ABSTRACT
Background: Acute diarrheal disease caused by viral, bacterial and parasitic infections are a major global health problem with substantial mortality and morbidity in children under five years of age in lower and middle income countries. However, a number of these infections also impact large segments of populations in upper income countries, as well as individuals who travel overseas for work, business or pleasure. Campylobacter has been and continues to be a leading cause of disease burden globally across all income countries.
Aims: The aim of this review is to describe recent understanding in burden of disease, consider the current landscape of Campylobacter vaccine development, and address the challenges that need to be overcome.
Sources: Relevant data from the literature as well as clinical trials described in European and US registries were used to conduct this review.
Content: Despite advances in population health, food security, improved sanitation, water quality and the reduction of poverty, Campylobacter infections continue to plague global populations. The emerging recognition of chronic health consequences attributed to this pathogen is changing the potential valuation of preventive interventions. Advancing development of new vaccines is a present opportunity and holds promise.
Keywords: Campylobacter, jejuni, vaccine, capsule, traveler, diarrhea
Introduction
Acute enteric infections causing diarrhea and gastroenteritis are a global public health problem with high mortality and morbidity, particularly among children of the developing world. Each year, approximately 500,000 children under five years old die from severe, dehydrating diarrhoea and dysentery worldwide, and millions more are hospitalized, mostly in low-resource countries.1 In addition, growing evidence finds that children are also susceptible to long-term physical and cognitive health consequences secondary to these frequent infections.2
While the overwhelming burden due to these infections is in the developing world, acute enteric infections are also a frequent cause for outpatient visits and hospitalization throughout the developed world with substantial societal and economic impact. For example, Scallan and colleagues published recently updated estimates for foodborne illness in the United States, which indicated that each year 31 major pathogens acquired in the United States caused 9.4 million episodes of diarrhoeal illness, 55,961 hospitalizations, and 1,351 deaths.3 While the acute consequences would appear to be of global significance and drive science and public health efforts to mitigate the problem, there is growing evidence linking such infections with a myriad of chronic health consequences including neurological, haematological and rheumatological systems.4 Furthermore, as with many other global infections, antibiotic resistance concerns among bacterial infections, particularly Campylobacter spp., have brought alarm to the potential worsening impact of these infections with decreasing effectiveness of commonly used antibacterials.
To fully address this problem, a multifaceted approach is needed including improvements in sanitation and hygiene, safe water, food security, breast-feeding, and adequate nutrition, and vaccination. While vaccines against some enteric pathogens are available, most are currently used for limited purposes, although efforts are underway to expand their utilization. Additionally, novel vaccines are in development, which promise hope to improve the health of at-risk populations from the developed and developing worlds. Herein, we review the current public health gap, understanding of pathogenesis, historical efforts, and current status of vaccine development for Campylobacter.
Burden of disease
It is estimated that Campylobacter infection is associated with 7.5 million disability adjusted life years (DALYs) globally, second only to rotavirus as a causative agent of diarrheal disease, with varying epidemiology across multiple populations.5 The 2015 global burden of disease estimates indicate over 25,000 deaths annually in children under 5 years of age with the highest rates in parts of Africa and Asia (Figure 1). Recent studies on diarrheal disease in several low and middle income countries (LMICs) have continued to highlight Campylobacter as a significant pathogen in children under five years of age.6,7 Despite the global burden of disease, the acute illness is often confounded by high infection rates in asymptomatic age-matched children.
Figure 1.
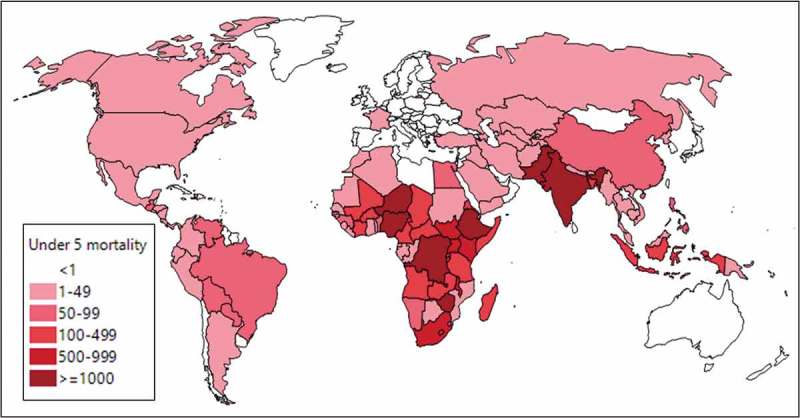
Campylobacter-attributable under 5 mortality (recreated from .
Two landmark studies have recently described the pathogens associated with acute enteric infections in children under 5 living in multiple LMICs. In the Global Enteric Multicenter Study (GEMS), C. jejuni was identified as a leading cause of moderate to severe diarrhea in children aged 0–11 months as well as in children 2–5 years of age across all study sites; however, rates appeared to be highest at study sites in Bangladesh, Pakistan and India.6 These data are somewhat limited in their ability to determine the true burden of campylobacteriosis due to case-control design. In particular, using mathematical models, Havelaar and Swart demonstrated that for pathogens in which the force of infection is high, disease odds ratios may be biased towards the null and estimates of the population attributable fraction may underestimate the proportion of cases attributable to the organism.8 Additionally, microbiological methods have hampered all prior estimates of Campylobacter morbidity. Specifically, studies relying on culture-based methods of pathogen identification likely underestimate pathogen-specific disease burden. In fact, a re-analysis of GEMS data, highlighted a significant increase in the attributable incidence of C. jejuni/coli (as well as other enteric pathogens) to moderate to severe diarrhea when molecular based methods were used to identify disease etiology.9
In the Malnutrition and Enteric Disease (MAL-ED) study, designed as a birth cohort study, Campylobacter was the most common pathogen, identified in approximately 35% of diarrheal stools and 25% of non-diarrheal stools in children < 1 year of age.7 By 1–2 years of age, that proportion had increased to 40% in diarrheal stools and 35% in non-diarrheal stools. Estimates of adjusted attributable fraction highlight Campylobacter as the third leading cause of diarrhea in children < 1 year of age in the populations with the highest rates in two sites in South America (Fortaleza, Brazil and Loreto, Peru) and in Venda, South Africa. By the second year of life, Campylobacter attributed to approximately 8% of all diarrheal cases, the highest of any diarrheal pathogen with the highest burden in Bhaktapur, Nepal and Loreto, Peru.
In addition to the morbidity associated with acute diarrheal illness in these populations, Campylobacter infection has been shown to be negatively associated with linear growth regardless of whether or not the infection is associated with acute illness.10 In children from the MAL-ED cohort study with Campylobacter infection, approximately 1 cm growth shortfall and an approximate 0.25 kg less weight gain was seen compared to children without Campylobacter; an effect that appeared to be most pronounced at 0–2 months of age.11 Perhaps even more important than the association between Campylobacter infection and growth faltering is the potential link to environmental enteric dysfunction as recently described by Schnee and Petri.12 If these associations are confirmed, current burden of disease estimates have likely grossly underestimated the morbidity associated with Campylobacter in children in LMICs.
