ABSTRACT
Shigella sonnei live vaccine candidate, WRSS1, which was previously evaluated in US, Israeli and Thai volunteers, was administered orally to Bangladeshi adults and children to assess its safety, clinical tolerability and immunogenicity. In a randomized, placebo-controlled, dose-escalation, age-descending study, 39 adults (18–39 years) and 64 children (5–9 years) were enrolled. Each adult cohort (n = 13) received one dose of 3x104, or three doses of 3 × 105 or 3 × 106 colony forming unit (CFU) of WRSS1 (n = 10) or placebo (n = 3). Each child cohort (n = 16) received one dose of 3x103, or three doses of 3x104, 3x105, or 3 × 106 CFU WRSS1 (n = 12) or placebo (n = 4). WRSS1 elicited mostly mild and transient reactogenicity events in adults and children. In the 3 × 106 dose group, 50% of the adults shed the vaccine; no shedding was seen in children. At the highest dose, 100% of adults and 40% of children responded with a ≥ 4-fold increase of S. sonnei LPS-specific IgA antibody in lymphocyte supernatant (ALS). At the same dose, 63% of adults and 70% of children seroconverted with IgA to LPS, while in placebo, 33% of adults and 18% of children seroconverted. Both the vaccinees and placebos responded with fecal IgA to LPS, indicating persistent exposure to Shigella infections. In conclusion, WRSS1 was found safe up to 106 CFU dose and immunogenic in adults and children in Bangladesh. These data indicate that live, oral Shigella vaccine candidates, including WRSS1 can potentially be evaluated in toddlers and infants (<2 years of age), who comprise the target population in an endemic environment.
KEYWORDS: Shigella sonnei vaccine, WRSS1, phase I trial, adult, children, endemic region, Bangladesh
Introduction
Diarrheal disease is the fourth leading cause of death in under 5 children, with 499,000 deaths in 2015.1 Furthermore, the incidence of moderate-to-severe diarrhea in infants and children correlates with increased risk of mortality, stunting of physical growth and lowered cognitive abilities.2 Accordingly, the development of vaccines against diarrhea remains a major global health focus, particularly for children in low-resource countries. Unfortunately, even licensed vaccines such as those for rotavirus and cholera elicit poor responses in this target group.3,4 This suggests that even good vaccines may be poorly immunogenic unless strategies can be devised to improve the performance of orally-administered vaccines in children living in endemic countries.
Live attenuated or inactivated oral bacterial vaccines mimic natural infection, are administered needle-free, are cheaper to manufacture than subunit vaccines and are convenient for compliance rates if multiple doses are needed. Hence, oral vaccines, even with reduced efficacy, could be of great public health benefit in resource-poor countries. For example, licensed oral rotavirus vaccines, even with 50–60% efficacy, have significantly reduced global rates of diarrhea-related childhood hospitalization.5-8
Shigella was the second leading cause of diarrhea-related deaths in 2016 among all ages.9 Among children aged 0–2 years, based on the multisite Malnutrition and Enteric Disease (MAL-ED) cohort, Shigella possessed the highest overall burden among ten pathogens accounting for 95.7% of attributable diarrhea.10 Furthermore, the Global Enteric Multicenter Study attributed Shigella to be the cause of the second largest proportion of moderate-to-severe diarrhea in toddlers, and the largest contributor in 24–59 months old children.2 Moreover, Shigella along with enteroaggregative E coli, Campylobacter, and Giardia, was substantially associated with sustained linear growth faltering during the first 2 years of life, and in some cases even for 5 years.11 Historically Shigella flexneri has been the dominant Shigella serogroup in endemic populations, while S. sonnei predominates in high-resource countries. With improved living conditions and availability of clean water in low-resource countries including Bangladesh, S. sonnei is slowly replacing S. flexneri.12-14 At the Dhaka Hospital of icddr,b, prevalence of S. sonnei increased from 39.3% in 2013 to 51.4% in 2016, whereas prevalence of S. flexneri remained the same (icddr,b surveillance data). Additionally, in 2016, of the Shigella isolates identified at the Dhaka Hospital of icddr,b, 68% were resistant to ciprofloxacin, 66% to cotrimoxazole and 50% to azithromycin, further emphasizing the need for a licensed Shigella vaccine. Such a vaccine, would be most effective in children <2 years of age, who are the target group.
Attenuated Shigella strains have been developed in the process of vaccine construction, which offers the opportunity to safely study immune responses to them in children living in low and middle income countries and to test strategies for improving these responses. A S. sonnei vaccine candidate WRSS1 has undergone sufficient clinical testing to be used to probe vaccination strategies in children. WRSS1 lacks the ability to spread from cell to cell due to loss of VirG (or IcsA).15 VirG-based vaccine strains such as S. flexneri 2a SC602, S. dysenteriae 1 WRSd1, and WRSS1 have demonstrated safety at low doses, significant immunogenicity, and in some cases efficacy in naïve US volunteers.16-18 WRSS1 was also found to be safe in Israeli and Thai adult volunteers.19,20
The current study was designed to evaluate the safety, clinical tolerability and immunogenicity of an oral vaccine, WRSS1 in Bangladesh, where Shigella is an important cause of moderate-to-severe diarrhea in children. This study was undertaken using WRSS1 as a tool to increase our understanding of how such a vaccine can be used in different age groups in an endemic area. In the future, data from this and other such studies will provide meaningful strategies for optimization of immune responses with live oral vaccines in toddlers and infants, who are the primary target population in Bangladesh.
Results
Study population
Among 252 screened participants, 39 adults and 64 children were enrolled based on inclusion and exclusion criteria (Supplementary Table 1). The CONSORT diagrams depicts screening, enrollment, allocation of vaccine/placebo, dose completion, and follow-up completion status of adults and children participants (Figure 1(a,b)). Demographic data of study participants by treatment groups are given in Supplementary Table 2.
Figure 1.
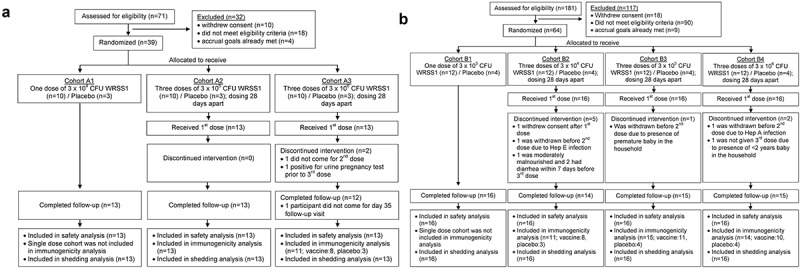
CONsolidated Standards of Reporting Trials (CONSORT) diagram showing screening, enrollment, allocation of vaccine/placebo, completion of intervention, and follow-up status of Bangladeshi (a) Adults and (b) Children participants. Each cohort of 13 adults (10 vaccinees and 3 placebo recipients) received either one dose of 3 × 104 (cohort A1) or three doses of 3 × 105 (cohort A2) or 3 × 106 (cohort A3) CFU of WRSS1 vaccine or placebo. Each cohort of 16 children (12 vaccinees and 4 placebos) received either one dose of 3 × 103 (cohort B1) or three doses of 3 × 104 (cohort B2), 3 × 105 (cohort B3) or 3 × 106 (cohort B4) CFU of vaccine or placebo.
