Abstract
Background
Fetal fibronectin (FFN) is an extracellular matrix glycoprotein localized at the maternal‐fetal interface of the amniotic membranes, between chorion and decidua, where it is concentrated in this area between decidua and trophoblast. In normal conditions, FFN is found at very low levels in cervicovaginal secretions. Levels greater than or equal to 50 ng/mL at or after 22 weeks have been associated with an increased risk of spontaneous preterm birth. In fact, FFN is one of the best predictors of preterm birth in all populations studied so far, and can help in selecting which women are at significant risk for preterm birth. This is an update of a review first published in 2008.
Objectives
To assess the effectiveness of management based on knowledge of FFN testing results for preventing preterm birth.
Search methods
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register (7 September 2018), ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (7 September 2018), and reference lists of retrieved studies.
Selection criteria
Randomized controlled trials of pregnant women screened with FFN for risk of preterm birth. Studies included are based exclusively on knowledge of FFN results versus no such knowledge, and we have excluded studies including women with only positive or only negative FFN results.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data, and checked them for accuracy. The quality of the evidence was assessed using the GRADE approach.
Main results
We identified 16 trials, of which six were eligible for inclusion. The six included studies randomized 546 women with singleton gestations and threatened preterm labor (PTL) at 23 0/7 to 34 6/7 weeks. A total of 277 women were randomized to knowledge and 269 to no knowledge of FFN. No trials were identified on asymptomatic women or multiple gestations.
The risk of bias of included studies was mixed. For selected important outcomes, preterm birth before 37, 34, and 32 weeks, and maternal hospitalization, we graded the quality of the evidence and created a 'Summary of findings' table. For these outcomes, the evidence was graded as mainly low quality due to the imprecision of effect estimates.
Management based on knowledge of FFN results may reduce preterm birth before 37 weeks (20.7%) versus controls without such knowledge (29.2%) (risk ratio (RR) 0.72, 95% confidence interval (CI) 0.52 to 1.01; 5 trials; 434 women; low‐quality evidence). However, management based on knowledge of FFN results may make little or no difference to preterm birth before 34 (RR 1.09, 95% CI 0.54 to 2.18; 4 trials; 357 women; low‐quality evidence) or maternal hospitalization (RR 1.06, 95% CI 0.79 to 1.43; 5 trials; 441 women; low‐quality evidence). The evidence for preterm birth before 32 weeks is uncertain because the quality was found to be very low (average RR 0.79, 95% CI 0.16 to 3.96; 4 trials; 357 women; very low‐quality evidence).
For all other outcomes, for which there were available data (preterm birth less than 28 weeks; gestational age at delivery (weeks); birthweight less than 2500 g; perinatal death; tocolysis; steroids for fetal lung maturity; time to evaluate; respiratory distress syndrome; neonatal intensive care unit (NICU) admission; and NICU days), knowledge of FFN results may make little or no difference to the outcomes.
Authors' conclusions
The evidence from this review suggests that management based on knowledge of FFN results may reduce preterm birth before 37 weeks. However, our confidence in this result is limited as the evidence was found to be of low quality. Effects on other substantive outcomes are uncertain due to serious concerns in study design, inconsistency, and imprecision of effect estimates. No trials were identified on asymptomatic women, or multiple gestations.
Future studies are needed that include specific populations (e.g. singleton gestations with symptoms of preterm labor), a study group managed with a protocol based on the FFN results, and that report not only maternal but also important perinatal outcomes. Cost‐effectiveness analyses are also needed.
Plain language summary
Fetal fibronectin testing for reducing the risk of preterm birth
What is the issue?
To assess the effectiveness of management of pregnant women based on a knowledge of fetal fibronectin test results for preventing preterm birth, compared with not having that knowledge. Fetal fibronectin (FFN) acts as a ‘glue’ between the pregnancy and the uterus. Normally very low levels of FFN can be found in secretions of the vagina and cervix. Raised levels at or after 22 weeks have been associated with an increased risk of spontaneous preterm birth.
Why is this important?
Preterm birth before 37 weeks is the main cause of sickness and death for newborn infants. Most women who give birth preterm have preterm labor symptoms such as contractions but many of the women with symptoms go on to deliver at term (37 weeks or more). Fetal fibronectin (FFN) is a test that can identify the women with symptoms of preterm labor who are most at risk for preterm birth. The level of FFN is measured in secretions from the vagina or cervix.
What evidence did we find?
We found six randomised controlled studies involving 546 women who were pregnant with one baby and were showing signs of preterm labor at between 23 to 34 weeks' gestation. We graded the following evidence as mainly low quality because of the low number of women in the studies and a wide variation in findings. We found that the number of births before 37 weeks may be slightly reduced when women and their doctors know the results of the FFN test (20.7% versus 29.2%; 5 trials; 434 women). However, knowledge of FFN results may make little or no difference for the other outcomes with available data, including: maternal hospitalization (5 trials; 441 women); use of uterine relaxants (tocolysis) to try to prevent labor; earlier preterm births; women’s gestational age at delivery; babies with a birthweight less than 2500 g; newborn deaths; the number of babies with respiratory distress syndrome; giving steroids to mature the unborn babies’ lungs; and number of days in a neonatal intensive care unit (NICU).
What does this mean?
This review of six studies did not find enough evidence to say whether or not the FFN test should be used in the management of women showing signs of preterm labor. A screening test such as FFN can only be considered effective if interventions based on the screening results, such as giving drugs to relax the uterus, reduce the number of preterm births. Further research should be encouraged.
Summary of findings
Summary of findings for the main comparison. Main comparison (FFN knowledge versus no knowledge).
| FFN knowledge versus no knowledge in reducing the risk of preterm birth | ||||||
| Patient or population: women with singleton pregnancies and threatened preterm labor (PTL) between 23 to 35 weeks Setting: hospital settings in United Kingdom and United States Intervention: FFN knowledge Comparison: no knowledge | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no knowledge | Risk with FFN knowledge | |||||
| Preterm birth < 37 weeks | Study population | RR 0.72 (0.52 to 1.01) | 434 (5 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 292 per 1.000 | 211 per 1.000 (152 to 295) | |||||
| Preterm birth < 34 weeks | Study population | RR 1.09 (0.54 to 2.18) | 357 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 3 | ||
| 79 per 1.000 | 86 per 1.000 (43 to 172) | |||||
| Preterm birth < 32 weeks | Study population | Average RR 0.79 (0.16 to 3.96) | 357 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 4 5 | ||
| 56 per 1.000 | 36 per 1.000 (14 to 98) | |||||
| Maternal hospitalization | Study population | RR 1.06 (0.79 to 1.43) | 441 (5 RCTs) | ⊕⊕⊝⊝ LOW 1 3 | ||
| 268 per 1.000 | 284 per 1.000 (211 to 383) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; OR: odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded (1) level for serious limitations in study design due to some studies having unclear risk of bias for several domains and one study being at high risk of detection bias
2 We downgraded (1) level for serious imprecision due to wide CI just crossing the line of no effect
3 We downgraded (1) level for serious imprecision due to wide CI crossing the line of no effect
4 We downgraded (2) levels for very serious imprecision due to wide CI crossing the line of no effect and a small number of events
5 We downgraded (1) level for serious inconsistency due to evidence of statistical heterogeneity I2 = 46%
Background
Description of the condition
Preterm birth is defined by the World Health Organization (WHO) as birth between 20 and 36 6/7 weeks. Its incidence is about 5% to 12% in most low‐, middle‐, and high‐income countries. This incidence is increasing in many countries, including low‐income countries. Preterm birth is the main cause of neonatal morbidity and mortality in most countries, especially in high‐ and middle‐income countries. In the USA, 75% of perinatal mortality occurs in preterm babies; more than two‐thirds of perinatal mortality (60% of total) occurs in infants born at less than 32 weeks. Mortality and morbidity are inversely associated with gestational age at birth. Morbidities include respiratory distress syndrome, bronchopulmonary dysplasia, intraventricular haemorrhage, necrotizing enterocolitis, sepsis, retinopathy, etc. All members of a family in which a preterm birth occurs suffer greatly, in several aspects, including medically, socially, psychologically, and financially (Berghella 2016).