In contrast to LMICs, the rate of asymptomatic infection is low; however, industrialized countries globally have documented a high rate of campylobacteriosis associated with consuming contaminated food and drink. In 2017 Campylobacter was the most commonly identified foodborne pathogen in the United States with approximately 10,000 cases confirmed in the 10 FoodNet sites performing active surveillance.13 In Europe, Campylobacter was also the leading cause of foodborne disease with over 200,000 cases in 2013, approximately 2.5 times the number of cases of Salmonella, another common foodborne pathogen.14 Similarly, data from OzFoodNet sites, Australia’s foodborne disease surveillance system, have highlighted Campylobacter as the leading cause of foodborne disease with an estimated 116 notifications per 100,000 population.15 While these data highlight the importance of Campylobacter, it is important to underscore the fact that these studies all likely underestimate the true incidence of disease due to the surveillance methods utilized. For example, in the United States, active surveillance as part of FoodNet is established for 10 representative locations around the country accounting for approximately 14% of the US population. Despite the ‘active’ nature of this surveillance, case identification is dependent on subjects seeking care. A 2011 report by the US Centers for Disease Control and Prevention highlighted the under-reporting inherent with this type of surveillance. For Campylobacter, it was estimated that for every 1 case of campylobacteriosis clinically diagnosed, over 30 cases occurred in the community.16
In travelers to LMICs, Campylobacter is a leading cause of travelers’ diarrhea (TD) second only to diarrheagenic Escherichia coli.17–19 Geographically, the incidence of Campylobacter-attributable TD varies with by far the highest rates observed in travelers to Southeast Asia. In addition to being a common cause of travelers’ diarrhea, Campylobacter is often associated with a more severe disease. In separate studies, Porter et al and Sanders et al found that approximately 50% of Campylobacter-attributed cases were unable to work as a result of their illness compared to only approximately 25% similarly affect cases attributed to other pathogens, highlighting the increased severity of illness in Campylobacter cases of TD.20,21 Clinically, Campylobacter cases tend to more commonly have fever, myalgia, arthralgia, and are more likely to have a sub-optimal response to treatment compared to TD cases of other etiology.21
In addition to the acute illness, Campylobacter infection is associated with numerous neurological, gastrointestinal and rheumatological post-infectious sequelae which further magnify the overall morbidity of infection.22 In particular, Campylobacter is the most common infection associated with Guillian-Barré Syndrome, a disorder in which the body’s immune system attacks the peripheral nerve cells causing an acute, ascending flaccid paralysis, a process initiated by anti-ganglioside antibodies directed to the Campylobacter infection.23,24 The association between Campylobacter infection and several chronic gastrointestinal outcomes including irritable bowel syndrome, functional dyspepsia, inflammatory bowel disease and celiac disease was recently reviewed and highlights the significant costs and impact on quality of life measures associated with this pathogen.25 Campylobacter is also one of several bacterial enteropathogens associated with reactive arthritis, a post-infectious sterile inflammation of joints and tissues that, in a significant proportion of cases, can be long lasting with significant associated morbidity.26,27
When considering the overall burden of campylobacteriosis, in addition to the corresponding acute morbidity and mortality described above as well as the long-term post-infectious sequelae, a consideration must also be made regarding anti-microbial resistance (AMR). In a recent study of international travelers, rates of ciprofloxacin resistance appeared to increase over time with almost 75% of the strains resistant by 2014.28 In addition to the rate of ciprofloxacin resistance, 4% were also resistant to erythromycin. The concern over AMR has led the US Centers for Disease Control and Prevention to designate antibiotic resistant Campylobacter as a serious threat to public health.29 Additionally, the World Health Organization recently labeled Campylobacter as a high priority pathogen for novel antibiotic development due to the rates of ciprofloxacin resistance.30 While the majority of Campylobacter cases do not require antibiotic treatment, the rising rates of AMR threatens to yield few treatment options in those at high risk of more serious outcomes or with more severe disease.
Advances in understanding of pathogenesis
Current knowledge on C. jejuni infectious process has revealed that the bacteria colonize the intestine via penetration of the intestinal mucus layer, invade intestinal epithelial cells leading to damages and ultimately disease manifestations. A number of mechanisms have been attributed to C. jejuni virulence including, cell adhesion molecules, cytolethal distending toxin (CDT), lipooligosaccharide (LOS), antimicrobial efflux pumps, capsular polysaccharide (CPS), invasion proteins, flagella, N- and O-glycosylation,31–34 and a Type VI secretion system that has been recently discovered in some C. jejuni strains.35 Unfortunately, the major mechanisms of virulence and human disease are currently unclear due to the lack of cell and animal models that accurately convey human infection and disease. Several small animal models have been used including new-born piglet,36 ferret,37 genetically modified mice38-40 and even insects.41 Nevertheless, each of those models require large C. jeuni doses and do not recapitulate the symptoms of human campylobacteriosis. Perhaps overcoming prior limitations, Giallourou et al. induced bloody and inflammatory diarrhea following C. jejuni infection in antibiotic-treated mice fed a low zinc diet.42 This novel animal model of campylobacteriosis may further elucidate mechanisms of C. jejuni virulence.
During the infectious process, C. jejuni is actively evading the host immune defenses through the action of various cell surface structures, including N- and O-linked glycosylation, multiple CPS and LOS serotypes and structures between strains, and molecular mimicry.34 Much of the current research on C. jejuni pathogenesis has focused on these cell structure mechanisms, modifications, and phase variations with the hope of developing vaccines that are unaffected by mechanisms of immune system escape.
The C. jejuni CPS highly contributes to immune system evasion. C. jejuni CPS, which was originally thought to be lipopolysaccharide (LPS), was first discovered upon identification of a putative kps locus of the first published C. jejuni genome sequence.43 Importantly, CPS was found to be the key antigenic determinant in the Penner heat-stable (heat-stable, HS) serotyping scheme.44 The Penner serotyping system was the most widespread method of C. jejuni strain typing and based on antiserum raised against heat-stable C. jejuni antigens.45 To date, 47 Penner serotypes have been described. Considering that CPS is a key determinant of the Penner serotyping scheme,44 CPS represented a key antigenic determinant and hence a good potential vaccine target. Further studies revealed a key role of CPS in pathogenesis. Specifically, CPS was shown to promote resistance to complement-mediated cell death by normal human serum.46 A recent study also indicated that CPS plays an essential role in promoting systemic infection and abortion in sheep which was demonstrated to be partially due to CPS-mediated serum resistance.47 Importantly, C. jejuni CPS mutants were shown to be significantly attenuated in a ferret model of diarrheal disease and nearly completely attenuated in a Galleria mellonella model of C. jejuni disease.46,48 Additionally, mutants lacking CPS expression were shown to be defective in chicken colonization, the major reservoir for human infection, although infected chickens do not develop diarrheal disease or other major disease symptoms.49 CPS expression and structure may also influence C. jejuni adhesion to and invasion of host epithelial cells, which are both believed to be key steps in the C. jejuni infectious process, although the results have been inconsistent between studies.46,48–51 This inconsistency between studies was most likely been due to differences in the C. jejuni strain, CPS serotype, and/or the cell line used for these experiments. Taken together, these results suggest a major role of CPS in C. jejuni pathogenesis and immune evasion.
Phase-variable modifications play a key role in the mechanisms of CPS pathogenesis and immune evasion. Phase variation in C. jejuni occurs via slipped-strand mispairing, where high frequency introduction/deletion of C/G residues can occur during replication at stretches of 8 or more C/G residues. This mechanism introduces an early stop codon resulting in a truncated nonfunctional product.43 One major phase-variable modification involved in C. jejuni pathogenesis is the unique O-methylphosphoramidate (MeOPN) modification of CPS. An 81–176 mutant defective in MeOPN biosynthesis was as sensitive to normal human serum killing as a non-capsulated mutant,52 highlighting the major role of the MeOPN modification in immune system evasion, although an 81–176 mutant deficient in MeOPN expression on CPS showed no significant defect in chicken colonization after 6 days of infection, although the long-term survival was not tested.48 This result suggests that the MeOPN modification may be important to survival within humans and possibly other hosts but not chickens, at least in the short term. Intriguingly, indirect evidence suggests that the phase-variable MeOPN modification in the HS2 serotype strain NCTC11168 may actually reduce normal human serum resistance,51 although more detailed studies using specific mutants in the MeOPN transferase genes are needed to confirm this observation. In addition to immune evasion, the MeOPN phase variation may also play a role in pathogenesis since 81–176 mutants deficient in MeOPN biosynthesis or attachment to CPS surprisingly displayed increased Caco-2 cell invasion but not adherence vs. wild-type 81–176.48 Therefore, cycling of MeOPN on 81–176 may play a multifaceted role in C. jejuni pathogenesis. Importantly, both the galactose 4-OH MeOPN transferase gene (cjj1420) and the galactose 2,6-OH MeOPN transferase gene (cjj1435) of 81–176 contain poly(C) tracts that can undergo slipped-strand mispairing, resulting in phase variable gene expression during infection.48,53 This mechanism likely promotes optimal C. jejuni virulence and fitness during infection.
Another major cell surface modification is LOS, the major structural component of the C. jejuni outer membrane. C. jejuni LOS consists of an endotoxin, core oligosaccharide, and a single O-chain oligosaccharide structure rather than the repeating oligosaccharide moiety observed in LPS. Importantly, a number of C. jejuni LOS O-chain oligosaccharide structures have similarity to ganglioside oligosaccharide structures found on human and animal cells.54,55,56 More recent studies also identified strains displaying structural determinants at the non-reducing end of the LOS oligosaccharide similar to those found on human glycoconjugates including P blood group antigens, Type I N-acetyllactosamine, and α2-3-sialylated Type I N-acetyllactosamine.57 This molecular mimicry may be useful for C. jejuni evasion of antibody recognition by displaying self-antigens; moreover, ganglioside-like LOS structures are also subject to phase-variable expression,58–60 which may promote antibody and T cell escape. C. jejuni strains expressing ganglioside-like LOS structures are strongly associated with the autoimmune disorder Guillain-Barré syndrome (GBS), which is believed to occur via antibody generation towards the ganglioside mimics that then damage host tissues and ultimately GBS symptoms.54 It is possible that C. jejuni LOS structural mimics may contribute to other autoimmune disease sequelae associated with C. jejuni such as RA and IBD, but this remains to be studied. For these reasons, LOS-based or whole cell vaccines against C. jejuni do not appear practical or fruitful, as discussed in more detail in the following sections.