Safety and clinical tolerability of WRSS1 in adults and children
In order to evaluate the safety of the WRSS1 vaccine, all participants were monitored for reactogenicity events, unexpected adverse events (AEs) and serious adverse events (SAEs) based on the definitions described in Materials and Methods. WRSS1 was generally well tolerated by Bangladeshi adults with reactogenicity symptoms occurring mostly after the first dosing in each dose group. Among 10 adults in each dose group, 4 (40%) in the 4-log, 5 (50%) in the 5-log and 8 (80%) in the 6-log dose groups experienced at least one reactogenicity event, showing a dose-dependent increase in the frequency of events. Five of 9 placebo recipients (56%) also experienced the same. The most common symptoms among vaccinees were headache, abdominal pain and bloating (Table 1). The majority of the symptoms were mild except for one moderate event each of abdominal pain (5-log dose group), and arthralgia and chills (6-log dose group), which resolved without sequelae within one to two days after the occurrence. Two participants in the 4-log and one in the 5-log dose group had loose stool for one day. One participant in the 6-log dose group experienced mild diarrhea for one day accompanied by mild abdominal cramps and mild headache. One adult each in 5-log and 6-log dose group had mild and transient fever, with the latter having co-existing symptoms of nausea, bloating, chills and arthralgia. The most common events in the placebo group were headache and nausea followed by bloating and abdominal pain (Table 1). There was one moderate event of abdominal cramps and one mild event of loose stool for one day among adult placebo recipients.
Table 1.
Reactogenicity events in Bangladeshi adults and children receiving WRSS1 or placebo.
| Adults (18–39 years) |
Children (5–9 years) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reactogenicity Events | 3x104 CFU dose group n = 10 |
3x105 CFU dose group n = 10 |
3x106 CFU dose group n = 10 |
Placebo n = 9 |
3x103 CFU dose group n = 12 |
3x104 CFU dose group n = 12 |
3 × 105 CFU dose group n = 12 |
3x106 CFU dose group n = 12 |
Placebo n = 16 |
| Abdominal Pain | 0 | 3a (30%) | 2 (20%) | 2 (22%) | 1(8%) | 1(8%) | 0 | 3 (25%) | 0 |
| Abdominal Cramps | 0 | 0 | 2 (20%) | 1a (11%) | 1(8%) | 0 | 0 | 2 (17%) | 0 |
| Bloating | 0 | 0 | 3 (30%) | 2 (22%) | 0 | 0 | 0 | 0 | 0 |
| Diarrhea | 0 | 0 | 1(10%) | 0 | 0 | 1(8%) | 0 | 0 | 0 |
| Chills | 0 | 0 | 2 a (20%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Fever | 0 | 1 (10%) | 1 (10%) | 0 | 0 | 3 (25%) | 0 | 1 (8%) | 1 (6%) |
| Headache | 2 (20%) | 0 | 6 (60%) | 4 (44%) | 0 | 0 | 0 | 1 (8%) | 1 (6%) |
| Lightheadedness | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (8%) | 1 (6%) |
| Loose stools | 2 (20%) | 1 (10%) | 0 | 1 (11%) | 0 | 2 (17%) | 3 (25%) | 1 (8%) | 0 |
| Malaise | 0 | 0 | 1 (10%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Myalgia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (8%) | 1 (6%) |
| Arthralgia | 0 | 0 | 1a (10%) | 0 | 0 | 0 | 0 | 1 (8%) | 0 |
| Nausea | 0 | 0 | 2 (20%) | 3 (33%) | 1(8%) | 0 | 0 | 1 (8%) | 0 |
| Vomiting | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (13%) |
Data are presented as number (%) of participants. aOne subject had moderate symptoms, otherwise all events were mild.
In children, fewer reactogenicity events were seen compared to adults and all events were mild in nature. Among 12 participants in each dose group, the number (proportion) of participants experiencing a minimum of one reactogenicity event were 2 (17%) in the 3-log, 5 (42%) in the 4-log, 3 (25%) in the 5-log and 7 (58%) in the 6-log dose groups. Among placebo recipients, 19% (3 out of 16) experienced at least one reactogenicity event. The most common symptoms among vaccine recipients were loose stool, abdominal pain and fever (Table 1). In the 4-log dose group, two children had loose stool for one day, while one child developed mild diarrhea for two days accompanied by fever for one day after the 1st dose. Two other children in the 4-log dose group also had mild and transient fever after the 1st dose. Three children in the 5-log dose group had loose stool for one day with each having it after the 1st, 2nd and 3rd dose, respectively. In the 106 CFU dose group, one participant had loose stools for one day after the 3rd dose. Another child in the same dose group had mild fever for one day followed by transient abdominal pain. None of these children required antibiotic treatment. One placebo recipient had mild fever for 3 days after the 1st dose. Two children of the placebo group vomited, one after the 1st dose and another after the 2nd dose (Table 1). The proportion of child participants with abdominal pain was higher in the 6-log dose group (p = 0.034) while the percentage of participants with loose stool was greater (p = 0.034) in the 5-log dose group compared to placebo.
Besides reactogenicity, there were several unexpected AEs in both age groups, ranging from mild to moderate in adults and mild to severe in children, which were clinically judged as vaccine-unrelated (see Materials & Methods for vaccine unrelated AEs and SAEs). In adults, elevated levels of liver enzymes (alanine transaminase (ALT) and aspartate transaminase (AST)) were the most frequent unsolicited AEs, with epigastric pain, upper respiratory tract infection and back pain being the other common AEs. The most common vaccine-unrelated AEs across all cohorts of children were fever and upper respiratory tract infection.
Four SAEs were reported in one adult and three children, none of which were assessed as related to the vaccine. Incomplete abortion in a female adult was considered as SAE, which was eventually resolved after further medication. One child was hospitalized for 2 days due to suspected enteric fever, 21 days after the first dosing; stool culture did not show presence of either WRSS1 vaccine strain or other tested enteric pathogens (e.g. Shigella, Salmonella, Escherichia coli, Vibrio, Campylobacter, Aeromonas, Plesiomonas, Yersinia). Tonsillectomy was performed in another child 9 days after the third dosing. Among placebos, one child participant was hospitalized ten days after the first dose due to diarrhea and dehydration; enteroaggegative E. coli was found in the stool by culture that was confirmed by PCR. Although the child was released from the hospital just after 5 hours from admission, the event was considered as SAE upon discretion of internal protocol safety team.
Shedding of vaccine strain
Shedding of WRSS1 in stool specimens/rectal swab was assessed on day 0 (the day of first dosing) and on days 1, 2, 3, 7, 28 (all cohorts), and additionally on days 35, 56, 63 and 84 (multi-dose cohorts) (Figure 2) by culture and confirmed by PCR assay. One adult in the 3 × 104 CFU dose group, one in the 3 × 105 dose group, and five in the 3 × 106 dose group shed the vaccine for one day after the first dosing of the vaccine. No further shedding was seen after subsequent doses. There was no shedding of WRSS1 in children.
Figure 2.
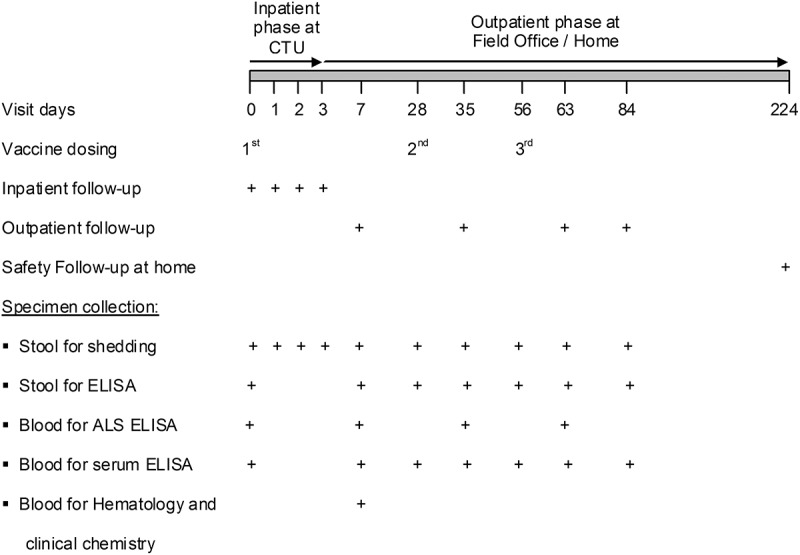
Outline for administration of vaccine/placebo, follow-up and specimen collection. Schedule for multi-dose cohorts (cohorts A2, A3, B2, B3 and B4) is given here. First vaccination and immediate inpatient safety evaluation for 72h was performed at the Clinical Trials Unit (CTU) of icddr,b. The second and third doses were given on an outpatient basis at the field office. Outpatient follow-up at day 7, 35, 63 and 84 were carried out at the field office. Long term safety follow-up took place at day 224 at the participants’ home. cohorts A1 and B1were given single dose on day 0, followed-up on day 7 and 28, and long term safety follow-up was made on day 168.
