ABSTRACT
Following the introduction of universal immunization against rotavirus, concerns were raised regarding pathogen-replacement of rotavirus by norovirus. The study aim was to examine the incidence and characteristics and norovirus gastroenteritis before and after the introduction of universal rotavirus immunization in Israel. We studied 1179 stool samples collected between November 2007 and December 2014 for a prospective hospital-based surveillance study of children aged 0–59 months hospitalized for gastroenteritis. A real-time RT-PCR assay was used to identify genogroup II (GII) norovirus in extracted fecal RNA samples. Overall, the weighted percentage of norovirus positive patients was 10.9%. Norovirus positivity was similar in the pre-universal rotavirus immunisation years (2008–2010) and the universal years (2011–2014), the respective average annual incidence of norovirus gastroenteritis was 1.6 (95% CI 0.6–2.3) per 1000 and 1.1 (95% CI 0.8–1.4) per 1000 children. Rotavirus was detected in 36.8% and 19.6% of the patients in the pre-vaccine years and the universal vaccine years, with an estimated incidence of 5.5 (95% CI 3.4–7.6) per 1000 and 2.1 (95% CI 1.6–2.7) per 1000 children, respectively. Most patients (59.1%) with norovirus gastroenteritis were infants aged 0–11 months. Norovirus was detected all year round with a significant 3-month peak from September through November. In conclusion, norovirus continues to be a leading cause of acute gastroenteritis associated with hospitalizations in young children. Future norovirus vaccines should target young infants. There was no evidence of pathogen-replacement by norovirus following the introduction of universal rotavirus immunization in Israel.
KEYWORDS: Norovirus, acute gastroenteritis, Real-time RT-PCR, incidence, children, hospitalizations
Introduction
Noroviruses belong to the Caliciviridae family. There are six established norovirus genogroups designated as genogroup I (GI) to GVI, and a seventh genogroup (GVII) has been proposed; these are subdivided into genotypes.1 Noroviruses from GI (nine genotypes), GII (22 genotypes), and GIV (one genotype) are known to be able to infect humans,2,3 while other norovirus genogroups infect animals.1,2
Norovirus is the main cause of gastroenteritis outbreaks4,5 and sporadic morbidity in children6,7 and adults,8,9 in both community settings9 and health-care facilities5 (reviewed in2,3). Noroviruses of GII genogroup cause 96% of norovirus gastroenteritis cases in children globally, especially GII.4 genotype which caused 60% of the illnesses10 and of all norovirus outbreaks globally since 2001.11,12 Infection with norovirus can be asymptomatic13, but some people develop gastroenteritis after a short incubation period.14 Norovirus gastroenteritis is mostly a mild illness of short duration, characterized by vomiting, diarrhea, nausea, and abdominal pain.4,5,15 Severe norovirus gastroenteritis may occur, especially in hospitalized patients and in the elderly.5,16 Characteristics of the virus, namely the small inoculum (~100 viral particles) needed to induce illness, prolonged viral shedding and the capacity to survive in the environment, enhance the transmissibility of norovirus3 through various modes including foodborne, person-to-person transmission and contact with contaminated objects.15
Recent estimates of the Global Burden Disease Study showed that norovirus causes 139 million diarrhea episodes and 19,496 deaths globally in all age groups. About 10,629 (55%) norovirus attributable deaths occur in children younger than 5 y of age and 3693 (19%) among persons aged 70 y or older.17 Most norovirus diarrheal episodes and deaths occur in developing countries.18 The annual global economic burden associated with norovirus gastroenteritis was estimated at $4.2 billion in direct cost to the health system and $60.3 billion in societal costs.19 In the United States, norovirus gastroenteritis was estimated to cause annually 14,000, 281,000, and 627,000 hospitalizations, emergency department visits and outpatient visits, respectively, in children less than 5 y of age.6 About 570–800 norovirus-associated deaths occur in the United States annually, in all age group of which 90% occur among persons aged 65 y or older20
Following the introduction of universal rotavirus immunization programs, concerns were raised regarding replacement of rotavirus by norovirus as the leading cause of severe diarrhea in children. In the United States, norovirus was the leading cause of diarrhea in children aged less than 5 y seeking medical care in the universal rotavirus immunization era, detected in 21% of patients, compared to 12% for rotavirus.6 However such differences were not noted in other countries.21,22 Given advances in the development of norovirus vaccines,23,24 understanding the epidemiological characteristics of norovirus gastroenteritis becomes an important public health need. The study aim was to examine the incidence and characteristics of norovirus gastroenteritis among children in Israel. Our primary hypothesis was that the incidence of norovirus gastroenteritis might differ before and after the introduction of universal rotavirus immunization.
Results
Between November 2007 and December 2014, 6621 children aged 0–59 months (mean age 17.8 months, standard deviation [SD] 13.9) were hospitalized for gastroenteritis in one of three study hospitals in northern Israel. Stool samples from 3722 (56.2%) were submitted to the hospital laboratory, and norovirus RNA was tested for in 1179 (17.8%) of the hospitalizations (Figure 1). The percentage of stool samples that were available for norovirus testing (Table 1) was higher in infants aged 0–5 months (24.5%) than older children (16.8%-21.1%), in Arab vs. Jewish children (24.1% vs. 18.4%), and in those from low socioeconomic status towns as opposed to children from intermediate and high socioeconomic status towns (22.5%, 15.5%, and 14.3%), respectively. The percentage of available samples differed by year and month of admission (Table 1).
Figure 1.
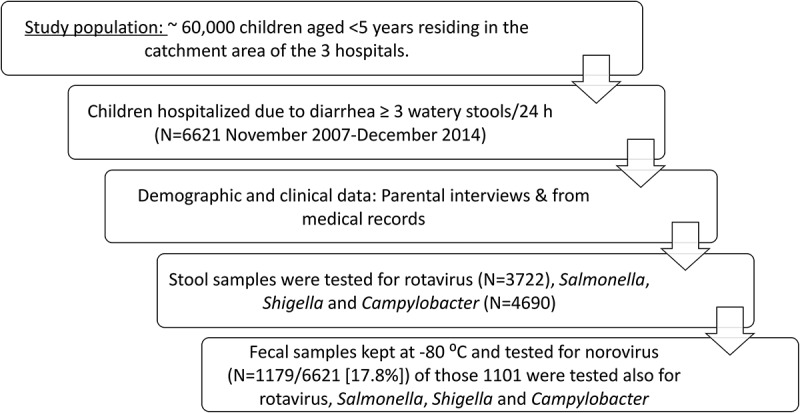
Flow chart, number of children enrolled and tested for enteric pathogens.
Table 1.
