ABSTRACT
CD4+ and CD8+ T subsets are essential components of the adaptive immune system which act in concert at the site of infections to effectively protect against pathogens. Very limited data is available in humans regarding the relationship between CD4+ and CD8+ S. Typhi responsive cells in the terminal ileum mucosa (TI) and peripheral blood following Ty21a oral typhoid immunization. Here, we compared TI lamina propria mononuclear cells (LPMC) and peripheral blood CD4+ and CD8+ T memory (TM) subsets responses and their relationship by Spearman’s correlation following Ty21a immunization in volunteers undergoing routine colonoscopy. We observed that Ty21a immunization (i) influences the homing and accumulation of both CD4+ and CD8+ T cells in the TI, particularly integrin α4β7+ CCR9+ CD8+ T cells, (ii) elicits significantly higher frequencies of LPMC S. Typhi-responsive CD8+ T multifunctional (CD107a, IFNγ, IL-17A and/or MIP1β) cells than their CD4+ T counterparts, and (iii) results in the correlation of LPMC CD4+ Teffector/memory (TEM) S. Typhi responses (CD107a, IFNγ, TNFα, IL-17A and/or MIP1β) to their LPMC CD8+ TEM counterparts. Moreover, we demonstrated that these positive correlations between CD4+ and CD8+ TEM occur primarily in TI LPMC but not in PBMC, suggesting important differences in responses between the mucosal and systemic compartments following oral Ty21a immunization. This study provides the first demonstration of the correlation of S. Typhi-specific CD4+ and CD8+ TM responses in the human terminal ileum mucosa and provides valuable information regarding the generation of mucosal and systemic immune responses following oral Ty21a-immunization which might impact future vaccine design and development.
KEYWORDS: Lamina propria mononuclear cells, S. Typhi specific, PBMC, T cells, Ty21a immunization
Introduction
The etiological agent of typhoid fever, Salmonella enterica serovar Typhi (S. Typhi), is a human restricted pathogen that rapidly and efficiently infects the intestinal mucosa. S. Typhi infection constitutes a major global health threat with an estimated 20.6 million cases of typhoid fever annually resulting in approximately 223,000 deaths worldwide.1-4 Furthermore, the emergence of multi-drug resistant5 and extensively drug-resistant (XDR) strains (e.g. H58 S. Typhi strain6) in various parts of the world requires renewed efforts to tackle the disease. In addition, given its potential to cause high morbidity and mortality via contaminated food and water supplies, S. Typhi could potentially be used as a bio-terror agent. Thus, the development of more effective typhoid vaccines is urgently needed and paramount to thwart these threats. All of the currently licensed typhoid vaccines have limitations. Ty21a is the only licensed live attenuated oral vaccine which generates only modest immunogenicity, but confers a moderate level of long-lived protection (60–80%, 5–7 years).7-9 On the other hand, the purified Vi capsular polysaccharide vaccine is well tolerated but moderately immunogenic.4,10 Of significance, both vaccines are not recommended for young children, a particularly susceptible age group.11,12 Recently, a Vi-tetanus toxoid (Vi-TT) conjugate vaccine was prequalified by the World Health Organization for introduction into areas of high incidence of typhoid fever13,14 because Vi-TT have the ability to induce immunity even in young children (<2 years).14 However, the effectiveness of the Vi-TT vaccine in the field have just started to be evaluated and nothing is known regarding the longevity of the elicited immune responses. Moreover, this vaccine will not be effective in protection from S. Paratyphi A and S. Paratyphi B, the causative agents of Paratyphoid fevers, which do not express Vi. Therefore, it is important to continue the development of effective new typhoid vaccines that will provide durable, long-lasting protection in vulnerable populations and that also confers broad-spectrum protection against other enteric Salmonella infections such as S. Paratyphi A, for which no vaccines are available. However, the development of novel vaccines have been hampered by an incomplete lack of understanding of the immune effector and memory responses responsible for protection (correlates of protection – CoP), particularly in the gut microenvironment mucosa (site of infection).
One of the most serious complications of typhoid fever is intestinal perforation which occurs mainly in the terminal ileum (TI) (in ~78% of perforation cases). Duodenal and appendiceal (<2% of cases) perforations have rarely been reported.15 These clinical data suggest that the human TI is the favored intestinal active invasion site for S. Typhi.16,17 While the human gastrointestinal tract exemplifies an area of high antigenic exposure and contains a vast amount of total body lymphocytes (~60%), our understanding of the intestinal mucosal immunity is poor, particularly with respect to the events in the generation and maintenance of CD4 and CD8 T cells after oral immunization in humans. This gap in knowledge is hampering the rational development of new oral vaccines, not only for S. Typhi, but also other enteric fevers (e.g., S. Paratyphi A, S. Paratyphi B), non-typhoidal Salmonella (e.g., S. Typhimurium, S. enteriditis), Shigella, Enterotoxigenic E. coli (ETEC) and other enteric bacteria.
The induction of humoral and CMI responses have been comprehensively studied in peripheral blood mononuclear cells (PBMC) obtained from healthy volunteers following immunization with 4 doses of Ty21a.18-27 These studies revealed that live oral S. Typhi vaccines elicited both CD4+ and CD8+ T cell responses, including IFNγ, cytotoxic T cells (CTL), proliferation, and multifunctional (MF) antigen-specific cytokine-producing cells,18,20,25,27-30 which might play an important role in long term immunity. We have also reported that Ty21a elicits S. Typhi-specific CD4+ and CD8+ T cell responses in PBMC by various T memory (TM) cell subsets, including T central/memory (TCM), T effector/memory (TEM), and CD45RA+ TEM (TEMRA).21,31 These responses were predominantly in the TEM and TEMRA subsets with low magnitude responses also observed in CD4+ and CD8+ TCM subsets.27,30,31 Furthermore, S. Typhi-specific MF cells were increased in both CD4+ and CD8+ TEM and TEMRA subsets post-vaccination albeit with differences in response between subsets. However, these detailed CMI responses were all evaluated in peripheral blood.
Limited information is available regarding the induction of CD4+ and CD8+ T cells to S. Typhi in the human intestinal mucosa.32,33 Recently, we have reported that live oral Ty21a immunization elicits CD4+ TM34 and CD8+ TM35 S. Typhi-specific responses in human terminal ileum specimens. These studies showed that oral Ty21a vaccination elicited significantly increased lamina propria mononuclear cells (LPMC) CD4+ and CD8+ T cell responses, including IFNγ, IL-17A, cytotoxic T cells (CTL), and multifunctional (MF) antigen-specific cytokine-producing LPMC.34,35 We also observed that Ty21a-immunization induced S. Typhi responsive LPMC CD4+ and CD8+ T cells in all major TM subsets albeit with some unique properties (e.g., IFNγ and IL-17A in CD4+ TEM; IFNγ and macrophage inflammatory protein (MIP)-1β in CD4+ TCM; and IL-2 in CD4+ TEMRA). Furthermore, we found that LPMC CD4+ and CD8+ TEM responses were mostly MF, except for cells exhibiting the characteristics associated with IL-17A (for CD4+ and CD8+), TNFα (for CD4+) and CD107a cytotoxic (for CD8+) responses. Finally, we reported that the characteristics of the S. Typhi-specific responses for both CD4+ and CD8+ TEM in the mucosa only partially overlap with their systemic counterparts.34,35 However, to our knowledge, there is sparse data in blood and no data in the TI mucosa detailing the correlation of CD4+ and CD8+ TM subsets induction on an individual basis in both tissues following immunization with the live attenuated oral vaccine Ty21a (henceforth Ty21a).
For these reasons, in this study we compared and correlated the expression of homing molecules on CD4+ and CD8+ T obtained from TI-lamina propria mononuclear cells (LPMC) and blood (PBMC) from Ty21a-vaccinated and unvaccinated volunteers. We next compared and correlated CD4+ and CD8+ TEM single and multifunctional S. Typhi-specific responses in LPMC and PBMC obtained from the two groups of volunteer following stimulation with autologous target cells infected with or without wt S. Typhi. Finally, we determined the correlation between CD4+ and CD8+ TCM and TEMRA responses following Ty21a immunization. These comparisons provide unique insights into the similarities and differences in the induction of CD4+ and CD8+ TM cells in the mucosal and systemic compartments in humans.
Results
Oral Ty21a-immunization induces differential homing and retention of CD4+ and CD8+ T cells
The impact of oral Ty21a immunization on the induction of both mucosal and systemic CD4+ and CD8+ T cells, and the relationship of their induction in each individual volunteer has not been determined. To directly address this issue, we first characterized CD8+ and CD4+ T cells subsets from freshly isolated TI LPMC (Figure 1(a)) and PBMC (Figure 1(b)) obtained from concurrent biopsies and blood, respectively, of Ty21a vaccinated (n = 12) and unvaccinated (n = 18) volunteers. A representative example of the gating strategy for these studies is shown in Figure 1. We observed that effector memory T cells (TEM) (CD62L-CD45RA-) constitute the predominant TM population in both LPMC CD4+ (~75%) and CD8+ (~65%) present at the TI mucosa (Figure 1(a)). Terminally differentiated effector memory T cells (TEMRA) (CD62L-CD45RA+) and central memory T cells (TCM) (CD62L+CD45RA-) represent relatively minor populations in LPMC CD4+ and CD8+ T subsets in the TI mucosa (Figure 1(a)). Figure 1(b) shows a representative distribution of CD4+ and CD8+ T memory subsets in PBMC, in which lower levels of CD4+ TEM (~25.1%) and higher CD8+ TEM (~35.5%) are typically present (Figure 1(b)). We also determined the expression of CD69, an activation/retention marker36 on the CD4+ and CD8+ TM subsets in both tissues (Figure 1(a,b)). We observed high levels of expression of CD69 in both CD4+ and CD8+ TM subsets obtained from terminal ileum LPMC, but not in those from PBMC (Figure 1(a,b)).