C. jejuni also harbors N- and O-glycosylation systems. While protein glycosylation in bacteria is being more widely recognized and appreciated, C. jejuni is quite unique in that it displays numerous classes of glycoconjugates and glycosylation systems including protein N-glycosylation, protein O-glycosylation, LOS, CPS, and peptidoglycan.54 The protein N-glycosylation synthesizes and attaches a unique heptasaccharide unit to target proteins that is not further modified,61 except that the terminal GalpNAc residue is sometimes modified with a phosphoethanolamine (PEtN) by the eptC gene product.62,63 The role of this N-glycan PEtN modification is currently unclear since eptC also adds PEtN to LOS and the flagellar rod protein FlgG.63,64 Expression of this N-linked heptasaccharide has been shown to promote resistance to cell death by host digestive protease enzymes such as trypsin,65 slightly reduce IL-6 production by human dendritic cells,66 and promote C. jejuni adhesion to and invasion of epithelial cells as well as colonization of the mouse intestine.67,68 In all these cases though, it is unclear if the N-glycan heptasaccharide directly mediates these effects or if N-glycosylation is merely required for full activity of N-glycosylated protein virulence factors such as Peb1 and JlpA. Taken together, the N-linked glycosylation system of C. jejuni may play an important role in C. jejuni host survival and infection.
Another major C. jejuni virulence factor is flagella. C. jejuni cells express bipolar flagella that promote cellular motility, and flagellar motility contributes to C. jejuni virulence.69 Additional roles of the flagella in pathogenesis, including host cell adhesion and as a virulence factor secretion system, have been previously reviewed.70 C. jejuni flagellin, the major protein of the flagellar filament, is also modified by O-glycosylation. In fact, the protein O-glycosylation system of C. jejuni seems to be limited to glycosylation of the flagellin protein subunits of the flagella. The flagellin O-glycan structures are monosaccharide units consisting of one of two rare sugars, pseudaminic acid (Pse5Ac7Ac) or legionaminic acid (Leg5Am7Ac), which can be further modified with small functional groups including acetate, acetamidine, and N-acetylglutamine (reviewed in54,70,71). These monosaccharide functional group modifications may be important in virulence as strains defective in O-glycan biosynthesis or modification showed significantly reduced adherence to and invasion of epithelial cells72 anti-inflammatory dendritic cell responses,73 colonization of chickens,74 and incidence of diarrheal disease in ferrets.72 The mechanisms by which O-glycosylation contributes to virulence though is unclear since, depending on the locations of the glycosylation sites, the O-linked glycans in strain 81–176 may be involved in either flagellar assembly and motility or autoagglutination, the latter of which may be important in C. jejuni biofilm formation.75 Importantly, the flagellum is one of the major Campylobacter antigens,76,77 with the O-glycosylated region of flagellin presumably being the most antigenic region.78–82 The variable monosaccharide structures and degree of O-glycosylation may thus assist in antibody and T cell escape. Additionally, C. jejuni flagellin is relatively unique in that it is not recognized by human or chicken Toll-Like Receptor 5 with or without O-glycosylation, even though Toll-like Receptor-5 is known to recognize flagellins from other bacteria to initiate and promote immune responses.83,84 Therefore, the antigenic C. jejuni flagellum utilizes multiple mechanisms to evade the immune system. However, considering the strong antigenicity towards the C. jejuni flagellum, development of a Campylobacter flagellar subunit vaccine has been of considerable interest, as discussed in more detail below.
Historical vaccine development and lessons learned
Development of an effective vaccine for the prevention of campylobacteriosis has been ongoing for over 20 years (Figure 2). The process has been hindered by multiple factors including the lack of small animal of disease, presence of LOS ganglioside mimics that can induce auto-immune diseases such as GBS, the lack of understanding of C. jejuni virulence factors, and the lack of known immunological correlates of disease protection. Poultry meat is considered the major source of infection, and much attention has been made in the veterinary field to develop a vaccine that would successfully control C. jejuni colonization in broilers.85,86 This review is focused on vaccines against Campylobacter that were processed into phase 1 human clinical trial. There is a multitude of C. jejuni antigens that were tested in animal models but were not tested in or even intended for humans. For example, there is a tremendous effort to reduce colonization of chicken in broiler setting via vaccination. Neal-McKinney et al. previously reviewed the control of Campylobacter jejuni colonization of poultry via vaccination.87 One of the most recent approaches was made by Szymanski et al., using the conserved N-glycan heptasaccharide conjugated to Tox protein. This conjugate vaccine provided an impressive reduction of C. jejuni colonization of the chicken gastrointestinal tract.88 This promising vaccine candidate may thus be worth testing in humans to assess its potential against campylobacteriosis.
Figure 2.
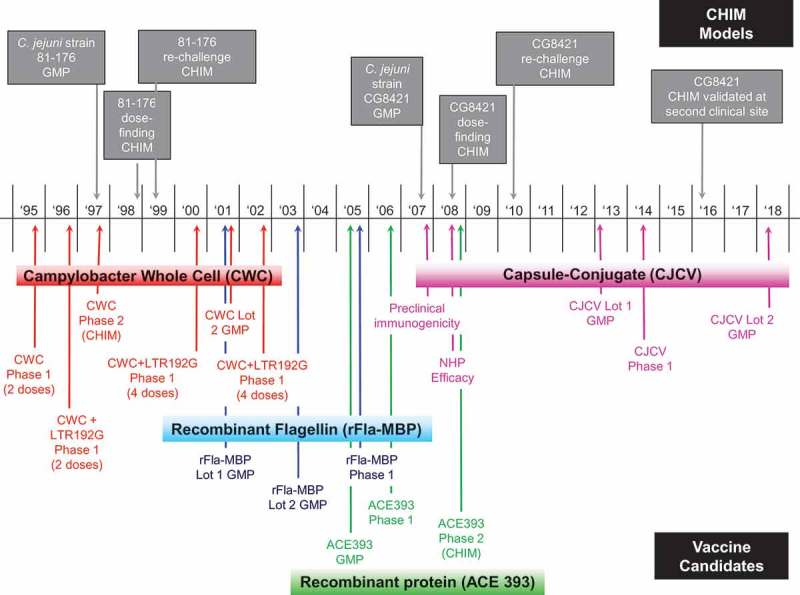
Campylobacter vaccine development history.
Controlled Human Infectious Model (CHIM) Good Manufacturing Practice (GMP)
Killed whole cell vaccine
The first effort toward the development of a C. jejuni vaccine was started in the early 1990’s using a killed whole cell approach. Inactivated whole cell bacteria is an attractive method of vaccination: They represent an economical process for large scale production and allows the inclusion of a myriad of antigens. Attenuated/killed whole cell vaccination has been successful for preventing disease induced by other enteric pathogens including Salmonella typhi and Vibrio cholerae. For C. jejuni, a killed whole cell vaccine represented an attractive approach that relieved costly and time-consuming fundamental research to understand mechanisms of protection as well as eliminated the need of the production of recombinant protective antigens.
The C. jejuni whole cell vaccine (CWC) was derived from the C. jejuni strain 81–176, isolated in 1981 from a child presenting acute diarrhea due to consumption of contaminated raw milk during a school field trip in Minnesota.89 In pre-clinical studies using 0.2% formalin-killed 81–176 cells, robust prevention of C. jejuni colonization in mice was seen when delivered orally alongside the E.coli heat labile toxin (LT) as an adjuvant.90 It was further demonstrated that the vaccination stimulated production of local and systemic IgA and IgG to Campylobacter antigens in rhesus monkeys that received the adjuvanted vaccine.91 These studies warranted the transition of the vaccine to safety and immunogenicity testing in a human phase 1 clinical trial.
The first human study of the CWC was performed using a vaccination regimen consisting of three vaccinations delivered 2 weeks apart at doses of 105, 107, or 109 cells, or 109 cells administered orally with 25 μg of LT(R192G) as adjuvant.92 The isogenic attenuated mutant of LT, LT (R192G), was used to reduce LT enterotoxicity but preserve adjuvanticity as demonstrated in animal studies. This first clinical evaluation demonstrated that the vaccine was safe in humans but failed to prevent campylobacteriosis during a homologous controlled human infection model (CHIM) with C. jejuni 81–176. The CWC approach thus needed further development.
In the meantime, a ferret animal model of disease was developed and demonstrated to mimic human campylobacteriosis symptoms.93 Studies in the ferret model led to the modification of the dose, number of immunizations, and schedule for oral vaccination; specifically, the next phase 1 CWC human clinical trial used a 1010 cell dose with 25 μg of LT(R192G) adjuvant given orally 4 times, 2 days apart. The new vaccination regimen demonstrated to be superior by inducing a higher level of fecal IgA compared to the previous human vaccination, but the vaccine was unsuccessful to protect against disease in a Phase 2b CHIM when receiving the homologous strain.92 It has to be noted that the hefty infectious dose of 1010 CFU may have overwhelmed the host immune system the vaccine may have been more effective had a lower challenge dose been used. The CWC approach was abandoned in early 2000’s and has not been further refined. The major motive for no longer pursuing this CWC was that the strain C. jejuni 81–176 used in the vaccine was found to present sialylated LOS that resemble GM2 and GM3 human gangliosides,59 which could potentially lead to the induction of GBS.