Immunogenicity
Only cohorts receiving three doses were included in the immunogenicity analyses (A2-A3 and B2-B4). Immunogenicity data are presented as responder frequency (proportion of participants with a ≥ 4-fold increase of antigen-specific antibody titers from prevaccination), fold rise (≥4-fold) of antibody titers in individual participants, and geometric mean (GM) of antibody titers with 95% confidence interval (CI).
(i) ALS antibody responses. LPS-specific IgA and IgG antibody in lymphocyte supernatant (ALS) was assessed before immunization and 7 days after each vaccination (Figure 2). In adults, the proportion of ALS IgA as well as IgG responders to LPS at any time after vaccination was 70% and 100% in the 5-log and the 6-log dose groups, respectively (Table 2), with the highest frequency obtained after the first vaccination (Supplementary Table 3) as expected in a primed population. Fold increase of antibody (post/pre-vaccination titers) in individual adult participants was as high as 310.7 for IgA and 1370.5 for IgG in the highest dose group (Supplementary Table 4). These responses were vaccine-specific since no placebo recipients had ≥4-fold increase in IgA or IgG ALS titers to LPS (Table 2).
Table 2.
Proportion (%) of participants with ≥4-fold increase in S.sonnei LPS-specific antibody titers from baseline at any time post oral administration of WRSS1 vaccine or placebo in Bangladeshi adults and children.
| ALS |
Serum |
Stool |
||||
|---|---|---|---|---|---|---|
| Dose Group | IgA | IgG | IgA | IgG | IgA | |
| Adult | 3 x 105 CFU (n = 10) | 70 | 70 | 50 | 0 | 30 |
| 3 x 106 CFU (n = 8) | 100 | 100 | 63 | 25 | 25 | |
| Placebo (n = 6) | 0 | 0 | 33 | 0 | 50 | |
| Children | 3 x 104 CFU (n = 8) | 0 | 0 | 25 | 0 | 25 |
| 3 x 105 CFU (n = 11) | 27 | 18 | 45 | 0 | 36 | |
| 3 x 106 CFU (n-10) | 40 | 50 | 70 | 20 | 40 | |
| Placebo (n = 11) | 0 | 0 | 18 | 9 | 36 | |
In children, ALS antibody responses were notably weaker. In the 6-log dose group, the proportion of LPS-specific IgA and IgG responders after any vaccination were 40% and 50%, respectively (Table 2); multiple doses were required to achieve these responses (supplementary Table 3). At the individual level, the maximum fold increase of ALS IgA was 32.5 and that of ALS IgG was 28.3 (Supplementary Table 4). As in adults, these responses were vaccine specific in children, since placebos did not have ≥4 fold increase of LPS-specific ALS IgA and IgG (Table 2).
(ii) Serum antibody responses. LPS-specific IgA and IgG antibody titers were measured in serum before vaccination and on day 7 and 28 after each vaccination (Figure 2).In adults, LPS-specific serum IgA responder rates were 50% and 63%, respectively in the 5-log and the 6-log dose groups. Among placebo recipients, the responder frequency was 33% (Table 2). Highest responder frequencies in the adult vaccinees were obtained after the first vaccination (Supplementary Table 5). When comparisons of LPS-specific serum IgA titers (Figure 3) between vaccine and placebo recipients were made, significant increases were observed at day 63 (p = 0.007) and day 84 (p = 0.016) in the 5-log dose group and at day 63 (p = 0.016) in the 6-log dose group (Table 3).
Figure 3.
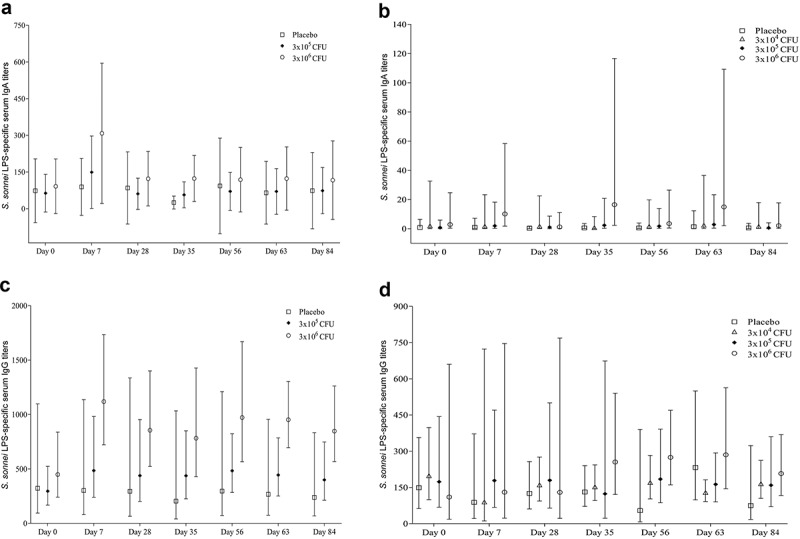
S. sonnei LPS-specific serum IgA and IgG antibody titers before and after administration of WRSS1vaccine or placebo in Bangladeshi adults (panels A, C) and children (panels B, D). Data are presented as Geometric mean with 95% confidence interval.
Table 3.
Multivariate regression analysis of S.sonnei LPS-specific serum antibody responses in vaccine groups compared to placebo recipients among Bangladeshi adults.
| LPS-IgA |
LPS-IgG |
|||||
|---|---|---|---|---|---|---|
| Placebo (n = 6) | 3x105 CFU (n = 10) | 3x106 CFU (n = 8) | 3x105 CFU (n = 10) | 3x106 CFU (n = 8) | ||
| Day 7 | Unadjusted | Ref. | 0.47 (−0.98, 1.92) | 0.97 (−0.52, 2.46) | 0.21 (−0.32, 0.73) | 0.57 (0.10, 1.04)* |
| Adjusteda | Ref. | 1.15 (−0.13, 2.43) | 1.32 (−0.17, 2.80) | 0.44 (0.14, 0.73)** | 0.73 (0.28, 1.17)** | |
| Day 28 | Unadjusted | Ref. | 0.16 (−1.37, 1.70) | 0.76 (−0.77, 2.29) | 0.17 (−0.42, 0.76) | 0.46 (−0.06, 1.00) |
| Adjusteda | Ref. | 0.75 (−0.60, 2.10) | 0.99 (−0.40, 2.38) | 0.43 (0.11, 0.75)* | 0.66 (0.24, 1.08)** | |
| Day 35 | Unadjusted | Ref. | 0.28 (−1.43, 2.00) | 1.13 (−0.43, 2.69) | 0.33 (−0.21, 0.87) | 0.58 (0.05, 1.11)* |
| Adjusteda | Ref. | 0.44 (−1.14, 2.02) | 0.91 (−0.72, 2.54) | 0.44 (0.10, 0.77)* | 0.79 (0.29, 1.28)** | |
| Day 56 | Unadjusted | Ref. | 0.90 (−0.42, 2.22) | 1.07 (−0.47, 2.61) | 0.21 (−0.30, 0.69) | 0.51 (0.004, 1.02)* |
| Adjusteda | Ref. | 1.16 (−0.15, 2.47) | 0.93 (−0.62, 2.49) | 0.37 (0.05, 0.69)* | 0.62 (0.26, 0.97)** | |
| Day 63 | Unadjusted | Ref. | 0.79 (−0.63, 2.20) | 1.45 (0.21, 2.68)* | 0.22 (−0.24, 0.69) | 0.56 (0.13, 0.98)* |
| Adjusteda | Ref. | 1.43 (0.48, 2.39)** | 1.34 (0.31, 2.37)* | 0.43 (0.06, 0.80)* | 0.63 (0.27, 1.00)** | |
| Day 84 | Unadjusted | Ref. | 0.58 (−0.92, 2.09) | 1.09 (−0.36, 2.54) | 0.22 (−0.26, 0.70) | 0.55 (0.12, 0.98)* |
| Adjusteda | Ref. | 1.33 (0.30, 2.36)* | 1.00 (−0.31, 2.31) | 0.34 (−0.01, 0.70) | 0.60 (0.35, 0.86)*** | |
Data are given as β-coefficient (Confidence Interval). aadjusted by age, sex and pre-existing titers. *p < 0.05, **p < 0.01, ***p < 0.001.