Figure 1.
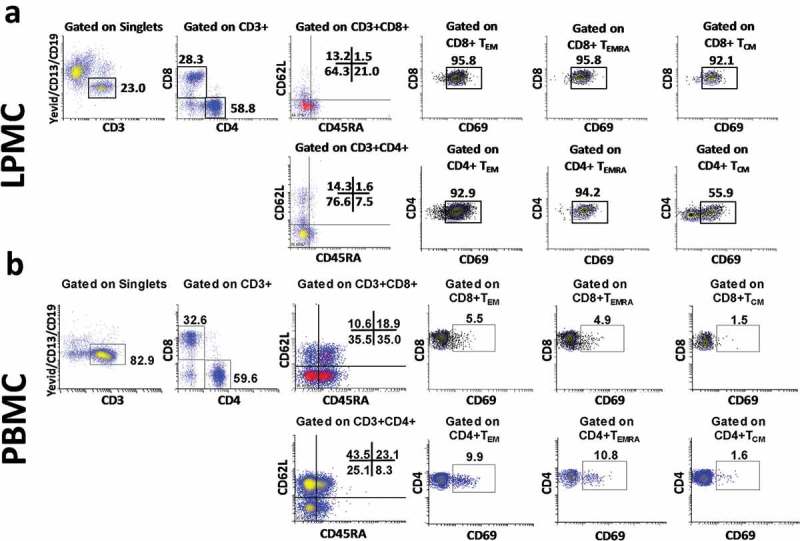
Gating Strategy to detect CD8+ and CD4+ TM subsets (CD4+ TM subsets [TCM (CD62L+CD45RA-), TEM (CD62L-CD45RA-), TEMRA (CD62L-CD45RA+) and Tnaive (CD62L+CD45RA+)] in (a) terminal ileum LPMC and (b) in PBMC isolated from a representative Ty21a-vaccinated volunteer. CD69 expression was determined for CD4 and CD8 TM subsets obtained from LPMC (a) and PBMC (b).
Recently, we have reported that oral Ty21a immunization influences the ex vivo expression of homing molecules on peripheral blood CD4+34) and CD8+ T cells,35 and their subsequent retention in the terminal ileum mucosa (site of infection). However, the relationship between the homing pattern of CD4+ and CD8+ T in blood and potential retention at the TI mucosa following Ty21a immunization had not yet been explored. To enable a direct comparison and explore the relationship of the induction of CD4+ and CD8+ TM subsets, we sampled simultaneously blood and TI biopsies from each individual and characterized the expression of homing and activation molecules, as well as the functional properties of LPMC and PBMC. To this end, we first compared the ex vivo frequencies of CD4+ and CD8+ T cells obtained concurrently from TI biopsies and their corresponding PBMC expressing the homing markers integrin α4β7, CCR9 and CCR6. Interestingly, no significant differences in the frequencies of CD4+ T cells and CD8+ T cells expressing integrin α4β7 were detected between these tissues (TI LPMC and PBMC) regardless of vaccination status (Figure 2(a)). Moreover, no significant differences in the frequencies of CCR9+ between CD4+ and CD8+ T cells were observed in PBMC from unvaccinated volunteers (Figure 2(b)). However, we noted a trend (p = 0.09) towards increased frequencies of CCR9+ CD8+ T cells when compared to CD4+ T cells in the TI mucosa in unvaccinated volunteers (Figure 2(b)). This difference was accentuated following Ty21a-immunization as evidenced by significantly higher frequencies of CCR9+ in CD8+ when compared to those in CD4+ T cells, suggesting that CD8+ T accumulate in the TI mucosa to a larger extent than CD4+ T cells (Figure 2(b)). Note that no significant differences between CD4+ and CD8+ T cells in the frequencies of cells expressing CCR9 in peripheral blood were detected following Ty21a immunization (Figure 2(b)). We subsequently compared the frequencies of integrin α4β7+ CCR9+ double positive cells between CD4+ and CD8+ T cells in LPMC and PBMC in both groups of volunteers. Similar to what we observed with CCR9 expression (Figure 2(b)), no significant differences in the frequencies of integrin α4β7+ CCR9+ between CD4+ and CD8+ T cells were found in peripheral blood of unvaccinated volunteers (Figure 2(c)), although a trend (p = 0.06) to increased frequencies in integrin α4β7+ CCR9+ on CD8+ T cells over those in CD4+ T cells was observed in the TI mucosa in unvaccinated volunteers (Figure 2(c)). Following Ty21a immunization, we observed significantly higher frequencies of integrin α4β7+ CCR9+ expressing cells in CD8+ than in CD4+ T cells present in the TI mucosa (Figure 2(c)). We also noted that there were no significant difference in CCR9 and integrin α4β7 co-expression between CD4+ and CD8+ T cells in peripheral blood following Ty21a immunization (Figure 2(c)). Finally, we also compared the frequencies of CD4+ and CD8+ T cells expressing the homing marker CCR6 in PBMC and LPMC obtained from both groups. No significant differences were observed in the frequencies of CD4+ and CD8+ T cells expressing CCR6 in LPMC regardless of Ty21a immunization (Figure 2(d)), although we noted in the unvaccinated cohort that CD8+ T cells expressed significantly higher CCR6 than CD4+ T cells in peripheral blood (Figure 2(d)), a finding that was not present following Ty21a immunization (Figure 2(d)). Taken together, these data suggest that CD8+ T cells either expressing CCR9 or co-expressing integrin α4β7 and CCR9 may accumulate in higher proportions than their CD4+ counterparts in the local TI mucosa following Ty21a immunization.
Figure 2.
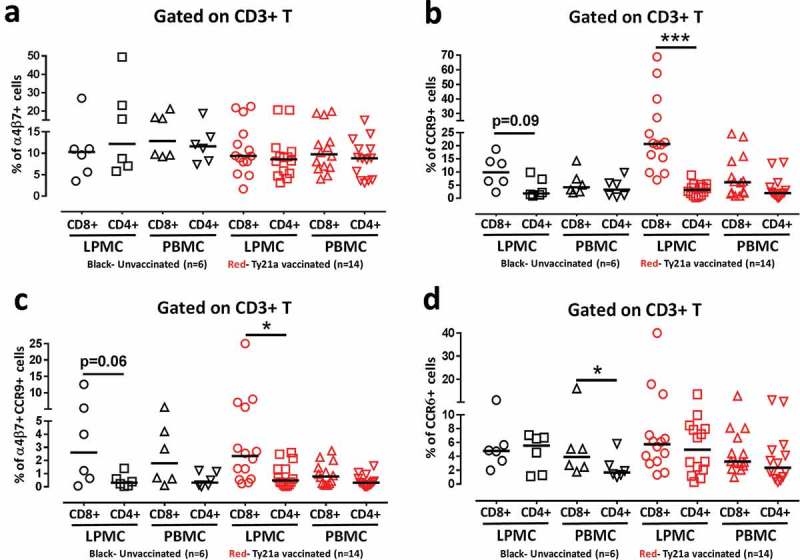
Comparison of ex vivo mucosal and systemic homing phenotypes of total CD4+ and CD8+ T cells following Ty21a oral vaccination. Ex-vivo percentages of (a) integrin α4β7+, (b) CCR9+, (c) integrin α4β7+ CCR9+ and (d) CCR6+ were evaluated in LPMC and PBMC total CD3+ CD4+ and CD3+ CD8+ T cells isolated from terminal ileum biopsies and blood of Ty21a vaccinated (red symbols; n = 14) and unvaccinated (black symbols; n = 6) volunteers. Significant differences between CD4+ and CD8+ T cells in vaccinated and unvaccinated volunteers are denoted as *P < 0.05, and ***P < 0.0005. Trends are denoted by their p value. Median values for each group are represented as horizontal black bars.
