Introduction
Diarrheal diseases remain a leading cause of mortality globally.1 Recent estimates of the Global Burden Disease study showed that nearly 1.65 million diarrheal diseases deaths occurred in 2016 in all ages globally, of which 446,000 deaths occurred among children aged under 5 years. More than 85% of diarrheal diseases deaths occurred in South Asia and sub-Saharan Africa.1 Rotavirus was the leading cause of diarrheal diseases deaths, being responsible for 228,000 deaths in all age groups, followed by Shigella causing 212,000 deaths, Vibrio cholerae (107,000 deaths), adenovirus (93,000 deaths), non-typhoidal Salmonella (NTS) (87,000 deaths), Campylobacter (75,000 deaths), Cryptosporidium (57,000 deaths), and enterotoxigenic Escherichia coli (ETEC) (51,000 deaths).1
There are various reasons that make enteric vaccines relevant globally (Figure 1).
Figure 1.
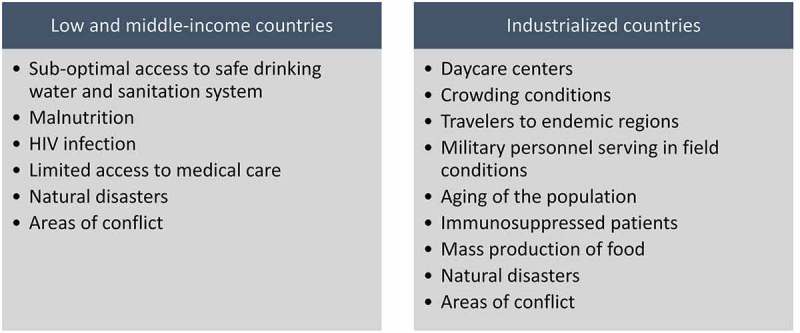
Risk factors of enteric diseases and target groups for enteric vaccines.
The incidence and mortality rates of enteric diseases are high in low- and middle-income countries (LMICs), where access to safe drinking water and sanitation systems is sub-optimal.2 Hence, improving access to safe drinking water supply and sanitation is essential for the prevention. Although progress has been documented in this area, achieving the universal access to safe drinking water and improved sanitation is far from being reached in the near future.3 Therefore, using vaccines can accelerate the reduction in diarrheal disease morbidity and mortality in LMICs.4 Moreover, some enteric pathogens cause illness and substantial burden in both industrialized countries and LMICs. A main example is rotavirus that caused substantial burden to the health care systems of developed countries, in terms of hospitalizations and clinic visits,5,6 in the years that preceded the licensure of the monovalent, vaccine, Rotarix™ (GSK Biologicals, Rixensart),7 and pentavalent vaccine, RotaTeq ™ (Merck & Co., Whitehouse, Pennsylvania)8 about a decade ago.
In industrialized countries, with increasing life expectancy and aging of the population, the proportion of the elderly individuals is rising. Additionally, in these settings, there is growing use in immunosuppressive treatments. The elderly, immunocompromised persons and patients with complex health conditions were shown to be vulnerable to enteric diseases, including NTS causing invasive disease,9,10 Clostridium difficile-associated diarrhea11,12 and norovirus illness.13,14
Enteric pathogens with low-infectious dose that enables efficient person-to-person transmission such as Shigella and noroviruses pose major challenge, especially in crowded conditions, even in industrialized countries. For example, children in daycare centers represent a main high-risk group for shigellosis,15,16 as well as communities characterized by high household crowding conditions such as the ultraorthodox Jewish population.15,17
Travelers from industrialized countries to LMICs and endemic regions and soldiers serving in field conditions, where sanitation might be sub-optimal, comprise major risk groups for diarrheal diseases, including ETEC, Campylobacter- and Shigella-associated diarrhea.18–20
Special consideration in industrialized countries is the large-mass production, processing and distribution of food. Contaminated food with enteric pathogens can cause large, multistate and multicounty food-born outbreaks such as salmonellosis21,22 and Shiga-toxin Escherichia coli (STEC) of serotype O104:H4 in the Europe in 2011.23
Following natural disasters, flooding and in areas of conflict, typically the existing infrastructures collapse, thus providing an ideal ground for the spread of enteric infections such as cholera24,25 and typhoid fever.26
Taken together, these points argue for the need to invest in the development of enteric vaccines and therapeutics and advocate for using the already-existing enteric vaccines. The current Special Issue on enteric vaccines includes original and review articles summarizing major achievements and remaining challenges in the field of enteric vaccines development.
The Special Issue includes articles on current licensed vaccines, as well as new vaccines in development, against rotavirus, Vibrio cholerae and Salmonella Typhi. In addition, novel vaccines against NTS, ETEC, Shigella, and norovirus in development are described. Challenges to the development and utilization of global vaccines are discussed.
1. Licensed vaccines available and development of improved generations
Rotavirus vaccines
Two internationally available oral live-attenuated rotavirus vaccines were licensed around 13 years ago.7,8 Rotarix™ (GSK Biologicals, Rixensart), which is a monovalent (G1P8) human rotavirus strain, is given in a two-dose schedule.7 RotaTeq ™ (Merck & Co., Whitehouse, Pennsylvania), which is a pentavalent bovine-human reassortant vaccine, carries neutralization epitopes against the common human rotavirus genotypes (G1, G2, G3, G4 and P8).8 These vaccines showed high efficacy in preventing severe rotavirus gastroenteritis in clinical trials conducted among infants from Europe, the US and Latin America,7,8 as well as acceptable safety profile. Clinical trials conducted in Africa and Asia showed lower efficacy (up to 65%).27–29 These vaccines have been prequalified by the World Health Organization (WHO) in 2009; the WHO recommended the introduction of universal rotavirus immunization globally.30 To date, nearly 100 countries have introduced a rotavirus vaccine into their national immunization programs,31 mostly utilizing Rotarix. This was followed by extensive post-marketing evaluations assessing impact, effectiveness and safety of rotavirus vaccines in real world. A monovalent rotavirus vaccine, ROTAVAC™ (Bharat Biotech, Hyderabad, India), was recently prequalified by WHO, and will be introduced in India. In this Special Focus issue, Steele et al.32 provide a comprehensive and thorough review on the experience with rotavirus vaccines globally. The authors present the various estimates of the rotavirus-related deaths worldwide, review the progress of introducing rotavirus vaccines into national immunization programs, the substantial impact in terms of decreased burden of rotavirus gastroenteritis, and discuss in depth the challenges with the globally available rotavirus vaccines.32 Interestingly, despite cost-effectiveness assessments supporting the introduction of universal rotavirus immunization, the approval by Gavi, the Vaccine Alliance, to support the introduction of these vaccines, many countries in South Asia, where the burden of rotavirus deaths is high, has not yet enrolled rotavirus vaccination in their national immunization programs.32 This review32 underscores the low efficacy of rotavirus vaccines in developing countries and indicates the advances in research exploring potential explanations and modalities to improve the impact of these vaccines in LMICs. Possible methods that may improve the performance of rotavirus vaccines in infants living in LMICs, include changing the immunization schedule of Rotarix from 2-dose to 3-dose schedule, giving a booster at age 9 months, developing neonatal rotavirus vaccines and micro-supplementation of rotavirus vaccine with zinc and probiotics.32
Dai et al.33 performed a meta-analysis using data from three clinical trials that assessed the immunogenicity of Rotarix administered in three doses given at age 6, 10 and 14 weeks vs. two doses given at age 10 and 14 weeks. The original trials were conducted in South Africa, Malawi,34 Pakistan35 and Ghana.36 The pooled analysis showed no significant difference in the seroconversion of anti-rotavirus IgA in the two-dose schedule vs. the three-dose schedule. This study highlights the scarcity of trials that have assessed the effect of different vaccine schedules on the immunogenicity of rotavirus vaccines in LMICs.33 Carvalho and Gill in their review published in this issue37 also underscore the need to overcome the limited efficacy of rotavirus vaccines in LMICs, including the need to better understanding of mucosal immunity of rotavirus, exploring alternative vaccine delivery routes (e.g., intranasal), delivery systems and mucosal adjuvants.37