Description of the intervention
Fetal fibronectin is an extracellular matrix glycoprotein. Fetal fibronectin in biologic fluids is produced by amniocytes and by cytotrophoblast. It is present throughout gestation in all pregnancies. It is not subject to genetic polymorphism. There are very high levels in amniotic fluid (100 µg/mL) in the second trimester, and 30 µg/mL at term. It is localized at the maternal‐fetal interface of the amniotic membranes, between chorion and decidua, where it is concentrated in this area between decidua and trophoblast. Here it acts as a 'glue' between the pregnancy and the uterus. Concentration of fetal fibronectin protein found in blood is 1/5th that found in amniotic fluid; it is not present in urine. In normal conditions, this glycoprotein remains in this area between chorion and decidua, and very low levels are found in cervicovaginal secretions after 22 weeks (less than 50 ng/mL). Levels above this value (greater than or equal to 50 ng/mL) at or after 22 weeks in the cervicovaginal secretions collected by a swab have been associated with an increased risk of spontaneous preterm birth (Berghella 2016). The fetal fibronectin test assesses risk of preterm birth by measuring amount of fetal fibronectin in cervicovaginal secretions. In fact, fetal fibronectin is one of the best predictors of preterm birth in all populations studied so far, including low‐ and high‐risk women without preterm labor, twins, and women in preterm labor (Leitich 1999). The overall sensitivity and specificity are 56% and 84% for preterm before 37 weeks, respectively, but vary according to gestational age at collection, population studied, prevalence of preterm birth, single versus multiple screening, etc. (Leitich 1999). Its positive predictive value varies from about 9% to 46% depending on the incidence of preterm labor in the population studies (Leitich 1999). Even at 13 to 22 weeks, higher (using 90th percentile) fetal fibronectin levels are associated with a two‐ to three‐fold increase risk in subsequent spontaneous preterm labor.
How the intervention might work
The majority of women presenting with symptoms of preterm labor, such as contractions, back pain, and increase in discharge, do not deliver preterm. Fetal fibronectin has been shown to predict which women would deliver preterm among those presenting with symptoms of preterm labor (threatened preterm labor). By predicting better which women to target interventions such as tocolysis on, fetal fibronectin screening of women with threatened preterm labor could decrease the incidence of preterm birth.
Why it is important to do this review
Preterm birth remains one of the main problems in obstetrics, given its association with perinatal morbidity and mortality, and high costs to care for these sick neonates. Half of preterm birth is preceded by preterm labor. The management of women with threatened preterm labor is controversial, with great difficulty in identifying the subgroup (about 30%) of women presenting with this condition who indeed deliver preterm. Being able to show that screening with fetal fibronectin decreases preterm birth would contribute to potentially save and/or ameliorate millions of lives of neonates worldwide every year. On the contrary, should this study show no benefit from fetal fibronectin screening, this screening, currently used in many units around the world, could be stopped, resulting in significant savings in time and money (Berghella 2016). This is an update of a review first published in 2008.
Objectives
To assess the effectiveness of management based on knowledge of FFN testing results for preventing preterm birth.
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished randomized and quasi‐randomized controlled trials. Cluster‐randomized trials were also eligible for inclusion, though none were identified for this update. Abstracts were eligible for inclusion if sufficient information was provided to judge the quality and potential for bias of these trials.
Types of participants
Pregnant women between the gestational ages of 22 and 34 weeks screened with fetal fibronectin for risk of preterm birth.
Types of interventions
A screening test such as fetal fibronectin can only be considered effective if interventions based on fetal fibronectin screening results reduce the outcome of preterm birth. Interventions based on fetal fibronectin screening results can also be classified as:
interventions based on knowledge of fetal fibronectin results (e.g. fetal fibronectin is collected on all women, but women are randomized so that in 50% of them the result is available to them and the managing obstetrician, while in 50% the fetal fibronectin is blind to them and the managing obstetrician; or fetal fibronectin screening is done only on half of the women);
interventions based on positive fetal fibronectin;
interventions based on negative fetal fibronectin.
This review focuses exclusively on (1), i.e. interventions based on knowledge of fetal fibronectin results.
Types of outcome measures
Primary outcomes
(1) Preterm birth (less than 37 weeks)
Secondary outcomes
(2) Preterm birth less than 34 weeks (3) Preterm birth less than 32 weeks (4) Preterm birth less than 28 weeks (5) Gestational age at delivery (6) Birthweight less than 2500 g (7) Perinatal death (fetal death and neonatal death) (8) Maternal hospitalization (9) Tocolysis (10) Steroids for fetal maturity (11) Time to evaluate (time from arrival to hospital for evaluation of preterm labor to decision regarding admission, discharge, or extended monitoring) (12) Respiratory distress syndrome (13) Intraventricular haemorrhage (14) Necrotizing enterocolitis (15) Sepsis (16) Neonatal intensive care unit (NICU) admission (17) NICU days (18) Maternal well‐being (e.g. stress level, etc.) (19) Economic analysis (cost‐effectiveness, cost utility)
We will report outcomes 1, 2, 3, 4, 5, 8, 9, 10, 11, 18, and 19 with 'mothers' as the denominator. We will report outcomes 6, 7, 12, 13, 14, 15, 16, and 17 with 'fetuses/neonates' as the denominator.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (7 September 2018).
The Register is a database containing over 24,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings; and the list of journals reviewed via the current awareness service; please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (7 September 2018) using the search methods detailed in Appendix 1.
Searching other resources
We reviewed the reference list of all articles, in particular trials and review articles. We contacted all researchers of included trials to provide actual databases and any pertinent further information. We contacted experts in the field for additional and ongoing trials.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeBerghella 2008.
For this update, the following methods were used for assessing the two trials that were identified as a result of the updated search.
Selection of studies
The two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion. Vincenzo Berghella is a co‐author on one of the excluded trials (Ness 2007) and was not involved in the eligibility assessment for this trial as this was assessed by Gabriele Saccone.
Data extraction and management
We designed a form to extract data. For eligible studies, the two authors extracted the data using the agreed form. We resolved discrepancies through discussion. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
The two authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomization);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings.
Assessment of the quality of the evidence using the GRADE approach
For this update, the quality of the evidence was assessed using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes for the main comparison (FFN knowledge versus no knowledge):
Preterm birth < 37 weeks
Preterm birth < 34 weeks
Preterm birth < 32 weeks
Maternal hospitalization
GRADEpro Guideline Development Tool was used to import data from Review Manager 5.3 (RevMan 2014) in order to create a ’Summary of findings’ table. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between trials. In future updates, if necessary, we will use the standardised mean difference to combine trials that measured the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomized trials
We planned to include cluster‐randomized trials in the analyses along with individually‐randomized trials, however all included studies were individually‐randomized trials.
Cross‐over trials
We planned to exclude cross‐over trials. However, no cross‐over trials were found during the search process.