Testing for norovirus according to background characteristics of hospitalizations for acute gastroenteritis.
| Number of gastroenteritis hospitalizations | Testing for norovirus, n (%) | |
|---|---|---|
| Total | 6621 | 1179 |
| Age (months) | ||
| 0–5 | 1189 | 291 (24.5%) |
| 6–11 | 1474 | 311 (21.1%) |
| 12–17 | 1259 | 241 (19.1%) |
| 18–23 | 894 | 150 (16.8%) |
| 24–59 | 1773 | 183 (16.8%) |
| Missing | 32 | 3 (9.4%) |
| Sex | ||
| Males | 3539 | 631 (17.8%) |
| Females | 3070 | 547 (17.8%) |
| Missing | 11 | 1 (9.1%) |
| Population group | ||
| Jews | 4132 | 760 (18.4%) |
| Arabs | 1737 | 419 (24.1%) |
| Missing | 752 | 0 (0%) |
| Residential socioeconomic rank | ||
| Low (1–4) | 1939 | 437 (22.5%) |
| Intermediate (5–6) | 3286 | 509 (15.5%) |
| High (7–10) | 848 | 121 (14.3%) |
| Missing | 436 | 112 (25.7%) |
| Year of admission | ||
| November–December 2007 | 312 | 75 (24.0%) |
| 2008 | 1211 | 310 (25.6%) |
| 2009 | 719 | 42 (5.8%) |
| 2010 | 1259 | 208 (16.5%) |
| 2011 | 691 | 80 (11.6%) |
| 2012 | 835 | 199 (23.8%) |
| 2013 | 828 | 173 (20.9%) |
| 2014 | 766 | 92 (12.0%) |
| Month of admission | ||
| January | 708 | 148 (20.9%) |
| February | 451 | 107 (23.7%) |
| March | 441 | 96 (21.8%) |
| April | 382 | 76 (19.9%) |
| May | 460 | 92 (20.0%) |
| June | 511 | 69 (13.5%) |
| July | 512 | 66 (12.9%) |
| August | 413 | 60 (14.5%) |
| September | 513 | 85 (16.6%) |
| October | 471 | 62 (13.2%) |
| November | 739 | 144 (19.5%) |
| December | 1020 | 174 (17.1%) |
The incidence of norovirus, rotavirus and all-cause gastroenteritis before and after universal rotavirus immunization
Overall, norovirus tested positive in 140 (11.9%) of 1179 tested samples; the weighted percentage of norovirus positivity was 10.9%. During the study period, the mean annual incidence of norovirus gastroenteritis hospitalization was estimated at 1.3 (95% CI 1.2–1.4) per 1000 children aged 0–59 months. The mean annual incidence was estimated at 1.6 (95% CI 0.6–2.3) per 1000 children during the pre-universal rotavirus immunization years (2008–2010) compared to 1.1 (95% CI 0.8–1.4) per 1000 children during the universal rotavirus immunization years (2011–2014). Rotavirus was detected in 36.8% of the patients in the pre-vaccine years and 19.6% in the universal vaccine years, with an estimated incidence of 5.5 (95% CI 3.4–7.6) per 1000 children and 2.1 (95% CI 1.6–2.7) per 1000 children, respectively. The corresponding incidence of all-cause gastroenteritis was 15.0 per 1000 children (95% CI 12.0–18.8) and 10.9 per 1000 children (95% CI 10.1–12.0).
An analysis using interrupted time series showed that the predicted incidence of rotavirus gastroenteritis and all-cause gastroenteritis declined significantly following the introduction of universal rotavirus immunization program, compared to the predicted incidence using the pre-vaccine monthly incidence data only (Figure 2(a and b)). The predicted and observed incidences of rotavirus gastroenteritis and all-cause gastroenteritis were overlapping when using the full data series (Figure 2(a and b)). For rotavirus gastroenteritis, the variable “study period” (before or after the introduction of universal rotavirus immunization) was a significant (P = 0.001) predictor of rotavirus gastroenteritis incidence (coefficient −0.54 [standard error 0.15]). In contrast, for norovirus gastroenteritis, the observed and predicted monthly incidences were overlapping showing continuous activity of norovirus with peaks during September–November (Figure 2(c)), but the variable study period was not statistically significant.
Figure 2.
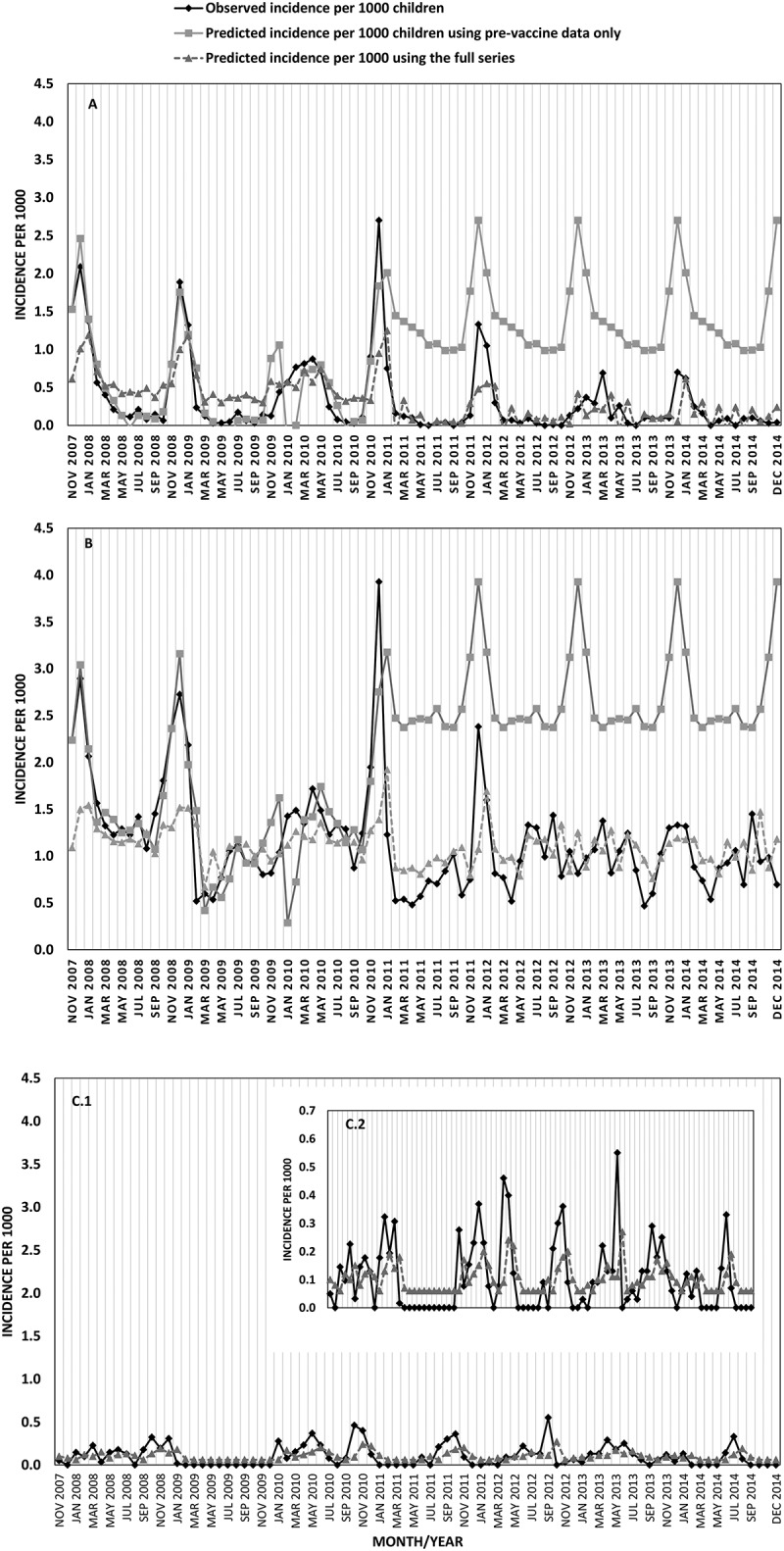
Observed and predicted monthly incidence (per 1000 children) of rotavirus gastroenteritis (A), all-cause gastroenteritis (B) and norovirus gastroenteritis (C), November 20,017-December 2014.