The introduction of rotavirus vaccines was followed by impressive reduction in the rotavirus disease burden,32 including reduction in diarrheal disease mortality that was seen in Mexico.38 Interestingly, Rotarix was used in the national immunization program in Mexico from 2006 to 2011 and from 2011, RotaTeq is being used. In this issue, Luna-Casas et al.39 compared the vaccine coverage of Rotarix among infants of the 2010 birth cohort and RotaTeq among infants of the 2012 birth cohort in Mexico. Completing the full vaccine series was higher for Rotarix than RotaTeq.39
Also in this issue, Lopez et al.40 nicely describe the experience of the Philippines with policy-making regarding the introduction of rotavirus vaccine into the national immunization program and provide essential evidence that could expedite this process in other countries as well. Despite the progress, enrolment of rotavirus immunization in national immunization programs remains incomplete in Europe.31,41 Heggie and colleagues describe the cost-impact of rotavirus immunization in Scotland42 following the introduction of universal rotavirus immunization in 2013. In Latvia such a program was introduced only in 2015, a decision that was strengthened by the substantial disease burden of rotavirus gastroenteritis in Latvia, as demonstrated in this issue by Tafalla et al.43
The success of rotavirus vaccines in reducing the burden of diarrheal diseases opens new era toward the prevention of diarrheal diseases caused by other enteric viruses, such as norovirus. Indeed, there are currently several norovirus vaccines in clinical testing including an oral monovalent vaccine44 and a bivalent vaccine given intramuscularly.45 Hallowell et al. in their commentary published in this issue describes the epidemiologic challenges facing the development of norovirus vaccines and suggest potential solutions,46 while focusing on vaccine effectiveness and defining target populations. We have examined the incidence of norovirus gastroenteritis hospitalization in young children before and after the introduction of universal rotavirus immunization in Israel.47 While no change in the incidence was found between the two periods, we documented the substantial burden and pointed to possible norovirus vaccine target groups.47
Cholera vaccines
Cholera remains a major public health problem, with an estimated burden of 2.86 million cases and 95,000 deaths in endemic countries in Asia and Africa.48 Over the past few years, we witnessed the rapid spread and fatal outcomes of cholera in areas of conflict such as Yemen,49 and following natural disasters, such as the earthquake in Haiti.50 In this Special Issue, Wierzba provides51 an overview of oral cholera vaccines and their impact on the global burden of disease.
There are three licensed and WHO prequalified cholera vaccines. All inactivated whole-cell vaccines given orally in multiple doses. The first vaccine was Dukoral that contains the cholera toxin B-subunit (CTB), given with buffer. The other two vaccines Shanchol and Euvichol-plus showed good effectiveness and were prequalified by the WHO in 2011 and 2015, respectively. In the past few years, cholera vaccines have been available for emergencies through a global stockpile funded by Gavi.51
In Bangladesh, cholera causes considerable burden. In this issue. Khan et al.52 assessed the feasibility of using the existing government infrastructure to provide oral cholera vaccine to residents in rural communities in Bangladesh. They demonstrated high vaccine coverage for the first and second vaccine doses of 90% and 83%, respectively.52
Interestingly, an oral live-attenuated vaccine CVD 103-HgR (VAXCHORA™) given in a single dose was licensed by the US FDA for US travelers to areas of ongoing cholera transmission. The vaccine was well-tolerated and provided high efficacy (90%) which was evident 10 days post-vaccination in a human challenge study.53
Salmonella Typhi vaccines
The etiological agent of typhoid fever, Salmonella enterica serovar Typhi (S. Typhi), is a human-restricted pathogen transmitted primarily via contaminated food and water.
Salmonella Typhi constitutes a major global health threat with an estimated 20.6 million cases of typhoid fever annually resulting in approximately 223,000 deaths worldwide.54
Several licensed vaccines are currently used to prevent typhoid fever, three injectable (one dose) subunit Vi polysaccharide-based vaccines Typhim Vi® (Sanofi), Typherix® (GSK), and Typbar® (Bharat Biotech) and one WHO prequalified single-dose Vi-conjugate vaccine (Typbar®-TCV (Bharat Biotech) and the live-attenuated Ty21a strain of S. Typhi, commercialized as Vivotif (Emergent Biosolutions).
In this Special Issue, Griffin et al.55 described the manufacture, release, characterization, and preclinical studies evaluating the safety and immunogenicity of Typhax, an investigational Vi antigen-based typhoid fever vaccine synthesized using Protein Capsular Matrix Vaccine (PCMV) technology as an alternative construct to the new Vi conjugate vaccine.56 In Typhax, Vi polysaccharide antigen purified from S. Typhi is entrapped in a glutaraldehyde-catalyzed matrix of cross-linked α-poly-L-lysine (α-PLL) and CRM197 protein. Data of a series of immunogenicity studies in mice, rabbits, and non-human primates show a robust immunogenicity of this subunit vaccine platform presented. In the non-human primate study, 100% seroconversion was attained at both 2.5 µg and 10 µg dose levels after the first injection.55 A phase-I clinical trial of Typhax is planned in view of these findings and previous successful experience with this the PCMV.57
2. Enteric vaccines in clinical development and supportive experimental and observational studies
Shigella vaccines
Shigellosis is common worldwide and hyperendemic in LMICs, with approximately 250 million cases annually.58 About 13% of all diarrheal diseases deaths are attributed to Shigella, which translates to 212,438 deaths in all age groups globally.58 Around 2 million shigellosis cases occur annually in industrialized countries.58,59 Shigellosis is associated with impaired linear growth and malnutrition60 and there is an increasing spread of antibiotic-resistant Shigella. Progress in Shigella vaccine development has been challenging for various reasons, such as the lack of suitable animal models, a limited understanding of the mechanism governing protection and the prominent capability of Shigella to subvert the host innate and adaptive immune response.
Shigella pathogenicity is strongly associated with the operation of a Type III secretion system (T3SS) which injects the virulence molecules into the host cell leading to promotion of intracellular bacterial survival and dissemination side by side with the subversion of host innate and adaptive immune defenses. Brunner et al.61 reviewed the capabilities of Shigella to adapt to the intestinal environment of its restricted human host and to impair the host defensive mechanisms. This may explain the relatively short-lived immunity conferred by natural Shigella infection and the need of subsequent protection against homologous serotypes.61
In particular, the authors emphasize the new paradigm of Shigella pathogenicity by means of invasion-independent T3SS effector-mediated targeting of activated human lymphocytes. This results in either effector interaction with cell-surface receptors or effector delivery into the host cells not followed by cell invasion.
It is hypothesized that the live-attenuated Shigella vaccine strains with altered metabolic functions or impaired cell to cell propagation in the colon epithelium maintain their subversion capabilities of the innate and adaptive B- and T-cell response immune response. Part of these vaccine candidates showed satisfactory mucosal and systemic immunogenicity in phase I–II studies, and significant protection against dysentery in a challenge study conducted among North American volunteers62 but they did not replicate and were poorly immunogenic in adults and children in Bangladesh, a highly endemic country.63 Namely, these trials assessed the SC602 Shigella flexneri 2a attenuated oral vaccine candidate. This strain had deletions in plasmid-borne virulence gene icsA that mediates intra- and intercellular spread, and the chromosomal locus iuc, encoding aerobactin. It is assumed that this is the result of a negative synergistic effect of Shigella immuno-suppressive effectors not knocked down in such vaccine candidates, the host intestinal barrier involving gut enteropathy, microbiome composition and other host factors.