Because significant proportions of both CD4+ and CD8+ T cells expressed integrin α4β7, CCR9 and CCR6 which are influenced by oral Ty21a immunization in both tissues, albeit differently and particularly in the gut microenvironment, we explored the relationship of the expression of homing molecules between CD4+ and CD8+ T cells in each individual volunteer. To this end, we performed Spearman correlation tests of the expression of homing molecules between CD4+ and CD8+ T cells in both tissues obtained from Ty21a vaccinated and unvaccinated volunteers. We observed that in unvaccinated volunteers, the frequencies of LPMC and PBMC expression of homing markers (integrin α4β7, and/or CCR9) expressed on CD4+ T cells were not correlated with their CD8+ T cells counterparts (Table 1). However, the frequencies of CCR6+ CD4+ were positively correlated to their CD8+ T cells counterparts in LPMC (trend) and PBMC (significant) (Table 1). Interestingly, following Ty21a vaccination, the frequencies of LPMC homing molecules (integrin α4β7 and CCR6) expressed on CD4+ T cells were significantly positively correlated to their CD8+ T cells counterparts (Table 1). In contrast, no significant correlations were observed between PBMC CD4+ and CD8+ T cells expressing integrin α4β7+ or CCR9+, integrin α4β7+ CCR9+ or CCR6+ following Ty21a vaccination (Table 1).
Table 1.
Spearman correlation analysis of total CD3+ CD4+ T and total CD3+ CD8+ T cell expression of homing markers in LPMC and PBMC obtained from unvaccinated (n=6) and Ty21a-vaccinated (n=14) volunteers.
| CD4+ vs CD8+ T cells (Spearman r) |
||||
|---|---|---|---|---|
| Expression of homing markers | ||||
| Unvaccinated |
Ty21a vaccinated |
|||
| LPMC | PBMC | LPMC | PBMC | |
| α4β7+ | –0.086 | 0.143 | 0.459 | 0.139 |
| CCR9+ | 0.143 | –0.029 | –0.284 | 0.117 |
| CCR6+ | 0.543 | 0.995 | 0.547 | 0.235 |
| CCR9+α4β7+ | 0.143 | 0.086 | –0.286 | –0.161 |
| Green p<0.005 | Red P<0.05 | |||
Differences in systemic and mucosal CD4+ and CD8+ T S. Typhi responsive cells following Ty21a immunization
We have recently demonstrated that oral Ty21a immunization elicits significantly higher single and multifunctional (MF) S. Typhi responsive cells from CD4+ TEM34 and CD8+ TEM35 obtained from terminal ileum biopsies and peripheral blood. However, to date, the relationship between the elicited specific cell mediated responses of CD4+ and CD8+ TEM in blood and TI mucosa following Ty21a immunization has not been explored. To address this key issue, we compared the CD4+ and CD8+ TEM S. Typhi-specific net responses in each individual volunteer, whether Ty21a-vaccinated (n = 12) or unvaccinated (n = 18), to determine the relationship between the induction of these key cell types and subsets following Ty21a immunization. In these studies we sampled simultaneously blood and TI biopsies from each individual and stimulated the cells using identical conditions (S. Typhi-infected and uninfected EBV) and measured cells expressing a single function as well as the multifunctionality of the responses using Winlist’s FCOM function. To this end, LPMC and PBMC CD4+ and CD8+ TEM responses were analyzed for the production of multiple cytokines/chemokines (IFNγ, TNFα, IL-2, IL-17A and MIP1β) and/or CD107a expression as shown by the representative cytograms in Figure 3. Higher levels of cytokines (IFNγ and IL-17A) and higher expression of cytotoxic marker CD107a were observed following stimulation with S. Typhi-infected targets than uninfected targets in both CD4+ and CD8+ TEM in the tissues (LPMC and PBMC) (Figure 3). S. Typhi-specific responding cells were characterized as either single cytokine producers/CD107a expressors (S; e.g., IFNγ+ but negative for the other 5 functions evaluated) or multifunctional (MF; defined as the sum of all combinations of at least two or more functions from a total of 64 possible combinations for the 6 functions evaluated, e.g., IFN-γ+ IL-17A+ CD107a+ MIP-1β+ quadruple positive cells). First, we analyzed and compared PBMC CD4+ and CD8+ TEM responses associated with expression of CD107a, a cytotoxic marker37 (Figure 4(a)). Interestingly, we observed significantly higher CD107a+CD8+ TEM MF than CD107a+CD4+ TEM MF responses in peripheral blood regardless of Ty21a immunization (Figure 4(a)). Similarly, we observed that the frequencies of CD107a+CD8+ TEM MF in LPMC were significantly higher than in their CD4+ TEM counterparts regardless of Ty21a immunization (Figure 4(b)).
Figure 3.
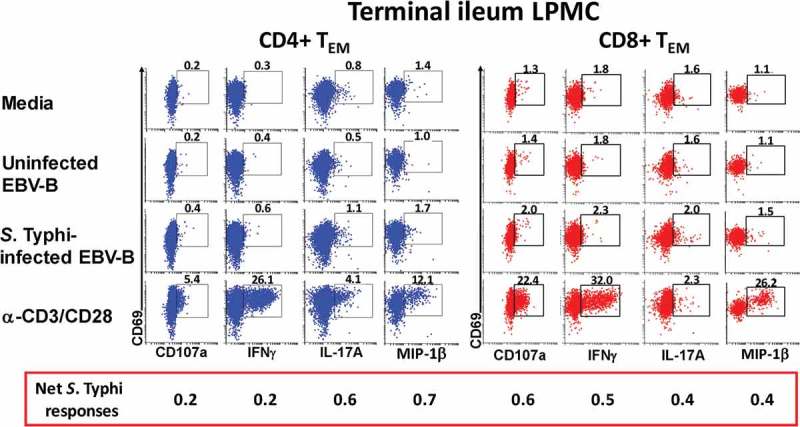
S. Typhi-specific responses by LPMC CD4+ and CD8+ T cells isolated from the terminal ileum of a Ty21a-vaccinated volunteer. LPMC from a representative Ty21a vaccinated volunteer showing up-regulation of CD107a expression and the induction of cytokine/chemokine production (IFNγ, IL-17A and MIP1β) in CD4+ (a) and CD8+ (b) TEM cells following stimulation by uninfected or S. Typhi-infected autologous EBV-B cells. Anti (α)-CD3/CD28 and media were used as positive and negative controls, respectively. The percentage of positive cells in the gated regions is shown above the corresponding black boxes. The net increases in the percentages of positive cells in the presence of S. Typhi-infected targets minus uninfected targets is shown in the red box below the x axis.
Figure 4.
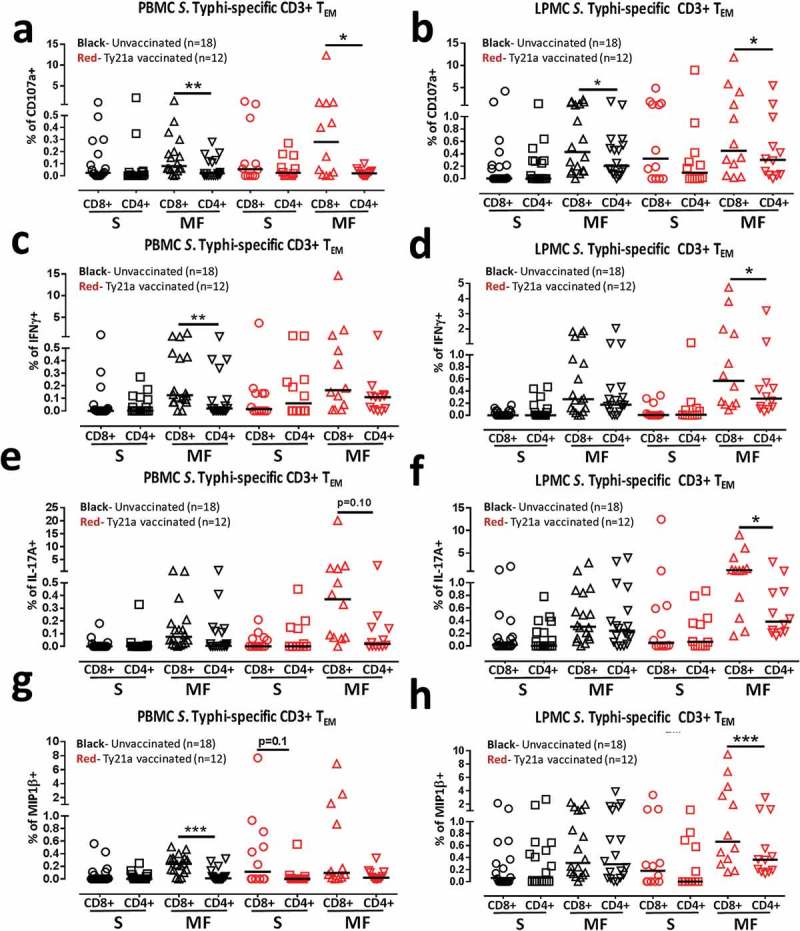
Comparison of single and multifunctional net S. Typhi-specific CD4+ and CD8+ TEM responses from LPMC and PBMC isolated from Ty21a-vaccinated and unvaccinated volunteers. Net S. Typhi-specific CD3+ CD4+ and CD3+ CD8+ TEM responses were calculated using the FCOM function of Winlist and stratified into multifunctional cells (MF) and single-positive effectors (S) following stimulation with autologous S. Typhi-infected and uninfected EBV-B. Comparison between CD4+ and CD8+ TEM S. Typhi-specific (a) PBMC CD107a+; (b) LPMC CD107a+; (c) PBMC INFγ+, (c) LPMC INFγ+, (e) PBMC IL-17A+; (f) LPMC IL-17A+ ; (g) PBMC MIP1β+ and (h) LPMC MIP1β+ MF and Si in Ty21a-vaccinated (n = 12; red symbols) and unvaccinated volunteers (n = 18; black symbols) with significant differences shown (*P < 0.05; **P < 0.005). p values for trends were also indicated. Horizontal black bars represent median values.