Dealing with missing data
For included studies, levels of attrition were noted. In future updates, if more eligible studies are included, the impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomized to each group in the analyses. The denominator for each outcome in each trial was the number randomized minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identified substantial heterogeneity (I² above 30%), we planned to explore it by prespecified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials. If we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
Had we identified substantial heterogeneity, we planned to investigate it using subgroup analyses and to consider whether an overall summary was meaningful, and if it was, to use a random‐effects analysis to produce it.
We planned to restrict subgroup analyses to primary outcomes for the following subgroups:
Symptomatic women with threatened preterm labor versus asymptomatic women
Women with multiple gestations versus women with singleton gestations
We also planned to assess subgroup differences by interaction tests available within RevMan (RevMan 2014) and to report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Given that all included trials enrolled only singleton gestations with threatened preterm labor, no subgroup analyses were performed.
Sensitivity analysis
We carried out sensitivity analyses for the primary outcome to explore the impact of trial quality assessed by concealment of allocation, high attrition rates, or both. Poor‐quality studies were excluded from the analyses in order to assess whether this made any difference to the overall result.
Results
Description of studies
Results of the search
See: Figure 1
1.
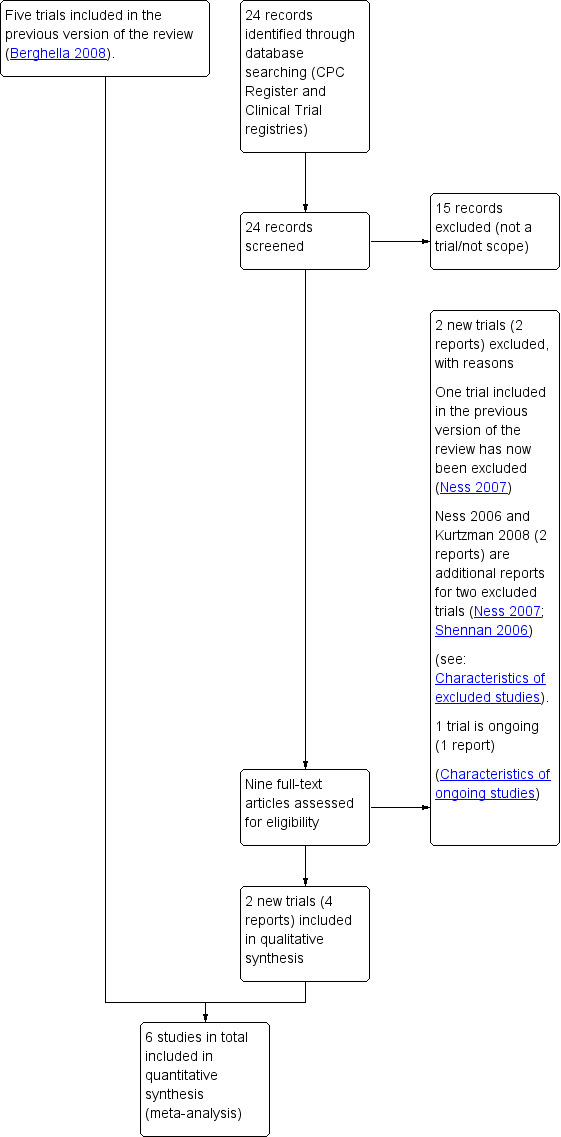
Study flow diagram.
For this update, we identified nine trial reports to assess. We included two new trials (Dutta 2011; Lee 2013) (four reports). We excluded two new trials (Gallot 2018; Osório 2010) (two reports) and added one trial report to ongoing (NCT01431885) (one report). Two reports were additional reports for two excluded trials (Ness 2007; Shennan 2006). One trial previously included has been excluded in this update (Ness 2007).
All women in the included studies had singleton gestations with threatened preterm labor (PTL) between 22 and 34 weeks. There were no trials comparing knowledge to no knowledge of fetal fibronectin (FFN) in asymptomatic women or women with multiple gestations. We identified no quasi‐randomized trials.
Included studies
The six included studies randomized 546 women, of which 277 were randomized to knowledge and 269 to no knowledge of FFN.
Settings
All trials were based in hospital settings. One trial took place in the United Kingdom (Dutta 2011); all the other trials took place in the United States. Dutta 2011 and Plaut 2003 were multicenter and the remaining four trials were based in single hospitals.
Participants
All trials included women with singleton gestations and threatened PTL from around 23 to around 35 weeks' gestation. All the trials excluded multiple pregnancies apart from Plaut 2003. We requested patient‐level databases from all authors, and obtained them from Grobman 2004, Lee 2013, Lowe 2004, and Nguyen 2002. Dutta 2011 and Plaut 2003 also replied to our query, but had no additional data to what they had already published. Our analysis was based only on singleton gestations. We avoided repeated entries of the same patients, which was possible since we had patient‐level data for the one trial where this occurred (Plaut 2003).
Interventions and comparisons
Of 546 women, 277 were randomized to knowledge of FFN and 269 to no knowledge of FFN. Intervention groups had the FFN following signs of PTL. Time taken to obtain results was not reported in most trials. In Lowe 2004, it took less than one hour, and Plaut 2003 took between one and two hours. Dutta 2011 and Nguyen 2002 did not perform FFN on women in the control group. The other trials performed FFN on participants in the control group but did not disclose the results. Only Lee 2013 and Nguyen 2002 had a protocol for positive FFN tests.
Funding
Two trials were supported by affiliated universities (Dutta 2011; Lowe 2004), and two trials did not disclose their funding sources (Lee 2013; Nguyen 2002). Two were sponsored by Adeza Biomedical which produced the FFN tests used in the trials (Grobman 2004; Plaut 2003).
Declarations of interest
Dutta 2011 and Lee 2013 declared that no authors had any conflicts of interest. None of the other trials reported whether or not there were conflicts of interest.
Excluded studies
We excluded seven of the 11 excluded studies because they only included women with positive (six trials) (Andrews 2001; Bisits 2004; Goldenberg 2001; Hauth 2001; ISRCTN43735180; Shennan 2006) or only with negative (one trial) (Elliott 2005) FFN results. One excluded study (Kalchbrenner 1999) compared speculum versus digital collection of FFN, with no management reported based on FFN results. Another study was excluded because it used different diagnostic tests, and only partly used FFN (Gallot 2018) and one study was published only as an abstract with insufficient information provided to judge the quality and risk of bias (Osório 2010). Another excluded study used mainly transvaginal ultrasound cervical length, and not FFN, in the algorithm studied (Ness 2007).
Risk of bias in included studies
2.
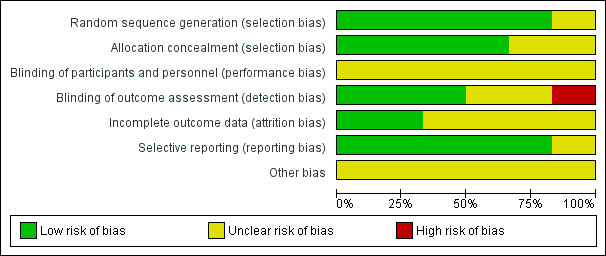
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
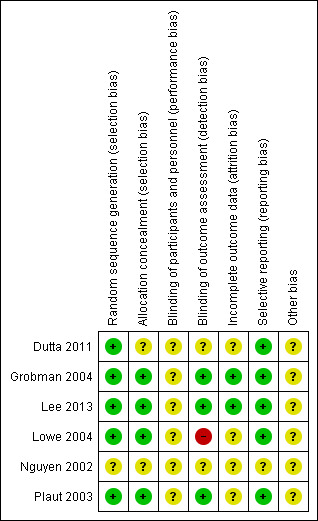
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Risk of selection bias was considered low in four of the six included trials. These four trials used computer‐generated random sequence generation and sealed opaque envelopes to conceal allocation (Grobman 2004; Lee 2013; Lowe 2004; Plaut 2003). One study (Dutta 2011) used telephonic random number generation but did not describe allocation concealment. The sixth study (Nguyen 2002) did not describe random sequence generation or allocation concealment (only the abstract of this study has been published).