The results of a phase-I study with the WRSS1, a Shigella sonnei live oral vaccine attenuated by a 212-bp deletion in the icsA plasmid virulence gene and carried out in adults and children in Bangladesh, are also reported in this special issue.64 At the highest dose of the vaccine 3 × 106 colony forming units (ingested three times on days 0, 28, and 56) shedding of the vaccine was evident in 50% of the adult vaccine recipients, but no shedding was seen in children. At this highest dose, 100% of adults and 40% of children responded with a ≥ 4-fold increase of S. sonnei LPS-specific IgA antibody in lymphocyte supernatant (ALS). At the same dose, 63% of adults and 70% of children seroconverted with IgA to LPS, while in the placebo groups, 33% of adults and 18% of children seroconverted. Interestingly, a similar immune response to that observed in adults in this study was seen in Israeli adult volunteers albeit following just one oral dose of 2-log lower colony forming units. In view of the lower rate and short time of vaccine shedding in Bangladeshi adults and absence of shedding in children, the authors suggest that in an endemic population, with prior exposure to Shigella, robust colonization of oral live vaccines may not occur or necessarily influence the elicitation of immune responses. They also propose employing the ALS assay, which would circumvent the difficulty in detecting significant rises in serum antibody titers due to high pre-vaccination levels.
Besides orally administered, live, rationally attenuated vaccine strains, a different approach relies on the development of parenterally delivered subunit vaccines. This strategy is based on the notion that specific bacterial surface polysaccharides such as the O-antigen membrane lipopolysaccharide (LPS) are the primary targets of the antibody response associated with serotype-specific protection.
Seroepidemiological studies conducted in Israel among soldiers serving under field conditions and highly exposed to Shigella showed that pre-existent serum IgG antibodies to S. sonnei or S. flexneri 2a LPS were strongly associated with protection against homologous but not heterologous Shigella infections.65,66 In view of these findings, other observations and the successful experience with the development of the pioneering H. influenza type b conjugate vaccine, injectable glycoconjugates incorporating detoxified LPS from S. flexneri 2a or S. sonnei and S. dysenteriae type 1 (Shiga), linked to carrier proteins, were developed at the National Institutes of Health by John Robbins and Rachel Schneerson.67,68 These vaccines induced high levels of serum IgG anti-homologous LPS in phase-1 and -2 studies in healthy volunteers.69,70 Protective efficacy of a S. sonnei conjugate against S. sonnei shigellosis was demonstrated in young adults (74%)71 and children older than 3 years of age (71%)72 in field trials. However, the S. sonnei conjugate was not immunogenic enough and failed to protect against homologous shigellosis in children under 3 years of age.72
Based on these promising results, several Shigella vaccine candidates are now under development with the goal of attaining good immunogenicity and protection in very young children and particularly in those living in LMICs where shigellosis is highly endemic and one of the leading causes of diarrheal diseases deaths.
In their review, Barel and Mulard73 summarize the different concepts and strategies toward a carbohydrate-based conjugate vaccine against Shigella. They encompass the knowledge accumulated on first generation of detoxified LPS based Shigella glycoconjugates and lessons learnt from the performance of these conjugates in preclinical and clinical studies. The comprehensive review also covers the development of the second generation of Shigella conjugates including the bioconjugates and synthetic oligosaccharide protein conjugates.73
ETEC vaccines
Levine et al.74 provide an extensive review on the contribution of volunteer challenge studies to the development of ETEC vaccine candidates in a historical detailed perspective. Although focused on the case of ETEC vaccines, this article constitutes a paradigm of how the human experimental approach, currently named Controlled Human Infection Models, can support the development of other enteric vaccines.
Pioneering volunteer challenge studies conducted at the Center for Vaccine Development at University of Maryland (CVD) established that an initial clinical ETEC infection with an LT/ST strain confers ~79% protection against the occurrence of diarrhea upon subsequent exposure to the homologous ETEC strain. Studies conducted thereafter at CVD, University of Texas Medical School and Johns Hopkins University School of Public Health elucidated key aspects and virulence factors in the pathogenesis of ETEC, the relative importance of the heat-labile enterotoxin (LT) and heat-stable enterotoxin (ST), and fimbriae/colonization factors as protective antigens, inactivation or attenuation strategies of ETEC vaccine prototypes and delivery routes. The volunteer challenge studies provided the means by which a wide range of immunological parameters at the mucosal site and in peripheral blood could be measured in relation to the protective efficacy of the candidate vaccines supporting the identification of potential correlates of protection.74
The Global Enteric Multicenter Study (GEMS)75 and the multisite birth cohort study (MAL-ED)76 found that ETEC strains producing ST, with or without LT, were strongly associated with diarrhea among children under 5 years of age in LMICs, while LT-only ETEC strains contribute only marginally to diarrhea burden in these pediatric populations. Research efforts are underway to develop standing alone ETEC ST toxoid vaccines and to explore further ways to include them in vaccine formulations to achieve a broad protection against ETEC.
In their article, Zegeye and colleagues77 mentioned the barriers that stand in the way of developing ETEC ST-toxoid vaccines, the poor immunogenicity of ST, its toxicity and the safety concerns related to potential for immunological cross-reactivity with the human gastrointestinal peptides (guanylin and uroguanylin). The authors review the recent efforts to overcome these obstacles focusing on two strategies for making ST immunogenic by coupling it to a protein carrier (either by ST-protein carrier fusion or ST-carrier protein bioconjugation), and on abolishing toxicity and reducing the risk for unwanted immunological cross-reaction by mutation.77 To induce a good mucosal immune response in main target populations in LMICs, the authors suggest co-administration of the ST-toxoid with the dmLT adjuvant, which has the ability to redirect parenterally administered vaccines to an immune response in the gut as shown in a previous study.78 Zegeye and colleagues also emphasize the urgent need to develop a human challenge model for ETEC that only express STh toward the clinical development stages of this vaccine prototype.77
Campylobacter vaccines
Campylobacter is a major cause of diarrheal diseases globally. Campylobacter is a significant diarrheal pathogen in children under 5 years of age in LMICs.75,79 It is also a leading cause of foodborne enteric diseases in industrialized countries, sometimes more common than NTS80,81 and ranked second after ETEC, as traveler’s diarrhea etiologic agent.82,83 There is growing evidence on the involvement of Campylobacter jejuni in chronic morbidity of neurological, hematological, and rheumatological systems.84 The antimicrobial resistance of C. jejuni is emerging having usually multiresistance to antibiotics acquired from animal reservoir level (e.g., poultry).
On the background of the global burden of campylobacteriosis, Poly and coauthors85 review the advances in the research of the pathogenesis and virulence mechanisms of Campylobacter as basis for vaccine development. They list the reasons of the process hindering including the lack of small animal models of disease, presence of lipooligosaccharide ganglioside mimics that can induce autoimmune diseases such as Gillian Bare Syndrome, the incomplete understanding of C. jejuni virulence factors, and the lack of known immunological correlates of protection. The authors review the main vaccine candidates developed and tested in phase-I studies in the last two decades with mostly disappointing results, and point out the promising profile of a parenteral capsular polysaccharide conjugate to be tested shortly in a CHIM study.85
3. Correlates of protection
The identification of correlates of protection or immunological parameters that can predict a reduced risk of homologous disease are of paramount importance in the development, licensure, and monitoring of vaccine effectiveness.
Adapting previous definitions for correlates of protection Cohen et al.86 listed a set of conditions that a correlate of protection against shigellosis should fulfill. A necessary, but insufficient condition, is that natural Shigella infection triggers an increase in the level of such immunological marker. Another necessary condition is that this immunological marker is associated with protection against shigellosis caused by the homologous Shigella serotype, under natural conditions of exposure and by a Shigella candidate vaccine, either in field efficacy trials or in human challenge studies, while demonstrating functional capabilities.
