (2015). [Google Scholar]
- 18.Skrzypczak T et al. , Plant Science View on Biohybrid Development. Front. Bioeng. Biotechnol. 5, 1–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takemura R, Hoshino T, Akiyama Y, Morishima K, in 2010 International Symposium on Micro-NanoMechatronics and Human Science: From Micro and Nano Scale Systems to Robotics and Mechatronics Systems, MHS 2010, Micro-Nano GCOE 2010, Bio-Manipulation 2010 (2010), vol. 8588, pp. 485–490. [Google Scholar]
- 20.Inoue N, Shimizu M, Hosoda K, in Living Machines 2014 (2014), pp. 402–404. [Google Scholar]
- 21.Webster VA, Hawley EL, Akkus O, Chiel HJ, Quinn RD, Effect of actuating cell source on locomotion of organic living machines with electrocompacted collagen skeleton. Bioinspir. Biomim. 11, 36012 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Park J et al. , Real-time measurement of the contractile forces of self-organized cardiomyocytes on hybrid biopolymer microcantilevers. Anal. Chem. 77, 6571–80 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Park J et al. , Fabrication of complex 3D polymer structures for cell–polymer hybrid systems. J. Micromechanics Microengineering. 16, 1614–1619 (2006). [Google Scholar]
- 24.Xi J, Schmidt JJ, Montemagno CD, Self-assembled microdevices driven by muscle. Nat. Mater. 4, 180–4 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Kim J et al. , Establishment of a fabrication method for a long-term actuated hybrid cell robot. Lab Chip. 7, 1504–8 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Chan V et al. , Development of miniaturized walking biological machines. Sci. Rep. 2 (2012), doi: 10.1038/srep00857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feinberg AW et al. , Muscular thin films for building actuators and powering devices. Science (80-. ). 317, 1366–70 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Holley MT, Nagarajan N, Danielson C, Zorlutuna P, Park K, Development and characterization of muscle-based actuators for self-stabilizing swimming biorobots. Lab Chip. 16, 3473–3484 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Nagarajan N, Holley MT, Danielson C, Park K, Zorlutuna P, Cardiac Muscle Cell-based Actuator and Self-stabilizing Biorobot - Part 2. J. Vis. Exp., e55643 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holley M, Nagarajan N, Danielson C, Zorlutuna P, Park K, Cardiac Muscle-cell Based Actuator and Self-stabilizing Biorobot - PART 1. J. Vis. Exp., e55642 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams BJ, V Anand S, Rajagopalan J, a Saif MT, A self-propelled biohybrid swimmer at low Reynolds number. Nat. Commun. 5 (2014), doi: 10.1038/ncomms4081. [DOI] [PubMed] [Google Scholar]
- 32.Nawroth JC et al. , A tissue-engineered jellyfish with biomimetic propulsion. Nat. Biotechnol. 30, 792–7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park S-J et al. , Phototactic guidance of a tissue-engineered soft-robotic ray. Science (80-. ). 353, 158–162 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J et al. , in IEEE International Conference on Robotics and Automation (Pasadena, CA, USA, 2008), pp. 880–885. [Google Scholar]
- 35.Knight M, Drew N, McCarthy L, Grosberg A, Emergent Global Contractile Force in Cardiac Tissues. Biophys. J. 110, 1615–24 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Legant WR et al. , Microfabricated tissue gauges to measure and manipulate forces from 3D microtissues. PNAS. 106, 10097–10102 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das M, Wilson K, Molnar P, Hickman JJ, Differentiation of skeletal muscle and integration of myotubes with silicon microstructures using serum-free medium and a synthetic silane substrate. Nat. Protoc. 2, 1795–801 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Shimizu K et al. , Assembly of skeletal muscle cells on a Si-MEMS device and their generative force measurement. Biomed. Microdevices. 