Blinding
Women and staff were aware of the intervention they were allocated to due to its nature but it is unclear if this affected results.
Laboratory personnel who performed the FFN test were blinded to women's characteristics and outcomes in Grobman 2004, Lee 2013, and Plaut 2003. One trial was unblinded (Lowe 2004). It was unclear in the remaining trials whether outcome assessors were blinded.
Incomplete outcome data
Two studies were assessed as having low risk of attrition bias because they reported no loss to follow‐up (Grobman 2004; Lee 2013). In the remaining four trials, there was insufficient detail in the reports to permit a judgement of low or high risk and so these trials were assessed as being at unclear risk (Dutta 2011; Lowe 2004; Nguyen 2002; Plaut 2003). See Characteristics of included studies for further details.
Selective reporting
Risk of reporting bias was considered low in five of the six included studies; no deviations from original protocols were noted. The sixth study (Nguyen 2002) did not describe reporting details (only the abstract of this study has been published).
Other potential sources of bias
The included studies did not have enough information to adequately assess the presence of other forms of bias and, as such, were deemed to have an unclear risk of bias. Nguyen 2002 was published only as abstract. Two studies (Grobman 2004; Plaut 2003) were sponsored by Adeza Biomedical which manufactures the FFN tests, however the results from these studies did not seem to be skewed favourably toward the FFN tests.
Effects of interventions
See: Table 1
See: Table 1 the comparison (i.e. FFN knowledge versus no knowledge) and for selected important outcomes, we graded the quality of the evidence and created 'Summary of findings' tables. For all these outcomes, the evidence was graded as mainly low quality due to the imprecision of effect estimates, limitations in study design, and inconsistency of results. We included six trials (involving 546 women) in this review. All women included had singleton gestations and threatened PTL.
Fetal fibronectin knowledge versus no knowledge
Primary outcome
Preterm birth before 37 weeks appeared to favour management based on knowledge of FFN results (20.7%) compared to controls without such knowledge (29.2%) though the confidence intervals (CIs) just crossed the line of no effect so we could not be certain of this effect (risk ratio (RR) 0.72, 95% CI 0.52 to 1.01; 5 trials; 434 women; low‐quality evidence; Analysis 1.1). Three out of five trials had RRs less than one, with no heterogeneity.
1.1. Analysis.
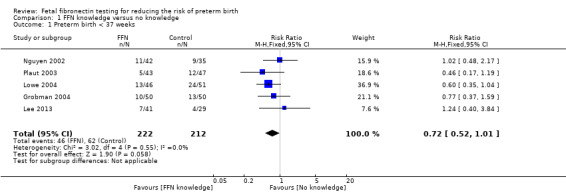
Comparison 1 FFN knowledge versus no knowledge, Outcome 1 Preterm birth < 37 weeks.
We performed a sensitivity analysis for the primary outcome, by removing Nguyen 2002 which was assessed to be at unclear risk of allocation concealment. Removing this data from the analysis indicated that knowledge of FFN may result in fewer preterm births before 37 weeks (19.4% compared to 29.9%) with CI below the line of no effect (RR 0.67, 95% CI 0.46 to 0.97; 4 trials; 357 women; Analysis 2.1).
2.1. Analysis.
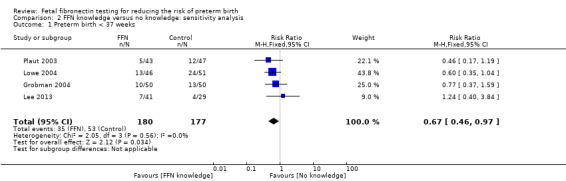
Comparison 2 FFN knowledge versus no knowledge: sensitivity analysis, Outcome 1 Preterm birth < 37 weeks.
Secondary outcomes
Effects on other secondary outcomes are uncertain due to serious concerns in study design, inconsistency, and imprecision of effect estimates:
preterm birth less than 34 weeks (RR 1.09, 95% CI 0.54 to 2.18; 4 trials; 357 women; low‐quality evidence; Analysis 1.2)
preterm birth less than 32 weeks (average RR 0.79, 95% CI 0.16 to 3.96; 4 trials; 357 women; Tau² = 0.93; I² = 46%; very‐low quality evidence; Analysis 1.3)
preterm birth less than 28 weeks (RR 0.63, 95% CI 0.15 to 2.59; 4 trials; 357 women; Analysis 1.4)
gestational age at delivery (weeks) (mean difference (MD) 0.14, 95% CI ‐0.36 to 0.63; 5 trials; 456 neonates; Analysis 1.5)
birthweight less than 2500 g (no events in either group; one trial; 68 neonates; Analysis 1.6)
perinatal death (RR 2.21, 95% CI 0.09 to 52.27; 2 trials; 164 neonates; Analysis 1.7)
maternal hospitalization (RR 1.06, 95% CI 0.79 to 1.43; 5 trials; 441 women; low‐quality evidence; Analysis 1.8)
tocolysis (RR 0.97, 95% CI 0.75 to 1.24; 6 trials; 531 women; Analysis 1.9)
steroids for fetal lung maturity (RR 1.04, 95% CI 0.79 to 1.38; 5 trials; 441 neonates; Analysis 1.10)
time to evaluate (time from arrival to hospital for evaluation of PTL to decision regarding admission, discharge, or extended monitoring (hours)) (MD 0.55, 95% CI ‐0.39 to 1.50; random‐effects; 6 trials; 528 women; Tau² = 0.55; I² = 52%; Analysis 1.11)
respiratory distress syndrome (RR 0.91, 95% CI 0.06 to 14.06; 2 trials; 148 neonates; Analysis 1.12)
NICU admission (RR 2.36, 95% CI 0.92 to 6.07; 2 trials; 124 neonates; Analysis 1.13)
economic analysis (hospitalization charges): Nguyen 2002 reported data regarding hospitalization charges and found that management based on FFN test required higher hospitalization charges (US dollars) (MD 153.00, 95% CI 24.01 to 281.99; Analysis 1.14).
1.2. Analysis.
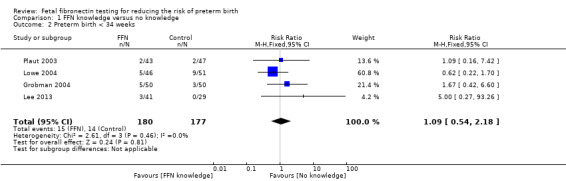
Comparison 1 FFN knowledge versus no knowledge, Outcome 2 Preterm birth < 34 weeks.
1.3. Analysis.
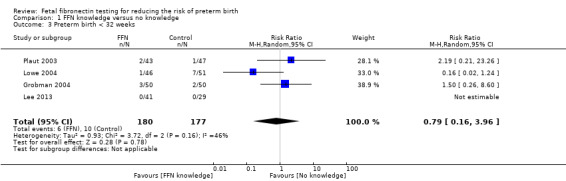
Comparison 1 FFN knowledge versus no knowledge, Outcome 3 Preterm birth < 32 weeks.