The authors bring evidence accumulated over the last four decades, which indicates that serum IgG antibody level to Shigella LPS fulfills all these conditions and can be defined as an immunological correlate of protection against shigellosis. Notably, there is a clear correlation between levels of ELISA-detected IgG to Shigella LPS and bactericidal activity pointing out that high levels of serum IgG can predict functionabilty of these antibodies.86
In another article of this theme in the Special Issue, Booth et al.87 used immunization with the oral live-attenuated Ty21a vaccine as a model for a protective immune response against typhoid involving CD4+ and CD8+T subsets against S. Typhi, an invasive organism causing systemic infection. CD4+ and CD8+T subsets are essential components of the adaptive immune system, which act in concert. The authors examined vaccines and non-vaccines undergoing routine colonoscopy. They found important differences in responses between the mucosal and systemic CD4+ and CD8+T subsets following oral Ty21a immunization with good correlation of S. Typhi-specific memory CD4+ and CD8+T responses in the human terminal ileum mucosa, the favored invasion site of S. Typhi, but not in the peripheral blood.87 It is believed that these findings will contribute to better understanding of the immune effector and memory responses responsible for protection against typhoid, which might influence future vaccine design and development.
4. Vaccines in preclinical development
The Global Burden Disease study estimated that 87,000 of diarrheal diseases deaths are caused by NTS.1 NTS causes invasive disease and bacteremia in the elderly and in immunocompromised patients,9,10,88 and hence the burden attributed to NTS is even higher. Over the past few decades, the importance of NTS as a leading cause of invasive blood stream disease has been recognized, especially in sub-Saharan Africa, where the prevalence of HIV infection and malnutrition is high.89 In the current issue, Balasubramanian et al.90 presented the global burden and epidemiology of invasive NTS infections. Balasubramanian et al. provided a comprehensive review of the estimated burden of invasive NTS disease in sub-Saharan Africa, and main risk groups. They also presented gaps in knowledge leading to under-recognition of the true burden of invasive NTS disease in sub-Saharan Africa, as well as in Asia and Latin America. The most prevalent S. enterica serovars, Typhimurium, Enteritidis and Dublin as causes of invasive NTS in sub-Saharan Africa are being targeted in vaccine development. Balasubramanian et al. also provided insights regarding antibiotic resistance of NTS.90
Vaccines against S. Typhimurium and S. Enteritidis that cause the majority of invasive NTS disease are under development, including live-attenuated and conjugate vaccines as well as vaccines employing the Generalized Modules for Membrane Antigens (GMMA) technology, which presents surface polysaccharides and outer membrane proteins in their native conformation, reviewed in.91–93 Little attention was given to the development of a human vaccine for sero-group C Salmonella, despite being a leading cause of salmonellosis, for example, in the US.94 In this issue, two articles described preclinical development of two vaccines to protect against Salmonella C2–C3 infection using different strategies: a live-attenuated vaccine strain of S. Newport95 and a glycoconjugate of S. Newport Core-O polysaccharide (COPS).96 Fuche et al.95 showed that the S. Newport-based live-attenuated vaccine candidate CVD 1966 was achieved by deletions of the genes guaBA and htrA. This vaccine elicited strong antibody responses against several antigens when administered intraperitoneally or orally to BALB/c mice and showed 50% protection following lethal challenge with the parental virulent strain of S. Newport.95
Schuster et al.96 used the approach of sub-unit vaccines. They described the development of a candidate serogroup C2–C3 glycoconjugate vaccine based on COPS and phase-1 flagellin (FliC). S. Newport COPS and FliC were purified from genetically engineered reagent strains, and conjugated at the polysaccharide-reducing end to FliC protein. The new vaccine candidate was immunogenic in mice and conferred protection against lethal challenge with wild-type S. Newport strain.96 Collectively these articles described substantial progress toward the development of NTS vaccines to be further tested in humans.
5. Other interventions against enteric diseases
Cryptosporidium, a highly infectious protozoan that causes diarrhea in immunocompetent and immunocompromised subjects,97,98 was shown to be a leading cause of childhood diarrhea in LMICs.75,79 It was estimated that 57,000 deaths of diarrheal diseases deaths are caused by Cryptosporidium.1 Cryptosporidium hominis was detected in 77.8% and C. parvum in 9.9% among patients with moderate-to-severe diarrhea who tested positive for Cryptosporidium in the GEMS.99 Currently there is no licensed vaccine against Cryptosporidium. Nitazoxanide is an FDA-approved drug for treating Cryptosporidium disease in persons above 1 year of age; however, its efficacy is limited in young children and immunocompromised persons (reviewed in97), and therefore development of Cryptosporidium vaccines and treatments should be highly prioritized. In their comprehensive review presented in this issue, Lee et al.100 described their successful experience in using the gnotobiotic piglet model to study Cryptosporidium, which is important since the piglet is susceptible to C. hominis and C. parvum. Their review focused on the usefulness of the gnotobiotic piglet model of acute diarrhea for exploring the human C. hominis species to assess the efficacy of therapeutic candidates.100 The TU502 C. hominis strain, originally isolated from an infant with diarrhea in Uganda, and the gnotobiotic piglet model currently are the only available preclinical tools to evaluate therapeutics targeting C. hominis. This unique model can be very helpful in drug discovery and testing vaccine candidates against cryptosporidiosis.100 Interestingly, the piglet model was also used to study acute and chronic C. difficile illness101 and the impact of systemic administration of C. difficile IgG anti-toxin antibodies during infection with C. difficile.102 C. difficile infection is a major health-care-associated challenge, usually causing diarrhea following antibiotic use, especially among the elderly and patients with multi-morbidity and complex health conditions.103 In their review published in this issue, Eliakim-Raz and Bishara104 reviewed the latest advances in treating and preventing C. difficile infection. Currently, there is no licensed vaccine against C. difficile. Treatment of C. difficile with metronidazole and vancomycin may affect the intestinal microbiota and recurrence of the infection. New treatments focus on limiting the destruction of the intestinal microbiota or restoring the microbiota to its pre-destructed state, including new drugs, fecal microbiota transplantation, competitive inhibition with non-toxigenic strains of C. difficile, and monoclonal antibodies against C. difficile toxins.104
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Collaborators GBDDD Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Infectious Diseases. 2018;18(11):1211–28. doi: 10.1016/S1473-3099(18)30362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNICEF/WHO Diarrhoea: why children are still dying and what can be done;2009.