In children, the responder frequency to serum LPS-specific IgA was dose dependent, and reached 70% in the highest dose group. However, 18% of the placebo recipients also responded to LPS (Table 2). In contrast to adult participants, responder frequencies in children were maximum after the 2nd or 3rd dose of the vaccine (Supplementary Table 5). After adjustment for age, sex and pre-existing titers, a significant increase in LPS-specific serum IgA titers was seen at day 7, 35, 56 and 63 in the highest dose group compared to placebo (Table 4). When serum IgA response in individual participants was considered, the magnitude of fold increase was much higher in children compared to adults (supplementary Table 6).
Table 4.
Multivariate regression analysis of S.sonnei LPS-specific serum IgA response in Bangladeshi children receiving WRSS1 compared to placebo recipients.
| Placebo (n = 11) | LPS-IgA |
||||
|---|---|---|---|---|---|
| 3x104 CFU (n = 8) | 3x105 CFU (n = 11) | 3x106 CFU (n = 10) | |||
| Day 7 | Unadjusted | Ref. | 0.30 (−1.04, 1.64) | 0.36 (−0.88, 1.60) | 1.06 (−0.08, 2.20) |
| Adjusteda | Ref. | 0.27 (−0.60, 1.13) | 0.49 (−0.35, 1.33) | 1.34 (0.50, 2.17)** | |
| Day 28 | Unadjusted | Ref. | 0.72 (−0.48, 1.91) | 0.52 (−0.57, 1.61) | 0.56 (−0.54, 1.69) |
| Adjusteda | Ref. | 0.69 (−0.10, 1.48) | 0.63 (−0.02, 1.29) | 0.75 (−0.19, 1.70) | |
| Day 35 | Unadjusted | Ref. | 0.15 (−1.05, 1.35) | 0.63 (−0.53, 1.79) | 1.47 (0.37, 2.57)* |
| Adjusteda | Ref. | 0.15 (−0.78, 1.07) | 0.70 (−0.27, 1.67) | 1.52 (0.49, 2.56)** | |
| Day 56 | Unadjusted | Ref. | 0.44 (−0.80, 1.68) | 0.47 (−0.67, 1.62) | 0.78 (−0.35, 1.91) |
| Adjusteda | Ref. | 0.39 (−0.43, 1.22) | 0.56 (−0.05, 1.17) | 0.94 (0.01, 1.88)* | |
| Day 63 | Unadjusted | Ref. | 0.22 (−1.18, 1.63) | 0.31 (−0.92, 1.53) | 1.02 (−0.20, 2.23) |
| Adjusteda | Ref. | 0.15 (−1.12, 1.42) | 0.38 (−0.65, 1.41) | 1.14 (0.07, 2.20)* | |
| Day 84 | Unadjusted | Ref. | 0.49 (−0.75, 1.73) | 0.06 (−1.06, 1.17) | 0.59 (−0.58, 1.76) |
| Adjusteda | Ref. | 0.45 (−0.33, 1.24) | 0.16 (−0.29, 0.61) | 0.72 (−0.25, 1.70) | |
Data are given as β-coefficient (Confidence Interval). aadjusted by age, sex and pre-existing titers. *p < 0.05, **p < 0.01.
Both adults and children had high pre-existing S. sonnei LPS-specific serum IgG titers (Figure 3) reflecting prior exposure. Due to high baseline antibody titers, seroconversion of LPS-specific IgG antibodies was seen only in 25% of adults receiving the highest dose (Table 2). Despite poor responder rates in adults, WRSS1 showed significantly higher LPS-specific serum IgG titers compared to placebo (Table 3). In the 5-log dose group, significant difference were observed at day 7 (p = 0.008), day 28 (p = 0.014), day 35 (p = 0.017), day 56 (p = 0.027) and day 63 (p = 0.026) after controlling the post-vaccination titers for age, sex and pre-existing titers. In the highest dose group, LPS-specific serum IgG titers in vaccinees were significantly higher than placebo throughout the study period.
In children, 20% of the 6-log dose group and 9% of the placebo recipients showed IgG response to LPS at any time after vaccination (Table 2). After adjusting for preexisting titers, significant increases in antigen-specific serum IgG titers over placebo was seen in the children’s 6-log dose group on day 56 and day 84 (Table 5).
Table 5.
Multivariate regression analysis of S.sonnei LPS-specific serum IgG response in Bangladeshi children receiving WRSS1 compared to placebo recipients.
| Placebo (n = 11) | LPS-IgG |
||||
|---|---|---|---|---|---|
| 3x104 CFU (n = 8) | 3x105 CFU (n = 11) | 3x106 CFU (n = 10) | |||
| Day 7 | Unadjusted | Ref. | 0.01 (−0.97, 1.00) | 0.30 (−0.42, 1.03) | 0.17 (−0.74, 1.08) |
| Adjusteda | Ref. | 0.14 (−0.83, 1.11) | 0.33 (−0.21, 0.87) | 0.37 (−0.50, 1.24) | |
| Day 28 | Unadjusted | Ref. | 0.11 (−0.30, 0.51) | 0.16 (−0.35, 0.66) | 0.02 (−0.71, 0.74) |
| Adjusteda | Ref. | 0.19 (−0.18, 0.57) | 0.17 (−0.23, 0.56) | −0.10 (−0.83, 0.63) | |
| Day 35 | Unadjusted | Ref. | 0.07 (−0.27, 0.41) | −0.03 (−0.73, 0.68) | 0.29 (−0.10, 0.68) |
| Adjusteda | Ref. | 0.12 (−0.20, 0.45) | −0.01 (−0.67, 0.65) | 0.32 (−0.05, 0.70) | |
| Day 56 | Unadjusted | Ref. | 0.50 (−0.52, 1.52) | 0.53 (−0.36, 1.43) | 0.70 (−0.21, 1.62) |
| Adjusteda | Ref. | 0.59 (−0.33, 1.52) | 0.56 (−0.22, 1.35) | 0.99 (0.20, 1.79)* | |
| Day 63 | Unadjusted | Ref. | −0.26 (−0.74, 0.23) | −0.15 (−0.59, 0.28) | 0.09 (−0.37, 0.55) |
| Adjusteda | Ref. | −0.13 (−0.54, 0.28) | −0.15 (−0.58, 0.27) | 0.19 (−0.17, 0.56) | |
| Day 84 | Unadjusted | Ref. | 0.34 (−0.42, 1.11) | 0.33 (−0.38, 1.03) | 0.44 (−0.25, 1.14) |
| Adjusteda | Ref. | 0.40 (−0.26, 1.07) | 0.36 (−0.21, 0.92) | 0.69 (0.09, 1.28)* | |
Data are given as β-coefficient (Confidence Interval). aadjusted by age, sex and pre-existing titers. *p < 0.05.