Next, we examined the IFN-γ responses for multi-functionality in Ty21a-vaccinees and controls. Interestingly, we observed that in unvaccinated volunteers, PBMC IFNγ+ CD8+ TEM MF frequencies were higher than their PBMC CD4+ TEM counterparts (Figure 4(c)). However, following Ty21a immunization, no significant differences were observed in PBMC IFNγ+ MF between CD8+ and CD4+ TEM (Figure 4(c)). In contrast, in the TI mucosa, no significant differences were noted for LPMC IFNγ+ S and MF between CD8+ and CD4+ TEM in unvaccinated volunteers (Figure 3(d)). However, following Ty21a immunization, we observed that there was significant (p < 0.05) increase in LPMC IFNγ+ MF in CD8+ than in CD4+ TEM (Figure 3(d)).
We then evaluated multifunctional IL-17A responses in both CD4+ and CD8+ TEM in Ty21a vaccinees and controls. Remarkably, the responses associated with IL-17A production followed somewhat similar patterns as those of IFNγ responses. No significant differences were observed in PBMC IL-17A+ S and MF between CD8+ and CD4+ TEM in unvaccinated volunteers (Figure 3(e)). However, we observed a trend (p = 0.1) towards increased responses in PBMC IL-17A+ CD8+ TEM MF than in their CD4+ TEM counterparts following Ty21a immunization (Figure 3(e)). Similarly, in the TI mucosa, no significant differences were noted for LPMC IL-17A S and MF between CD8+ and CD4+ TEM in unvaccinated volunteers (Figure 3(f)). However, following Ty21a immunization, we observed that there were significant (p < 0.05) increases in the frequencies of LPMC IL-17A+ CD8+ TEM MF compared with their CD4+ TEM counterparts (Figure 3(f)).
Finally, we determined CD4+ and CD8+ TEM responses associated with MIP1β in LPMC and PBMC obtained from Ty21a-vaccinees and unvaccinated volunteers. Interestingly, we observed that the frequencies of PBMC MIP1β+ CD8+ TEM MF was significantly higher than their CD4+ TEM counterparts (Figure 3(g)) in unvaccinated volunteers. Following Ty21a immunization, we noted a trend in the frequencies of PBMC MIP1β+ CD8+ TEM S to show higher response than their CD4+ TEM counterparts. No significant differences were noted in PBMC MIP1β+ MF between the two subsets following vaccination (Figure 3(g)). In contrast, in the TI mucosa, no significant differences were noted for LPMC MIP1β S and MF between CD8+ and CD4+ TEM in unvaccinated volunteers (Figure 3(h)). However, following Ty21a immunization, we observed that there were significant (p < 0.0005) increases in the frequency of LPMC MIP1β+ CD8+ TEM MF when compared to their CD4+ TEM counterparts (Figure 3(h)). Taken together, these data suggest that there are marked differences in the responses between the systemic and mucosal compartments.
Correlation between CD4+ and CD8+ TM S. Typhi responsive cells following Ty21a immunization in the systemic and mucosal compartments
Understanding the generation and maintenance of antigen-specific CD4+ and CD8+ TM subsets at the site of infection and in peripheral blood is important given the complexity in obtaining pristinely isolated mucosal T cells in vaccinated and unvaccinated healthy humans. Because CD4+ and CD8+ TM subsets are elicited specifically following oral Ty21a immunization in both the lamina propria and blood compartments, with particular characteristics, we explored the relationship between the generation of S. Typhi specific immune responses between CD4+ and CD8+ TEM in each individual volunteer. To this end we performed Spearman correlation tests between CD4+ TEM and CD8+ TEM S and MF responses in both tissues obtained from Ty21a vaccinated (n = 12) and unvaccinated (n = 18) volunteers.
Interestingly, we observed that in unvaccinated volunteers, the frequencies of LPMC CD4+ TEM MF (CD107a, IFN-γ, TNF-α and MIP1β), but not S, responses were significantly positively correlated with those of their CD8+ TEM counterparts (Table 2). However, following Ty21a immunization, the frequencies of LPMC CD4+ TEM S (CD107a and MIP1β) and MF (IFN-γ, TNF-α, IL-17A and MIP1β) responses were significantly positively correlated with those observed in CD8+ TEM (Table 2). In contrast, we observed that in unvaccinated volunteers, the frequencies of PBMC CD4+ TEM S (IFN-γ) and MF (CD107a and IFN-γ) responses were significantly positively correlated to those of CD8+ TEM (Table 2). However, following Ty21a immunization, the frequencies of PBMC CD4+ TEM S (IFN-γ, positively) and MF (TNF-α; negatively) responses were significantly correlated to those observed in CD8+ TEM (Table 2). Taken together, these data showed remarkable similarities, as well as differences, in CD4+ and CD8+ responses in the systemic and mucosal compartments.
Table 2.
Spearman correlation analysis of CD4+ TEM and CD8+ TEM S. Typhi-specific single (S) or multifunctional (MF) responses in LPMC and PBMC obtained from unvaccinated (n=18) and Ty21a-vaccinated (n=12) volunteers.
| Net S. Typhi-specific responses | CD4+ vs CD8+ TEM cells (Spearman r) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Unvaccinated |
Ty21a vaccinated |
|||||||
| LPMC |
PBMC |
LPMC |
PBMC |
|||||
| S | MF | S | MF | S | MF | S | MF | |
| CD107A+ | –0.155 | 0.568 | 0.046 | 0.444 | 0.708 | 0.451 | –0.023 | –0.213 |
| IFNγ+ | –0.166 | 0.446 | 0.560 | 0.502 | 0.049 | 0.665 | 0.562 | 0.184 |
| TNFα+ | 0.223 | 0.702 | 0.162 | 0.108 | –0.084 | 0.784 | 0.146 | –0.608 |
| IL-17A+ | –0.021 | –0.034 | 0.179 | –0.131 | –0.156 | 0.663 | 0.516 | 0.182 |
| IL-2+ | 0.194 | 0.101 | 0.140 | –0.139 | 0.107 | 0.346 | 0.381 | 0.107 |
| MIP1β+ | –0.284 | 0.520 | 0.129 | –0.004 | 0.606 | 0.886 | –0.160 | –0.455 |
| Orange p<0.0005 | Green p<0.005 | Red P<0.05 | ||||||
Because both, CD4+ and CD8+ have two relatively minor TM population (TCM and TEMRA) that also responded specifically to Ty21a immunization, we also explored the relationship between the generation of S. Typhi specific immune responses between CD4+ and CD8+ TCM and TEMRA in each individual volunteer. To this end we performed Spearman correlation tests between CD4+ TCM and CD8+ TCM and CD4+ TEMRA and CD8+ TEMRA responses in both tissues obtained from Ty21a vaccinated (n = 12) and unvaccinated (n = 18) volunteers.
Interestingly, we observed that in unvaccinated volunteers, the frequencies of LPMC CD4+ TCM (IFN-γ, TNF-α and IL-17A) responses exhibited trends to be positively correlated to those in CD8+ TCM (Table 3(a)). However, following Ty21a immunization, the frequencies of LPMC CD4+ TCM were correlated to their CD8+ TCM counterparts for IFN-γ and IL-2 (trend; positively), TNF-α (significant; positively) and for IL-2 (significant; positively) and IL-17A (significant; negatively) responses (Table 3(a)). Remarkably, we observed that in unvaccinated volunteers, no significant correlations were apparent between the frequencies of LPMC CD4+ TEMRA and their CD8+ TEMRA counterparts (Table 3(b)). However, following Ty21a immunization, the frequencies of LPMC CD4+ TEMRA producing IFN-γ responses were negatively correlated to their CD8+ TEMRA counterparts (Table 3(b)). These data evidenced marked differences in CD4+ and CD8+ TM subset responses in the human mucosa microenvironment.
Table 3.