- 3.World Health Organization (WHO) UNCsFU Progress on drinking water, sanitation and hygiene: 2017 update and SDG baselines. Geneva, Switzerland: World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF), 2017. Licence: CC BY-NC-SA 3.0 IGO; 2017. [Google Scholar]
- 4.Fischer Walker CLF, Friberg IK, Binkin N, Young M, Walker N, Fontaine O, Weissman E, Gupta A, Black RE, Osrin D.. Scaling up diarrhea prevention and treatment interventions: a lives saved tool analysis. PLoS Medicine. 2011;8(3):e1000428. doi: 10.1371/journal.pmed.1000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9:565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malek MA, Curns AT, Holman RC, Fischer TK, Bresee JS, Glass RI, Steiner CA, Parashar UD. Diarrhea- and rotavirus-associated hospitalizations among children less than 5 years of age: United States, 1997 and 2000. Pediatrics. 2006;117(6):1887–92. doi: 10.1542/peds.2005-2351. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354(1):11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 8.Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354(1):23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 9.Zaidenstein R, Peretz C, Nissan I, Reisfeld A, Yaron S, Agmon V, Weinberger M. The epidemiology of extraintestinal non-typhoid Salmonella in Israel: the effects of patients‘ age and sex. Eur J Clin Microbiol. 2010;29(9):1103–09. doi: 10.1007/s10096-010-0968-1. [DOI] [PubMed] [Google Scholar]
- 10.Hohmann EL. Nontyphoidal salmonellosis. Clinical Infectious Diseases: an Official Publication of the Infectious Diseases Society of America. 2001;32(2):263–69. doi: 10.1086/318457. [DOI] [PubMed] [Google Scholar]
- 11.Na‘Amnih W, Adler A, Miller-Roll T, Cohen D, Carmeli Y. Incidence and risk factors for community and hospital acquisition of Clostridium difficile infection in the Tel Aviv sourasky medical center. Infection Control and Hospital Epidemiology. 2017;38(8):912–20. doi: 10.1017/ice.2017.82. [DOI] [PubMed] [Google Scholar]
- 12.Hunter JC, Mu Y, Dumyati GK, Farley MM, Winston LG, Johnston HL, Meek JI, Perlmutter R, Holzbauer SM, Beldavs ZG, et al. Burden of nursing home-onset Clostridium difficile infection in the United States: estimates of incidence and patient outcomes. Open Forum Infectious Diseases. 2016;3(1):ofv196. doi: 10.1093/ofid/ofv196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris JP, Edmunds WJ, Pebody R, Brown DW, Lopman BA. Deaths from norovirus among the elderly, England and Wales. Emerging Infectious Diseases. 2008;14(10):1546–52. doi: 10.3201/eid1410.080188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopman BA, Reacher MH, Vipond IB, Sarangi J, Brown DW. Clinical manifestation of norovirus gastroenteritis in health care settings. Clinical Infectious Diseases: an Official Publication of the Infectious Diseases Society of America. 2004;39(3):318–24. doi: 10.1086/421948. [DOI] [PubMed] [Google Scholar]
- 15.Garrett V, Bornschlegel K, Lange D, Reddy V, Kornstein L, Kornblum J, Agasan A, Hoekstra M, Layton M, Sobel J. A recurring outbreak of Shigella sonnei among traditionally observant Jewish children in New York City: the risks of daycare and household transmission. Epidemiol Infect. 2006;134(6):1231–36. doi: 10.1017/S0950268806006182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohle-Boetani JC, Stapleton M, Finger R, Bean NH, Poundstone J, Blake PA, Griffin PM. Communitywide shigellosis: control of an outbreak and risk factors in child day-care centers. American Journal of Public Health. 1995;85:812–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen D, Bassal R, Goren S, Rouach T, Taran D, Schemberg B, Peled N, Keness Y, Ken-Dror S, Vasilev V, et al. Recent trends in the epidemiology of shigellosis in Israel. Epidemiology and Infection. 2014;142(12):2583–94. doi: 10.1017/S0950268814000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porter CK, Olson S, Hall A, Riddle MS. Travelers‘ diarrhea: an update on the incidence, etiology, and risk in military deployments and similar travel populations. Military Medicine. 2017;182(S2):4–10. doi: 10.7205/MILMED-D-17-00064. [DOI] [PubMed] [Google Scholar]
- 19.Cohen D, Sela T, Slepon R, Yavzori M, Ambar R, Orr N, Robin G, Shpielberg O, Eldad A, Green M. Prospective cohort studies of shigellosis during military field training. European Journal of Clinical Microbiology & Infectious Diseases: Official Publication of the European Society of Clinical Microbiology. 2001;20(2):123–26. doi: 10.1007/s100960000428. [DOI] [PubMed] [Google Scholar]
- 20.Zboromyrska Y, Hurtado JC, Salvador P, Alvarez-Martinez MJ, Valls ME, Mas J, Marcos MA, Gascón J, Vila J. Aetiology of traveller‘s diarrhoea: evaluation of a multiplex PCR tool to detect different enteropathogens. Clinical Microbiology and Infection: the Official Publication of the European Society of Clinical Microbiology and Infectious Diseases. 2014;20(10):O753–759. doi: 10.1111/1469-0691.12621. [DOI] [PubMed] [Google Scholar]
- 21.Cavallaro E, Date K, Medus C, Meyer S, Miller B, Kim C, Nowicki S, Cosgrove S, Sweat D, Phan Q, et al. Salmonella typhimurium infections associated with peanut products. N Engl J Med. 2011;365(7):601–10. doi: 10.1056/NEJMoa1011208. [DOI] [PubMed] [Google Scholar]
- 22.Hormansdorfer S, Messelhausser U, Rampp A, Schonberger K, Dallman T, Allerberger F, Kornschober C, Sing A, Wallner P, Zapf A. Re-evaluation of a 2014 multi-country European outbreak of Salmonella Enteritidis phage type 14b using recent epidemiological and molecular data. Eurosurveillance. 2017;22(50):13–19. doi: 10.2807/1560-7917.ES.2017.22.50.17-00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchholz U, Bernard H, Werber D, Bohmer MM, Remschmidt C, Wilking H, Deleré Y, An der Heiden M, Adlhoch C, Dreesman J, et al. German outbreak of Escherichia coli O104: h4associated with sprouts. N Engl J Med. 2011;365(19):1763–70. doi: 10.1056/NEJMoa1106482. [DOI] [PubMed] [Google Scholar]
- 24.Raad II, Chaftari AM, Dib RW, Graviss EA, Hachem R. Emerging outbreaks associated with conflict and failing healthcare systems in the Middle East. Infection Control and Hospital Epidemiology. 2018;39(10):1230–36. doi: 10.1017/ice.2018.177. [DOI] [PubMed] [Google Scholar]
- 25.Rieckmann A, Tamason CC, Gurley ES, Rod NH, Jensen PKM. Exploring droughts and floods and their association with cholera outbreaks in sub-saharan africa: a register-based ecological study from 1990 to 2010. Am J Trop Med Hyg. 2018;98(5):1269–74. doi: 10.4269/ajtmh.17-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu ZD, Lao JH, Zhang Y, Liu YY, Zhang J, Wang H, Jiang B. Association between floods and typhoid fever in Yongzhou, China: effects and vulnerable groups. Environ Res. 2018;167:718–24. doi: 10.1016/j.envres.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 27.Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, Binka FN, Steele AD, Laserson KF, Ansah NA, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):606–14. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 28.Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, Ngwira B, Victor JC, Gillard PH, Cheuvart BB, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362(4):289–98. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 29.Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, Podder G, Vu DT, Le TPM, Luby SP, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):615–23. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 30.Rotavirus vaccines:an update. Wkly Epidemiol Rec. 2009. Dec 18;84(50):533–40. [PubMed] [Google Scholar]