Subunit vaccines
Following the abandonment of the CWC approach, C. jejuni vaccinology efforts switched to the use of a recombinant C. jejuni antigens. Based on the findings of other successful enteric pathogen vaccines, the ideal antigen candidate should be surface exposed/accessible to the host immune system and able to elicit significant seroconversion of IgG as well as mucosal and serological IgA. In addition, the subunit vaccine should ultimately induce long-term immunological memory. In the early 2000’s, the Naval Medical Research Center, Silver Spring, USA initiated the production of a recombinant C. jejuni flagellin vaccine.94 Flagellin has well-documented proof of immunogenicity in various animal models and also in humans.94,95 Indeed, early CHIM studies demonstrated that volunteers challenged with C. jejuni 81–176 developed a robust immune response against the flagella.94In addition, the immunological level of response against the flagellin correlated with the protection against disease as demonstrated during re-challenge studies.94To thus generate a recombinant flagellin-based vaccine, the conserved region of FlaA from C. coli VC167 was fused with the maltose binding protein (MBP) of E.coli.94 The vaccine (rFlaA-MBP) was tested for immunological response and protective efficacy in a nasal mouse model developed in house.94 Encouraging results were made: intestinal secretory IgA response and protection against heterologous colonization and disease by C. jejuni 81–176 were established when the rFlaA-MBP was adjuvanted with LT(R192G).
The rFlaA-MBP vaccine was tested in a human clinical trial for safety and immunogenicity (ClinicalTrials.gov Identifier: NCT00124865). The vaccine was dispensed via intranasal delivery at one of four doses (25, 125, 625, or 1000 μg), with 3 total immunizations delivered at 2 week intervals. Interestingly, no adjuvant was administered, in contrast to the mouse model.92 The vaccine was deemed safe but only mildly immunogenic at the systemic and mucosal level. In part due to this disappointing result, the rFla-MBP C. jejuni vaccine was abandoned.
In the mid 2000’s ACE BioSciences, now Zymenex, ventured into the development of a travelers’ diarrhea vaccine.96 This endeavor led to the development of a subunit vaccine, ACE393, a proprietary cell surface exposed C. jejuni protein. Following successful reduction of colonization in vaccinated mice, the vaccine was rapidly transitioned into a Phase 1 and extended Phase 1 for safety, immunogenicity and role in the prevention of disease (ClinicalTrials.gov Identifier: NCT00859716). The vaccine, consisting of 250 μg of recombinant protein and 500 μg Alhydrogel® (aluminum hydroxide adjuvant), was administered twice intra-muscularly 3 weeks apart. Volunteers were challenged with a newly established C. jejuni strains, CG8421, which lack sialylated LOS and by consequence unable to trigger auto immune sequelae including GBS.97 The vaccination regimen had low/no effect on prevention campylobacteriosis and has not been further pursued to this date.
Glycoconjugate vaccine
Protein-polysaccharide conjugate vaccines are superior to polysaccharide vaccines the protein carrier, provides the ability to induce T-cell responses towards the polysaccharide. Conjugated polysaccharide vaccines are immunogenic in young children98 and induce long term immune protection compared to unconjugated polysaccharide vaccines.99 Three licensed conjugated polysaccharide vaccines targeting encapsulated strains of the pathogens Haemophilus influenzae (type b), Neisseria meningitidis, and Streptococcus pneumoniae are available on the market. Based on the success of conjugated polysaccharide vaccines and the recent characterization of a polysaccharide capsule in C. jejuni described above, NMRC initiated a pilot vaccine study using the capsule of C. jejuni conjugated to CRM197 as protein carrier. The choice of CRM197 as protein carrier for the prototype vaccine was motivated for the reasons that CRM197, a non-toxic diphtheria toxin used in the pneumococcal conjugate vaccine, has been demonstrated to be safe, is commercially available, and is amenable to conjugation to oxidized polysaccharide.
For pre-clinical studies, the strain C. jejuni 81–176 was chosen. This strain harbors a serotype HS23/36 capsule that is composed of repeating units of the following core trisaccharide [→3)-β-D-GlcpNAc-(1→3)-α-D-Galp-(1→2)-D-glycero-α-D-altro-Hepp-(1→]n.100,101 As described above, the CPS structure also contains non-stoichiometric modifications including methyl phosphoramidate (MeOPN) occurring on the Galactose residue at the 2-OH, 4-OH, or 6-OH group.53 Those MeOPN are believed to be immunodominant and required for vaccine efficacy. To prevent potential auto-immune reactions resulting from trace contamination with the 81–176 sialylated LOS structures, the 81–176 strain was genetically manipulated to prevent expression of ganglioside-like mimics.
The CPS was extracted and purified directly from the cell paste of C. jejuni 81–176. The glycoconjugate vaccine was then synthesized by CPS oxidation with sodium periodate to yield an aldehyde functional group at the non-reducing end, allowing the conjugation to the lysine residues of CRM197 via reductive amination.102
Dose ranging and pre-clinical studies were performed in a mouse intranasal vaccination colonization model and validated via subcutaneous vaccination in a non-human primate (NHP) Aotus nancymaae model. NHP were vaccinated with 1, 5, or 25 μg of glycoconjugate with alum adjuvant administered in three doses of vaccine or PBS at 6-week intervals. The highest dose resulted in 100% protection against disease during homologous challenge in the NHP model 9 weeks post last vaccination.102 This encouraging result led to development of a cGMP grade vaccine that was tested for safety and immunogenicity in a phase 1 clinical trial that occurred in 2014 (ClinicalTrials.gov Identifier: NCT02067676). This study enrolled a total of 48 volunteers that received two intramuscular (IM) vaccinations, 4 weeks apart, of CJCV1 of 2μg, 5μg, or 10μg with or without 125μg Alhydrogel®. The vaccine was deemed safe but did not result in a significant serum immunological response (both IgA and IgG) against the CPS moiety. This disappointing result was retrospectively hypothesized to be due to the lack on the immunodominant MeOPN epitopes in CJCV1 vaccine. Both the strain used and the manufacturing process can be incriminated. Unfortunately, the transferase enzymes responsible for the attachment of the MeOPN on the galactose residues are known to be affected by a mechanism of phase variation.53
Based on this observation, a natural variant of C. jejuni 81–176 presenting both MeOPN transferases in the “phase on” configuration was selected and demonstrated to be stable during the cell production for CPS extraction. In addition, the extraction protocol was optimized and now utilizes a more gentle extraction procedure that minimizes MeOPN hydrolysis and loss. The current iteration of the C. jejuni CPS glycoconjugate vaccine, CJCV2, is currently under manufacture and will be shortly tested in a Phase 1 clinical trial for safety and immunogenicity (Table 1).
Table 1.
Comparison of manufacturing parameters and vaccination regimen between C. jejuni CPS conjugate vaccine CJCV1 and CJCV2.
| CJCV1 | CJCV2 | |
|---|---|---|
| Strain | 81–176 | 81–176 o/o(a) |
| Growth medium | Animal-based | Non-Animal-based |
| Capsule extraction | Phenol | DOC (b) |
| Endotoxin removal | Long acetic acid treatment | Short acetic acid treatment |
| Oxidation | Sodium metaperiodate | Sodium metaperiodate |
| Conjugation | Sodium cyanoborohydride | Sodium cyanoborohydride |
| Protein Carrier | CRM197 | CRM197 |
| Adjuvant | Alum hydroxide | TBD |
| Number of dose | 2 doses 4 weeks apart | TBD |
| Amount of CPS/dose | 2, 5 and 10 mg | TBD |
(a) C. jejuni 81–176 strain stably expressing both phase-variable MeOPN transferases.
(b) Sodium deoxycholate
Summary and looking towards the future
Momentum has been building in both the public and private sectors around research and development of new diarrheal disease interventions, including rotavirus, cholera, typhoid, ETEC, Shigella, and Campylobacter vaccines. Public funding for diarrheal diseases from high-income country governments and multinational organizations has increased substantially. Global agencies have made greater commitments to understanding diarrheal disease burden and the impact of specific pathogens. Opportunities to leverage private markets for the public good through implementation of tiered pricing schemes allow companies to achieve a return on investment in profitable markets (i.e. travelers and emerging economies), while providing products at lower cost in the developing world. While industry funding would likely be able to take a vaccine for the high-income country market, further development in low- and middle-income country markets would likely require funding and initiative from a range of sources, including vaccine-manufacturing partners in potential target markets, national governments, and global public health nonprofit organizations. The emerging epidemiology and understanding of campylobacter-attributable burden (both acute and chronic) in both traveler and global populations, combined with promising development in vaccines, leads to the consideration of the possibility for a global vaccine against C. jejuni. Based on the epidemiological, immunological, and virulence studies reviewed here and described elsewhere, the targets and goals for such a C. jejuni vaccine are shown in Table 2.