Universal rotavirus immunization was introduced on December 2010.
Norovirus positivity according to demographic factors
Infants aged 0–5 comprised 19.0% of patients with norovirus, compared to 40.1%, 23.4%, 10.9%, and 6.6% in the age groups 6–11, 12–17, 18–23, and 24–59 months, respectively. The respective percentages among patients who tested negative for norovirus were 27.3%, 24.1%, 20.5%, 12.8%, and 15.9%. The percentage of males among norovirus positive patients tended to be higher than in patients who tested negative for norovirus: 61.3% vs. 52.5% (P = 0.052). Norovirus gastroenteritis patients comprised a higher percentage of Jewish children than those who tested negative for norovirus (76.1% vs. 63.5%, P = 0.004). An association was found between norovirus gastroenteritis and residential socioeconomic status (P = 0.026); the percentage of children who lived in towns of low, intermediate, and high socioeconomic status was 29.8%, 54.0%, and 16.1%, respectively, in the norovirus positive group, while the corresponding figures were 42.4%, 46.9%, and 10.7% in the norovirus negative (Table 2). In a multivariable analysis, children aged 6–11 months had 2.49-fold higher likelihood to be positive for norovirus compared to children aged 0–5 months (P = 0.001), while the likelihood increased by 1.6-fold in males vs. females (P = 0.018) and by 1.83-fold in Jewish vs. Arab patients (P = 0.005). The variables population group and residential socioeconomic rank were highly correlated (Phi coefficient 0.84, P < 0.001, i.e. Arab children lived in towns of lower socioeconomic rank). Therefore, a separate model was fitted, in which the variable “residential socioeconomic rank” was included instead of “population group”. This model showed that children who lived in towns of high socioeconomic rank had twofold increased likelihood to be norovirus positive than children who lived in towns of low socioeconomic rank (P = 0.018), and a similar but non-significant trend (P = 0.072) was found in children who lived in towns of intermediate socioeconomic rank (Table 2).
Table 2.
Associations between sociodemographic factors and norovirus positivity among hospitalized patients with gastroenteritis a.
| Norovirus positive, n (%) | Norovirus negative, n (%) | Pb | Unadjusted OR (95% CI)e |
Adjusted OR (95% CI)f | P j | |
|---|---|---|---|---|---|---|
| Total | 138 | 1021 | ||||
| Age (months)c | df = 4 | <0.001 | df = 4 | df = 4 | 0.001 | |
| 0–5 | 26 (19.0%) | 278 (27.3%) | Reference | Reference | ||
| 6–11 | 55 (40.1%) | 246 (24.1%) | 2.39 (1.45–3.93) | 2.49 (1.45–4.26) | 0.001 | |
| 12–17 | 32 (23.4%) | 209 (20.5%) | 1.64 (0.95–2.83) | 1.56 (0.85–2.84) | 0.14 | |
| 18–23 | 15 (10.9%) | 124 (12.2%) | 1.29 (0.66–2.52) | 1.23 (0.59–2.56) | 0.5 | |
| 24–59 | 9 (6.6%) | 162 (15.9%) | 0.59 (0.27–1.30) | 0.64 (0.28–1.44) | 0.2 | |
| Missing | 1 (0.7%) | 2 (0.2%) | ||||
| Sex | 0.052 | |||||
| Males | 84 (61.3%) | 536 (52.5%) | 1.43 (1.00–2.07) | 1.61 (1.08–2.40) | 0.018 | |
| Females | 53 (38.7%) | 485 (47.5%) | Reference | Reference | ||
| Missing | 1 (0.7%) | 0 (%) | ||||
| Population group | 0.004 | |||||
| Jews | 105 (76.1%) | 648 (63.5%) | 1.83 (1.21–2.76) | 1.83 (1.20–2.79) | 0.005 | |
| Arabs | 33 (23.9%) | 373 (36.5%) | Reference | Reference | ||
| Residential socioeconomic rank d | df = 2 | 0.026 | df = 2 | df = 2 | 0.044 | |
| Low (1–4) | 37 (29.8%) | 389 (42.4%) | Reference | Reference | ||
| Intermediate (5–6) | 65 (54.0%) | 435 (47.0%) | 1.57 (1.03–2.41) | 1.49 (0.96–2.31) | 0.072 | |
| High (7–10) | 20 (16.1%) | 101 (10.9%) | 2.08 (1.16–3.74) | 2.08 (1.14–3.81) | 0.018 | |
| Missing | 16 (11.5%) | 96 (9.4%) |
aThis analysis is based on 1159 children (138 positive for norovirus and 1021 negative for norovirus). Each child was included only once in this analysis. For children with recurrent hospitalizations, we analyzed data of the first hospitalization in which the child was tested for norovirus.
bP obtained by chi-square test. cdf = degrees of freedom, P for trend = 0.12, dP for trend = 0.007.
eOdds ratio (OR) and 95% confidence intervals (CI).
fAdjusted for the variables in the table. The variables population group and socioeconomic rank were highly correlated (Phi coefficient 0.84, P < 0.001), therefore they were included in separate models. jP obtained from multivariable logistic regression models.