(iii) Fecal IgA responses. LPS-specific IgA titers in stool were measured prior to vaccination and 7 and 28 days after each vaccination (Figure 2). Antigen-specific IgA responses in stool were normalized with total IgA. Among adults, responder frequencies for LPS-specific fecal IgA were 30% in the 5-log dose group and 25% in the 6-log dose group, while that in placebo recipients was 50% (Table 2). In children, responder frequencies for LPS-specific fecal IgA were 25%, 36% and 40% in the 4-log, 5-log and 6-log dose groups, respectively (Table 2). Among child placebo recipients, 36% responded to LPS. There were no significant differences in fecal IgA titers between vaccinees and placebo in both age groups even after adjusting for pre-existing titers.
Effects of shedding and reactogenicity on immune responses
To assess the effect of shedding of WRSS1 on immune response, LPS-specific antibody titers in ALS and serum were compared between shedders and non-shedders in the highest dose group of adults (Table 6). Multivariate regression analysis of antibody responses did not show any significant difference between shedders and non-shedders (data not shown). Furthermore, the effect of reactogenicity events on immune response profile was evaluated. No significant difference was found in the immunogenicity response between vaccinees with and without reactogenicity events in either adults or children (data not shown).
Table 6.
Fold increase (post-/pre-vaccination)a of S.sonnei LPS-specific ALS and serum antibodies on day 7 among vaccine shedders and non-shedders in the 6-log dose group of adults.
| ALS |
Serum |
||||
|---|---|---|---|---|---|
| Participant# | WRSS1 Shedder/Non-shedder | LPS-IgA | LPS-IgG | LPS-IgA | LPS-IgG |
| 1 | Non-shedder | 63.7 | 19.4 | - | - |
| 2 | Shedder | 39.1 | 55.2 | 6.8 | - |
| 3 | Non-shedder | - | - | - | - |
| 4 | Non-shedder | 81.3 | 126.2 | 6.8 | - |
| 5 | Shedder | 15 | 64.3 | - | - |
| 6 | Shedder | 269.4 | 97.4 | 36.4 | 5.7 |
| 7 | Non-shedder | 310.7 | 1370.5 | 7.6 | 9.6 |
| 8 | Non-shedder | 244.6 | 179.5 | 1876 | - |
| 9 | Shedder | 109.9 | 170.9 | - | 6.9 |
| 10 | Shedder | 33.4 | 40.5 | 4.7 | - |
Data for each participant is given (intention to treat). a ≥ 4-fold increase was shown only; <4-fold increase in titers was indicated by ‘-’.
Discussion
This is the first Phase I trial of WRSS1, conducted in adults and children 5–9 years of age in Bangladesh, who represent different levels of immunologic experience in an endemic environment. Earlier trials with WRSS1 were conducted in naive US adults, Israeli soldiers and Thai adults, where a single dosing schedule was used.17,19,20 In this study, up to three doses, spaced one month apart, were tested to maximize immune responses. The findings indicate that WRSS1 was safe and immunogenic in both age groups. Overall, children elicited lower mucosal immune responses than adults, but showed relatively higher systemic IgA responses, indicating that oral vaccine candidates can be evaluated in future studies, where infants and toddlers constitute the primary target.
WRSS1 up to the highest dose tested (106 CFU) demonstrated good safety profile in both adults and children in Bangladesh. In more naive US and Israeli adults, >30% of the participants receiving WRSS1 at doses above 104 CFU exhibited mild to severe constitutional symptoms including mild to moderate diarrhea; the symptoms increased with increasing doses.17,19 In contrast, only 10% of the Bangladeshi adults in the 6-log dose group and 8% of the children in the 4-log dose group had mild dirrahea; fever and other constitutional symptoms were mostly mild (in children, all symptomps were mild) and transient. A similar difference between naive adults, and endemic adults and children was seen for SC602 a S. flexneri 2a oral vaccine candidate.21,22 These findings suggest that adults and children older than 5 years in endemic environments are immunologically more experienced than naïve non-exposed adults. This is also borne out by preexisting serum antibody titers to Shigella antigens in this population.
An important characteristic of live oral vaccines, especially against invasive microorganisms, is the association between vaccine shedding and immune responses, vaccine shedding being a marker for intestinal colonization. In US or Israeli adults, 50–90% of the volunteers receiving 3-log to 6-log doses of WRSS1 or SC602 shed the vaccine strain robustly for 5–7 days and mounted vigorous immune responses to LPS.17,19,21 In Thailand, where S. sonnei is endemic, a single dose of 104 CFU of WRSS1 in adults led to much lower shedding and immune responses than observed in US and Israeli adults.20 Even then, a moderate correlation between shedding and immune responses was demonstrated both post vaccination and post challenge. In Bangladesh, 50% of the adults at the highest dose shed WRSS1 for only one day post 24h vaccination, but no difference was seen in immunogenicity response between shedders and non-shedders. One could also argue that the vaccine shedding seen in Bangladeshi adults merely reflects the passage of the vaccine strain through the gut and is not an actual colonization of WRSS1. Still, 88% of the adults demonstrated S. sonnei LPS-specific ALS IgA and IgG responses after the first dose, indicating an anamnestic response in what would be considered primed individuals living in an endemic environment. Earlier studies have shown that primed adults mount higher immune responses to natural Shigella infection compared to naïve adults and children.23-27 Antigen-specific memory B cells probably have a major role in this rapid response.28 Although none of the Bangladeshi children shed the vaccine, they were still able to induce high systemic immunity. When SC602 was tested in Bangladeshi adults and children, LPS-specific serum IgA response was found only in the 4-log dose group which did not shed the vaccine.22 These findings suggest that in an endemic population, with prior exposure to Shigella, robust colonization of oral live vaccines may not occur or necessarily influence the elicitation of immune responses.
In Bangladeshi children, where incidences of Shigella infection is presumably lower than in adults, multiple doses of WRSS1 was needed to increase the LPS-specific mucosal response though the magnitude of the response was lower than observed in Bangladeshi adults. Earlier studies by Mel et al have shown that multiple doses of a streptomycin-dependent live Shigella vaccine, administered at shorter intervals (separated by 3 days) to children living in an endemic environment helped achieve improved immunity.29 In Bangladesh, the dosing of WRSS1 was planned to match the Expanded Program of Immunization (EPI) schedule. Even with the longer interval (28 days), fold increase in serum IgA in children was mostly seen after the 2nd and 3rd doses. Importantly, the systemic IgA response in children was better than that of adults. The generally lower responder frequency with respect to LPS-specific serum IgG in both adults and children may be partially explained by the presence of high pre-existing IgG titers resulting from previous and sustained exposure. This observation of high circulating IgG titers directed to Shigella antigens in endemic regions is well-known.23,30,31 After adjusting for preexisting titers, significant increase in post-vaccination IgG response was seen in both adults and children, which indicates that previous exposures can blunt the response to an oral vaccine. High proportion of placebo recipients responding with fecal IgA to S. Sonnei LPS also indicated high prevalence of S. sonnei infections in the community. From the present results, it is apparent that in children, antigen-specific serum IgA antibody was a more sensitive biomarker of oral vaccination than ALS IgA or IgG and fecal IgA responses. Similar findings were reported in children and naive adults receiving oral ETEC vaccines.32,33
Lack of colonization and modest immunogenicity in endemic populations have been seen with other live oral vaccines such as polio, cholera, and rotavirus vaccines, that also require higher and repeated doses to be effective.8,34-38 Reasons for colonization resistance in children are complex, multifactorial and poorly understood. These include breastfeeding, genetic risk factors (histo blood group antigens, variants within the HLA locus), malnutrition, micronutrient deficiencies, exposure to a wider variety of enteric organisms and higher pre-vaccination antibody titers.39 Additionally, environmental enteropathy and the gut microbiome may play significant roles in colonization resistance.39-46 Comparison of stool microbiota and stool metabolome between naive and primed subjects may help to elucidate the nature of these differences.