Table 2.
Target product profile of a capsule conjugated Campylobacter or other injectable vaccine for global use.
| Variable | Traveler population to LMI Countries | Children up to 5 years of age in LMI Countries |
|---|---|---|
| Indication | Prevention of moderate to severe illness due to Campylobacter jejuni in travelers from high income countries | Prevention of moderate to severe diarrhea due to Campylobacter jejuni in children less than two years of age. |
| Target Population | Adults 18 – 65 years | Children up to 5 years of age. |
| Schedule and Route of Administration* | Optimal: 1 dose IM route. Acceptable: 2 dose IM route 28 days or less |
Optimal: EPI schedule – 1 dose IM route. Acceptable: EPI schedule: 2 or 3 dose + booster IM route. |
| Safety | Safety and reactogenicity profile should be clinically acceptable. Contraindications should be restricted to know hypersensitivity to any of the vaccine components | |
| Efficacy* | 70% efficacy against moderate to severe illness caused by all C. jejuni strains | Optimal: 70% efficacy against moderate to severe illness caused by all C. jejuni strains Acceptable: 50% efficacy against moderate to severe illness caused by C. jejuni strains in the vaccine |
| Duration of Protection | Optimal: lifetime Acceptable: 2 years |
Optimal: To 5 years. Acceptable: 2 years, w/boosting possible to extend protection |
| Cost per regimen | $75 – 100 | $1 – 3 |
| Co-administration | With travel vaccines without interference | With EPI vaccines without interference |
| Vaccine volume | 0.5 ml/dose | |
| Target Countries | US, Europe, Japan, Australia, New Zealand and other HDI countries | GAVI-eligible and LMIC |
| Onset of immunity | 1–2 weeks after primary series | 2 weeks after vaccine series |
| Indirect protection | No | Yes, ideally |
| Protection against chronic health sequelae | Yes (functional bowel disorders, reactive arthritis, GBS) | Yes (environmental enteropathy, GBS) |
A capsule-conjugate vaccine approach against C. jejuni is promising but several questions still need to be addressed. As mentioned previously, highly effective conjugate vaccines have been developed for other mucosal pathogens, one of which, Streptococcus pneumoniae, has more capsular types (~ 90) than C. jejuni (~ 35). There have been numerous prevalence studies of capsule serotypes in the developed world,77,95 but few studies from developing countries where the disease incidence is higher. Alternative vaccine approaches, such as recombinant protein subunits, have not yet demonstrated feasibility but are likely to advance with improved understanding of Campylobacter pathogenesis.
The CHIM for C. jejuni is likely to be utilized in future vaccine down/up-selection decisions and a brief review of its current status is warranted here. Briefly, the currently CHIM utilizes an HS23/36 strain, named CG8421, isolated from a US solder in Thailand with acute febrile dysentery in 199997 The strain has been safely administered to 68 “naïve” subjects at 3 clinical sites at doses ranging from 2 × 104 to 1 × 10 colony forming units.103–105 In the most recent randomized, double-blind, placebo-controlled trial, the strain induced campylobacteriosis in 85.7% (24/28) of all subjects.103 Importantly, microbial recrudescence has been observed with this strain, a phenomenon that may be specific to this model or may be reflective of natural Campylobacter infection.103,104,106,107 While the CG8421 appears to be sufficiently robust to assess current vaccine candidates, other vaccine strategies (or other capsule-types) may require the development of new C. jejuni CHIMs.
More research is needed on Campylobacter-host interactions and epidemiology to identify adequate coverage of a capsule conjugate or protein subunit vaccine. The identification and characterization of a correlate of protection is important and could be informed indirectly through seroepidemiological studies. Favorable results from early phase clinical trials in adults from the high-income countries (e.g., travelers or other high-risk populations) would provide hope that such a vaccine might be effective in low- and middle-income country populations.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Authors’ contributions
All authors contributed to this manuscript equally.
Disclaimers
The views expressed in this article do not necessarily reflect the official policy or position of the Uniformed Services University, Department of the Navy, Department of Defense, nor the U.S. Government. This is a partial US Government work. There are no restrictions on its use. This work was supported by work unit number 6000.RAD1.DA3.A0308.
Copyright Statement
M.S.R is an employee of the U.S. Government and a military service member. F.P. and C.K.P are employees of the U.S. Government. This work was prepared as part of official duties. Title 17 U.S.C. §105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
References
- 1.GBD Diarrhoeal Diseases Collaborators. Estimates of Global, Regional, and National Morbidity, Mortality, and Aetiologies of Diarrhoeal Diseases: A Systematic Analysis for The Global Burden of Disease Study 2015. Lancet Infect Dis 2017;17(9):909––948.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nataro JP, Guerrant RL.. Chronic consequences on human health induced by microbial pathogens: growth faltering among children in developing countries. Vaccine. 2017;35(49Pt A):6807–6812. doi: 10.1016/j.vaccine.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 3.Scallan E, Hoekstra RM, Mahon BE, Jones TF, Griffin PM. An assessment of the human health impact of seven leading foodborne pathogens in the United States using disability adjusted life years. Epidemiol Infect. 2015;143(13):2795–2804. doi: 10.1017/S0950268814003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verdu EF, Riddle MS. Chronic gastrointestinal consequences of acute infectious diarrhea: evolving concepts in epidemiology and pathogenesis. Am J Gastroenterol. 2012;107(7):981–989. doi: 10.1038/ajg.2012.65. [DOI] [PubMed] [Google Scholar]
- 5.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA Abdalla S, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012; (9859):2197–2223. [DOI] [PubMed]
- 6.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 7.Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, McCormick BJ, McGrath M, Olortegui MP, Samie A, et al. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). The Lancet Global Health. 2015;3(9):e564–75. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Havelaar AH, Swart A. Impact of waning acquired immunity and asymptomatic infections on case-control studies for enteric pathogens. Epidemics. 2016;17:56–63. doi: 10.1016/j.epidem.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C, Operario DJ, Uddin J, Ahmed S, Alonso PL, et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet. 2016;388(10051):1291–1301. doi: 10.1016/S0140-6736(16)31529-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amour C, Gratz J, Mduma E, Svensen E, Rogawski ET, McGrath M, Seidman JC, McCormick BJ, Shrestha S, Samie A, et al. Epidemiology and impact of campylobacter infection in children in 8 low-resource settings: results from the MAL-ED study. Clin Infect Dis. 2016;63(9):1171–1179. doi: 10.1093/cid/ciw542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Investigators M-EN. Relationship between growth and illness, enteropathogens and dietary intakes in the first 2 years of life: findings from the MAL-ED birth cohort study. BMJ Glob Health. 2017;2(4):e000370. doi: 10.1136/bmjgh-2017-000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schnee AE, Petri WA Jr.. Campylobacter jejuni and associated immune mechanisms: short-term effects and long-term implications for infants in low-income countries. Curr Opin Infect Dis. 2017;30(3):322–328. doi: 10.1097/QCO.0000000000000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marder EP, Griffin PM, Cieslak PR, Dunn J, Hurd S, Jervis R, Lathrop S, Muse A, Ryan P, Smith K, et al. Preliminary incidence and trends of infections with pathogens transmitted commonly through food - foodborne diseases active surveillance network, 10 U.S. Sites, 2006-2017. MMWR Morb Mortal Wkly Rep. 2018;67(11):324–328. doi: 10.15585/mmwr.mm6711a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jorgensen K. [Prevalence of Campylobacter fetus ssp. jejuni in Danish dogs (author’s transl)]. NordVetMed. 1981;33(1):42–48. [PubMed] [Google Scholar]