Seasonality
Norovirus was detected all year around, with a significant 3-month peak from September to November, in which 37.5% of the cases occurred (Ratchet circular test for short seasonal peak: test statistics = 2.75, P < 0.05). In comparison, rotavirus gastroenteritis showed a different seasonal pattern (Figure 3), peaking between November and March, during which 84.7% of the cases occurred (P = 0.015 by Hewitt‘s rank sum test for 5-month seasonal peak).
Figure 3.
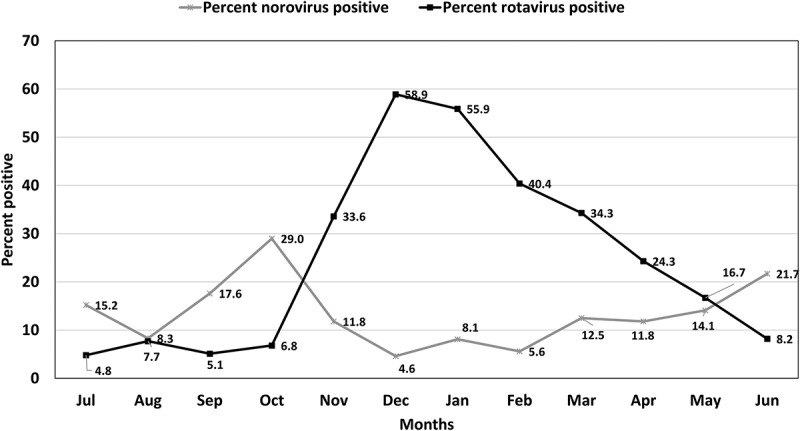
–Prevalence (percent positive) of norovirus and rotavirus among hospitalized children with gastroenteritis by months, 2007–2014.
Overall, 3722 samples were tested for rotavirus and 1179 for norovirus. Percent positive was calculated by division of the number of positive samples the total number of samples tested in the month multiplied by 100.
Detection of enteric pathogens
This analysis was limited to 1101 children who were tested for norovirus, rotavirus, Shigella, Salmonella, and Campylobacter. Overall, 628 (57.0%) tested positive for at least one pathogen. Norovirus was the only pathogen detected in 117 (10.6%) patients and in 7 (0.6%), 4 (0.4%) and 3 (0.3%) patients it was detected together with rotavirus, Salmonella, and Campylobacter, respectively. Norovirus was the only detected pathogen in 61/585 (10.4%) of the patients before universal rotavirus vaccination period compared to 56/516 (10.9%) in the universal vaccination years (chi-square = 0.05, P = 0.8). Rotavirus was the only detected pathogen in 338/1101 (30.7%) patients; more often 235/585 (40.2%) before the universal vaccination years vs. 203/516 (20.0%) during the universal vaccination years (chi-square = 52.6, P < 0.001). Shigella, Salmonella, and Campylobacter were detected as the only pathogen in 30 (2.7%), 20 (1.8%), and 78 (7.1%) children, respectively (Table 3).
Table 3.
Detection of enteric pathogens in children aged 0–59 months hospitalized for gastroenteritis between November 2007 and December 2014.
| Pathogen | n | % |
|---|---|---|
| Total number of patients tested to all pathogens a | 1101 | 100.0 |
| Positive for any pathogen | 628 | 57.0 |
| Negative for all tested pathogens | 473 | 43.0 |
| Total number of norovirus positive patients | 131 | 11.9 |
| Distribution of patients with identified pathogens | ||
| Patients with viruses | ||
| Norovirus | 117 | 10.6 |
| Rotavirus | 338 | 30.7 |
| Norovirus and rotavirus | 7 | 0.6 |
| Patients with bacterial pathogen | ||
| Shigella | 30 | 2.7 |
| Salmonella | 20 | 1.8 |
| Campylobacter | 78 | 7.1 |
| Shigella and Campylobacter | 1 | 0.1 |
| Patients with mixed viral and bacterial pathogens | ||
| Norovirus and Salmonella | 4 | 0.4 |
| Norovirus and Campylobacter | 3 | 0.3 |
| Rotavirus and Shigella | 1 | 0.1 |
| Rotavirus and Salmonella | 6 | 0.5 |
| Rotavirus and Campylobacter | 23 | 2.1 |
aOnly 1101/1179 (94.4%) children who were tested for norovirus were also tested for the presence of rotavirus, Shigella, Salmonella, and Campylobacter.
Clinical symptoms
Fever was less common in patients who had norovirus than patients who had rotavirus or other enteric pathogens: 58.1%, 80.5%, and 80.8%, respectively (P < 0.001). The percentage of patients with norovirus who had five or more watery stools/24 h was lower (65.8%) compared to children with rotavirus (75.9%) or other pathogens (74.9%), but the difference was not significant (P = 0.09). The percentage of children with vomiting was similar among children with norovirus and rotavirus (85.6% and 93.6%) compared to 70.2% in children with other enteric pathogens. The respective percentages of children who vomited four times or more in 24 h was 58.7%, 58.8%, and 31.1%.