Spearman correlation analysis of CD3+ CD4+ and CD3+ CD8+ (A) TCM and (B) TEMRA S. Typhi-specific responses in LPMC and PBMC obtained from unvaccinated (n=18) and Ty21a-vaccinated (n=12) volunteers.
| CD4+ vs CD8+ TCM (Spearman r) |
CD4+ vs CD8+ TEMRA (Spearman r) |
||||
|---|---|---|---|---|---|
| Net S. Typhi-specific responses |
LPMC |
Net S. Typhi-specific responses |
LPMC |
||
| Unvaccinated | Ty21a vaccinated | Unvaccinated | Ty21a vaccinated | ||
| CD107A+ | 0.537 | 0.177 | CD107A+ | 0.188 | 0.241 |
| IFNγ+ | 0.312 | 0.384 | IFNγ+ | 0.240 | –0.353 |
| TNFα+ | 0.399 | 0.521 | TNFα+ | 0.235 | 0.235 |
| IL-2+ | 0.110 | 0.425 | IL-2+ | 0.186 | 0.046 |
| IL-17A+ | 0.350 | –0.380 | IL-17A+ | 0.186 | 0.046 |
| MIP1β+ | 0.224 | –0.089 | MIP1β+ | 0.048 | 0.048 |
| Green P<0.005 | Red P<0.05 | Blue p<0.1 | |||
Discussion
One of the critical components of the adaptive immune system are T lymphocytes which orchestrate both cellular and humoral immune responses at various sites of infection providing long term protection against pathogens. CD4+ and CD8+ T subsets constitute the major players and are comprised of multiple helper, memory and effector subsets which in concert contribute to effectively protect the host. Thus, it is vital to understand CD4+ and CD8+ T cell-mediated immunity (T-CMI) at the site of infection and systemically, as well as the relationship between these two subsets as part of vaccine development efforts. Here, we determined the relationship between CD4+ and CD8+ T cells obtained from human terminal ileum biopsies and peripheral blood following oral immunization with the attenuated oral typhoid vaccine Ty21a.
We observed that Ty21a immunization (i) influences the homing and accumulation of both CD4+ and CD8+ effectors in the TI, particularly CD8+ T cells co-expressing integrin α4β7+ and CCR9+, (ii) results in significant positive correlations in the expression of integrin α4β7 and CCR6 between LPMC CD4+ and CD8+ T cells; (iii) elicits significantly higher frequencies of LPMC S. Typhi responsive CD8+ T multifunctional (CD107a, IFNγ, IL-17A and/or MIP1β) cells than their CD4+ T counterparts in the TI, the preferred site of infection for S. Typhi17,38; and (iv) results in the positive correlation of LPMC CD4+ TEM S. Typhi responses (CD107a, IFNγ, TNFα, IL-17A and/or MIP1β) to their LPMC CD8+ TEM counterparts. Moreover, we showed that the positive correlation between CD4+ TEM and CD8+ TEM occur primarily in TI LPMC rather than in peripheral blood following Ty21a immunization, suggesting that the TI LPMC CD4+ and CD8+ T subsets are different than those present in circulation. Based on these data, we surmise that oral Ty21a immunization elicits, as we have previously reported,34,35 both CD4+ and CD8+ S. Typhi-specific TM subsets in the terminal ileum mucosa and blood and hypothesized that these subsets are induced in concert and coordinate the host mucosal and systemic immune responses likely to contribute to effective protective (e.g., TC1, TH1, TC17 and TH17) immunity against S. Typhi.
In order to design effective vaccines capable of eliciting broad T-CMI against intracellular pathogens such as S. Typhi, a clear understanding of the required signals for the development and maintenance of memory CD4+ and CD8+ T cells at the site of infection (e.g., TI) is needed. CD8+ T cells play an important role in immunity to intracellular pathogens (e.g., S. Typhi) through multiple mechanisms including killing of infected targets, as well as the secretion of cytokines such as IFNγ and TNFα.20,27,35 CD8+ T cells are activated through the recognition of specific peptides presented by classical and non-classical MHC class I on antigen-presenting cells (APCs) and by co-stimulatory signals and cytokines provided by APCs. In addition to CD8+ cells, CD4+ T cells also play an important role in immunity by providing help and coordination of both cellular and humoral immune responses to pathogens, including S. Typhi.28,39 In fact, CD4+ T cells are essential for the induction of memory CD8+ T cells following infection or immunization.40 CD4+ T cells are activated through the recognition of specific peptides presented by MHC class II on APCs and by co-stimulatory signals and cytokines. Both CD4 and CD8 T cells acting in concert can effectively coordinate immune responses to effectively protect the host. Here, we showed that there are multiple positive correlations (either trends or significant) between CD4+ and CD8+ T cell (expression of CD107a and/or production of IFNγ, TNFα, IL-17A and MIP1β) responses (particularly MF) in the TI mucosa and PBMC even before immunization, likely to be the result of cross-reactivity in memory responses elicited by previous exposure to other Salmonella serovars41-43 or other Enterobacteriaceae, including those present in the normal gut microbiota.27,44-46 However, following oral Ty21a immunization, these positive correlations between the responses of the two subsets are more accentuated (e.g., IFNγ MF). In addition, we observed that the positive correlation of responses between CD4+ and CD8+ T cells are much stronger in the TI mucosa than in peripheral blood. These results argue that oral Ty21a immunization elicits both CD4+ and CD8+ specific T cells and that these responses are compartmentalized, with stronger responses localized in the gut microenvironment, as shown by major differences in correlations between TI LPMC and PBMC. Taken together, these results suggest that the signal requirements for eliciting and maintaining interactions between CD4+ and CD8+ T cells may be dependent on the microenvironment at the site of infection, hence, they are likely to be tissue specific.
Interestingly, the homing patterns (integrin α4β7, CCR9 and CCR6) displayed on CD4+ and CD8+ T cells obtained from PBMC were not significantly different following Ty21a immunization. In contrast, in the TI mucosa, CD8+ T cells expressing either CCR9 alone or co-expressing integrin α4β7 and CCR9 were significantly higher than their CD4+ T cells counterparts following Ty21a immunization. Moreover, we noted that there were significant positive correlations of the expression of integrin α4β7 and CCR6 between CD4+ and CD8+ T cells following Ty21a immunization. Taken together, these observations argue in favor of recruitment and retention of specific CD8+ and CD4+ T cells at the mucosa, particularly CCR9+ and integrin α4β7+ CCR9+ cells. Thus, we hypothesized that oral Ty21a immunization results in an accumulation of effectors at the site of infection, likely by an expansion of local T cells, as well as recruitment of S. Typhi-specific responsive cells from circulation. Of note, these CD4 and CD8 T effector cells that accumulate in the mucosa exhibit a multifunctional phenotype which could be a major determinant in protection against S. Typhi. This observation adds further support to our recent report that S. Typhi–specific CD8+ MF responses correlate with protection against typhoid and delayed disease onset in humans challenged with wt S. Typhi.47 Taken together, these results suggest that the development of a highly efficacious Salmonella vaccine might need to induce MF CD4+ and CD8+ TEM cells in the mucosal microenvironment. These observations also provide novel insights to advance the development of oral vaccines for enteric pathogens other than S. Typhi, including Shigella and ETEC.
Remarkably, we also noted that there is no significant correlation between CD4+ and CD8+ TEM or TEMRA IL-2 responses in either the mucosa or PBMC regardless of vaccination status. However, we observed a positive correlation regarding IL-2 production by LPMC CD4+ and CD8+ TCM from Ty21a vaccinated participants. We speculate that IL-2 produced by TCM, and perhaps other subsets, is important in the local microenvironment to support the expansion of antigen-specific CD4+ and CD8+ TEM cell populations and maintenance of regulatory T cells (TREG) following Ty21a-immunization or challenge with wt S. Typhi.48 We also observed that correlation of responses between CD4+ and CD8+ T cells from different TM subsets varies substantially in the mucosa following Ty21a immunization. For example, TEMRA subset showed no correlations in the responses between CD4+ and CD8+ T cells. This may suggest that these terminally activated effectors play unique roles following Ty21a immunization in the terminal ileum. Finally, we noted that the correlation of TNFα responses between CD4+ and CD8+ TEM are positively correlated in LPMC but negatively correlated in PBMC. This observation provide further evidence of differences in elicitation of CD4+ and CD8+ T cells between the systemic and mucosal compartments.
There are few limitations in this study. In particular, due to the invasive nature and difficulty in obtaining terminal ileum biopsies by colonoscopy in participants, we were limited to obtaining TI biopsies at a single time point. In future studies we will assess mucosal immunity at additional time points to obtain key information on the dynamics of the generation of S. Typhi responsive cells by both CD4 and CD8 T cells subsets because of the likelihood that CD4 and CD8 responses have different kinetics, as well as to study persistence of the responses. In addition, at present it is unclear whether the sampling time (14–21 days after oral Ty21a vaccination) of PBMC and LPMC specimens is the best to observe the effect of vaccination. Finally, we observed that CD8 T cell responses appear to be dominant following stimulation with S. Typhi-infected targets. One caveat in this interpretation is that T-CMI against S. Typhi mediated by CD4 and CD8 T cells is likely to depend on the nature of the stimulant, as we have previously shown.27,29,39,49 CD4 cells are more prone to respond to S. Typhi soluble antigens while CD8+ cells were more likely to be activated by S. Typhi-infected targets.39 Because of limitations in the numbers of LPMC cells available we could only use S. Typhi-infected targets as the stimulant. Thus, it is possible that this may have exerted some influence in the differences observed in the relative magnitudes of the responses between CD4 and CD8 cells. Nonetheless, this first observation of differences in the magnitudes and correlations of CD4 and CD8 T cell responses measured in the individual volunteers under identical in vitro stimulation conditions elicited by oral Ty21a immunization contribute much needed new information to advance our understanding of the generation of effector and memory CD4 and CD8 T cells in the mucosal and systemic compartments following oral Ty21a immunization.