- 15.OzFoodNet Working G Monitoring the incidence and causes of diseases potentially transmitted by food in Australia: annual report of the OzFoodNet network, 2011. Commun Dis Intell Q Rep. 2015;39(2):E236–64. [DOI] [PubMed] [Google Scholar]
- 16.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States–major pathogens. Emerg Infect Dis. 2011;17(1):7–15. doi: 10.3201/eid1701.091101p1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riddle MS, Sanders JW, Putnam SD, Tribble DR. Incidence, etiology, and impact of diarrhea among long-term travelers (U.S. military and similar populations): A systematic review. Am J Trop Med Hyg. 2006;74(5):891–900. [PubMed] [Google Scholar]
- 18.Shah N, DuPont HL, Ramsey DJ. Global etiology of travelers’ diarrhea: systematic review from 1973 to the present. Am J Trop Med Hyg. 2009;80(4):609–614. [PubMed] [Google Scholar]
- 19.Porter CK, Olson S, Hall A, Riddle MS. Travelers’ Diarrhea: an update on the incidence, etiology, and risk in military deployments and similar travel populations. Mil Med. 2017;182(S2):4–10. doi: 10.7205/MILMED-D-17-00064. [DOI] [PubMed] [Google Scholar]
- 20.Porter CK, Riddle MS, Tribble DR, Putnam SD, Rockabrand DM, Frenck RW, Rozmajzl P, Kilbane E, Fox A, Ruck R, et al. The epidemiology of travelers’ diarrhea in Incirlik, Turkey: a region with a predominance of heat-stabile toxin producing enterotoxigenic Escherichia coli. Diagn Microbiol Infect Dis. 2010;66(3):241–247. doi: 10.1016/j.diagmicrobio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Sanders JW, Isenbarger DW, Walz SE, Pang LW, Scott DA, Tamminga C, Oyofo BA, Hewitson WC, Sanchez JL, Pitarangsi C, et al. An observational clinic-based study of diarrheal illness in deployed United States military personnel in Thailand: presentation and outcome of Campylobacter infection. Am J Trop Med Hyg. 2002;67(5):533–538. [DOI] [PubMed] [Google Scholar]
- 22.Keithlin J, Sargeant J, Thomas MK, Fazil A. Systematic review and meta-analysis of the proportion of Campylobacter cases that develop chronic sequelae. BMC Public Health. 2014;14:1203. doi: 10.1186/1471-2458-14-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodfellow JA, Willison HJ. Guillain-Barre syndrome: a century of progress. Nat Rev Neurol. 2016;12(12):723–731. doi: 10.1038/nrneurol.2016.172. [DOI] [PubMed] [Google Scholar]
- 24.Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barre syndrome. Lancet. 2016;388(10045):717–727. doi: 10.1016/S0140-6736(16)00339-1. [DOI] [PubMed] [Google Scholar]
- 25.Riddle MS, Gutierrez RL, Verdu EF, Porter CK. The chronic gastrointestinal consequences associated with campylobacter. Curr Gastroenterol Rep. 2012;14(5):395–405. doi: 10.1007/s11894-012-0278-0. [DOI] [PubMed] [Google Scholar]
- 26.Ajene AN, Fischer Walker CL, Black RE. Enteric pathogens and reactive arthritis: a systematic review of Campylobacter, salmonella and Shigella-associated reactive arthritis. J Health Popul Nutr. 2013;31(3):299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stavropoulos PG, Soura E, Kanelleas A, Katsambas A, Antoniou C. Reactive arthritis. J Eur Acad Dermatol Venereol. 2015;29(3):415–424. doi: 10.1111/jdv.12741. [DOI] [PubMed] [Google Scholar]
- 28.Post A, Martiny D, van Waterschoot N, Hallin M, Maniewski U, Bottieau E, Van Esbroeck M, Vlieghe E, Ombelet S, Vandenberg O, et al. Antibiotic susceptibility profiles among Campylobacter isolates obtained from international travelers between 2007 and 2014. Eur J Clin Microbiol Infect Dis. 2017;36(11):2101–2107. doi: 10.1007/s10096-017-3032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kosunen TU, Ponka A, Kauranen O, Martio J, Pitkanen T, Hortling L, Aittoniemi S, Penttila O, Koskimies S. Arthritis associated with Campylobacter jejuni enteritis. ScandJRheumatol. 1981;10(2):77–80. [DOI] [PubMed] [Google Scholar]
- 30.Ponka A, Kosunen TU. Pancreas affection in association with enteritis due to Campylobacter fetus ssp. jejuni. Acta MedScand. 1981;209(3):239–240. [DOI] [PubMed] [Google Scholar]
- 31.Young KT, Davis LM, Dirita VJ. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol. 2007;5(9):665–679. doi: 10.1038/nrmicro1718. [DOI] [PubMed] [Google Scholar]
- 32.Poly F, Guerry P. Pathogenesis of Campylobacter. Curr Opin Gastroenterol. 2008;24(1):27–31. doi: 10.1097/MOG.0b013e3282f1dcb1. [DOI] [PubMed] [Google Scholar]
- 33.Dasti JI, Tareen AM, Lugert R, Zautner AE, Gross U. Campylobacter jejuni: a brief overview on pathogenicity-associated factors and disease-mediating mechanisms. Int J Med Microbiol. 2010;300(4):205–211. doi: 10.1016/j.ijmm.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Guerry P, Poly F, Riddle M, Maue AC, Chen YH, Monteiro MA. Campylobacter polysaccharide capsules: virulence and vaccines. Front Cell Infect Microbiol. 2012;2:7. doi: 10.3389/fcimb.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lertpiriyapong K, Gamazon ER, Feng Y, Park DS, Pang J, Botka G, Graffam ME, Ge Z, Fox JG. Campylobacter jejuni type VI secretion system: roles in adaptation to deoxycholic acid, host cell adherence, invasion, and in vivo colonization. PLoS One. 2012;7(8):e42842. doi: 10.1371/journal.pone.0042842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Babakhani FK, Bradley GA, Joens LA. Newborn piglet model for campylobacteriosis. InfectImmun. 1993;61(8):3466–3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox JG, Ackerman JI, Taylor N, Claps M, Murphy JC. Campylobacter jejuni infection in the ferret: an animal model of human campylobacteriosis. AmJVetRes. 1987;48(1):85–90. [PubMed] [Google Scholar]
- 38.Mansfield LS, Bell JA, Wilson DL, Murphy AJ, Elsheikha HM, Rathinam VA, Fierro BR, Linz JE, Young VB. C57BL/6 and congenic interleukin-10-deficient mice can serve as models of Campylobacter jejuni colonization and enteritis. InfectImmun. 2007;75(3):1099–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stahl M, Graef FA, Vallance BA. Mouse Models for Campylobacter jejuni Colonization and Infection. Methods Mol Biol. 2017;1512:171–188. doi: 10.1007/978-1-4939-6536-6_15. [DOI] [PubMed] [Google Scholar]
- 40.Fox JG, Rogers AB, Whary MT, Ge Z, Taylor NS, Xu S, Horwitz BH, Erdman SE. Gastroenteritis in NF-kappaB-deficient mice is produced with wild-type Camplyobacter jejuni but not with C. jejuni lacking cytolethal distending toxin despite persistent colonization with both strains. Infect Immun. 2004;72(2):1116–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Champion OL, Karlyshev AV, Senior NJ, Woodward M, La Ragione R, Howard SL, Wren BW, Titball RW. Insect infection model for Campylobacter jejuni reveals that O-methyl phosphoramidate has insecticidal activity. J Infect Dis. 2010;201(5):776–782. doi: 10.1086/650494. [DOI] [PubMed] [Google Scholar]
- 42.Giallourou N, Medlock GL, Bolick DT, Medeiros PH, Ledwaba SE, Kolling GL, Tung K, Guerry P, Swann JR, Guerrant RL. A novel mouse model of Campylobacter jejuni enteropathy and diarrhea. PLoS Pathog. 2018;14(3):e1007083. doi: 10.1371/journal.ppat.1006797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parkhill J, Wren BW, Mungall K, Ketley JM, Churcher C, Basham D, Chillingworth T, Davies RM, Feltwell T, Holroyd S, et al. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000;403(6770):665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 44.Karlyshev AV, Linton D, Gregson NA, Lastovica AJ, Wren BW. Genetic and biochemical evidence of a Campylobacter jejuni capsular polysaccharide that accounts for Penner serotype specificity. Mol Microbiol. 2000;35(3):529–541. [DOI] [PubMed] [Google Scholar]
- 45.Penner JL, Hennessy JN. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. J Clin Microbiol. 1980;12(6):732–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bacon DJ, Szymanski CM, Burr DH, Silver RP, Alm RA, Guerry P. A phase-variable capsule is involved in virulence of Campylobacter jejuni 81-176. Mol Microbiol. 2001;40(3):769–777. [DOI] [PubMed] [Google Scholar]