Discussion
In this 7-y prospective study, the incidence and characteristics of norovirus gastroenteritis were examined before and after the introduction of universal rotavirus immunization in young children in Israel. The average annual incidence estimate of norovirus gastroenteritis of 1.3 per 1000 children in our study, is comparable with other reports from Israel25, but it is somewhat lower than that found in the United States.6 In our study, norovirus was the second leading cause of gastroenteritis hospitalizations in children aged less than 5 y, found in 10.6% of the patients. This percentage was similar before and after the introduction of universal rotavirus immunization in Israel. A substantial 62% reduction was evident in the incidence of rotavirus gastroenteritis hospitalizations during the universal immunization years26-28 compared to the pre-universal immunization years, but rotavirus remained the leading cause of gastroenteritis hospitalizations, being detected in 40.2% and 20.0% before and after the introduction of universal immunization. The coverage of three doses of rotavirus vaccine in Israel is estimated at ~80%,29 which together with good vaccine effectiveness30 explain the reduction in hospitalizations for rotavirus gastroenteritis. Herd immunity possibly explains the 36% reduction in the incidence of rotavirus gastroenteritis hospitalizations in children aged 24–59 who were not eligible for a rotavirus vaccine in the framework of the universal vaccination program.27,28,31
A 1-y study after the introduction of universal rotavirus immunization in Finland showed that norovirus was detected in 25% of children aged less than 15 y with acute gastroenteritis (both inpatient and outpatient settings), while rotavirus was found in 24%.22 In agreement with our findings, a reduction in rotavirus gastroenteritis was observed after introducing universal rotavirus immunization compared to the preceding period in Finland, however, as in our study, the percentage of norovirus gastroenteritis was similar between the two periods.22,32 Another study from Finland with longer follow-up periods following the introduction of rotavirus immunization, showed a slight decrease in the number of norovirus gastroenteritis cases, but the proportion of norovirus positive patients increased from 26% in the pre-vaccine years 2006–2008 to 34% universal vaccine years (2009–2011), compared to 52% and 26% for rotavirus, respectively.33 In Colombia, rotavirus remained the leading cause of gastroenteritis found in 33.7% of children less than 5 y of age hospitalized for acute gastroenteritis, with norovirus being found in 22.6% of the patients.21 A study from the United States6 during the universal rotavirus immunization years showed that norovirus was detected in 21% of children seeking medical care for gastroenteritis, compared to only 12% for rotavirus. Our findings together with others’21,22,32–34 suggest that norovirus is a leading cause of gastroenteritis associated with seeking medical care and hospitalizations in young children, regardless of the introduction of the universal rotavirus immunization program. Despite the challenges facing the development of a norovirus vaccine,23 prevention of norovirus gastroenteritis through vaccination is especially attractive in view of the success of rotavirus immunization in preventing rotavirus gastroenteritis and child mortality due to diarrheal diseases globally.35 Currently, several norovirus vaccines are in development,23,24,36,37 including a bivalent GI.1/GII.4 vaccines based on virus-like particles (VLPs) given intramuscularly and an oral vaccine based on non-replicating adenovirus vector expressing VP1 gene from GI.1 norovirus,37 which have already been tested in clinical trials. Most (83%) norovirus positive patients in our study were children aged less than 18 months, about 40% were infants aged 6–11 months, in agreement with previous reports.22,38 Therefore a future norovirus vaccine should likely target infants less than six months of age, before the peak incidence at ages 6–18 months. The elderly and patients in nursing homes should also be targeted in future norovirus vaccination programs, based on evidence of severe norovirus disease, of longer duration5 and even of death in this group.39
The strengths of our study include the prospective study design with 7-y data on a well-defined population. Our study has also limitations. Only 1179 (18%) stool samples were available for norovirus testing among all gastroenteritis hospitalizations that occurred during the study period in three hospitals in northern Israel. Children tested for norovirus differed in demographic factors and year/season of admission compared to those who were not tested for norovirus. To account for these differences we performed a weighted analysis, to estimate the number of norovirus hospitalizations in the study catchment area. Second, we examined only GII noroviruses and excluded nosocomial infections from the analysis; thus, the incidence of norovirus gastroenteritis in our might have been underestimated. However, it was shown that noroviruses of GII genogroup cause 96% of norovirus gastroenteritis cases in children globally.10 Overall all-cause gastroenteritis decreased after the introduction of universal rotavirus immunization; thus, our findings do not support the possibility of “emergence” of other untested pathogens such as GI noroviruses. Since the original study was planned to assess the burden of rotavirus gastroenteritis,40 our case definition was based on having three or more watery stools in a 24/hour period. Therefore, it is possible that we missed some norovirus patients who presented with vomiting only.
In summary, norovirus continues to be a leading cause of acute gastroenteritis hospitalizations in young children in the universal rotavirus immunization period in Israel. Future norovirus vaccines should target infants less than six months of age before the peak disease incidence at ages 6–18 months. There was no evidence for an increase in the incidence of hospitalizations for norovirus gastroenteritis following the introduction of universal rotavirus immunization in Israel.
Materials and methods
Study population and design
A prospective hospital-based surveillance study was undertaken in northern Israel to assess the incidence of rotavirus gastroenteritis before and after the introduction of the universal rotavirus immunization program.26,27,40,41 This platform was utilized to assess the incidence of norovirus gastroenteritis.7 Details of the study design have been reported.7,26,27,40,41 In brief, the study target population included children 0–59 months of age residing in the catchment area of three hospitals: Carmel in Haifa, Hillel Yaffe in Hadera, and Laniado in Netanya.40,41 The current analysis was limited to hospitalizations that occurred from November 2007 until December 2014, the period during which we tested for norovirus.
The sampling frame included children aged 0–59 months who were hospitalized in the three hospitals with diarrhea (three or more watery stools during a 24-h period). Information was collected about each child on age (in months, categorized as 0–5, 6–11, 12–17, 18–23, and 24–59) sex, population group (Arabs or Jews) and clinical symptoms (number of stools per day, vomiting, bloody stools) through parental interviews and from medical records, and body temperature was measured. Fever was defined as a measurement of ≥38°C. Socioeconomic status of place of residence defined by the Central Bureau of Statistics was used a proxy for socioeconomic status.42 Towns with ranks of 1–4, 5–6, and 7–10 were considered as low, intermediate and high socioeconomic status, respectively.
Laboratory methods
A stool sample was collected from children hospitalized for diarrhea within the first 48 h of hospital admission. Stool samples were refrigerated and transported to the hospital laboratories within a few hours after collection where they were tested for the presence of rotavirus antigen by immunochromatography (Rotavirus Dipsticks, Hylabs Rehovot & Novamed, Jerusalem, Israel). The sensitivity of specificity of immunochromatographic tests compared to RT-PCR30 was estimated at 99% and 96%, respectively. There was a high agreement between the immunochromatographic kits used in our study compared with DAKO ELISA kit (Dakopatts A/S. Denmark) 95% for Novamed kit and 93% for Hylabs kits.41 Stool culture was performed following standard microbiologic methods to isolate Salmonella, Shigella, and Campylobacter species. A portion of each sample was stored at −80°C until norovirus testing was performed.
Norovirus GII testing
We focused on GII noroviruses, since they were shown to cause the majority of norovirus illnesses.10 Real-time RT-PCR was employed for the detection of norovirus genotype GII RNA on extracted fecal RNA by TaqMan technology using the Cog 2F (caR gaR BcN atg tty agR tgg atg ag) and Cog 2R (tcg acg cca tct tca ttc aca) primers and Ring 2 probe (FAM-tgg gag ggc gat cgc aat ct-BHQ) developed at the Centers for Disease Control and Prevention (CDC), Atlanta, Georgia, USA by Trujillo et al.43 The primers and probe were synthesized by Hylabs, Rehovot, Israel. The assay was validated using serial dilutions of norovirus GII RNA (positive control) extracted from a stool sample containing GII norovirus kindly supplied by the CDC, Atlanta, Georgia, USA, and RNA extracted from a pool of two norovirus GII-positive stool suspensions. Cycle threshold (Ct) for the norovirus GII qRT-PCR assay of RNA extracted from the pooled stool suspensions were linear over a 105-fold serial dilution. A 334 nt segment of the norovirus capsid protein, sequenced using GII norovirus-specific primers G2SK (CTGCCCGAATTYGTAAATGA) and G2SKR (CCAACCCARCCATTRTACA) primers as described by Kojima et al.44 confirmed qRT-PCR results with Ct values ≤29 for RNAs from a group of 17 norovirus GII qRT-PCR-positive stool suspensions from pediatric patients and 25 stool suspensions from adult patients. The specificity of the qRT-PCR was validated using norovirus GI RNA extracted from a stool sample containing GI norovirus also kindly supplied by US CDC, and nucleic acids extracted from 15 different RNA viruses and 9 different DNA viruses. All were negative, e.g. there was no cross-reactivity. qRT-PCR for MS2 was performed after adding a known amount of MS2 RNA to the RNA extracted from stool suspensions to ensure that no inhibitors of PCR were coextracted with the RNA.45 Quality control for the assay was monitored according to Westgard Rules (https://www.westgard.com/mltirule.htm) comparing the Ct values of aliquots of positive control RNA (stored frozen at −70°C) that were included in each test.