- 31.World Health Organization I Vaccines and Biologicals. Vaccine in National Immunization Programme Update; 2019. [Google Scholar]
- 32.Steele AD, Victor JC, Carey ME, Tate JE, Atherly DE, Pecenka C, Diaz Z, Parashar UD, Kirkwood CD. Experiences with rotavirus vaccines: can we improve rotavirus vaccine impact in developing countries? Human Vaccines & Immunotherapeutics. 2019;1215–1227. doi: 10.1080/21645515.2018.1553593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai X, Bai R, Jian M, Ji Z, Ding Z, Wang F, Bi Y, Manzamaesso A, Chen T, Luo L, et al. Immunogenicity of different dosing schedules of the human live attenuate rotavirus vaccine (RV1) in infants and children: a meta-analysis. Human Vaccines & Immunotherapeutics. 2018;1228–1236. doi: 10.1080/21645515.2018.1537742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madhi SA, Kirsten M, Louw C, Bos P, Aspinall S, Bouckenooghe A, Neuzil KM, Steele AD. Efficacy and immunogenicity of two or three dose rotavirus-vaccine regimen in South African children over two consecutive rotavirus-seasons: a randomized, double-blind, placebo-controlled trial. Vaccine. 2012;30 Suppl 1(Suppl 1):A44–51. doi: 10.1016/j.vaccine.2011.08.080. [DOI] [PubMed] [Google Scholar]
- 35.Ali SA, Kazi AM, Cortese MM, Fleming JA, Parashar UD, Jiang B, McNeal MM, Steele D, Bhutta Z, Zaidi A. Impact of different dosing schedules on the immunogenicity of the human rotavirus vaccine in infants in Pakistan: a randomized trial. The Journal of Infectious Diseases. 2014;210(11):1772–79. doi: 10.1093/infdis/jiu335. [DOI] [PubMed] [Google Scholar]
- 36.Armah G, Lewis KD, Cortese MM, Parashar UD, Ansah A, Gazley L, Victor JC, McNeal MM, Binka F, Steele AD. A randomized, controlled trial of the impact of alternative dosing schedules on the immune response to human rotavirus vaccine in Rural Ghanaian infants. The Journal of Infectious Diseases. 2016;213(11):1678–85. doi: 10.1093/infdis/jiw023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carvalho MF, Gill D. Rotavirus vaccine efficacy: current status and areas for improvement. Human Vaccines & Immunotherapeutics. 2018;1237–1250. doi: 10.1080/21645515.2018.1520583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richardson V, Hernandez-Pichardo J, Quintanar-Solares M, Esparza-Aguilar M, Johnson B, Gomez-Altamirano CM, Parashar U, Patel M. Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. N Engl J Med. 2010;362(4):299–305. doi: 10.1056/NEJMoa0905211. [DOI] [PubMed] [Google Scholar]
- 39.Luna-Casas G, Juliao P, Carreño-Manjarrez R, Castañeda-Prado A, Cervantes-Apolinar MY, Navarro-Rodriguez R, Sánchez-González G, Cortés-Alcalá R, DeAntonio R. Vaccine coverage and compliance in Mexico with the two-dose and three-dose rotavirus vaccines. Human Vaccines & Immunotherapeutics. 2019;1251–1259. doi: 10.1080/21645515.2018.1540827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez AL, Raguindin PF, Silva MWT. Prospects for rotavirus vaccine introduction in the Philippines: bridging the available evidence into immunization policy. Human Vaccines & Immunotherapeutics. 2019;1260–1264. doi: 10.1080/21645515.2018.1551673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parez N, Giaquinto C, Du Roure C, Martinon-Torres F, Spoulou V, Van Damme P, Vesikari T. Rotavirus vaccination in Europe: drivers and barriers. The Lancet Infectious Diseases. 2014;14(5):416–25. doi: 10.1016/S1473-3099(14)70035-0. [DOI] [PubMed] [Google Scholar]
- 42.Heggie R, Murdoch H, Cameron C, Smith-Palmer A, McIntosh E, Bouttell J. Cost-impact study of rotavirus vaccination programme in Scotland. Human Vaccines & Immunotherapeutics. 2019;1265–1271. doi: 10.1080/21645515.2018.1543522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tafalla M, Gardovska D, Gopala K, Kozlovska L. Primary care-based surveillance to estimate the proportion of rotavirus gastroenteritis among Latvian children below 5 years of age with acute gastroenteritis. Human Vaccines & Immunotherapeutics. 2019;1272–1278. doi: 10.1080/21645515.2018.1534515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim L, Liebowitz D, Lin K, Kasparek K, Pasetti MF, Garg SJ, Gottlieb K, TragerG, Tucker SN. Safety and immunogenicity of an oral tablet norovirus vaccine, a phase I randomized, placebo-controlled trial. JCI Insight. 2018;3(13). doi: 10.1172/jci.insight.97941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bernstein DI, Atmar RL, Lyon GM, Treanor JJ, Chen WH, Jiang X, Vinjé J, Gregoricus N, Frenck RW, Moe CL, et al. Norovirus vaccine against experimental human GII.4 virus illness: a challenge study in healthy adults. The Journal of Infectious Diseases. 2015;211(6):870–78. doi: 10.1093/infdis/jiu497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hallowell BD, Parashar UD, Hall AJ. Epidemiologic challenges in norovirus vaccine development. Human Vaccines & Immunotherapeutics. 2018;1279–1283. doi: 10.1080/21645515.2018.1553594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muhsen K, Kassem E, Rubenstein U, Goren S, Ephros M, Shulman LM, Cohen D. No evidence of increase in the incidence of norovirus gastroenteritis hospitalizations in young children after the introduction of universal rotavirus immunization in Israel. Human Vaccines & Immunotherapeutics. 2019;1284–1293. doi: 10.1080/21645515.2019.1599522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ali M, Nelson AR, Lopez AL, Sack DA, Remais JV. Updated global burden of cholera in endemic countries. PLoS Neglected Tropical Diseases. 2015;9(6):e0003832. doi: 10.1371/journal.pntd.0003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kennedy J, Harmer A, McCoy D. The political determinants of the cholera outbreak in Yemen. The Lancet Global Health. 2017;5(10):e970–e971. doi: 10.1016/S2214-109X(17)30332-7. [DOI] [PubMed] [Google Scholar]
- 50.Barzilay EJ, Schaad N, Magloire R, Mung KS, Boncy J, Dahourou GA, Mintz ED, Steenland MW, Vertefeuille JF, Tappero JW. Cholera surveillance during the Haiti epidemic–the first 2 years. N Engl J Med. 2013;368(7):599–609. doi: 10.1056/NEJMoa1204927. [DOI] [PubMed] [Google Scholar]
- 51.Wierzba TF. Oral cholera vaccines and their impact on the global burden of disease. Human Vaccines & Immunotherapeutics. 2019;1294–1301. doi: 10.1080/21645515.2018.1504155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khan AI, Khan IA, Siddique SA, Rahman A, Islam MT, Bhuiya MAI, Saha NC, Biswas PK, Saha A, Chowdhury F, et al. Feasibility, coverage and cost of oral cholera vaccination conducted by icddr,b using the existing national immunization service delivery mechanism in rural setting Keraniganj, Bangladesh. Human Vaccines & Immunotherapeutics. 2019;1302–1309. doi: 10.1080/21645515.2018.1528833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levine MM, Chen WH, Kaper JB, Lock M, Danzig L, Gurwith M. PaxVax CVD 103-HgR single-dose live oral cholera vaccine. Expert Review of Vaccines. 2017;16(3):197–213. doi: 10.1080/14760584.2017.1291348. [DOI] [PubMed] [Google Scholar]