Although both SC602 and WRSS1 have now been tested in Bangladeshi adults and children, the immune responses elicited by WRSS1 are greater than previously seen with SC602. Administration of lower volumes of bicarbonate buffer in this study may have contributed to the improved immune response. A recent study has shown that much lower volumes of bicarbonate buffer than traditionally used can neutralize gastric acidity and be more optimal since Shigella is fairly acid resistant and the viability of Shigella is lower in bicarbonate solution.47 Other means of improving the immune responses could be the use of an adjuvant such as the double mutant heat-labile toxin (dmLT) that was shown to increase the efficacy of ACE527, a live attenuated ETEC vaccine candidate.48Additional studies such as serum bactericidal activity and cytokine responses with WRSS1 cohort samples are ongoing, which may help to identify protective immune mechanisms in endemic populations.
Some of the limitations of this study are the lack of optimization of the dose, the number of doses, the dosing schedule and the bicarbonate volume to be ingested. Also, in order to be effective a Shigella vaccine must have more than one serotype. Currently a quadrivalent vaccine appears to be the goal, consisting of S. flexneri 2a, 3a, 6 and S. sonnei which are the prevalent circulating serotypes worldwide. It remains to be seen how such a mixture of serotypes will be combined to be effective in an endemic environment, realizing that there may be different levels of preexisting antibodies to the various Shigella serotypes. In addition, the real target of such an oral vaccine are infants and toddlers less than 5 years of age, since children 5–9 years of age are apparently quite resistant to Shigella. Nonetheless, this study provides a starting point for future work in this area, and suggests that a live oral vaccine can be safely tested in a target population where it is most needed
Materials and methods
WRSS1 vaccine strain
WRSS1 was manufactured in 1997 under current good manufacturing practice at the Walter Reed Army Institute of Research (WRAIR) Pilot Bioproduction Facility.15 Lyophilized vaccine vials (lot#0451) are removed periodically from −80° storage and tested for viability and stability of the Form I phenotype. Since its manufacture, WRSS1 has maintained its viability (~1–2 x1010 CFU/vial) and its stability with >90% Form I phenotype, i.e., round smooth-edged colonies indicating retention of the large virulence plasmid that encodes the genes for epithelial cell invasion as well as for LPS O-antigen synthesis. The lyophilized vaccine was shipped from WRAIR to icddr,b on dry ice where it was maintained at −65°C to −85°C.
Study design
This study was designed as a double-blinded, randomized, placebo-controlled, dose-escalating, age-descending study, starting out with healthy adults and sequentially moving into school-age children. The study was conducted between August 2013 and March 2016 at icddr,b, Dhaka, Bangladesh. Thirty nine healthy adults (18–39 years) and 64 healthy children (5–9 years) who met the eligibility criteria (Supplementary Table 1) were enrolled at the urban field site in Mirpur, a suburb of Dhaka. Some of the important inclusion criteria were (a) absence of obvious health problems as determined by clinical examination and medical history, (b) normal bowel habits (defined by <three grade 1 or 2 stools each day; ≥one grade 1 or 2 stools every 2 days) and (c) negative pregnancy test. Exclusion criteria included (a) significant medical or psychiatric abnormalities or any condition that might jeopardize the safety of study participants or interfere with the evaluation of the study objectives, (b) significant abnormalities on physical examination and in screening hematology and serum chemistry, (c) febrile illness within 48 hours prior to vaccination, (d) diarrhea within 7 days before vaccination, (e) receipt of antimicrobials within 7 days before vaccination, and (f) prior receipt of any Shigella vaccine. Written informed consent was obtained from adult participants and from guardians of children. Adults were randomized in 3 cohorts (cohorts A1-A3) and children in 4 cohorts (cohorts B1-B4). Each cohort of 13 adults (10 vaccinees and 3 placebo recipients) received either one dose of 3 × 104 (cohort A1) or three doses of 3 × 105 (cohort A2) or 3 × 106 (cohort A3) CFU of WRSS1 vaccine or placebo. Each cohort of 16 children (12 vaccinees and 4 placebos) received either one dose of 3 × 103 (cohort B1) or three doses of 3 × 104 (cohort B2), 3 × 105 (cohort B3) or 3 × 106 (cohort B4) CFU of vaccine or placebo. In each cohort, administration of the first dose and immediate inpatient safety evaluation for 72h were performed at the Clinical Trial Unit of icddr,b; the second and third vaccinations and all follow-up visits on days 7 and 28 for single-dose cohorts and days 7, 35, 63 and 84 for multi-dose cohorts were conducted at the field office (Figure 2). Long term safety follow-up took place at day 168 (single dose cohorts) and 224 (multi-dose cohorts) at the participants’ home.
Administration of vaccine, placebo and bi-carbonate buffer
Lyophilized WRSS1 vaccine was reconstituted in 5 ml of sterile water and serially diluted in saline to the appropriate dose. One ml of the vaccine was mixed with 30 ml (adults) or 15 ml (children) of normal saline for ingestion. Inoculum concentration was verified before and within 2 hours after dosing by viable-cell counting of the diluted vaccine. The viability and stability of WRSS1 vaccine strain remained similar at pre- and post-vaccination time points. Placebo recipients received the same volume of normal saline. To neutralize gastric acidity, vaccination was preceded by an oral administration of 100 ml (adults) and 50 ml (children) of 0.15M bicarbonate buffer. The buffer volumes used here were lower than in previous trials with S. flexneri SC60222 and WRSS1,17,19,20 where 150 ml of bicarbonate in adults and 50–75 ml in children were used. Participants fasted for 90 minutes before and after ingestion of the vaccine/placebo.
Participants were randomized to receive vaccine or placebo using sequential, simple random sampling. The sequence was generated in SAS® (North Carolina, USA) and codes were assigned to participants using individual, sealed, and sequentially numbered envelopes.
Safety evaluation
For evaluation of safety, AEs including reactogenicity, and SAEs were assessed according to the following definitions. Adverse event (AE) is any unintended medical occurrence (new events or worsening of pre-existing conditions) in clinical trial participants during the conduct of the trial. AEs may or may not be associated with the study drug. Reactogenicity are those events that according to the current Investigator’s Brochure and investigational plan/protocol are known to be caused by the study drug. Based on the prior studies, WRSS1 vaccine may result in symptoms similar to infection with Shigella, including loose stool, diarrhea, dysentery, nausea, vomiting, abdominal pain, abdominal cramps, bloating, constipation, fever, chills, headache, lightheadedness, generalized myalgia, malaise, decreased appetite, excess flatulence, reactive arthritis and arthralgia. In the current protocol, these symptoms were defined as reactogenicity events only when these occurred immediately after vaccination, i.e. up to 6 days after the first vaccination and 72h post second and third vaccination. If these events were experienced by the participants outside of the protocol-defined period, clinical judgment was applied to find out whether they were related to WRSS1. Events outside of the above list, irrespective of their occurrence time were also clinically judged to be related or unrelated to WRSS1. SAE is any AE that leads to (1) hospitalization or prolongation of existing hospitalization, (2) immediate risk of death (life-threatening), (3) persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions, (4) congenital abnormality or birth defect, (5) a medically important event that may jeopardize the participant or may require intervention to prevent one of the other outcomes listed above (e.g. intensive treatment at home for allergic bronchospasm; blood dyscrasia, or convulsions that do not result in hospitalization), (6) death.