The Ct value from the real-time RT-PCR, which represents the number of PCR reactions until the appearance of the amplification signal, was used as a proxy measure of fecal viral load. The lower the Ct values, the greater the viral RNA content in fecal material. In a pilot study, the real-time PCR assays were run for 60 cycles using RNA extracted from stool samples of 27 healthy children, without signals of amplification.7 The real-time RT-PCR was run for 50 cycles and if no amplification was seen at this level, it was considered negative. The distribution of Ct values of children having “potentially positive” norovirus test (i.e. Ct value <50) who also tested negative for other pathogens was used to determine the cutoff of Ct value for norovirus positive test (Supplementary figure 1). Samples with Ct values lower than the 75th percentile of this distribution, i.e., Ct values <32 were considered positive for norovirus. Other norovirus studies using real-time PCR have used Ct values ranging from 21.4 to 35.3 to define norovirus-associated diarrheal episode.9,46–49
Statistical analysis
The percentage of available samples for norovirus testing was examined according to age group, population group, year and month of admission. To estimate the total number of children with norovirus gastroenteritis, a weighted analysis was applied, in which the weights were determined as the inverse of norovirus testing proportion (equivalent to sampling fraction), as described elsewhere.27 We calculated the annual incidence (per 1000 children) of norovirus gastroenteritis hospitalization using the estimated number of norovirus gastroenteritis hospitalizations in the numerator and the number of children aged 0–59 months living in the study catchment area as the denominator. Data on the population size was estimated using the Central Bureau of Statistics reports on population size by sub-district.50 The pediatric population receiving hospitalization services in these hospitals was estimated at 80%-90% of the Hadera sub-district, 25% of the Haifa sub-district and 60%-70% of the HaSharon sub-district, respectively. The yearly average number of children 0–59 months of age served by these hospitals during the study period was estimated at ~66,000. The 95% confidence intervals (CIs) of the incidence proportions were estimated as the 2.5th and 97.5th percentiles from MonteCarlo simulations with 1000 runs. Similar analyses were performed for rotavirus gastroenteritis and all-cause gastroenteritis. Additionally, we performed interrupted time series analyses for the incidence of each of these outcomes (incidence of all-cause, rotavirus and norovirus gastroenteritis).51 Initially, using Autoregressive integrated moving average (ARIMA) models and monthly data obtained between November 2007 and December 2010, we predicted the monthly incidence of each of these outcomes for the universal vaccination period as if there was no universal immunization program. Additionally, we predicted the monthly incidence of each of these outcomes using ARIMA models and full data series. We further performed multivariable ARIMA, in which the variable “period” (before or after the introduction of universal rotavirus immunization) was included as a predictor of the incidence of each outcome. The observed incidence was plotted against the predicted incidence throughout the study period.
The presence of seasonal pattern of norovirus positivity was tested using Ratchet circular test for short seasonal peak and Hewitt‘s rank-sum test for longer peaks. Differences between norovirus positive and negative in demographic factors and clinical symptoms were examined using chi-square test. In the analyses of demographic associations with norovirus positivity, bivariate and multivariable logistic regression models were fitted. In these analyses, any subsequent hospitalizations of a patient for recurrent gastroenteritis during the study period were excluded to avoid dependence between observations. A two-sided P < 0.05 was considered statistically significant. Data were analyzed using SPSS version 25 (IBM, New York, United States) and Winpepi software.52
Funding Statement
The study was funded by the World Health Organization, Department of Immunization, Vaccination and Biologicals (V27-181-190) in the first year, and by the Israel National Institute for Health Policy and Research (grant 2011/154A) in the remaining years.
Acknowledgments
We would like to thank the research staff at the participating medical centers: Meissa Yunes, Fatma Abu Rakia, Sarit Primer, Tali Zim, Pninit Shaked-Mishan, and Ana Rimer, as well as Ms. Ilana Zilberstient from the Central Virology Laboratory. We would also like to thank Ms. Anya Bialik and Mr. Michael Brik from Tel Aviv University for technical assistance in conducting the study.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Ethical considerations
The study protocol was approved by the Institutional Review Boards of all participating hospitals and by the Ministry of Health. Parents signed a written informed consent form.