1.4. Analysis.
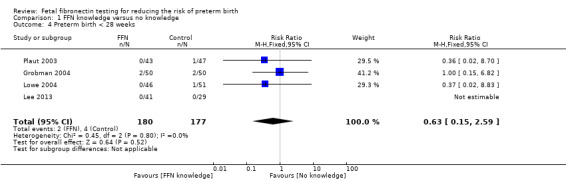
Comparison 1 FFN knowledge versus no knowledge, Outcome 4 Preterm birth < 28 weeks.
1.5. Analysis.
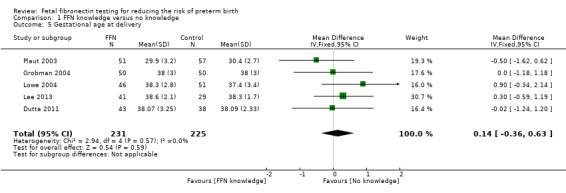
Comparison 1 FFN knowledge versus no knowledge, Outcome 5 Gestational age at delivery.
1.6. Analysis.
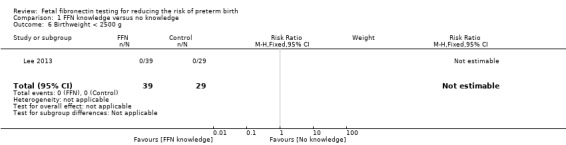
Comparison 1 FFN knowledge versus no knowledge, Outcome 6 Birthweight < 2500 g.
1.7. Analysis.
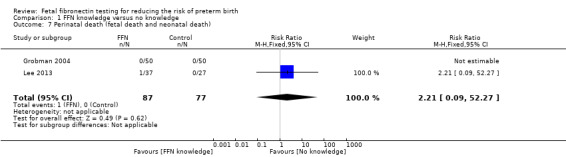
Comparison 1 FFN knowledge versus no knowledge, Outcome 7 Perinatal death (fetal death and neonatal death).
1.8. Analysis.
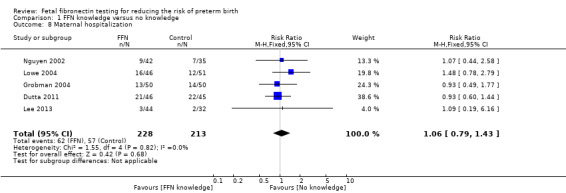
Comparison 1 FFN knowledge versus no knowledge, Outcome 8 Maternal hospitalization.
1.9. Analysis.
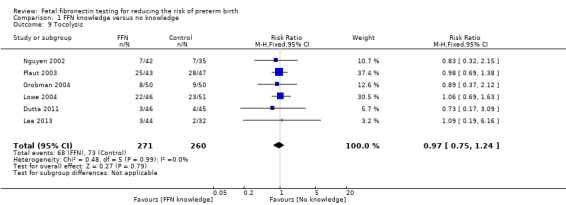
Comparison 1 FFN knowledge versus no knowledge, Outcome 9 Tocolysis.
1.10. Analysis.
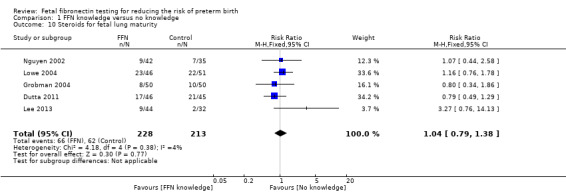
Comparison 1 FFN knowledge versus no knowledge, Outcome 10 Steroids for fetal lung maturity.
1.11. Analysis.
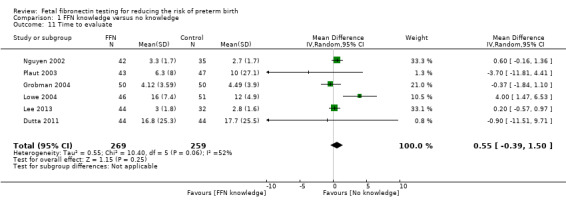
Comparison 1 FFN knowledge versus no knowledge, Outcome 11 Time to evaluate.
1.12. Analysis.
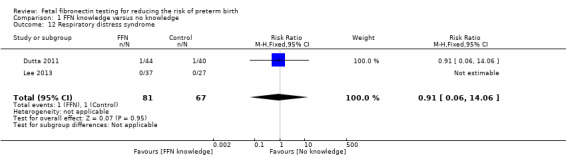
Comparison 1 FFN knowledge versus no knowledge, Outcome 12 Respiratory distress syndrome.
1.13. Analysis.
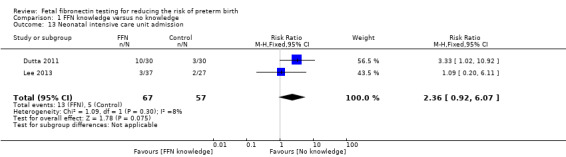
Comparison 1 FFN knowledge versus no knowledge, Outcome 13 Neonatal intensive care unit admission.
1.14. Analysis.
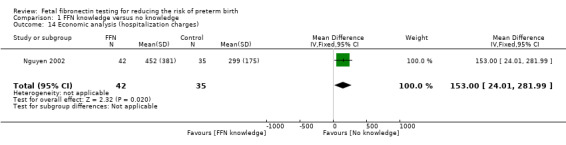
Comparison 1 FFN knowledge versus no knowledge, Outcome 14 Economic analysis (hospitalization charges).
Intraventricular haemorrhage, necrotizing enterocolitis, sepsis, NICU days, and maternal well‐being were not reported in any trial.
Discussion
Summary of main results
Management based on knowledge of FFN results may reduce preterm birth before 37 weeks (20.7%) versus controls without such knowledge (29.2%). However, management based on knowledge of FFN results may make little or no difference to preterm birth before 34 weeks or maternal hospitalization. The evidence for preterm birth before 32 weeks is uncertain because the quality was found to be very low.
For all other outcomes, for which there were available data (preterm birth less than 28 weeks; gestational age at delivery (weeks); birthweight less than 2500 g; perinatal death; tocolysis; steroids for fetal lung maturity; time to evaluate; respiratory distress syndrome; neonatal intensive care unit (NICU) admission; and NICU days), knowledge of FFN results may make little or no difference to the outcomes.
Therefore, further research is necessary before fetal fibronectin testing can be routinely recommended. There are no randomized trials comparing knowledge versus no knowledge of fetal fibronectin in asymptomatic pregnant women, or in women with multiple gestations.
Overall completeness and applicability of evidence
We attempted to be as inclusive as possible in the search strategy. Nonetheless, the studies reported are predominantly from the US, which may limit the external validity of these results. The evidence from the included studies is also based on the cutoff for the fFN bedside test, also known as Quickchack fFN (50 ng/L). It should be noted, that the use of quantitative fFN which has since become available, may affect any future conclusions of this review.
It is still unclear which interventions are most beneficial once fetal fibronectin results are known. The Ness positive study (Ness 2007) reported a detailed protocol of management based on cervical length mainly (and fetal fibronectin results for a small subgroup), and could be replicated in further research trials. Our review did not include by design assessment of effectiveness of interventions based on positive fetal fibronectin testing, or negative fetal fibronectin testing. We identified no trials on women without signs or symptoms of labor.
Quality of the evidence
For the main comparison (fetal fibronectin knowledge versus no knowledge) and for most important outcomes (preterm birth less than 37 weeks; preterm birth less than 34 weeks; preterm birth less than 32 weeks; maternal hospitalization), we graded the quality of evidence using the GRADE approach. For most of these outcomes, the evidence was graded as low‐quality mainly due to the imprecision of effect estimates and limitations in study design. We graded preterm birth less than 32 weeks as very low‐quality due to evidence of inconsistency in results, in addition to imprecision of effect estimates and limitations in study design (Table 1).