12, 247–252 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Wilson K, Das M, Wahl KJ, Colton RJ, Hickman J, Measurement of contractile stress generated by cultured rat muscle on silicon cantilevers for toxin detection and muscle performance enhancement. PLoS One. 5, e11042 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Das M et al. , A defined system to allow skeletal muscle differentiation and subsequent integration with silicon microstructures. Biomaterials. 27, 4374–80 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Baryshyan AL, Woods W, Trimmer BA, Kaplan DL, Isolation and maintenance-free culture of contractile myotubes from Manduca sexta embryos. PLoS One. 7, e31598 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baryshyan AL, Domigan LJ, Hunt B, Trimmerb BA, Kaplan DA, Self-assembled insect muscle bioactuators with long term function under a range of environmental conditions. R. Soc. Chem. Adv. 75, 39962–39968 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakar MS et al. , Formation and optogenetic control of engineered 3D skeletal muscle bioactuators. Lab Chip. 12, 4976–85 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neal D, Asada HH, in 35th Annual International Conference of the IEEE EMBS (Osaka, Japan, 2013), vol. 2013, pp. 326–9. [DOI] [PubMed] [Google Scholar]
- 45.Cvetkovic C et al. , Three-dimensionally printed biological machines powered by skeletal muscle. PNAS. 111, 10125–10130 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herr H, Dennis RG, A swimming robot actuated by living muscle tissue. J. Neuroeng. Rehabil. 1 (2004), doi: 10.1186/1743-0003-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akiyama Y et al. , Rapidly-moving insect muscle-powered microrobot and its chemical acceleration. Biomed. Microdevices. 14, 979–986 (2012). [DOI] [PubMed] [Google Scholar]
- 48.Webster VA et al. , in Biomimetic and Biohybrid Systems: 5th International Conference, Living Machines 2016, Edinburgh, UK, July 19–22, 2016. Proceedings, Lepora FN et al. , Eds. (Springer International Publishing, Cham, 2016; 10.1007/978-3-319-42417-0_33), pp. 365–374. [DOI] [Google Scholar]
- 49.Tanaka Y, Noguchi Y, Yalikun Y, Kamamichi N, Earthworm muscle driven bio-micropump. Sensors Actuators B Chem. 242, 1186–1192 (2016). [Google Scholar]
- 50.Kuwana Y, Shimoyama I, Miura H, Steering control of a mobile robot using insect antennae. IEEE/RSJ Int. Conf. Intell. Robot. Syst. Hum. Robot Interact. Coop. Robot 2, 530–535 (1995). [Google Scholar]
- 51.Du L et al. , Bioengineered olfactory sensory neuron-based biosensor for specific odorant detection. Biosens. Bioelectron. 40, 401–406 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Du L et al. , A novel biomimetic olfactory cell-based biosensor with DNA-directed site-specific immobilization of cells on a microelectrode array. Sensors Actuators, B Chem. 217, 186–192 (2015). [Google Scholar]
- 53.Raman R et al. , Optogenetic skeletal muscle-powered adaptive biological machines. Proc. Natl. Acad. Sci. 113, 3497–3502 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adamatzky A, Towards slime mould colour sensor: Recognition of colours by Physarum polycephalum. Org. Electron. physics, Mater. Appl. 14, 3355–3361 (2013). [Google Scholar]
- 55.Buselli E et al. , Development and characterization of a bio-hybrid skin-like stretchable electrode. Microelectron. Eng. 88, 1676–1680 (2011). [Google Scholar]
- 56.Cheneler D et al. , Bio-hybrid tactile sensor and experimental set-up for investigating and mimicking the human sense of touch. In. IEEE/RSJ Int. Conf. Intell. Robot. Syst. Work. Adv. tactile Sens. touch-based human-robot Interact, 2–4 (2012). [Google Scholar]
- 57.Cheneler D et al. , A bio-hybrid tactile sensor incorporating living artificial skin and an impedance sensing array. Sensors (Switzerland). 14, 23781–23802 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salgarella AR et al. , A bio-hybrid mechanotransduction system based on ciliate cells. Microelectron. Eng. 144, 51–56 (2015). [Google Scholar]
- 59.Adamatzky A, Slime mould tactile sensor. Sensors Actuators, B Chem. 188, 38–44 (2013). [Google Scholar]
- 60.Adamatzky A, Tactile bristle sensors made with slime mold. IEEE Sens. J. 14, 324–332 (2014). [Google Scholar]