References
- 1.Vinje J. Advances in laboratory methods for detection and typing of norovirus. J Clin Microbiol. 2015;53(2):373–81. doi: 10.1128/JCM.01535-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banyai K, Estes MK, Martella V, Parashar UD. Viral gastroenteritis. Lancet. 2018;392(10142):175–86. doi: 10.1016/S0140-6736(18)31128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robilotti E, Deresinski S, Pinsky BA. Norovirus. Clin Microbiol Rev. 2015;28(1):134–64. doi: 10.1128/CMR.00075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arness MK, Feighner BH, Canham ML, Taylor DN, Monroe SS, Cieslak TJ, Hoedebecke EL, Polyak CS, Cuthie JC, Fankhauser RL, et al. Norwalk-like viral gastroenteritis outbreak in U.S. army trainees. Emerg Infect Dis. 2000;6(2):204–07. doi: 10.3201/eid0602.009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopman BA, Reacher MH, Vipond IB, Sarangi J, Brown DW. Clinical manifestation of norovirus gastroenteritis in health care settings. Clin Infect Dis. 2004;39(3):318–24. doi: 10.1086/421948. [DOI] [PubMed] [Google Scholar]
- 6.Payne DC, Vinje J, Szilagyi PG, Edwards KM, Staat MA, Weinberg GA, Hall CB, Chappell J, Bernstein DI, Curns AT, et al. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med. 2013;368(12):1121–30. doi: 10.1056/NEJMsa1206589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muhsen K, Kassem E, Rubinstein U, Schachter Y, Kremer A, Goren S, Zilberstein I, Ephros M, Cohen D, Shulman LM. Incidence and characteristics of sporadic norovirus gastroenteritis associated with hospitalization of children less than 5 years of age in Israel. Pediatr Infect Dis J. 2013;32(6):688–90. doi: 10.1097/INF.0b013e318287fc81. [DOI] [PubMed] [Google Scholar]
- 8.Gastanaduy PA, Hall AJ, Curns AT, Parashar UD, Lopman BA. Burden of norovirus gastroenteritis in the ambulatory setting–United States, 2001–2009. J Infect Dis. 2013;207(7):1058–65. doi: 10.1093/infdis/jis942. [DOI] [PubMed] [Google Scholar]
- 9.Phillips G, Tam CC, Conti S, Rodrigues LC, Brown D, Iturriza-Gomara M, Gray J, Lopman B. Community incidence of norovirus-associated infectious intestinal disease in England: improved estimates using viral load for norovirus diagnosis. Am J Epidemiol. 2010;171(9):1014–22. doi: 10.1093/aje/kwq021. [DOI] [PubMed] [Google Scholar]
- 10.Hoa Tran TN, Trainor E, Nakagomi T, Cunliffe NA, Nakagomi O. Molecular epidemiology of noroviruses associated with acute sporadic gastroenteritis in children: global distribution of genogroups, genotypes and GII.4 variants. J Clin Virol. 2013;56(3):185–93. doi: 10.1016/j.jcv.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention Updated norovirus outbreak management and disease prevention guidelines. MMWR Recomm Rep. 2011;60(RR–3):1–18. [PubMed] [Google Scholar]
- 12.Siebenga JJ, Vennema H, Zheng DP, Vinjé J, Lee BE, Pang X-L, Ho ECM, Lim W, Choudekar A, Broor S, et al. Norovirus illness is a global problem: emergence and spread of norovirus GII.4 variants, 2001–2007. J Infect Dis. 2009;200(5):802–12. doi: 10.1086/605127. [DOI] [PubMed] [Google Scholar]
- 13.Phillips G, Tam CC, Rodrigues LC, Lopman B. Prevalence and characteristics of asymptomatic norovirus infection in the community in England. Epidemiol Infect. 2010;138(10):1454–58. doi: 10.1017/S0950268810000439. [DOI] [PubMed] [Google Scholar]
- 14.Lee RM, Lessler J, Lee RA, Rudolph KE, Reich NG, Perl TM, Cummings DA. Incubation periods of viral gastroenteritis: a systematic review. BMC Infect Dis. 2013;13:446. doi: 10.1186/1471-2334-13-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Graaf M, van Beek J, Koopmans MP. Human norovirus transmission and evolution in a changing world. Nat Rev Microbiol. 2016;14(7):421–33. doi: 10.1038/nrmicro.2016.48. [DOI] [PubMed] [Google Scholar]
- 16.Harris JP, Edmunds WJ, Pebody R, Brown DW, Lopman BA. Deaths from norovirus among the elderly, England and Wales. Emerg Infect Dis. 2008;14(10):1546–52. doi: 10.3201/eid1410.080188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collaborators GBDDD. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1211–28. doi: 10.1016/S1473-3099(18)30362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pires SM, Fischer-Walker CL, Lanata CF, Devleesschauwer B, Hall AJ, Kirk MD, Duarte ASR, Black RE, Angulo FJ, Selvey LA. Aetiology-specific estimates of the global and regional incidence and mortality of diarrhoeal diseases commonly transmitted through food. PLoS One. 2015;10(12). doi: 10.1371/journal.pone.0142927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartsch SM, Lopman BA, Ozawa S, Hall AJ, Lee BY. Global economic burden of norovirus gastroenteritis. PLoS One. 2016;11(4):e0151219. doi: 10.1371/journal.pone.0151219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall AJ, Lopman BA, Payne DC, Patel MM, Gastañaduy PA, Vinjé J, Parashar UD. Norovirus disease in the United States. Emerg Infect Dis. 2013;19(8):1198–205. doi: 10.3201/eid1908.130465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez-Medina E, Parra B, Davalos DM, Lopez P, Villamarin E, Pelaez M. Acute gastroenteritis in a pediatric population from Cali, Colombia in the post rotavirus vaccine era. Int J Infect Dis. 2018;73:52–59. doi: 10.1016/j.ijid.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Puustinen L, Blazevic V, Salminen M, Hamalainen M, Rasanen S, Vesikari T. Noroviruses as a major cause of acute gastroenteritis in children in Finland, 2009–2010. Scand J Infect Dis. 2011;43(10):804–08. doi: 10.3109/00365548.2011.588610. [DOI] [PubMed] [Google Scholar]
- 23.Hallowell BD, Parashar UD, Hall AJ. Epidemiologic challenges in norovirus vaccine development. Hum Vaccin Immunother. 2018. doi: 10.1080/21645515.2018.1553594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernstein DI, Atmar RL, Lyon GM, Treanor JJ, Chen WH, Jiang X, Vinjé J, Gregoricus N, Frenck RW, Moe CL, et al. Norovirus vaccine against experimental human GII.4 virus illness: a challenge study in healthy adults. J Infect Dis. 2015;211(6):870–78. doi: 10.1093/infdis/jiu497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leshem E, Givon-Lavi N, Vinje J, Gregoricus N, Parashar U, Dagan R. Differences in norovirus-associated hospital visits between Jewish and Bedouin children in southern Israel. Pediatr Infect Dis J. 2015;34(9):1036–38. doi: 10.1097/INF.0000000000000786. [DOI] [PubMed] [Google Scholar]
- 26.Muhsen K, Kassem E, Rubenstein U, Goren S, Ephros M, Cohen D, Shulman LM. Incidence of rotavirus gastroenteritis hospitalizations and genotypes, before and five years after introducing universal immunization in Israel. Vaccine. 2016;34(48):5916–22. doi: 10.1016/j.vaccine.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Muhsen K, Rubenstein U, Kassem E, Goren S, Schachter Y, Kremer A, Shulman LM, Ephros M, Cohen D. A significant and consistent reduction in rotavirus gastroenteritis hospitalization of children under 5 years of age, following the introduction of universal rotavirus immunization in Israel. Hum Vaccin Immunother. 2015;11(10):2475–82. doi: 10.1080/21645515.2015.1056951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muhsen K, Cohen D. Rotavirus vaccines in Israel: uptake and impact. Hum Vaccin Immunother. 2017;13(7):1722–27. doi: 10.1080/21645515.2017.1297908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Israel Central Bureau of Statistics. Statistical abstract of Israel 2018. State of Israel, Jerusalem. [Google Scholar]