Potential biases in the review process
We acknowledge that our analysis of preterm delivery is flawed as it was analyzed as four separate outcomes (< 37, < 34, < 32, < 28 weeks); and clearly these outcomes are not independent. A much better analysis would be to use the time to birth for each participant, but that would require individual patient data (IPD). This approach is not feasible without the availability of IPD. Vincenzo Berghella is a co‐author on one of the excluded trials (Ness 2007) and was not involved in the eligibility assessment for this trial as this was assessed by Gabriele Saccone.
Agreements and disagreements with other studies or reviews
The findings of this review largely agree with our prior Cochrane Review and other reviews on this topic, with now additional studies and larger sample size (Berghella 2008; Berghella 2016).
Authors' conclusions
Implications for practice.
The evidence from this review suggests that management based on knowledge of FFN results may reduce preterm birth before 37 weeks. However, our confidence in this result is limited as the evidence was found to be of low‐quality. Effects on other substantive outcomes are uncertain due to serious concerns in study design, inconsistency, and imprecision of effect estimates. No trials were identified on asymptomatic women, or multiple gestations. Currently, the only management protocol for screening of women with threatened preterm labor shown by randomized trial data to decrease preterm birth has been that based mainly on transvaginal ultrasound cervical length, with fetal fibronectin only in women with cervical length 20 mm to 29 mm (Berghella 2016; Ness 2007).
Implications for research.
Given that the evidence suggests that there may be a reduction of around 28% in the primary outcome, further research could be encouraged, minimizing attrition bias, to better understand whether and under what circumstances the predictive characteristics of the fetal fibronectin test can be translated into better clinical management. Future studies could include specific populations (e.g. singleton gestations with symptoms of preterm labor), a study group managed with a protocol based on the fetal fibronectin results, and report not only maternal but also significant perinatal outcomes. Cost‐effectiveness analyses are also needed.
What's new
| Date | Event | Description |
|---|---|---|
| 24 September 2019 | Amended | We have corrected an error in the reporting of the primary outcome 'preterm birth before 37 weeks'. The number of women and trials contributing data is 5 (434 women) and not 4 (357 women) as previously published in the Abstract, Plain language summary and Discussion. |
History
Protocol first published: Issue 4, 2007 Review first published: Issue 4, 2008
| Date | Event | Description |
|---|---|---|
| 7 September 2018 | New citation required but conclusions have not changed | Two new studies have been added (Dutta 2011; Lee 2013), but the conclusions have not changed. |
| 7 September 2018 | New search has been performed | Search updated and new trials added. A 'Summary of findings' table has been incorporated. |
| 16 December 2007 | Amended | Converted to new review format. |
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), and the Group's Statistical Adviser. The authors are grateful to the following peer reviewer for her time and comments: Dr Monika Skubisz, South Australian Health and Medical Research Institute (SAHMRI), Women and Kids Theme.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search methods for ICTRP, ClinicalTrials.gov and additional MEDLINE search
ICTRP
fetal AND fibronectin
ffn
ClinicalTrials.gov
fibronectin | Interventional Studies | pregnancy
fibronectin | Interventional Studies | preterm
Interventinal Studies | preterm | Fetal fibronectin
Data and analyses
Comparison 1. FFN knowledge versus no knowledge.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth < 37 weeks | 5 | 434 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.52, 1.01] |
| 2 Preterm birth < 34 weeks | 4 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.54, 2.18] |
| 3 Preterm birth < 32 weeks | 4 | 357 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.16, 3.96] |
| 4 Preterm birth < 28 weeks | 4 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.15, 2.59] |
| 5 Gestational age at delivery | 5 | 456 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.36, 0.63] |
| 6 Birthweight < 2500 g | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Perinatal death (fetal death and neonatal death) | 2 | 164 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [0.09, 52.27] |
| 8 Maternal hospitalization | 5 | 441 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.79, 1.43] |
| 9 Tocolysis | 6 | 531 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.75, 1.24] |
| 10 Steroids for fetal lung maturity | 5 | 441 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.79, 1.38] |
| 11 Time to evaluate | 6 | 528 | Mean Difference (IV, Random, 95% CI) | 0.55 [‐0.39, 1.50] |
| 12 Respiratory distress syndrome | 2 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.06, 14.06] |
| 13 Neonatal intensive care unit admission | 2 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [0.92, 6.07] |
| 14 Economic analysis (hospitalization charges) | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | 153.0 [24.01, 281.99] |
Comparison 2. FFN knowledge versus no knowledge: sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth < 37 weeks | 4 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.46, 0.97] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dutta 2011.
| Methods | Randomized controlled trial | |
| Participants | 24 0/7‐34 6/7 weeks singletons presenting to the hospital for the primary reason of uterine activity. Twins excluded. Number of participants = 93 (100 enrolled, but 7 excluded because they did not meet the inclusion criteria of which 4 were twins). | |
| Interventions | FFN testing (44) versus no testing (44) Time FFN results available: unknown Protocol for FFN knowledge group: no |
|
| Outcomes | Primary: hospital admission | |
| Notes | Unpublished data were not obtainable. Setting: 2 hospitals, Scotland Dates: December 2007 ‐ March 2009 Conflict of interest: none Funding source: the trial is registered with the University of Edinburgh which is a charitable body, registered in Scotland, with registration number SC005336. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Telephonic randomization |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is reported that those that did not follow the protocol were excluded from the study. No study flow diagram. In methods reports that 100 patients were enrolled, and 93 were available for analysis ‐ 44 in the control group and 49 in the test group ‐ but then denominator results differ ‐ sometimes both groups reported as 44 and then in other places reports 49. |
| Selective reporting (reporting bias) | Low risk | The trial was registered and there were no deviations from the original protocol in the final publication. |
| Other bias | Unclear risk | Insufficient reporting to determine presence of other form of bias |
Grobman 2004.
| Methods | Randomized controlled trial | |
| Participants | 24‐34 week singletons with > 6 contractions/hour. Twins excluded. Number of participants: 100 (50/50: knowledge/no knowledge) |
|
| Interventions | FFN knowledge or not Time FFN results available: unknown Protocol for FFN knowledge group: no |
|
| Outcomes | Primary: total costs | |
| Notes | Intention‐to‐treat; only singletons; no protocol Positive FFN test in each group: 5 (10%) in knowledge, 3 (6%) in no knowledge group Setting: hospital, United States Dates: "12 month period" dates unclear Conflict of interest: not mentioned Funding source: supported by a grant from Adeza Biomedical, Sunnyvale, Calif. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Computer‐generated, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Laboratory personnel who performed the FFN test were blinded to women's characteristics and outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. One woman randomized to the knowledge group ‐ her results were not available because of problems with the machine ‐ however it is reported that outcomes for this patient were analysed in the group to which she was randomized, suggesting they adhered to intention to treat analysis. |
| Selective reporting (reporting bias) | Low risk | The trial was registered and there were no deviations from the original protocol in the final publication. |
| Other bias | Unclear risk | Insufficient reporting to determine presence of other form of bias. |
Lee 2013.
| Methods | Randomized controlled trial | |
| Participants | 24 0/7‐33 6/7 weeks singletons with symptoms suggesting PTL Number of participants: 76 (44/32: knowledge/no knowledge) |
|
| Interventions | FFN knowledge versus no FFN knowledge Time FFN results available: unknown Protocol for FFN knowledge group: yes |
|
| Outcomes | Time required to evaluate participants in triage in Labor and Delivery | |
| Notes | Setting: medical centre, United States Dates: September 2006 ‐ December 2010 Conflict of interest: none Funding source: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Computer‐generated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Laboratory personnel who performed the FFN test were blinded to women's characteristics and outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | The trial was registered and there were no deviations from the original protocol in the final publication. |
| Other bias | Unclear risk | Insufficient reporting to determine presence of other form of bias |
Lowe 2004.