- 61.Lucarotti C, aria Oddo CM, Vitiello N, hiara Carrozza MC, Synthetic and bio-artificial tactile sensing: a review. Sensors (Basel). 13, 1435–1466 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang JV, Wang Y, Krapp HG, Wall Following in a Semi-closed-loop Fly-Robotic Interface. Biomim. Biohybrid Syst. 1, 268–279 (2015). [Google Scholar]
- 63.Huang JV, Krapp HG, Closed-Loop Control in an Autonomous Bio-hybrid Robot System Based on Binocular Neuronal Input. Lect. Notes Comput. Sci. 9222, 164–174 (2015). [Google Scholar]
- 64.Myrick AJ, Park K-C, Hetling JR, Baker TC, Real-time odor discrimination using a bioelectronic sensor array based on the insect electroantennogram. Bioinspir. Biomim. 3, 046006 (2008). [DOI] [PubMed] [Google Scholar]
- 65.Myrick AJ, Baker TC, Locating a compact odor source using a four-channel insect electroantennogram sensor. Bioinspir. Biomim. 6, 016002 (2011). [DOI] [PubMed] [Google Scholar]
- 66.Martinez D, Arhidi L, Demondion E, Masson J-B, Lucas P, Using insect electroantennogram sensors on autonomous robots for olfactory searches. J. Vis. Exp., e51704 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Q et al. , Extracellular potentials recording in intact olfactory epithelium by microelectrode array for a bioelectronic nose. Biosens. Bioelectron. 25, 2212–2217 (2010). [DOI] [PubMed] [Google Scholar]
- 68.Liu Q et al. , Olfactory epithelium biosensor: Odor discrimination of receptor neurons from a bio-hybrid sensing system. Biomed. Microdevices. 14, 1055–1061 (2012). [DOI] [PubMed] [Google Scholar]
- 69.Dong Q et al. , A novel bioelectronic nose based on brain-machine interface using implanted electrode recording in vivo in olfactory bulb. Biosens. Bioelectron. 49, 263–269 (2013). [DOI] [PubMed] [Google Scholar]
- 70.Lebedev MA, Nicolelis MAL, Brain-Machine Interfaces: From Basic Science to Neuroprostheses and Neurorehabilitation. Physiol. Rev. 97, 767–837 (2017). [DOI] [PubMed] [Google Scholar]
- 71.Pohlmeyer EA et al. , Beyond intuitive anthropomorphic control: recent achievements using brain computer interface technologies, 101941N (2017). [Google Scholar]
- 72.DeMarse TB, Wagenaar DA, Blau AW, Potter SM, The Neurally Controlled Animat: Biological Brains Acting with Simulated Bodies. Auton. Robots. 76, 211–220 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bakkum DJ et al. , in Embodied Artificial Intelligence (2004), pp. 130–145. [Google Scholar]
- 74.Novellino a et al. , Connecting neurons to a mobile robot: an in vitro bidirectional neural interface. Comput. Intell. Neurosci. 2007 (2007), doi: 10.1155/2007/12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Santos D et al. , A client-server architecture for remotely controlling a robot using a closed-loop system with a biological neuroprocessor. Rob. Auton. Syst. 58, 1223–1230 (2010). [Google Scholar]
- 76.Warwick K et al. , Controlling a Mobile Robot with a Biological Brain. Def. Sci. J. 60, 5–14 (2010). [Google Scholar]
- 77.Warwick K, Nasuto SJ, Whalley VMBBJ, Experiments with an in-vtro Robot Brain (2011). [Google Scholar]
- 78.Ben-ary G et al. , MEART : the semi-living artist MEART : The semi-living artist (2007), doi: 10.3389/neuro.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bakkum DJ et al. , Embodying cultured networks with a robotic drawing arm. Annu. Int. Conf. IEEE Eng. Med. Biol. - Proc, 2996–2999 (2007). [DOI] [PubMed] [Google Scholar]
- 80.Hayashi I, Kiyotoki M, Kiyohara A, Tokuda M, Kudoh SN, Fuzzy bio-interface: Indicating logicality from living neuronal network and learning control of bio-robot. Proc. Int. Jt. Conf. Neural Networks, 2417–2423 (2011). [Google Scholar]
- 81.Novellino A, Chiappalone M, Tessadori J, in Mobile Robots - Control Architectures, Bio-Interfacing, Navigation, Multi Robot Motion Planning and Operator Training (InTech, 2009), pp. 145–162. [Google Scholar]
- 82.Chiappalone M, Massobrio P, Martinoia S, Network plasticity in cortical assemblies. Eur. J. Neurosci. 28, 221–237 (2008). [DOI] [PubMed] [Google Scholar]