- 54.Crump JA, Mintz ED. Global trends in typhoid and paratyphoid Fever. Clinical Infectious Diseases. 2010;50(2):241–46. doi: 10.1086/649541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Griffin TJ,AT IV, Cartee RT, Mekalanos JJ. In vitro characterization and preclinical immunogenicity of typhax, a typhoid fever protein capsular matrix vaccine candidate. Human Vaccines & Immunotherapeutics. 2019;1310–1316. doi: 10.1080/21645515.2019.1599674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jin C, Gibani MM, Moore M, Juel HB, Jones E, Meiring J, Harris V, Gardner J, Nebykova A, Kerridge SA, et al. Efficacy and immunogenicity of a Vi-tetanus toxoid conjugate vaccine in the prevention of typhoid fever using a controlled human infection model of Salmonella Typhi: a randomised controlled, phase 2b trial. Lancet. 2017;390(10111):2472–80. doi: 10.1016/S0140-6736(17)32149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thanawastien A, Cartee RT, Griffin TJ, Killeen KP, Mekalanos JJ. Conjugate-like immunogens produced as protein capsular matrix vaccines. P Natl Acad Sci USA. 2015;112(10):E1143–E1151. doi: 10.1073/pnas.1425005112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khalil IA, Troeger C, Blacker BF, Rao PC, Brown A, Atherly DE, Brewer TG, Engmann CM, Houpt ER, Kang G, et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: the Global Burden of Disease Study 1990-2016. The Lancet. Infectious Diseases. 2018;18(11):1229–40. doi: 10.1016/S1473-3099(18)30475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pires SM, Fischer-Walker CL, Lanata CF, Devleesschauwer B, Hall AJ, Kirk MD, Duarte ASR, Black RE, Angulo FJ, Selvey LA. Aetiology-specific estimates of the global and regional incidence and mortality of diarrhoeal diseases commonly transmitted through food. PloS One. 2015;10(12):e0142927. doi: 10.1371/journal.pone.0142927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rogawski ET, Liu J, Platts-Mills JA, Kabir F, Lertsethtakarn P, Siguas M, Khan SS, Praharaj I, Murei A, Nshama R, et al. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED cohort study. The Lancet. Global Health. 2018;6(12):E1319–E1328. doi: 10.1016/S2214-109X(18)30351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brunner K, Samassa F, Sansonetti PJ, Phalipon A. Shigella-mediated immunosuppression in the human gut: subversion extends from innate to adaptive immune responses. Human Vaccines & Immunotherapeutics. 2019;1317–1325. doi: 10.1080/21645515.2019.1594132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coster TS, Hoge CW, VanDeVerg LL, Hartman AB, Oaks EV, Venkatesan MM, Cohen D, Robin G, Fontaine-Thompson A, Sansonetti PJ, et al. Vaccination against shigellosis with attenuated Shigella flexneri 2a strain SC602. Infection and Immunity. 1999;67:3437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rahman KM, El Arifeen S, Zaman K, Rahman M, Raqib R, Yunus M, Begum N, Islam MS, Sohel BM, Rahman M, et al. Safety, dose, immunogenicity, and transmissibility of an oral live attenuated Shigella flexneri 2a vaccine candidate (SC602) among healthy adults and school children in Matlab, Bangladesh. Vaccine. 2011;29(6):1347–54. doi: 10.1016/j.vaccine.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 64.Raqib R, Sarker P, Zaman K, Alam NH, Wierzba TF, Maier N, Talukder K, Baqui AH, Suvarnapunya AE, Qadri F, et al. A phase I trial of WRSS1, a Shigella sonnei live oral vaccine in Bangladeshi adults and children. Human Vaccines & Immunotherapeutics. 2019;1326–1337. doi: 10.1080/21645515.2019.1575165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cohen D, Green MS, Block C, Rouach T, Ofek I. Serum antibodies to lipopolysaccharide and natural immunity to shigellosis in an israeli military population. The Journal of Infectious Diseases. 1988;157:1068–71. [DOI] [PubMed] [Google Scholar]
- 66.Cohen D, Green MS, Block C, Slepon R, Ofek I. Prospective-study of the association between serum antibodies to lipopolysaccharide O-antigen and the attack rate of shigellosis. J Clin Microbiol. 1991;29:386–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robbins JB, Chu CY, Schneerson R. Hypothesis for Vaccine Development - Protective Immunity to Enteric Diseases Caused by Nontyphoidal Salmonellae and Shigellae May Be Conferred by Serum Igg Antibodies to the O-Specific Polysaccharide of Their Lipopolysaccharides. Clinical Infectious Diseases. 1992;15:346–61. [DOI] [PubMed] [Google Scholar]
- 68.Chu CY, Liu BK, Watson D, Szu SS, Bryla D, Shiloach J, Schneerson R, Robbins JB. Preparation, characterization, and immunogenicity of conjugates composed of the O-specific polysaccharide of Shigella dysenteriae type 1 (Shiga‘s bacillus) bound to tetanus toxoid. Infection and Immunity. 1991;59:4450–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taylor DN, Trofa AC, Sadoff J, Chu C, Bryla D, Shiloach J, Cohen D, Ashkenazi S, Lerman Y, Egan W. Synthesis, characterization, and clinical evaluation of conjugate vaccines composed of the O-specific polysaccharides of Shigella dysenteriae type 1, Shigella flexneri type 2a, and Shigella sonnei (Plesiomonas shigelloides) bound to bacterial toxoids. Infection and Immunity. 1993;61:3678–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cohen D, Ashkenazi S, Green M, Lerman Y, Slepon R, Robin G, Orr N, Taylor DN, Sadoff JC, Chu C, et al. Safety and immunogenicity of investigational Shigella conjugate vaccines in Israeli volunteers. Infection and Immunity. 1996;64:4074–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cohen D, Ashkenazi S, Green MS, Gdalevich M, Robin G, Slepon R, Yavzori M, Orr N, Block C, Ashkenazi I, et al. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet. 1997;349(9046):155–59. doi: 10.1016/S0140-6736(96)06255-1. [DOI] [PubMed] [Google Scholar]
- 72.Passwell JH, Ashkenzi S, Banet-Levi Y, Ramon-Saraf R, Farzam N, Lerner-Geva L, Even-Nir H, Yerushalmi B, Chu C, Shiloach J, et al. Age-related efficacy of Shigella O-specific polysaccharide conjugates in 1-4-year-old Israeli children. Vaccine. 2010;28(10):2231–35. doi: 10.1016/j.vaccine.2009.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barel LA, Mulard LA. Classical and novel strategies to develop a Shigella glycoconjugate vaccine: from concept to efficacy in human. Human Vaccines & Immunotherapeutics. 2019;1338–1356. doi: 10.1080/21645515.2019.1606972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Levine MM, Barry EM, Chen WH. A roadmap for enterotoxigenic Escherichia coli vaccine development based on volunteer challenge studies. Human Vaccines & Immunotherapeutics. 2019;1357–1378. doi: 10.1080/21645515.2019.1578922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–22. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 76.Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, McCormick BJ, McGrath M, Olortegui MP, Samie A, et al. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). The Lancet Global Health. 2015;3(9):e564–75. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zegeye ED, Govasli ML, Sommerfelt H, Puntervoll P. Development of an enterotoxigenic Escherichia coli vaccine based on the heat-stable toxin. Human Vaccines & Immunotherapeutics. 2019;1379–1388. doi: 10.1080/21645515.2018.1496768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Frederick DR, Goggins JA, Sabbagh LM, Freytag LC, Clements JD, McLachlan JB. Adjuvant selection regulates gut migration and phenotypic diversity of antigen-specific CD4(+) T cells following parenteral immunization. Mucosal Immunology. 2018;11(2):549–61. doi: 10.1038/mi.2017.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Platts-Mills JA, Liu J, Rogawski ET, Kabir F, Lertsethtakarn P, Siguas M, Khan SS, Praharaj I, Murei A, Nshama R, et al. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: a reanalysis of the MAL-ED cohort study. The Lancet. Global Health. 2018;6(12):E1309–E1318. doi: 10.1016/S2214-109X(18)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marder EP, Griffin PM, Cieslak PR, Dunn J, Hurd S, Jervis R, Lathrop S, Muse A, Ryan P, Smith K, et al. Preliminary incidence and trends of infections with pathogens transmitted commonly through food - foodborne diseases active surveillance network, 10 US sites, 2006-2017. Mmwr-Morbid Mortal W. 2018;67(11):324–28. doi: 10.15585/mmwr.mm6711a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bassal R, Lerner L, Valinsky L, Agmon V, Peled N, Block C, Keller N, Keness Y, Taran D, Shainberg B, et al. Trends in the epidemiology of campylobacteriosis in Israel (1999-2012). Foodborne Pathog Dis. 2016;13(8):448–55. doi: 10.1089/fpd.2015.2096. [DOI] [PubMed] [Google Scholar]