AEs were monitored via focused medical interview and physical examination during the inpatient period, and on scheduled and unscheduled (need-based) follow-up visits in the field office. On days 4–6 and 72h post second and third vaccination at home, adult participants or guardians of the children recorded the frequency of defecation and stool grade in a simple memory aid. Other symptoms including temperature were documented by field staff during this period or when necessary. Laboratory evaluation including haematological and biochemical parameters was performed 7 days after the first vaccination and as suggested by the study clinicians. Participants with AE including SAE were provided appropriate care and treatment.
Assessment of fecal shedding
Stool samples were transferred using swab sticks to vials containing buffered glycerol saline within 3 hours of collection before transportation to the laboratory at icddr,b. In absence of stool, rectal swabs were collected in buffered glycerol saline. Stool or rectal swab samples were plated on Hektoen Enteric Agar and incubated at 37°C for 18 hours. Non-lactose-fermenting colonies were tested by agglutination with S. sonnei antiserum (Denka Seiken, Tokyo, Japan). Isolated S. sonnei was then tested for the virG(icsA) deletion in WRSS1 by PCR assay as described previously.20
Immunogenicity evaluation
Venous blood was collected from participants in BD Vacutainer SST tubes (Becton Dickinson, Franklin Lakes, NJ, USA) to separate serum by centrifugation. For ALS responses, blood was collected in BD sodium heparin containing tubes (Becton Dickinson). Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation, incubated in cell culture media at a concentration of 1x107/mL at 37°C, 5% CO2 for 72 h without any antigenic stimulation and the supernatant was collected and stored at −80°C. To extract protein-rich fraction from stool, extraction buffer containing soybean trypsin inhibitor (0.1 mg/ml) (Sigma, cat#T9003), EDTA (0.05M) (Sigma, cat#E5234), PMSF (0.002M) (Sigma, cat#P7626) and Tween 20 (0.05%) (Sigma, cat#P1379) in PBS (pH 7.2) was added to stool samples (4 ml buffer per gram of stool). After thorough mixing, the suspension was filtered using gauze fabric to remove large debris. The filtrate was finally centrifuged at 12,000g for 30 min and the supernatant was collected and stored at −80°C.
Endpoint antibody titers in serum, ALS, and stool extract were determined by enzyme-linked immunosorbent assay (ELISA) as described previously.20 In brief, 96-well U-bottom polystyrene microtiter plates (medium binding; Thermo Scientific, Rochester, NY) were coated with 1 µg/well S. sonnei LPS. Non-specific antigen-binding sites were blocked with 2% casein filler (Sigma, cat#C5890) for ALS and serum specimens or 1% bovine serum albumin (Sigma, cat#A7906) in PBS for stool specimens. All samples were serially diluted (2-fold) and added into the wells in duplicate; only diluents were added as blank controls. Following incubation and washing, anti-human IgG (1:500) or IgA (1:500) conjugated with alkaline phosphatase (KPL, Gaithersburg, MD) were added for ALS and serum and subsequent color reaction was developed by adding para-nitrophenyl phosphate (pNPP) (Sigma, cat#71768) as substrate. For stool specimens, IgA conjugated with horseradish peroxidase (KPL) and 2,2ʹ-Azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt (ABTS)-peroxidase substrate system (KPL, cat#50-62-00) were applied. Microtiter plates were read at 450 nm in an automated ELISA reader (Thermo Scientific Multiskan EX, Thermo Fisher Scientific, Waltham, Massachusetts). Titers were calculated by interpolating the dilution of serum, ALS and stool specimens, which yielded an optical density (OD) ≥0.2. Pre-vaccine and post-vaccine sera were run in batches.
Total IgA level in stool was measured by biochemistry analyzer (Hitachi 902, Roche Diagnostics GmbH, Boehringer, Mannheim, Germany) and antigen-specific fecal IgA response was normalized to total IgA.
Ethics statement
The clinical trial was conducted under an Investigational New Drug application (IND 15335) to the United States Food and Drug Administration. Ethical clearance was obtained from the ethical review committee of icddr,b and the Western Institutional Review Board. The trial was carried out in accordance with the standards of the International Conference on Harmonization guidelines on Good Clinical Practices (ICH-GCP), and followed the ethical principles established in the Declaration of Helsinki. All adult participants and guardians of child participants provided written informed consent prior to enrollment. The trial was registered at www.clinicaltrials.gov as NCT01813071.
Statistical analysis
The statistical analyses were performed with Stata 13 (StataCorp, LP, College Station, Texas, USA) and Statistical package for the Social Science (SPSS) for Windows (version 20; Armonk, NY: IBM SPSS corp.; 2011). Figures with immunological data were prepared using the GraphPad Prism 7.0. For safety evaluation, intention-to-treat analyses were performed. Proportion of vaccine and placebo recipients with specific reactogenicity event was compared using chi-square test. For immunogenicity evaluation, per-protocol analyses were performed, which included participants completing all vaccination doses. When individual participant’s immunological data were shown, all participants (intention-to-treat) were included in Tables. Normal distributions of the residuals in all the models were checked with normal k-density curve, probability of skewness and kurtosis and q-q plots. IgA and IgG titers in serum and stool was log-transformed to normalize data. Multivariate regression analysis was performed adjusting for age, sex and pre-existing titers to compare the means of serum and stool antibody titers between vaccine and placebo recipients at each time point. Multivariate regression analysis was also used to evaluate the mean changes of serum, ALS and stool antibody titers between shedders and non-shedders as well as between participants with and without reactogenicity events. A p value <0.05 was regarded as significant.
Data availability
The data supporting the findings of this study are available from the corresponding author upon request.
Funding Statement
This research study was funded by PATH, an international non-profit organization that works to improve the health of people around the world by advancing technologies, strengthening systems and encouraging healthy behaviors. PATH participated in the design of the studies, interpretation of results and review of this manuscript. icddr,b acknowledges with gratitude PATH’s commitment to its research efforts. icddr,b is also grateful to the Governments of Bangladesh, Canada, Sweden and the UK for providing core/unrestricted support.
Acknowledgments
We are greatly indebted to the late Dr. Lillian Van de Verg for her significant contribution towards study design, implementation and support for this study. We acknowledge the study participants for their consent to participate in the study and cordially allowing the scheduled/unscheduled visits of the field staff to their house/home. We appreciate the tedious works of clinical, field, data management and laboratory staff as well as the unblinded randomization team to make the study successful. We thank the project manager, medical monitor, study monitors and data management team of the Contract Research Organization (Emmes Corporation) for their guidance and critical review of the study procedures, source documents and database. We thank Dr. Robert Kaminski at WRAIR for providing S. sonnei LPS. Special thanks to Mr. Ahsanul Haque for his assistance in data analysis for the manuscript.
Disclosure of potential conflicts of interest
RR is the principal investigator. RR, KZ, NM, AB, RW, AF, MV conceived and designed the study. RR, PS, KZ, NHA, TW, NM, KT, AB, AS, FQ, RW, AF, MV participated in interpretation of results. AS and MV evaluated viability and stability of the vaccine candidate. RR and PS were responsible for the immunological analyses, and KZ and NHA for the clinical assessments and analyses of adverse events. RR and PS wrote the manuscript. All coauthors contributed to the critical review and revision of the manuscript and have approved the final version. The authors report no conflict of interests.