| Methods | Randomized controlled trial | |
| Participants | 23‐34 week singletons/twins with contractions +/‐ cervical change Number of participants: 97 [89 singletons/8 twins] (46/51: knowledge/no knowledge) |
|
| Interventions | FFN knowledge or not Time FFN results available: < 1 hour Protocol for FFN knowledge group: no |
|
| Outcomes | Primary: length of stay | |
| Notes | 13/110 excluded post‐randomization: unclear whether intention‐to‐treat; included twins; no protocol Positive FFN test in each group: 11 (24%) in knowledge group; FFN test not performed in no knowledge group Setting: hospital, United States Dates: August 2000 ‐ May 2002 Conflict of interest: not mentioned Funding source: supported by Process Improvement Grant, University of Iowa |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Computer‐generated, opaque envelopes, blocks of 10 |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unblinded intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 110 were enrolled into the study, but 13 were not included (reasons were documented ‐ but did not present characteristics of these women or make it clear which group they had been randomized to) and so 97 women were available for the analysis. It is not clear exactly how many were initially randomised. |
| Selective reporting (reporting bias) | Low risk | No deviation from the original protocol |
| Other bias | Unclear risk | Insufficient reporting to determine presence of other form of bias |
Nguyen 2002.
| Methods | Randomized controlled trial | |
| Participants | 24‐35 weeks (unclear if twins included) with symptoms of PTL Number of participants: 77 (42/35: knowledge/no knowledge) |
|
| Interventions | FFN testing done (and knowledge available) versus not done (so knowledge not available) Time FFN results available: unknown Protocol for FFN knowledge group: yes |
|
| Outcomes | Primary: cost‐effectiveness | |
| Notes | Abstract only; unclear if intention‐to‐treat, twins included, protocol, etc. Positive FFN test in each group: not available in knowledge group; FFN not performed in no knowledge group Setting: hospital, United States Dates: not specified Conflict of interest: not mentioned Funding source: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description available (only abstract published) |
| Allocation concealment (selection bias) | Unclear risk | No description available (only abstract published) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description available (only abstract published) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description available (only abstract published) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only abstract available ‐ all enrolled appear to have been randomized |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | No description available (only abstract published) |
Plaut 2003.
| Methods | Randomized controlled trial | |
| Participants | 24‐34 6/7 week singletons/twins with 'symptoms of PTL' Number of participants: 114 (unclear from text) randomized [96 singleton, 12 twins] (analysed: 51/57: knowledge/no knowledge) |
|
| Interventions | FFN knowledge or not Time FFN results available: 1‐2 hours Protocol for FFN knowledge group: no |
|
| Outcomes | Primary: transport rates (not reported) | |
| Notes | 6/114 excluded post‐randomization: not intention‐to‐treat ‐ 6 patients were excluded; 8 participants entered in study twice; stopped trial prematurely; sponsored by Adeza; included twins; no protocol Positive FFN test in each group: 6 (12%) in knowledge, 6 (10.5%) in no knowledge group Setting: 4 community hospitals, United States Dates: September 2000 ‐ December 2001 Conflict of interest: not mentioned Funding source: supported by Adeza Biomedical, Sunnyvale, Calif. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Computerized opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Laboratory personnel who performed the FFN test were blinded to women's characteristics and outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Consent obtained from 114 patients, 6 patients were excluded (reasons given), this left 108 swabs from 100 different patients ‐ 8 patients were entered into the study twice. Results are presented for 108 swabs. Not clear number originally randomized into each group. |
| Selective reporting (reporting bias) | Low risk | No deviation from the original protocol |
| Other bias | Unclear risk | Insufficient reporting to determine presence of other form of bias |
FFN: fetal fibronectin
ITT: intention to treat PTL: preterm labor
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andrews 2001 | Only women with positive FFN were included. |
| Bisits 2004 | Only women with positive FFN were included. They were randomized to tocolysis or not. |
| Elliott 2005 | Only women with negative FFN were included. |
| Gallot 2018 | Registration protocol of a trial using different diagnostic tests, and only in part on FFN |
| Goldenberg 2001 | Only women with positive FFN were included (secondary analysis of NICHD BV/TV study). |
| Hauth 2001 | Only women with positive FFN were included. |
| ISRCTN43735180 | Only women with positive FFN were included. |
| Kalchbrenner 1999 | This was a trial comparing speculum versus digital collection of FFN. There were no data regarding the results of the FFN test, and no intervention was reported based on FFN results. |
| Ness 2007 | The algorithm used was based mostly on cervical length results, and only in part (for cervical lengths 20‐29 mm) on FFN. |
| Osório 2010 | Published only as abstract with no data available on methods or the outcomes of interest. |
| Shennan 2006 | Only women with positive FFN were included. |
FFN: fetal fibronectin
Characteristics of ongoing studies [ordered by study ID]
NCT01431885.
| Trial name or title | 2 methods of diagnosing preterm labor |
| Methods | Randomized controlled trial |
| Participants | Symptomatic women with more than 6 uterine contractions per hour |
| Interventions | Symptomatic preterm labor patients will be randomized to diagnosis of preterm labor by serial digital examination versus an algorithm incorporating transvaginal ultrasound measurement of cervical length and vaginal fetal fibronectin |
| Outcomes | Preterm birth < 37 weeks |
| Starting date | 2011 |
| Contact information | Conrad Chao; 001‐559‐499‐6548; cchao@fresno.ucsf.edu |
| Notes |
Differences between protocol and review
A 'Summary of findings' table has been incorporated in this update and we added in a search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned, and ongoing trial reports.
In the original review, we also ran a separate search of MEDLINE (January 1966 to December 2007) using the search strategy detailed below:
MEDLINE
#1 exp Obstetric labor, premature/ #2 Fibronectins/ #3 #1 and #2 #4 fetal adj3 fibronectin #5 #3 or #4
Contributions of authors
Vincenzo Berghella (VB), Gabriele Saccone:
helped with the initial idea of a fetal fibronectin review update;
met several times regarding all aspects of the development of the protocol and review;
contributed to the writing and the editing of the protocol and the review;
helped to revise and respond to the feedback received on the first draft of the protocol and the review.
VB prepared the first draft and finalised the revised draft of the protocol and the review in response to feedback.
VB is the guarantor of the review.
Sources of support
Internal sources
None, Other.
External sources
No sources of support supplied
Declarations of interest
Vincenzo Berghella: is a co‐author on one of the excluded trials (Ness 2007). VB was not involved in the eligibility assessment for this trial as this was assessed by Gabriele Saccone.
Gabriele Saccone: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Dutta 2011 {published data only}
- Dutta D, Norman JE. Pilot study into the efficacy of foetal fibronectin testing in minimising hospital admissions in women presenting with symptoms of preterm labour: a randomised controlled trial of obstetric and neonatal outcomes. Archives of Gynecology and Obstetrics 2011;284(3):559‐65. [DOI] [PubMed] [Google Scholar]
Grobman 2004 {published and unpublished data}
- Grobman W, Welshman E, Calhoun E. Does fetal fibronectin use in the diagnosis of preterm labor affect physician behavior and health care costs [abstract]. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S80. [DOI] [PubMed] [Google Scholar]
- Grobman WA, Welshman EE, Calhoun EA. Does fetal fibronectin use in the diagnosis of preterm labor affect physician behavior and health care costs? A randomized trial. American Journal of Obstetrics and Gynecology 2004;191:235‐40. [DOI] [PubMed] [Google Scholar]
Lee 2013 {published data only}
- Burwick R, York N, Lee GT, Ross MG, Kjos SL. Cervilenz assessment of cervical length is equivalent to fetal fibronectin in the prediction of preterm delivery. American College of Obstetricians and Gynecologists' Annual Meeting; 2010 May 17‐19; San Francisco, California, USA. 2010.