In conclusion, we have identified key differences and similarities in the S. Typhi-specific CD4+ and CD8+ TM responses elicited by oral Ty21a immunization in the systemic and mucosal compartments. Furthermore, our data offer major insights into the S. Typhi-specific CD4+ and CD8+ TM responses elicited in the TI mucosa and suggest that these responses are due to local immunomodulatory pathways capable of influencing T cell activation, expansion and differentiation, resulting in unique phenotypes and specificities which only partially overlap with those in the systemic compartment.
Materials and methods
Volunteers, immunization and sample collection
Volunteers undergoing routine colonoscopies who had no history of typhoid fever were recruited from the Baltimore-Washington metropolitan area and University of Maryland, Baltimore campus. Written informed consent was obtained from volunteers and all procedures were approved by the University of Maryland, Baltimore Institutional Review Board (IRB). Volunteers (demographics shown in Table 4) were assigned to two groups. The first group (n = 12) received the four recommended doses of Ty21a vaccine (Vivotif enteric-coated capsules; Crucell, Bern, Switzerland) while volunteers assigned to the second group were not vaccinated (control group) (n = 18) as shown in the study design (Figure 5). Blood samples were collected at least 21 days before colonoscopy (pre-immunization) and on colonoscopy day (day 0) together with TI biopsies using large capacity forceps (Figure 5). PBMC were isolated immediately after blood draws by density gradient centrifugation following standard techniques.30
Table 4.
Demographics of the participants included in this study.
| Characteristics | Unvaccinated | Ty21a vaccinated |
|---|---|---|
| Number of volunteers | 18 | 12 |
| Age, mean (range) | 56 (47–73) | 58 (50–73) |
| Sex, # female (%) | 14 (78 %) | 8 (67%) |
| Ethnicity, # of Caucasian (%) | 9 (50 %) | 9 (75%) |
| # of African American (%) | 8 (44 %) | 2 (17 %) |
| # of Asian (%) | 1 (6 %) | 1(8 %) |
Figure 5.
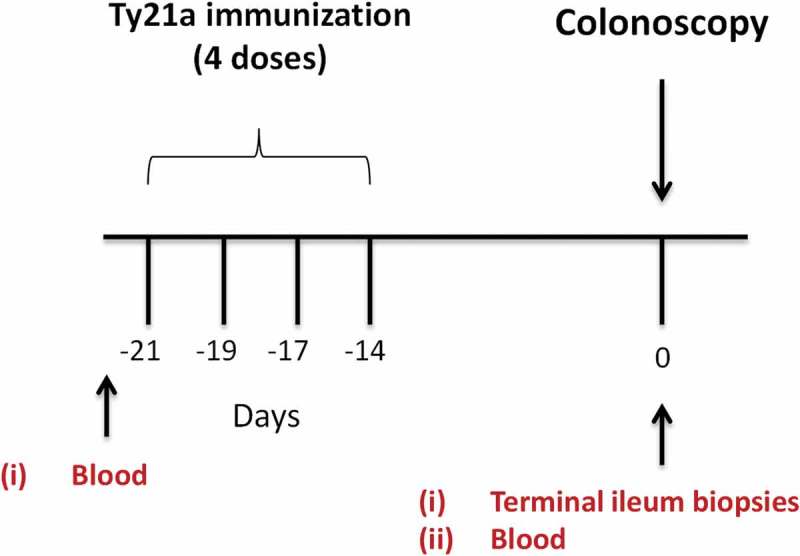
Study design. Oral typhoid vaccine Ty21a dose schedule (4 doses at −21 to −14 days) and time of collection of specimens (blood and terminal ileum (TI) biopsies) from volunteers undergoing routine screening colonoscopies. Autologous EBV-B cells were generated from pre-immunized blood.
Isolation of lamina propria mononuclear cells (LPMC) from terminal ileum biopsies
TI-LPMC were freshly isolated using an optimized procedure as previously described.35,50,51 Briefly, following collection of terminal ileum biopsies from routine colonoscopy volunteers, tissues were treated with HBSS (without CaCl2, MgCl2, MgSO4) (Gibco, Carlsbad, CA) and EDTA (1 mM; Ambion, Grand Island, NY) to remove intraepithelial cells (IEL). Then, the biopsies were enzymatically digested using collagenase D (100 μg/ml; Roche, Indianapolis, IN) and DNase I (10 μg/ml; Affymetrix, Cleveland, OH)), followed by homogenization with a Bullet Blender homogenizer (Next Advance Inc, Averill, NY). After homogenization, LPMC cells were then isolated by centrifugation, followed by a wash and resuspension in complete medium (cRPMI) (RPMI 1640 [Gibco Invitrogen, Carlsbad, CA] supplemented with 10% heat-inactivated fetal bovine serum [BioWhittaker, Walkersville, MD], 2 mM l-glutamine [HyClone, Logan, UT], 2.5 mM sodium pyruvate [Gibco], and 10 mM HEPES [Gibco], 100 U/ml penicillin [Sigma-Aldrich, St. Louis, MO], 100 μg/ml streptomycin [Sigma-Aldrich], and 50 μg/ml gentamicin [Gibco]) and counted using Kova Glastic Slides (Hycor Biomedical, CA). Isolated LPMC were either stained immediately for immune-phenotyping by flow cytometry or stimulated overnight with either S. Typhi-infected or uninfected targets or controls before staining with a 14-color flow cytometry panel and analyzed using a customized LSR-II instrument (BD, Franklin Lakes, NJ).
Target cell preparation
Autologous Epstein-Barr virus (EBV)-transformed lymphoblastoid cell line (EBV-B cells) were generated from each participant’s pre-immunization PBMC (Figure 1) as previously described.19,30,35 Briefly, autologous EBV-B cells were generated by incubation of PBMC with EBV-containing supernatant from the B95-8 cell line (ATCC CRL1612) and cyclosporine (0.5 μg/mL; Sigma-Aldrich, Saint-Louis, MO) at 37°C with 5% CO2. After transformation, EBV-B were kept in culture in cRPMI until used in the experiments.
S. Typhi infection of target cells
Autologous target cells (EBV-B) generated as described above were infected with wt-S. Typhi strain ISP1820 at a multiplicity of infection of 7:1 as previously described.19,30,35 Briefly, targets and bacteria were incubated for 3 h at 37°C in RPMI without antibiotics, washed three times with cRMPI and cultured overnight with cRPMI containing 150 μg/ml gentamicin. S. Typhi-infected and uninfected cells were gamma-irradiated (6,000 rad) for 6 mins before being used as “targets” for ex vivo TI LPMC and PBMC stimulation. The efficiency of the infection with S. Typhi-infected EBV-B was confirmed by staining with anti-Salmonella common structural Ag (CSA-1)-FITC (Kierkegaard and Perry, Gaithersburg, MD) and analysis by flow cytometry as previously described.18
Stimulation of PBMC and terminal ileum LPMC
Freshly isolated TI-LPMC and PBMC were used as effector cells as previously described.35,51 Briefly, LPMC and PBMC were co-cultured with either non-infected or S. Typhi-infected EBV-B (MOI of 7). LPMC and PBMC cultured with media only or in the presence of α-CD3/CD28 (Life technologies, Grand Island, NY) were used as negative and positive controls, respectively. At the time of stimulation, anti-human CD107a-FITC (5 μl; H4A3, BD, San Jose, CA) was added. The CD107a antibody is a functional marker for degranulation and considered a measure of cytotoxicity, a mechanism essential for the killing of S. infected targets by T cells.37 After 2 hours, 0.5 μl of Golgi Stop (Monensin, BD) and Golgi Plug (Brefeldin A, BD) were added and cultures continued overnight at 37°C in 5% CO2.
Surface and intracellular staining
Following stimulation, PBMC and TI-LPMC were stained for flow cytometry analysis as previously described.35,50 Briefly, PBMC and LPMC were stained for live/dead discrimination (YEVID) (Invitrogen, Carlsbad, CA) followed by blocking of Fc receptors using human immunoglobulin (3 µg/mL; Sigma). Next, the cells were surface stained with fluorescently labeled monoclonal antibodies (mAbs) directed to CD13-Pacific Orange (Conjugated in house), CD19-BV570 (HIB19, Biolegend), CD3-BV650 (OKT3, Biolegend), CD4-PE-Cy5 (RPA-T4, BD), CD8-PerCP-Cy5.5 (SK1, BD), CD45RA-biotin (HI100, BD), CD62L-APC-A780 (DREG-56, Ebioscience) and integrin α4β7-A647 (ACT1; conjugated in house) at 4°C for 30 min. Cells were washed with wash buffer and stained with streptavidin (SAV)-Qdot800 (Invitrogen) at 4°C for 30 min. Next, the cells were fixed and permeabilized using IC fixation and permeabilization buffers (8222/8333, eBioscience) according to the manufacturer’s recommendations. This was followed by intracellular staining with mAbs directed to IL-17A-BV421 (BL168, Biolegend), IFNγ-PE-Cy7 (B27, BD), CD69-ECD (TP1.55.3, Beckman Coulter, Danvers, MA), and MIP-1β-PE (IC271P, R&D) which was performed at 4°C overnight. After staining, cells were stored in 1% paraformaldehyde at 4°C until data collection. Using a customized LSRII flow cytometer (BD), the data were collected and analyzed using the WinList version 7 (Verity Software House, Topsham, ME) software package. S. Typhi-specific responses were expressed as net percentage of positive cells (background after stimulation with uninfected cells were subtracted from values obtained with S. Typhi-infected stimulators). This WinList package includes the FCOM function, a subroutine analysis whereby the multifunctionality of the responses on a single cell basis can be determined and thus enabling the classification of events based on combinations of selected gates. This function informs whether particular cells are single producing cells or produced two or more cytokines and/or express surface markers simultaneously.