- 83.Li Y, Sun R, Wang Y, Li H, Zheng X, A novel robot system integrating biological and mechanical intelligence based on dissociated neural network-controlled closed-loop environment. PLoS One. 11, 1–21 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pizzi RMR et al. , A cultured human neural network operates a robotic actuator. BioSystems. 95, 137–144 (2009). [DOI] [PubMed] [Google Scholar]
- 85.Webster VA et al. , in Biomimetic and Biohybrid Systems: 6th International Conference, Living Machines 2017, Stanford, CA, USA, July 26−−28, 2017, Proceedings, Mangan M et al. , Eds. (Springer International Publishing, Cham, 2017; 10.1007/978-3-319-63537-8_40), pp. 475–486. [DOI] [Google Scholar]
- 86.Hurwitz I, Neustadter D, Morton DW, Chiel HJ, Susswein AJ, Activity patterns of the B31/B32 pattern initiators innervating the I2 muscle of the buccal mass during normal feeding movements in Aplysia californica. J. Neurophysiol. 75, 1309–26 (1996). [DOI] [PubMed] [Google Scholar]
- 87.McManus JM, Lu H, Chiel HJ, An in vitro preparation for eliciting and recording feeding motor programs with physiological movements in Aplysia californica. J. Vis. Exp. (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wessberg J et al. , Real-time prediction of hand trajectory by ensembles of cortical neurons in primates. Nature. 408, 361–365 (2000). [DOI] [PubMed] [Google Scholar]
- 89.Rajangam S et al. , Wireless Cortical Brain-Machine Interface for Whole-Body Navigation in Primates. Sci. Rep. 6, 22170 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hochberg LR et al. , Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 485, 372–375 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huggins JE, Wren PA, Gruis KL, What would brain-computer interface users want? Opinions and priorities of potential users with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 12, 318–324 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kucukyildiz G, Ocak H, Karakaya S, Sayli O, Design and Implementation of a Multi Sensor Based Brain Computer Interface for a Robotic Wheelchair. J. Intell. Robot. Syst. 87, 247–263 (2017). [Google Scholar]
- 93.Galán F et al. , A brain-actuated wheelchair: Asynchronous and non-invasive Brain-computer interfaces for continuous control of robots. Clin. Neurophysiol. 119, 2159–2169 (2008). [DOI] [PubMed] [Google Scholar]
- 94.Palankar M et al. , Control of a 9-DoF wheelchair-mounted robotic arm system using a P300 brain computer interface: Initial experiments. 2008 IEEE Int. Conf. Robot. Biomimetics, ROBIO 2008, 348–353 (2008). [Google Scholar]
- 95.Bell CJ, Shenoy P, Chalodhorn R, Rao RPN, Control of a humanoid robot by a noninvasive brain–computer interface in humans. J. Neural Eng. 5, 214–220 (2008). [DOI] [PubMed] [Google Scholar]
- 96.Escolano C, Antelis JM, Minguez J, A telepresence mobile robot controlled with a noninvasive brain-computer interface. IEEE Trans. Syst. Man, Cybern. Part B Cybern. 42, 793–804 (2012). [DOI] [PubMed] [Google Scholar]
- 97.Li G, Zhang D, Brain-Computer Interface Controlled Cyborg: Establishing a Functional Information Transfer Pathway from Human Brain to Cockroach Brain. PLoS One. 11, e0150667 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim D, Liu A, Diller E, Sitti M, Chemotactic steering of bacteria propelled microbeads. Biomed. Microdevices. 14, 1009–1017 (2012). [DOI] [PubMed] [Google Scholar]
- 99.Park D et al. , Motility analysis of bacteria-based microrobot (bacteriobot) using chemical gradient microchamber. Biotechnol. Bioeng. 111, 134–143 (2014). [DOI] [PubMed] [Google Scholar]
- 100.Barroso Á et al. , Optical assembly of bio-hybrid micro-robots. Biomed. Microdevices. 17, 1–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stanton MM et al. , Biohybrid Microtube Swimmers Driven by Single Captured Bacteria. Small, 1603679 (2017). [DOI] [PubMed] [Google Scholar]
- 102.Martel S, Towards Fully Autonomous Bacterial Microrobots. Exp. Robot. 79, 775–784 (2014). [Google Scholar]
- 103.Stanton MM et al. , Magnetotactic Bacteria Powered Biohybrids Target E. coli Biofilms. ACS Nano, acsnano.7b04128 (2017). [DOI] [PubMed] [Google Scholar]