- Burwick RM, Zork NM, Lee GT, Ross MG, Kjos SL. Cervilenz assessment of cervical length compared to fetal fibronectin in the prediction of preterm delivery in women with threatened preterm labor. Journal of Maternal‐Fetal & Neonatal Medicine 2011;24(1):127‐31. [DOI] [PubMed] [Google Scholar]
- Lee GT, Burwick R, Zork N, Kjos SL. Does the use of fetal fibronectin in an algorithm for preterm labor reduce triage evaluation times?. Journal of Maternal‐Fetal & Neonatal Medicine 2013;26(7):706‐9. [DOI] [PubMed] [Google Scholar]
Lowe 2004 {published and unpublished data}
- Hansen W, Lowe MP, Zimmerman B. Effect of the fetal fibronectin assay on preterm labor management [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S136. [DOI] [PubMed] [Google Scholar]
- Lowe MP, Zimmerman B, Hansen W. Prospective randomized trial of fetal fibronectin on preterm labor management in a tertiary care center. American Journal of Obstetrics and Gynecology 2004;190:358‐62. [DOI] [PubMed] [Google Scholar]
Nguyen 2002 {published and unpublished data}
- Nguyen TCQ, Toy EC, Baker B. The cost‐effectiveness of fetal fibronectin testing in suspected preterm labor: a randomized trial. Obstetrics & Gynecology 2002;99(4 Suppl):97S. [Google Scholar]
Plaut 2003 {published and unpublished data}
- Plaut MM, Smith W, Kennedy K. Fetal fibronectin: the impact of a rapid test on the treatment of women with preterm labor symptoms. American Journal of Obstetrics and Gynecology 2003;188:1588‐95. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Andrews 2001 {published data only}
- Andrews WW, Sibai BM, Thom EA, Dudley D, Ernest JM, McNellis D, et al. Randomized clinical trial of metronidazole plus erythromycin to prevent spontaneous preterm delivery in fetal fibronectin‐positive women. Obstetrics & Gynecology 2003;101(5 Pt 1):847‐55. [DOI] [PubMed] [Google Scholar]
Bisits 2004 {published data only}
- Bisits A, Madsen G, Knox M, Gill A, Smith R, Yeo G, et al. The randomized nitric oxide tocolysis trial (RNOTT) for the treatment of preterm labor. American Journal of Obstetrics and Gynecology 2004;19(3):683‐90. [DOI] [PubMed] [Google Scholar]
Elliott 2005 {published data only}
- Elliott JP, Miller HS, Coleman S, Rhea D, Abril D, Hallbauer K, et al. A randomized multicenter study to determine the efficacy of activity restriction for preterm labor management in patients testing negative for fetal fibronectin. Journal of Perinatology 2005;25(10):626‐30. [DOI] [PubMed] [Google Scholar]
Gallot 2018 {published data only}
- NCT03608995. Combined detection of interleukin‐6 and insulin‐like growth factor binding protein‐1 total and native (premaquick) for the prediction of delivery in women with threatened preterm labor. clinicaltrials.gov/ct2/show/record/NCT03608995 (first received 1 August 2018).
Goldenberg 2001 {published data only}
- Goldenberg RL, Klebanoff M, Carey JC, Macpherson C. Metronidazole treatment of women with a positive fetal fibronectin test result. American Journal of Obstetrics and Gynecology 2001;185(2):485‐6. [DOI] [PubMed] [Google Scholar]
Hauth 2001 {published data only}
- Hauth JC, Cliver S, Hodgkins P, Andrews WW, Schwebke JR, Hook EW, et al. Mid‐trimester metronidazole and azithromycin did not prevent preterm birth in women at increased risk: a double‐blind trial [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S86. [Google Scholar]
ISRCTN43735180 {published data only}
- ISRCTN43735180. Randomised double blind placebo controlled trial of antimicrobial treatment in pregnant women at risk of preterm labour. www.isrctn.com/ISRCTN43735180 (first received 12 September 2003).
Kalchbrenner 1999 {published data only}
- Kalchbrenner M, Weisenborn B, Jacques D, Coleman S, Chyu J. A randomized comparison of specimen collection methods for the fetal fibronectin immunoassay [abstract]. Obstetrics & Gynecology 1999;93(4 Suppl):51S. [Google Scholar]
Ness 2007 {published and unpublished data}
- NCT00371046. The use of fetal fibronectin and transvaginal ultrasound cervical length in women with threatened preterm labor: a randomized trial. clinicaltrials.gov/ct2/show/NCT00371046 (first received 1 September 2006).
- Ness A, Visintine J, Ricci E, Berghella V. Does knowledge of cervical length and fetal fibronectin affect management of women with preterm labor? A randomized trial. American Journal of Obstetrics and Gynecology 2007;197(4):426.el‐426.e7. [DOI] [PubMed] [Google Scholar]
- Ness A, Visintine J, Ricci E, Boyle K, Berghella V. Use of fetal fibronectin and transvaginal ultrasound cervical length to triage women with suspected preterm labor: a randomized trial. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S67. [DOI] [PubMed] [Google Scholar]
Osório 2010 {published data only}
- Osório M, Neiva R, Montes L, Silva J, Pinelo S, Pinho M, et al. The impact of fetal fibronectin assay on preterm labor management: a randomized controlled trial. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23(Suppl 1):304‐5. [Google Scholar]
Shennan 2006 {published data only}
- Kurtzman J, Chandiramani M, Briley A, Poston L, Shennan A. Minimal quantitative levels of fetal fibronectin (1‐49 NG/ML) at 24‐27 weeks are associated with an increased risk of recurrent preterm delivery in asymptomatic patients with prior preterm birth. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S47. [DOI] [PubMed] [Google Scholar]
- Shennan A, Crawshaw S, Briley A, Hawken J, Seed P, Jones, G, et al. A randomised controlled trial of metronidazole for the prevention of preterm birth in women positive for cervicovaginal fetal fibronectin: the PREMET Study. British Journal of Obstetrics & Gynaecology 2006;113(1):65‐74. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01431885 {published data only}
- NCT01431885. Randomized controlled trial of two methods of diagnosing preterm labor. clinicaltrials.gov/ct2/show/NCT01431885 (first received 12 September 2011).
Additional references
Berghella 2016
- Berghella V, Saccone G. Fetal fibronectin testing for prevention of preterm birth in singleton pregnancies with threatened preterm labor: a systematic review and meta‐analysis of randomized controlled trials. American Journal of Obstetrics & Gynecology 2016;16:30136‐3. [DOI: 10.1016/j.ajog.2016.04.038] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Leitich 1999
- Leitich H, Egarter C, Kaider A, Hohlagschwandtner M, Berghammer P, Husslein P. Cervico‐vaginal fetal fibronectin as a marker for preterm delivery: a meta‐analysis. American Journal of Obstetrics and Gynecology 1999;180(5):1169‐76. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
References to other published versions of this review
Berghella 2008
- Berghella V, Hayes E, Visintine J, Baxter JK. Fetal fibronectin testing for reducing the risk of preterm birth. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD006843.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