Surface and intracellular staining for homing markers
Freshly isolated TI LPMC and PBMC were characterized for homing markers. Briefly, PBMC and LPMC were stained for live/dead discrimination (YEVID) (Invitrogen, Carlsbad, CA) followed by blocking of Fc receptors using human immunoglobulin (3 µg/mL; Sigma). Next, the cells were surface stained with fluorescently labeled mAbs directed to CD13-Pacific Orange (Conjugated in house), CD19-BV570 (HIB19, Biolegend), CD3-BV650 (OKT3, Biolegend), CD4-V450 (RPA-T4, BD), CD8-APC-H7 (SK1, BD), CCR9-APC (FAB1791A, R&D Systems), CCR6-biotin (11A9, BD), and integrin α4β7-A647 (ACT1; conjugated in house) at 4°C for 30 min. Cells were washed with wash buffer and stained with streptavidin (SAV)-Qdot800 (Invitrogen) at 4°C for 30 min. Next, the cells were fixed and permeabilized using IC fixation and permeabilization buffers (8222/8333, eBioscience) according to the manufacturer’s recommendations. This was followed by intracellular staining with mAbs directed to IL-17A-PerCP-Cy5.5 (N49-653, BD), IFNγ-PE-Cy7 (B27, BD), and CD69-ECD (TP1.55.3, Beckman Coulter, Danvers, MA), which was performed at 4°C overnight. After staining, cells were stored in 1% paraformaldehyde at 4°C until data collection. Using a customized LSRII flow cytometer (BD), the data were collected and analyzed using the WinList version 7 (Verity Software House, Topsham, ME) software package.
Statistical analysis
Data were analyzed using the statistical software GraphPad PrismTM version 6.03 (Graphpad, San Diego, CA, USA). We present the data as net responses by subtracting the responses to EBV. The net increases in the percentages of positive cells were calculated by subtracting the responses in the uninfected targets from those in the presence of S. Typhi-infected targets. Statistical differences in median values between two groups were determined using Mann–Whitney tests. Wilcoxon Matched pair tests were used to assess statistical differences between CD4+ and CD8+ paired responses. Correlations (CD4+ v/s CD8+ S Typhi–specific responses or homing markers expression) were evaluated using Spearman correlation tests.
Funding Statement
This work was funded by NIAID, NIH, DHHS grants R01-AI036525, U19-AI082655 (Cooperative Center for Human Immunology [CCHI]) and U19-AI109776 (Center of Excellence for Translational Research [CETR]). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Acknowledgments
We are indebted to the volunteers who allowed us to perform this study. We thank Mrs. Robin Barnes and the staff from the Clinical Studies Group of the Center for Vaccine Development for their help in collecting TI biopsies and blood specimens; Mr. Jeffery Floyd for isolating PBMC and Ms. Regina Harley and Catherine Storrer for excellent technical assistance in the performance of the flow cytometric determinations.
Author Contributions
JSB performed the experiments, contributed to study design, acquisition of data, analysis and drafting of the manuscript; EG and SAP performed endoscopies, obtained terminal ileum biopsies and reviewed the manuscript; BDG, performed endoscopies, obtained terminal ileum biopsies and reviewed the manuscript; MBS designed the study, supervised the work and drafted the manuscript.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Bhutta ZA, Threlfall J.. Addressing the global disease burden of typhoid fever. JAMA. 2009;302(8):898–99. doi: 10.1001/jama.2009.1259. [DOI] [PubMed] [Google Scholar]
- 2.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–53. [PMC free article] [PubMed] [Google Scholar]
- 3.Crump JA, Mintz ED. Global trends in typhoid and paratyphoid fever. Clin Infect Dis. 2010;50(2):241–46. doi: 10.1086/649541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine MM. Typhoid fever vaccines In: Plotkin SA, Orenstein WA, Offit PA, Edwards KM, editors. Plokin’s vaccines. 7th ed. Philadelphia, PA: Elsevier, Inc; 2018. p. 1114–44. [Google Scholar]
- 5.Mitchell DH. Ciprofloxacin-resistant salmonella typhi: an emerging problem. MedJAust. 1997;167:172. [DOI] [PubMed] [Google Scholar]
- 6.Klemm EJ, Shakoor S, Page AJ, Qamar FN, Judge K, Saeed DK, Wong VK, Dallman TJ, Nair S, Baker S, et al. Emergence of an extensively drug-resistant salmonella enterica serovar typhi clone harboring a promiscuous plasmid encoding resistance to fluoroquinolones and third-generation cephalosporins. mBio. 2018;9(1). doi: 10.1128/mBio.00105-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine MM, Ferreccio C, Abrego P, Martin OS, Ortiz E, Cryz S. Duration of efficacy of Ty21a, attenuated Salmonella typhi live oral vaccine. Vaccine. 1999;17:S22–S27. [DOI] [PubMed] [Google Scholar]
- 8.Black RE, Levine MM, Ferreccio C, Clements ML, Lanata C, Rooney J, Germanier R. Efficacy of one or two doses of Ty21a Salmonella typhi vaccine in enteric-coated capsules in a controlled field trial. Vaccine. 1990;8:81–84. [DOI] [PubMed] [Google Scholar]
- 9.Ferreccio C, Levine MM, Rodriguez H, Contreras R. Comparative efficacy of two, three, or four doses of Ty21a live oral typhoid vaccine in enteric-coated capsules: a field trial in an endemic area. J Infect Dis. 1989;159:766–69. [DOI] [PubMed] [Google Scholar]
- 10.Tacket CO, Ferreccio C, Robbins JB, Tsai CM, Schulz D, Cadoz M, Goudeau A, Levine MM. Safety and immunogenicity of two Salmonella typhi Vi capsular polysaccharide vaccines. J Infect Dis. 1986;154:342–45. [DOI] [PubMed] [Google Scholar]
- 11.Wain J, Hendriksen RS, Mikoleit ML, Keddy KH, Ochiai RL. Typhoid fever. Lancet. 2015;385(9973):1136–45. doi: 10.1016/S0140-6736(13)62708-7. [DOI] [PubMed] [Google Scholar]
- 12.Levine MP, Sztein MB, Pasetti MF. Salmonella enterica Serovar Typhi (typhoid) vaccines. Immunization, Vaccines and Biologicals. World Health Organization; 2011. r. http://apps.who.int/iris/bitstream/handle/10665/44752/9789241502610_eng.pdf;jsessionid=B7BCCD5D3658080E77798D156E7161D6?sequence=1
- 13.Jin C, Gibani MM, Moore M, Juel HB, Jones E, Meiring J, Harris V, Gardner J, Nebykova A, Kerridge SA, et al. Efficacy and immunogenicity of a Vi-tetanus toxoid conjugate vaccine in the prevention of typhoid fever using a controlled human infection model of Salmonella Typhi: a randomised controlled, phase 2b trial. Lancet. 2017;390(10111):2472–80. doi: 10.1016/S0140-6736(17)32149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health O Typhoid vaccines: WHO position paper, March 2018 - Recommendations. Vaccine. 2019;37(2):214–216. [DOI] [PubMed] [Google Scholar]
- 15.Golakai VK, Makunike R. Perforation of terminal ileum and appendix in typhoid enteritis: report of two cases. East Afr Med J. 1997;74:796–99. [PubMed] [Google Scholar]
- 16.Ukwenya AY, Ahmed A, Garba ES. Progress in management of typhoid perforation. Ann Afr Med. 2011;10(4):259–65. doi: 10.4103/1596-3519.87040. [DOI] [PubMed] [Google Scholar]
- 17.Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. Typhoid fever. N Engl J Med. 2002;347(22):1770–82. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- 18.Salerno-Goncalves R, Pasetti MF, Sztein MB. Characterization of CD8(+) effector T cell responses in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J Immunol. 2002;169(4):2196–203. doi: 10.4049/jimmunol.169.4.2196. [DOI] [PubMed] [Google Scholar]
- 19.Salerno-Goncalves R, Fernandez-Vina M, Lewinsohn DM, Sztein MB. Identification of a human HLA-E-restricted CD8+ T cell subset in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J Immunol. 2004;173(9):5852–62. doi: 10.4049/jimmunol.173.9.5852. [DOI] [PubMed] [Google Scholar]
- 20.Salerno-Goncalves R, Wahid R, Sztein MB. Immunization of volunteers with Salmonella enterica serovar Typhi strain Ty21a elicits the oligoclonal expansion of CD8+ T cells with predominant Vbeta repertoires. Infect Immun. 2005;73(6):3521–30. doi: 10.1128/IAI.73.6.3521-3530.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salerno-Goncalves R, Wahid R, Sztein MB. Ex Vivo kinetics of early and long-term multifunctional human leukocyte antigen E-specific CD8+ cells in volunteers immunized with the Ty21a typhoid vaccine. Clin Vaccine Immunol. 2010;17(9):1305–14. doi: 10.1128/CVI.00234-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kantele A. Antibody-secreting cells in the evaluation of the immunogenicity of an oral vaccine. Vaccine. 1990;8:321–26. [DOI] [PubMed] [Google Scholar]