- 104.Felfoul O et al. , Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat. Nanotechnol. 11, 941–947 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Itoh A, Tamura W, Object Manipulation by a Formation-controlled Euglena group. Bio-mechanisms Swim. Fly., 41–52 (2008). [Google Scholar]
- 106.Wong D, Beattie EE, Steager EB, Kumar V, Effect of surface interactions and geometry on the motion of micro bio robots. Appl. Phys. Lett. 103, 1–5 (2013). [Google Scholar]
- 107.Magdanz V, Sanchez S, Schmidt OG, Development of a sperm-flagella driven micro-bio-robot. Adv. Mater. 25, 6581–6588 (2013). [DOI] [PubMed] [Google Scholar]
- 108.Sato H, Maharbiz MM, Recent developments in the remote radio control of insect flight. Front. Neurosci. 4, 1–12 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sato H et al. , Cyborg beetles: The remote radio control of insect flight. Proc. IEEE Sensors, 1–4 (2010). [Google Scholar]
- 110.Sato H et al. , Remote radio control of insect flight. Front. Integr. Neurosci. 3 (2009), doi: 10.3389/neuro.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sanchez CJ et al. , Locomotion control of hybrid cockroach robots. J. R. Soc. Interface. 12, 20141363–20141363 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Latif T, Bozkurt A, Line following terrestrial insect biobots. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc EMBS, 972–975 (2012). [DOI] [PubMed] [Google Scholar]
- 113.Schwefel J et al. , Wireless Communication by an Autonomous Self-Powered Cyborg Insect. J. Electrochem. Soc. 161, H3113–H3116 (2014). [Google Scholar]
- 114.Zhu L, Liu H, Wang Z, Pi X, Zhou S, Preliminary study of an intestinal bio-robot system based on nerve stimulation. J. Neuroeng. Rehabil. 9, 68 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.West GB, The origin of allometric scaling laws in biology from genomes to ecosystems: towards a quantitative unifying theory of biological structure and organization. J. Exp. Biol. 208, 1575–1592 (2005). [DOI] [PubMed] [Google Scholar]
- 116.Herzog B, Wirth R, Swimming behavior of selected species of Archaea. Appl. Environ. Microbiol. 78, 1670–1674 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.in McGraw-Hill Encyclopedia of Science and Technology (McGraw Hill, New York, 1960). [Google Scholar]
- 118.The Mind of a Worm, (available at http://www.wormatlas.org/ver1/MoW_built0.92/description.html). [Google Scholar]
- 119.Hahm J-H et al. , C. elegans maximum velocity correlates with healthspan and is maintained in worms with an insulin receptor mutation. Nat. Commun. 6, 8919 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Drewes CD, Helical swimming and body reversal behaviors in Lumbriculus variegatus (Annelida: Clitellata: Lumbriculidae). Hydrobiologia. 406, 263–269 (1999). [Google Scholar]
- 121.Chugunova NI, (Izdatel’stvo Akademii Nauk SSSR, Moskva, 1959), p. 132. [Google Scholar]
- 122.Kottelat M, Freyhof J, Handbook of European freshwater fishes (Berlin, 2007). [Google Scholar]
- 123.Collette BB, in Fischer W, Krupp F, Schneider W, Sommer C, Carpenter KE and Niem V (eds.) Guia FAO para Identification de Especies para lo Fines de la Pesca. Pacifico Centro-Oriental; (1995), pp. 1521–1543. [Google Scholar]
- 124.Collette BB, Nauen CE, FAO Species Catalogue. Vol. 2. Scombrids of the world. An annotated and illustrated catalogue of tunas, mackerels, bonitos and related species known to date. FAO Fish. Synop. 125, 137 (1983). [Google Scholar]
- 125.Nichols JT, The freshwater fishes of China Natural history of Central Asia: Volume IX (The American Museum of Natural History, New York, USA, 1943). [Google Scholar]
- 126.Shen SC, Ed., Fishes of Taiwan (Department of Zoology, National Taiwan University, Taipei.). [Google Scholar]
- 127.Robins CR, Ray GC, A field guide to Atlantic coast fishes of North America (Houghton Mifflin Company, Boston, U.S.A, 1986). [Google Scholar]
- 128.Whitehead PJ, FAO Species Catalogue. Vol. 7. Clupeoid fishes of the world (suborder Clupeioidei). An annotated and illustrated catalogue of the herrings, sardines, pilchards, sprats, shads, anchovies and wolf-herrings. FAO Fish. Synop. 125, 1–303 (1985). [Google Scholar]