- 47.Sahin O, Terhorst SA, Burrough ER, Shen Z, Wu Z, Dai L, et al. Key role of capsular polysaccharide in the induction of systemic infection and abortion by hypervirulent campylobacter jejuni. Infect Immun. 2017;85(6). doi: 10.1128/IAI.00001-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Alphen LB, Wenzel CQ, Richards MR, Fodor C, Ashmus RA, Stahl M, Karlyshev AV, Wren BW, Stintzi A, Miller WG, et al. Biological roles of the O-methyl phosphoramidate capsule modification in Campylobacter jejuni. PloS one. 2014;9(1):e87051. doi: 10.1371/journal.pone.0087051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grant AJ, Coward C, Jones MA, Woodall CA, Barrow PA, Maskell DJ. Signature-tagged transposon mutagenesis studies demonstrate the dynamic nature of cecal colonization of 2-week-old chickens by Campylobacter jejuni. Appl Environ Microbiol. 2005;71(12):8031–8041. doi: 10.1128/AEM.71.12.8031-8041.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bachtiar BM, Coloe PJ, Fry BN. Knockout mutagenesis of the kpsE gene of Campylobacter jejuni 81116 and its involvement in bacterium-host interactions. FEMS Immunol Med Microbiol. 2007;49(1):149–154. doi: 10.1111/j.1574-695X.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- 51.Wong A, Lange D, Houle S, Arbatsky NP, Valvano MA, Knirel YA, Dozois CM, Creuzenet C. Role of capsular modified heptose in the virulence of Campylobacter jejuni. Mol Microbiol. 2015;96(6):1136–1158. doi: 10.1111/mmi.12995. [DOI] [PubMed] [Google Scholar]
- 52.Maue AC, Mohawk KL, Giles DK, Poly F, Ewing CP, Jiao Y, Lee G, Ma Z, Monteiro MA, Hill CL, et al. The polysaccharide capsule of Campylobacter jejuni modulates the host immune response. Infect Immun. 2013;81(3):665–672. doi: 10.1128/IAI.01008-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pequegnat B, Laird RM, Ewing CP, Hill CL, Omari E, Poly F, Monteiro MA, Guerry P. Phase-variable changes in the position of O-methyl phosphoramidate modifications on the polysaccharide capsule of campylobacter jejuni modulate serum resistance. J Bacteriol. 2017;199(14). doi: 10.1128/JB.00027-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guerry P, Szymanski CM. Campylobacter sugars sticking out. Trends Microbiol. 2008;16(9):428–435. doi: 10.1016/j.tim.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Prendergast MM, Moran AP. Lipopolysaccharides in the development of the Guillain-Barre syndrome and Miller Fisher syndrome forms of acute inflammatory peripheral neuropathies. J Endotoxin Res. 2000;6(5):341–359. [PubMed] [Google Scholar]
- 56.Penner JL, Aspinall GO. Diversity of lipopolysaccharide structures in Campylobacter jejuni. J Infect Dis. 1997;176(Suppl 2):S135–8. [DOI] [PubMed] [Google Scholar]
- 57.Houliston RS, Vinogradov E, Dzieciatkowska M, Li J, St Michael F, Karwaski MF, Brochu D, Jarrell HC, Parker CT, Yuki N, et al. Lipooligosaccharide of Campylobacter jejuni: similarity with multiple types of mammalian glycans beyond gangliosides. J Biol Chem. 2011;286(14):12361–12370. doi: 10.1074/jbc.M110.181750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Linton D, Gilbert M, Hitchen PG, Dell A, Morris HR, Wakarchuk WW, Gregson NA, Wren BW. Phase variation of a beta-1,3 galactosyltransferase involved in generation of the ganglioside GM1-like lipo-oligosaccharide of Campylobacter jejuni. Mol Microbiol. 2000;37(3):501–514. [DOI] [PubMed] [Google Scholar]
- 59.Guerry P, Szymanski CM, Prendergast MM, Hickey TE, Ewing CP, Pattarini DL, Moran AP. Phase variation of Campylobacter jejuni 81-176 lipooligosaccharide affects ganglioside mimicry and invasiveness in vitro. Infect Immun. 2002;70(2):787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Godschalk PC, Kuijf ML, Li J, St Michael F, Ang CW, Jacobs BC, Karwaski MF, Brochu D, Moterassed A, Endtz HP, et al. Structural characterization of Campylobacter jejuni lipooligosaccharide outer cores associated with Guillain-Barre and Miller Fisher syndromes. Infect Immun. 2007;75(3):1245–1254. doi: 10.1128/IAI.00872-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Young NM, Brisson JR, Kelly J, Watson DC, Tessier L, Lanthier PH, Jarrell HC, Cadotte N, St Michael F, Aberg E, et al. Structure of the N-linked glycan present on multiple glycoproteins in the Gram-negative bacterium, Campylobacter jejuni. J Biol Chem. 2002;277(45):42530–42539. doi: 10.1074/jbc.M206114200. [DOI] [PubMed] [Google Scholar]
- 62.Scott NE, Nothaft H, Edwards AV, Labbate M, Djordjevic SP, Larsen MR, Szymanski CM, Cordwell SJ. Modification of the Campylobacter jejuni N-linked glycan by EptC protein-mediated addition of phosphoethanolamine. J Biol Chem. 2012;287(35):29384–29396. doi: 10.1074/jbc.M112.380212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cullen TW, O'Brien JP, Hendrixson DR, Giles DK, Hobb RI, Thompson SA, Brodbelt JS, Trent MS. EptC of Campylobacter jejuni mediates phenotypes involved in host interactions and virulence. Infect Immun. 2013;81(2):430–440. doi: 10.1128/IAI.01046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cullen TW, Trent MS. A link between the assembly of flagella and lipooligosaccharide of the Gram-negative bacterium Campylobacter jejuni. Proc Natl Acad Sci U S A. 2010;107(11):5160–5165. doi: 10.1073/pnas.0913451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alemka A, Nothaft H, Zheng J, Szymanski CM. N-glycosylation of Campylobacter jejuni surface proteins promotes bacterial fitness. Infect Immun. 2013;81(5):1674–1682. doi: 10.1128/IAI.01370-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Sorge NM, Bleumink NM, van Vliet SJ, Saeland E, van der Pol WL, van Kooyk Y, van Putten JP. N-glycosylated proteins and distinct lipooligosaccharide glycoforms of Campylobacter jejuni target the human C-type lectin receptor MGL. Cell Microbiol. 2009;11(12):1768–1781. doi: 10.1111/j.1462-5822.2009.01370.x. [DOI] [PubMed] [Google Scholar]
- 67.Szymanski CM, Burr DH, Guerry P. Campylobacter protein glycosylation affects host cell interactions. Infect Immun. 2002;70(4):2242–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karlyshev AV, Everest P, Linton D, Cawthraw S, Newell DG, Wren BW. The Campylobacter jejuni general glycosylation system is important for attachment to human epithelial cells and in the colonization of chicks. Microbiology. 2004;150(Pt 6):1957–1964. doi: 10.1099/mic.0.26721-0. [DOI] [PubMed] [Google Scholar]
- 69.Morooka T, Umeda A, Amako K. Motility as an intestinal colonization factor for Campylobacter jejuni. J Gen Microbiol. 1985;131(8):1973–1980. doi: 10.1099/00221287-131-8-1973. [DOI] [PubMed] [Google Scholar]
- 70.Guerry P. Campylobacter flagella: not just for motility. Trends Microbiol. 2007;15(10):456–461. doi: 10.1016/j.tim.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 71.Merino S, Tomas JM. Gram-negative flagella glycosylation. Int J Mol Sci. 2014;15(2):2840–2857. doi: 10.3390/ijms15022840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guerry P, Ewing CP, Schirm M, Lorenzo M, Kelly J, Pattarini D, Majam G, Thibault P, Logan S. Changes in flagellin glycosylation affect Campylobacter autoagglutination and virulence. Mol Microbiol. 2006;60(2):299–311. doi: 10.1111/j.1365-2958.2006.05100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stephenson HN, Mills DC, Jones H, Milioris E, Copland A, Dorrell N, Wren BW, Crocker PR, Escors D, Bajaj-Elliott M. Pseudaminic acid on Campylobacter jejuni flagella modulates dendritic cell IL-10 expression via Siglec-10 receptor: a novel flagellin-host interaction. J Infect Dis. 2014;210(9):1487–1498. doi: 10.1093/infdis/jiu287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Howard SL, Jagannathan A, Soo EC, Hui JP, Aubry AJ, Ahmed I, Karlyshev A, Kelly JF, Jones MA, Stevens MP, et al. Campylobacter jejuni glycosylation island important in cell charge, legionaminic acid biosynthesis, and colonization of chickens. Infect Immun. 2009;77(6):2544–2556. doi: 10.1128/IAI.01425-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ewing CP, Andreishcheva E, Guerry P. Functional characterization of flagellin glycosylation in Campylobacter jejuni 81-176. J Bacteriol. 2009;191(22):7086–7093. doi: 10.1128/JB.00378-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nachamkin I, Hart AM. Western blot analysis of the human antibody response to Campylobacter jejuni cellular antigens during gastrointestinal infection. J Clin Microbiol. 1985;21(1):33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martin PM, Mathiot J, Ipero J, Kirimat M, Georges AJ, Georges-Courbot MC. Immune response to Campylobacter jejuni and Campylobacter coli in a cohort of children from birth to 2 years of age. Infect Immun. 1989;57(8):2542–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Logan SM, Trust TJ, Guerry P. Evidence for posttranslational modification and gene duplication of Campylobacter flagellin. J Bacteriol. 1989;171(6):3031–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alm RA, Guerry P, Power ME, Trust TJ. Variation in antigenicity and molecular weight of Campylobacter coli VC167 flagellin in different genetic backgrounds. J Bacteriol. 1992;174(13):4230–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Power ME, Guerry P, McCubbin WD, Kay CM, Trust TJ. Structural and antigenic characteristics of Campylobacter coli FlaA flagellin. J Bacteriol. 1994;176(11):3303–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Doig P, Kinsella N, Guerry P, Trust TJ. Characterization of a post-translational modification of Campylobacter flagellin: identification of a sero-specific glycosyl moiety. Mol Microbiol. 1996;19(2):379–387. [DOI] [PubMed] [Google Scholar]