- 82.Shah N, DuPont HL, Ramsey DJ. Global Etiology of Travelers‘ Diarrhea: systematic Review from 1973 to the Present. Am J Trop Med Hyg. 2009;80:609–14. [PubMed] [Google Scholar]
- 83.Riddle MS, Sanders JW, Putnam SD, Tribble DR. Incidence, etiology, and impact of diarrhea among long-term travelers (US military and similar populations): A systematic review. Am J Trop Med Hyg. 2006;74:891–900. [PubMed] [Google Scholar]
- 84.Verdu EF, Riddle MS. Chronic gastrointestinal consequences of acute infectious diarrhea: evolving concepts in epidemiology and pathogenesis. The American Journal of Gastroenterology. 2012;107(7):981–89. doi: 10.1038/ajg.2012.65. [DOI] [PubMed] [Google Scholar]
- 85.Poly F, Noll AJ, Riddle MS, Porter CK. Update on campylobacter vaccine development. Human Vaccines & Immunotherapeutics. 2019;1389–1400. doi: 10.1080/21645515.2018.1528410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cohen D, Meron-Sudai S, Bialik A, Asato V, Goren S, Ariel-Cohen O, Reizis A, Hochberg A, Ashkenazi S. Serum IgG antibodies to Shigella lipopolysaccharide antigens – a correlate of protection against shigellosis. Human Vaccines & Immunotherapeutics. 2019;1401–1408. doi: 10.1080/21645515.2019.1606971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Booth JS, Goldberg E, Patil SA, Greenwald BD, Sztein MB. Association between S. Typhi-specific memory CD4+ and CD8+ T responses in the terminal ileum mucosa and in peripheral blood elicited by the live oral typhoid vaccine Ty21a in humans. Human Vaccines & Immunotherapeutics. 2019;1409–1420. doi: 10.1080/21645515.2018.1564570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Katz D, Ben-Chetrit E, Sherer SS, Cohen D, Muhsen K. Correlates of non-typhoidal Salmonella bacteraemia: A case-control study. International Journal of Infectious Diseases: IJID: Official Publication of the International Society for Infectious Diseases. 2019;81:170–75. doi: 10.1016/j.ijid.2019.01.028. [DOI] [PubMed] [Google Scholar]
- 89.Uche IV, MacLennan CA, Saul A, Baker S. A systematic review of the incidence, risk factors and case fatality rates of invasive nontyphoidal salmonella (iNTS) disease in Africa (1966 to 2014). PLoS Negl Trop Dis. 2017;11(1):e0005118. doi: 10.1371/journal.pntd.0005118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Balasubramanian R, Im J, Lee JS, Jeon HJ, Mogeni OD, Kim JH, Rakotozandrindrainy R, Baker S, Marks F. The global burden and epidemiology of invasive non-typhoidal Salmonella infections. Human Vaccines & Immunotherapeutics. 2019;1421–1426. doi: 10.1080/21645515.2018.1504717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tennant SM, MacLennan CA, Simon R, Martin LB, Khan MI. Nontyphoidal salmonella disease: current status of vaccine research and development. Vaccine. 2016;34(26):2907–10. doi: 10.1016/j.vaccine.2016.03.072. [DOI] [PubMed] [Google Scholar]
- 92.Baliban SM, Curtis B, Toema D, Tennant SM, Levine MM, Pasetti MF, Simon R, Darton TC. Immunogenicity and efficacy following sequential parenterally-administered doses of Salmonella Enteritidis COPS:fliC glycoconjugates in infant and adult mice. PLoS Neglected Tropical Diseases. 2018;12(5):e0006522. doi: 10.1371/journal.pntd.0006522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baliban SM, Yang M, Ramachandran G, Curtis B, Shridhar S, Laufer RS, Wang JY, Van Druff J, Higginson EE, Hegerle N, et al. Development of a glycoconjugate vaccine to prevent invasive Salmonella Typhimurium infections in sub-Saharan Africa. PLoS Neglected Tropical Diseases. 2017;11(4):e0005493. doi: 10.1371/journal.pntd.0005493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fuche FJ, Sow O, Simon R, Tennant SM, Salmonella Serogroup C. Current status of vaccines and why they are needed. Clin Vaccine Immunol. 2016;23(9):737–45. doi: 10.1128/CVI.00243-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fuche FJ, Jones JA, Ramachandran G, Higginson EE, Simon R, Tennant SM. Deletions in guaBA and htrA but not clpX or rfaL constitute a live-attenuated vaccine strain of Salmonella Newport to protect against serogroup C2-C3 Salmonella in mice. Human Vaccines & Immunotherapeutics. 2019;1427–1435. doi: 10.1080/21645515.2018.1491499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schuster O, Sears KT, Ramachandran G, Fuche FJ, Curtis B, Tennant SM, Simon R. Immunogenicity and protective efficacy against Salmonella C2-C3 infection in mice immunized with a glycoconjugate of S. Newport Core-O polysaccharide linked to the homologous serovar FliC protein. Human Vaccines & Immunotherapeutics. 2019;1436–1444. doi: 10.1080/21645515.2018.1483808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Checkley W, White AC Jr., Jaganath D, Arrowood MJ, Chalmers RM, Chen XM, Fayer R, Griffiths JK, Guerrant RL, Hedstrom L, et al. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. The Lancet Infectious Diseases. 2015;15(1):85–94. doi: 10.1016/S1473-3099(14)70772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Snelling WJ, Xiao L, Ortega-Pierres G, Lowery CJ, Moore JE, Rao JR, Smyth S, Millar BC, Rooney PJ, Matsuda M, et al. Cryptosporidiosis in developing countries. Journal of Infection in Developing Countries. 2007;1:242–56. [PubMed] [Google Scholar]
- 99.Sow SO, Muhsen K, Nasrin D, Blackwelder WC, Wu Y, Farag TH, Panchalingam S, Sur D, Zaidi AK, Faruque AS. The burden of cryptosporidium diarrheal disease among children < 24 months of age in moderate/high mortality regions of sub-saharan africa and south asia, utilizing data from the global enteric multicenter study (GEMS). PLoS Neglected Tropical Diseases. 2016;10(5):e0004729. doi: 10.1371/journal.pntd.0004729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee S, Beamer G, Tzipori S. The piglet acute diarrhea model for evaluating efficacy of treatment and control of cryptosporidiosis. Human Vaccines & Immunotherapeutics. 2019;1445–1452. doi: 10.1080/21645515.2018.1498436. PubMedPMID: 30036127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Steele J, Feng H, Parry N, Tzipori S. Piglet models of acute or chronic Clostridium difficile illness. The Journal of Infectious Diseases. 2010;201(3):428–34. doi: 10.1086/649799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cohen OR, Steele JA, Zhang Q, Schmidt DJ, Wang Y, Hamel PES, Beamer G, Xu B, Tzipori S, Chang Y-F. Systemically administered IgG anti-toxin antibodies protect the colonic mucosa during infection with Clostridium difficile in the piglet model. PloS One. 2014;9(10):e111075. doi: 10.1371/journal.pone.0111075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Na‘Amnih W, Adler A, Miller-Roll T, Cohen D, Carmeli Y. Risk factors for recurrent Clostridium difficile infection in a tertiary hospital in Israel. European Journal of Clinical Microbiology & Infectious Diseases: Official Publication of the European Society of Clinical Microbiology. 2018;37(7):1281–88. doi: 10.1007/s10096-018-3247-1. [DOI] [PubMed] [Google Scholar]
- 104.Eliakim-Raz N, Bishara J. Prevention and treatment of Clostridium difficile associated diarrhea by reconstitution of the microbiota. Human Vaccines & Immunotherapeutics. 2019;1453–1456. doi: 10.1080/21645515.2018.1472184. [DOI] [PMC free article] [PubMed] [Google Scholar]