- 30.Wilhelmi I, Colomina J, Martin-Rodrigo D, Roman E, Sanchez-Fauquier A. New immunochromatographic method for rapid detection of rotaviruses in stool samples compared with standard enzyme immunoassay and latex agglutination techniques. Eur J Clin Microbiol Infect Dis. 2001;20:741–43. [DOI] [PubMed] [Google Scholar]
- 31.Givon-Lavi N, Ben-Shimol S, Cohen R, Greenberg D, Dagan R. Rapid impact of rotavirus vaccine introduction to the national immunization plan in southern Israel: comparison between 2 distinct populations. Vaccine. 2015;33(16):1934–40. doi: 10.1016/j.vaccine.2015.02.062. [DOI] [PubMed] [Google Scholar]
- 32.Rasanen S, Lappalainen S, Salminen M, Huhti L, Vesikari T. Noroviruses in children seen in a hospital for acute gastroenteritis in Finland. Eur J Pediatr. 2011;170(11):1413–18. doi: 10.1007/s00431-011-1443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hemming M, Rasanen S, Huhti L, Paloniemi M, Salminen M, Vesikari T. Major reduction of rotavirus, but not norovirus, gastroenteritis in children seen in hospital after the introduction of RotaTeq vaccine into the National Immunization Programme in Finland. Eur J Pediatr. 2013;172(6):739–46. doi: 10.1007/s00431-013-1945-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Operario DJ, Platts-Mills JA, Nadan S, Page N, Seheri M, Mphahlele J, Praharaj I, Kang G, Araujo IT, Leite JPG, et al. Etiology of severe acute watery diarrhea in children in the global rotavirus surveillance network using quantitative polymerase chain reaction. J Infect Dis. 2017;216(2):220–27. doi: 10.1093/infdis/jix294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burnett E, Jonesteller CL, Tate JE, Yen C, Parashar UD. Global impact of rotavirus vaccination on childhood hospitalizations and mortality from diarrhea. J Infect Dis. 2017;215(11):1666–72. doi: 10.1093/infdis/jix186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atmar RL, Bernstein DI, Harro CD, Al-Ibrahim MS, Chen WH, Ferreira J, Estes MK, Graham DY, Opekun AR, Richardson C, et al. Norovirus vaccine against experimental human Norwalk virus illness. N Engl J Med. 2011;365(23):2178–87. doi: 10.1056/NEJMoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim L, Liebowitz D, Lin K, Huey MG, Parker RE, Page LS, Hill AA, Wang X, Frye SV, Earp HS, et al. Safety and immunogenicity of an oral tablet norovirus vaccine, a phase I randomized, placebo-controlled trial. JCI Insight. 2018;3(13). doi: 10.1172/jci.insight.97941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farsi M, Roodbari F, Nejati B, Arashkia A, Jalilvand S, Nateghian A, Rahbarimanesh A, Marashi SM, Shoja Z. Prevalence and genetic diversity of norovirus genogroup II in children less than 5 years of age with acute gastroenteritis in Tehran, Iran. Med Microbiol Immunol. 2018;207(3–4):201–10. doi: 10.1007/s00430-018-0541-6. [DOI] [PubMed] [Google Scholar]
- 39.van Asten L, van den Wijngaard C, van Pelt W, van de Kassteele J, Meijer A, van der Hoek W, Kretzschmar M, Koopmans M. Mortality attributable to 9 common infections: significant effect of influenza A, respiratory syncytial virus, influenza B, norovirus, and parainfluenza in elderly persons. J Infect Dis. 2012;206(5):628–39. doi: 10.1093/infdis/jis415. [DOI] [PubMed] [Google Scholar]
- 40.Muhsen K, Shulman L, Rubinstein U, Kasem E, Kremer A, Goren S, Zilberstein I, Chodick G, Ephros M, Cohen D. Incidence, characteristics, and economic burden of rotavirus gastroenteritis associated with hospitalization of Israeli children <5 years="” of="” age,=""></5>. J Infect Dis. 2009;200(Suppl 1):S254–63. doi: 10.1086/605425. [DOI] [PubMed] [Google Scholar]
- 41.Muhsen K, Shulman L, Kasem E, Rubinstein U, Shachter J, Kremer A, Goren S, Zilberstein I, Chodick G, Ephros M, et al. Effectiveness of rotavirus vaccines for prevention of rotavirus gastroenteritis-associated hospitalizations in Israel: A case-control study. Hum Vaccin. 2010;6(6):450–54. [DOI] [PubMed] [Google Scholar]
- 42.Israel Central Bureau of Statistics Characterization and classification of local authorities by the socio-economic level of the population. State of Israel, Jerusalem; 2008. [Google Scholar]
- 43.Trujillo AA, McCaustland KA, Zheng DP, Hadley LA, Vaughn G, Adams SM, Ando T, Glass RI, Monroe SS. Use of TaqMan real-time reverse transcription-PCR for rapid detection, quantification, and typing of norovirus. J Clin Microbiol. 2006;44(4):1405–12. doi: 10.1128/JCM.44.4.1405-1412.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kojima S, Kageyama T, Fukushi S, Hoshino FB, Shinohara M, Uchida K, Natori K, Takeda N, Katayama K. Genogroup-specific PCR primers for detection of Norwalk-like viruses. J Virol Methods. 2002;100:107–14. [DOI] [PubMed] [Google Scholar]
- 45.Shulman LM, Hindiyeh M, Muhsen K, Cohen D, Mendelson E, Sofer D. Evaluation of four different systems for extraction of RNA from stool suspensions using MS-2 coliphage as an exogenous control for RT-PCR inhibition. PLoS One. 2012;7(7):e39455. doi: 10.1371/journal.pone.0039455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trang NV, Choisy M, Nakagomi T, Chinh NTM, Doan YH, Yamashiro T, Bryant JE, Nakagomi O, Anh DD. Determination of cut-off cycle threshold values in routine RT-PCR assays to assist differential diagnosis of norovirus in children hospitalized for acute gastroenteritis. Epidemiol Infect. 2015;143(15):3292–99. doi: 10.1017/S095026881500059X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C, Operario DJ, Uddin J, Ahmed S, Alonso PL, et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet. 2016;388(10051):1291–301. doi: 10.1016/S0140-6736(16)31529-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kageyama T, Kojima S, Shinohara M, Uchida K, Fukushi S, Hoshino FB, Takeda N, Katayama K. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J Clin Microbiol. 2003;41:1548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phillips G, Lopman B, Tam CC, Iturriza-Gomara M, Brown D, Gray J. Diagnosing norovirus-associated infectious intestinal disease using viral load. BMC Infect Dis. 2009;9:63. doi: 10.1186/1471-2334-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Israel Central Bureau of Statistics Statistical abstract of Israel 2014. No. 65 ed State of Israel, Jerusalem; 2015. [Google Scholar]
- 51.Kontopantelis E, Doran T, Springate DA, Buchan I, Reeves D. Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis. BMJ. 2015;350:h2750. doi: 10.1136/bmj.h2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abramson JH. WINPEPI updated: computer programs for epidemiologists, and their teaching potential. Epidemiol Perspect Innov. 2011;8(1):1. doi: 10.1186/1742-5573-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