- 82.Guerry P, Doig P, Alm RA, Burr DH, Kinsella N, Trust TJ. Identification and characterization of genes required for post-translational modification of Campylobacter coli VC167 flagellin. Mol Microbiol. 1996;19(2):369–378. [DOI] [PubMed] [Google Scholar]
- 83.Watson RO, Galan JE. Signal transduction in Campylobacter jejuni-induced cytokine production. Cell Microbiol. 2005;7(5):655–665. doi: 10.1111/j.1462-5822.2004.00498.x. [DOI] [PubMed] [Google Scholar]
- 84.de Zoete MR, Keestra AM, Wagenaar JA, van Putten JP. Reconstitution of a functional Toll-like receptor 5 binding site in Campylobacter jejuni flagellin. J Biol Chem. 2010;285(16):12149–12158. doi: 10.1074/jbc.M109.070227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hermans D, Pasmans F, Messens W, Martel A, Van Immerseel F, Rasschaert G, Heyndrickx M, Van Deun K, Haesebrouck F. Poultry as a host for the zoonotic pathogen Campylobacter jejuni. Vector Borne Zoonotic Dis. 2012;12(2):89–98. doi: 10.1089/vbz.2011.0676. [DOI] [PubMed] [Google Scholar]
- 86.de Zoete MR, van Putten JP, Wagenaar JA. Vaccination of chickens against Campylobacter. Vaccine. 2007;25(30):5548–5557. doi: 10.1016/j.vaccine.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 87.Neal-McKinney JM, Samuelson DR, Eucker TP, Nissen MS, Crespo R, Konkel ME. Reducing Campylobacter jejuni colonization of poultry via vaccination. PLoS One. 2014;9(12):e114254. doi: 10.1371/journal.pone.0114254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nothaft H, Perez-Munoz ME, Gouveia GJ, Duar RM, Wanford JJ, Lango-Scholey L, Panagos CG, Srithayakumar V, Plastow GS, Coros C, et al. Co-administration of the Campylobacter jejuni N-glycan based vaccine with probiotics improves vaccine performance in broiler chickens. Appl Environ Microbiol. 2017. doi: 10.1128/AEM.01523-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Korlath JA, Osterholm MT, Judy LA, Forfang JC, Robinson RA. A point-source outbreak of campylobacteriosis associated with consumption of raw milk. JInfectDis. 1985;152(3):592–596. doi: 10.1093/infdis/152.3.592. [DOI] [PubMed] [Google Scholar]
- 90.Baqar S, Applebee LA, Bourgeois AL. Immunogenicity and protective efficacy of a prototype Campylobacter killed whole-cell vaccine in mice. InfectImmun. 1995;63(9):3731–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baqar S, Bourgeois AL, Schultheiss PJ, Walker RI, Rollins DM, Haberberger RL, Pavlovskis OR. Safety and immunogenicity of a prototype oral whole-cell killed Campylobacter vaccine administered with a mucosal adjuvant in non-human primates. Vaccine. 1995;13(1):22–28. [DOI] [PubMed] [Google Scholar]
- 92.David R, Tribble SB, Thompson SA. Development of a Human Vaccine In: editor. Campylobacter. Irving Nachamkin CMSaMJB. 3rd Washington D.C.: ASM press; 2008. p. 429. [Google Scholar]
- 93.Burr DH, Rollins D, Lee LH, Pattarini DL, Walz SS, Tian JH, Pace JL, Bourgeois AL, Walker RI. Prevention of disease in ferrets fed an inactivated whole cell Campylobacter jejuni vaccine. Vaccine. 2005;23(34):4315–4321. doi: 10.1016/j.vaccine.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 94.Lee LH, Burg E, Baqar S, Bourgeois AL, Burr DH, Ewing CP, Trust TJ, Guerry P. Evaluation of a truncated recombinant flagellin subunit vaccine against Campylobacter jejuni. InfectImmun. 1999;67(11):5799–5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blaser MJ, Duncan DJ. Human serum antibody response to Campylobacter jejuni infection as measured in an enzyme-linked immunosorbent assay. InfectImmun. 1984;44(2):292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schrotz-King P, Prokhorova TA, Nielsen PN, Crawford JS, Morsczeck C. Campylobacter jejuni proteomics for new travellers’ diarrhoea vaccines. TravelMedInfectDis. 2007;5(2):106–109. [DOI] [PubMed] [Google Scholar]
- 97.Poly F, Read TD, Chen YH, Monteiro MA, Serichantalergs O, Pootong P, Bodhidatta L, Mason CJ, Rockabrand D, Baqar S, et al. Characterization of two Campylobacter jejuni strains for use in volunteer experimental-infection studies. Infect Immun. 2008;76(12):5655–5667. doi: 10.1128/IAI.00780-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rijkers GT, Sanders EA, Breukels MA, Zegers BJ. Infant B cell responses to polysaccharide determinants. Vaccine. 1998;16(14–15):1396–1400. [DOI] [PubMed] [Google Scholar]
- 99.Lesinski GB, Westerink MA. Novel vaccine strategies to T-independent antigens. J Microbiol Methods. 2001;47(2):135–149. [DOI] [PubMed] [Google Scholar]
- 100.Aspinall GO, McDonald AG, Pang H. Structures of the O chains from lipopolysaccharides of Campylobacter jejuni serotypes O:23 and O:36. Carbohydr Res. 1992;231:13–30. [DOI] [PubMed] [Google Scholar]
- 101.Karlyshev AV, Champion OL, Churcher C, Brisson JR, Jarrell HC, Gilbert M, Brochu D, St Michael F, Li J, Wakarchuk WW, et al. Analysis of Campylobacter jejuni capsular loci reveals multiple mechanisms for the generation of structural diversity and the ability to form complex heptoses. Mol Microbiol. 2005;55(1):90–103. doi: 10.1111/j.1365-2958.2004.04374.x. [DOI] [PubMed] [Google Scholar]
- 102.Monteiro MA, Baqar S, Hall ER, Chen YH, Porter CK, Bentzel DE, Applebee L, Guerry P. Capsule polysaccharide conjugate vaccine against diarrheal disease caused by Campylobacter jejuni. InfectImmun. 2009;77(3):1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rimmer JE, Harro C, Sack DA, Talaat KR, Gutierrez RL, DeNearing B, Brubaker J, Laird RM, Poly F, Maue AC, et al. Rifaximin fails to prevent campylobacteriosis in the human challenge model: A randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2018;66(9):1435–1441. doi: 10.1093/cid/cix1014. [DOI] [PubMed] [Google Scholar]
- 104.Kirkpatrick BD, Lyon CE, Porter CK, Maue AC, Guerry P, Pierce KK, Carmolli MP, Riddle MS, Larsson CJ, Hawk D, et al. Lack of homologous protection against Campylobacter jejuni CG8421 in a human challenge model. Clin Infect Dis. 2013;57(8):1106–1113. doi: 10.1093/cid/cit454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tribble DR, Baqar S, Carmolli MP, Porter C, Pierce KK, Sadigh K, Guerry P, Larsson CJ, Rockabrand D, Ventone CH, et al. Campylobacter jejuni strain CG8421: a refined model for the study of Campylobacteriosis and evaluation of Campylobacter vaccines in human subjects. Clin Infect Dis. 2009;49(10):1512–1519. doi: 10.1086/644622. [DOI] [PubMed] [Google Scholar]
- 106.Lindow JC, Poly F, Tribble DR, Guerry P, Carmolli MP, Baqar S, Porter CK, Pierce KK, Darsley MJ, Sadigh KS, et al. Caught in the act: in vivo development of macrolide resistance to Campylobacter jejuni infection. J Clin Microbiol. 2010;48(8):3012–3015. doi: 10.1128/JCM.00768-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baqar S, Tribble DR, Carmolli M, Sadigh K, Poly F, Porter C, Larsson CJ, Pierce KK, Guerry P, Campylobacter Study Team, et al. Recrudescent Campylobacter jejuni infection in an immunocompetent adult following experimental infection with a well-characterized organism. ClinVaccine Immunol. 2010;17(1):80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]