- 129.Billard R, Les poissons d’eau douce des rivières de France. Identification, inventaire et répartition des 83 espèces. [Google Scholar]
- 130.Kottelat M, European freshwater fishes. Biol. 52 5, 1–271 (1997). [Google Scholar]
- 131.Chao LN, Trewavas E, in Check-list of the fishes the eastern tropical Atlantic, Quero JC, Hureau JC, Karrer C, Post A, Saldanha L, Eds. (1990), pp. 813–826. [Google Scholar]
- 132.Iwai T, Hisada M, Fishes - Illustrated Book of Gakken (1998). [Google Scholar]
- 133.Nagai H, Fluiddynamics Learned from Dolphin (Ohm Books, 1999). [Google Scholar]
- 134.Lory P, Ophoff R, Nahmias J, Towards a unified nomenclature describing voltage-gated calcium channel genes. Hum Genet. 100, 149–50 (1997). [DOI] [PubMed] [Google Scholar]
- 135.Chiel HJ, Beer RD, The brain has a body: Adaptive behavior emerges from interactions of nervous system, body and environment. Trends Neurosci. 20, 553–557 (1997). [DOI] [PubMed] [Google Scholar]
- 136.Kier WM, The diversity of hydrostatic skeletons. J. Exp. Biol. 215, 1247–1257 (2012). [DOI] [PubMed] [Google Scholar]
- 137.Ting LH et al. , Neuromechanical principles underlying movement modularity and their implications for rehabilitation. Neuron. 86, 38–54 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Raman R, Cvetkovic C, Bashir R, A modular approach to the design, fabrication, and characterization of muscle-powered biological machines. Nat. Protoc. 12, 519–533 (2017). [DOI] [PubMed] [Google Scholar]
- 139.Kopell NJ, Gritton HJ, Whittington MA, Kramer MA, Beyond the connectome: The dynome. Neuron. 83, 1319–1328 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shoji K et al. , Insect biofuel cells using trehalose included in insect hemolymph leading to an insect-mountable biofuel cell. Biomed. Microdevices. 14, 1063–1068 (2012). [DOI] [PubMed] [Google Scholar]
- 141.Shoji K, Morishima K, in International Conference on Mechatronics and Automation (Harbin, China, 2016), pp. 629–634. [Google Scholar]
- 142.Philamore H, Rossiter J, Stinchcombe A, Ieropoulos I, Row-bot: An energetically autonomous artificial water boatman. IEEE Int. Conf. Intell. Robot. Syst 2015-Decem, 3888–3893 (2015). [Google Scholar]
- 143.Xu B et al. , Cell Generator: A Self-Sustaining Biohybrid System Based on Energy Harvesting from Engineered Cardiac Microtissues. Adv. Funct. Mater. 27, 1–9 (2017). [Google Scholar]
- 144.V Murphy S, Atala A, 3D bioprinting of tissues and organs. Nat. Biotechnol. 32, 773–785 (2014). [DOI] [PubMed] [Google Scholar]
- 145.Lovett M, Lee K, Edwards A, Kaplan DL, Vascularization strategies for tissue engineering. Tissue Eng. Part B. Rev. 15, 353–70 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cao F, Zhang C, Choo HY, Sato H, Insect-computer hybrid legged robot with user-adjustable speed, step length and walking gait. J. R. Soc. Interface. 13 (2016), doi: 10.1098/rsif.2016.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sato H et al. , Deciphering the Role of a Coleopteran Steering Muscle via Free Flight Stimulation. Curr. Biol. 25, 798–803 (2016). [DOI] [PubMed] [Google Scholar]
- 148.Kandhari A et al. , in Biomimetic and Biohybrid Systems: 5th International Conference, Living Machines 2016, Edinburgh, UK, July 19–22, 2016. Proceedings, Lepora NF et al. , Eds. (Springer International Publishing, Cham, 2016; 10.1007/978-3-319-42417-0_10), pp. 97–106. [DOI] [Google Scholar]
- 149.Beer RD, Chiel HJ, Quinn RD, Ritzmann RE, Biorobotic approaches to the study of motor systems. Curr. Opin. Neurobiol. 8, 777–782 (1998). [DOI] [PubMed] [Google Scholar]
- 150.Webb B, Consilvio T, Eds., Biorobotics (MIT Press, Cambridge, MA, USA, 2001). [Google Scholar]
- 151.Kim D-H et al. , Fabrication of patterned micromuscles with high activity for powering biohybrid microdevices. Sensors Actuators B. 117, 391–400 (2006). [Google Scholar]
- 152.Warwick K, Implications and consequences of robots with biological brains. Ethics Inf. Technol. 12, 223–234 (2010). [Google Scholar]
- 153.Ijspeert AJ, Biorobotics: using robots to emulate and investigate agile locomotion. Science. 346, 196–203 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