- 23.McArthur MA, Sztein MB. Heterogeneity of multifunctional IL-17A producing S. Typhi-specific CD8+ T cells in volunteers following Ty21a typhoid immunization. PLoS One. 2012;7(6):e38408. doi: 10.1371/journal.pone.0038408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindow JC, Fimlaid KA, Bunn JY, Kirkpatrick BD. Antibodies in action: role of human opsonins in killing Salmonella enterica serovar Typhi. Infect Immun. 2011;79(8):3188–94. doi: 10.1128/IAI.05081-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sztein MB. Cell-mediated immunity and antibody responses elicited by attenuated Salmonella enterica Serovar Typhi strains used as live oral vaccines in humans. Clin Infect Dis. 2007;45(Suppl 1):S15–9. doi: 10.1086/518140. [DOI] [PubMed] [Google Scholar]
- 26.Sztein MB. Is a human CD8 T-cell vaccine possible, and if so, what would it take? CD8 T-cell-mediated protective immunity and vaccination against enteric bacteria. Cold Spring Harbor perspectives in biology; 2018;10(9):pii:a029546. doi:10.1101. [DOI] [PMC free article] [PubMed]
- 27.Sztein MB, Salerno-Goncalves R, McArthur MA. Complex adaptive immunity to enteric fevers in humans: lessons learned and the path forward. Front Immunol. 2014;5:516. doi: 10.3389/fimmu.2014.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wahid R, Fresnay S, Levine MM, Sztein MB. Cross-reactive multifunctional CD4+ T cell responses against Salmonella enterica serovars Typhi, Paratyphi A and Paratyphi B in humans following immunization with live oral typhoid vaccine Ty21a. Clin Immunol. 2016;173:87–95. doi: 10.1016/j.clim.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wahid R, Salerno-Goncalves R, Tacket CO, Levine MM, Sztein MB. Generation of specific effector and memory T cells with gut- and secondary lymphoid tissue- homing potential by oral attenuated CVD 909 typhoid vaccine in humans. Mucosal Immunol. 2008;1(5):389–98. doi: 10.1038/mi.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sztein MB, Tanner MK, Polotsky Y, Orenstein JM, Levine MM. Cytotoxic T lymphocytes after oral immunization with attenuated vaccine strains of Salmonella typhi in humans. J Immunol. 1995;155:3987–93. [PubMed] [Google Scholar]
- 31.Wahid R, Fresnay S, Levine MM, Sztein MB. Immunization with Ty21a live oral typhoid vaccine elicits crossreactive multifunctional CD8+ T-cell responses against Salmonella enterica serovar Typhi, S. Paratyphi A, and S. Paratyphi B in humans. Mucosal Immunol. 2015;8(6):1349–59. doi: 10.1038/mi.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pennington SH, Thompson AL, Wright AK, Ferreira DM, Jambo KC, Wright AD, Faragher B, Gilmour JW, Gordon SB, Gordon MA. Oral typhoid vaccination with live-attenuated salmonella typhi strain Ty21a generates Ty21a-responsive and heterologous influenza virus-responsive CD4+ and CD8+ T cells at the human intestinal mucosa. J Infect Dis. 2016;213(11):1809–19. doi: 10.1093/infdis/jiw030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lundin BS, Johansson C, Svennerholm AM. Oral immunization with a Salmonella enterica serovar typhi vaccine induces specific circulating mucosa-homing CD4(+) and CD8(+) T cells in humans. Infect Immun. 2002;70:5622–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Booth JS, Goldberg E, Patil SA, Barnes RS, Greenwald BD, Sztein MB. Effect of live oral attenuated Typhoid vaccine, Ty21a, on systemic and terminal ileum mucosal CD4+ T memory responses in humans. Int Immunol. 2018. doi: 10.1093/intimm/dxy070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Booth JS, Patil SA, Ghazi L, Barnes R, Fraser CM, Fasano A, Greenwald BD, Sztein MB. Systemic and terminal ileum mucosal immunity elicited by oral immunization with the Ty21a typhoid vaccine in humans. Cell Mol Gastroenterol Hepatol. 2017;4(3):419–37. doi: 10.1016/j.jcmgh.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol. 2013;14(12):1285–93. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294(1–2):15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Sharma A, Sharma R, Sharma S, Sharma A, Soni D. Typhoid intestinal perforation: 24 perforations in one patient. Ann Med Health Sci Res. 2013;3(Suppl 1):S41–3. doi: 10.4103/2141-9248.121220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salerno-Goncalves R, Tettelin H, Lou D, Steiner S, Rezwanul T, Guo Q, Picking WD, Nene V, Sztein MB, Picardeau M. Use of a novel antigen expressing system to study the Salmonella enterica serovar Typhi protein recognition by T cells. PLoS Negl Trop Dis. 2017;11(9):e0005912. doi: 10.1371/journal.pntd.0005912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laidlaw BJ, Craft JE, Kaech SM. The multifaceted role of CD4(+) T cells in CD8(+) T cell memory. Nat Rev Immunol. 2016;16(2):102–11. doi: 10.1038/nri.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McClelland M, Sanderson KE, Spieth J, Clifton SW, Latreille P, Courtney L, Porwollik S, Ali J, Dante M, Du F, et al. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature. 2001;413(6858):852–56. doi: 10.1038/35101614. [DOI] [PubMed] [Google Scholar]
- 42.Wahid R, Simon R, Zafar SJ, Levine MM, Sztein MB. Live oral typhoid vaccine Ty21a induces cross-reactive humoral immune responses against Salmonella enterica serovar Paratyphi A and S. Paratyphi B in humans. Clin Vaccine Immunol. 2012;19(6):825–34. doi: 10.1128/CVI.00058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pakkanen SH, Kantele JM, Herzog C, Kantele A. Cross-reactive immune response elicited by parenteral Vi polysaccharide typhoid vaccine against non-typhoid Salmonellae. Vaccine. 2014;32(5):544–51. doi: 10.1016/j.vaccine.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–41. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferreira RB, Antunes LC, Finlay BB. Should the human microbiome be considered when developing vaccines? PLoS Pathog. 2010;6(11):e1001190. doi: 10.1371/journal.ppat.1000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eloe-Fadrosh EA, McArthur MA, Seekatz AM, Drabek EF, Rasko DA, Sztein MB, Fraser CM, Gilbert JA. Impact of oral typhoid vaccination on the human gut microbiota and correlations with s. Typhi-specific immunological responses. PLoS One. 2013;8(4):e62026. doi: 10.1371/journal.pone.0062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fresnay S, McArthur MA, Magder L, Darton TC, Jones C, Waddington CS, Blohmke CJ, Angus B, Levine MM, Pollard AJ, et al. Salmonella Typhi-specific multifunctional CD8+ T cells play a dominant role in protection from typhoid fever in humans. J Transl Med. 2016;14:62. doi: 10.1186/s12967-016-0867-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McArthur MA, Fresnay S, Magder LS, Darton TC, Jones C, Waddington CS, Blohmke CJ, Dougan G, Angus B, Levine MM, et al. Activation of Salmonella Typhi-specific regulatory T cells in typhoid disease in a wild-type S. Typhi challenge model. PLoS Pathog. 2015;11(5):e1004914. doi: 10.1371/journal.ppat.1004914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salerno-Goncalves R, Wyant TL, Pasetti MF, Fernandez-Vina M, Tacket CO, Levine MM, Sztein MB. Concomitant induction of CD4+ and CD8+ T cell responses in volunteers immunized with Salmonella enterica serovar typhi strain CVD 908-htrA. J Immunol. 2003;170(5):2734–41. doi: 10.4049/jimmunol.170.5.2734. [DOI] [PubMed] [Google Scholar]
- 50.Booth JS, Toapanta FR, Salerno-Goncalves R, Patil S, Kader HA, Safta AM, Czinn SJ, Greenwald BD, Sztein MB. Characterization and functional properties of gastric tissue-resident memory T cells from children, adults, and the elderly. Front Immunol. 2014;5:294. doi: 10.3389/fimmu.2014.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Booth JS, Salerno-Goncalves R, Blanchard TG, Patil SA, Kader HA, Safta AM, Morningstar LM, Czinn SJ, Greenwald BD, Sztein MB. Mucosal-associated invariant T cells in the human gastric mucosa and blood: role in helicobacter pylori infection. Front Immunol. 2015;6:466. doi: 10.3389/fimmu.2015.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]