The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the US Department of the Army or the Department of Defense. The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70–25.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Collaborators GBDDD Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:909–48. doi: 10.1016/S1473-3099(17)30276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–22. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 3.Burnett E, Parashar U, Tate J.. Rotavirus vaccines: effectiveness, safety, and future directions. Paediatr Drugs. 2018;20:223–33. doi: 10.1007/s40272-018-0283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riddle MS, Chen WH, Kirkwood CD, MacLennan CA. Update on vaccines for enteric pathogens. Clin Microbiol Infect. 2018;24:1039–45. doi: 10.1016/j.cmi.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 5.Burnett E, Jonesteller CL, Tate JE, Yen C, Parashar UD. Global impact of rotavirus vaccination on childhood hospitalizations and mortality from diarrhea. J Infect Dis. 2017;215:1666–72. doi: 10.1093/infdis/jix186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mujuru HA, Yen C, Nathoo KJ, Gonah NA, Ticklay I, Mukaratirwa A, Berejena C, Tapfumanei O, Chindedza K, Rupfutse M, et al. Reduction in diarrhea- and rotavirus-related healthcare visits among children <5 years of age after national rotavirus vaccine introduction in Zimbabwe. Pediatr Infect Dis J. 2017;36:995–99. doi: 10.1097/INF.0000000000001648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahajamanana VL, Raboba JL, Rakotozanany A, Razafindraibe NJ, Andriatahirintsoa E, Razafindrakoto AC, Mioramalala SA, Razaiarimanga C, Weldegebriel GG, Burnett E, et al. Impact of rotavirus vaccine on all-cause diarrhea and rotavirus hospitalizations in Madagascar. Vaccine. 2017. doi: 10.1016/j.vaccine.2017.08.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaman K, Sack DA, Neuzil KM, Yunus M, Moulton LH, Sugimoto JD, Fleming JA, Hossain I, Arifeen SE, Azim T, et al. Effectiveness of a live oral human rotavirus vaccine after programmatic introduction in Bangladesh: A cluster-randomized trial. PLoS Med. 2017;14:e1002282. doi: 10.1371/journal.pmed.1002282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khalil IA, Troeger C, Blacker BF, Rao PC, Brown A, Atherly DE, Brewer TG, Engmann CM, Houpt ER, Kang G, et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: the Global Burden of Disease Study 1990-2016. Lancet Infect Dis. 2018;18:1229–40. doi: 10.1016/S1473-3099(18)30475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Platts-Mills JA, Liu J, Rogawski ET, Kabir F, Lertsethtakarn P, Siguas M, Khan SS, Praharaj I, Murei A, Nshama R, et al. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: a reanalysis of the MAL-ED cohort study. Lancet Glob Health. 2018;6:e1309–e1318. doi: 10.1016/S2214-109X(18)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogawski ET, Liu J, Platts-Mills JA, Kabir F, Lertsethtakarn P, Siguas M, Khan SS, Praharaj I, Murei A, Nshama R, et al. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob Health. 2018;6:e1319–e1328. doi: 10.1016/S2214-109X(18)30351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das SK, Ahmed S, Ferdous F, Farzana FD, Chisti MJ, Leung DT, Malek MA, Talukder KA, Bardhan PK, Salam MA, et al. Changing emergence of Shigella sero-groups in Bangladesh: observation from four different diarrheal disease hospitals. PLoS One. 2013;8:e62029. doi: 10.1371/journal.pone.0062029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livio S, Strockbine NA, Panchalingam S, Tennant SM, Barry EM, Marohn ME, Antonio M, Hossain A, Mandomando I, Ochieng JB, et al. Shigella isolates from the global enteric multicenter study inform vaccine development. Clin Infect Dis. 2014;59:933–41. doi: 10.1093/cid/ciu468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ud-Din AI, Wahid SU, Latif HA, Shahnaij M, Akter M, Azmi IJ, Hasan TN, Ahmed D, Hossain MA, Faruque AS, et al. Changing trends in the prevalence of Shigella species: emergence of multi-drug resistant Shigella sonnei biotype g in Bangladesh. PLoS One. 2013;8:e82601. doi: 10.1371/journal.pone.0082601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartman AB, Venkatesan MM. Construction of a stable attenuated Shigella sonnei DeltavirG vaccine strain, WRSS1, and protective efficacy and immunogenicity in the guinea pig keratoconjunctivitis model. Infect Immun. 1998;66:4572–76. http://www.ncbi.nlm.nih.gov/pubmed/9712824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz DE, Coster TS, Wolf MK, Trespalacios FC, Cohen D, Robins G, Hartman AB, Venkatesan MM, Taylor DN, Hale TL. Two studies evaluating the safety and immunogenicity of a live, attenuated Shigella flexneri 2a vaccine (SC602) and excretion of vaccine organisms in North American volunteers. Infect Immun. 2004;72:923–30. http://www.ncbi.nlm.nih.gov/pubmed/14742537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotloff KL, Taylor DN, Sztein MB, Wasserman SS, Losonsky GA, Nataro JP, Venkatesan M, Hartman A, Picking WD, Katz DE, et al. Phase I evaluation of delta virG Shigella sonnei live, attenuated, oral vaccine strain WRSS1 in healthy adults. Infect Immun. 2002;70:2016–21. http://www.ncbi.nlm.nih.gov/pubmed/11895966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKenzie R, Venkatesan MM, Wolf MK, Islam D, Grahek S, Jones AM, Bloom A, Taylor DN, Hale TL, Bourgeois AL. Safety and immunogenicity of WRSd1, a live attenuated Shigella dysenteriae type 1 vaccine candidate. Vaccine. 2008;26:3291–96. doi: 10.1016/j.vaccine.2008.03.079. [DOI] [PubMed] [Google Scholar]
- 19.Orr N, Katz DE, Atsmon J, Radu P, Yavzori M, Halperin T, Sela T, Kayouf R, Klein Z, Ambar R, et al. Community-based safety, immunogenicity, and transmissibility study of the Shigella sonnei WRSS1 vaccine in Israeli volunteers. Infect Immun. 2005;73:8027–32. doi: 10.1128/IAI.73.12.8027-8032.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitisuttithum P, Islam D, Chamnanchanunt S, Ruamsap N, Khantapura P, Kaewkungwal J, Kittitrakul C, Luvira V, Dhitavat J, Venkatesan MM, et al. Clinical trial of an oral live Shigella sonnei vaccine candidate, WRSS1, in Thai adults. Clin Vaccine Immunol. 2016;23:564–75. doi: 10.1128/CVI.00665-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coster TS, Hoge CW, VanDeVerg LL, Hartman AB, Oaks EV, Venkatesan MM, Cohen D, Robin G, Fontaine-Thompson A, Sansonetti PJ, et al. Vaccination against shigellosis with attenuated Shigella flexneri 2a strain SC602. Infect Immun. 1999;67:3437–43. http://www.ncbi.nlm.nih.gov/pubmed/10377124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahman KM, Arifeen SE, Zaman K, Rahman M, Raqib R, Yunus M, Begum N, Islam MS, Sohel BM, Rahman M, et al. Safety, dose, immunogenicity, and transmissibility of an oral live attenuated Shigella flexneri 2a vaccine candidate (SC602) among healthy adults and school children in Matlab, Bangladesh. Vaccine. 2011;29:1347–54. doi: 10.1016/j.vaccine.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 23.Cam PD, Pal T, Lindberg AA. Immune response against lipopolysaccharide and invasion plasmid-coded antigens of shigellae in Vietnamese and Swedish dysenteric patients. J Clin Microbiol. 1993;31:454–57. http://www.ncbi.nlm.nih.gov/pubmed/8432838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ekwall E, Cam PD, Chan N, Phu LK, Trach DD, Lindberg AA. Shigella-flexneri O-antigen specific enzyme-immunoassay - a prospective-study of class-specific antibody-titers against lipopolysaccharide antigens in vietnamese children and adults with serotype-1b or serotype-2a dysentery. Serodiagnosis Immunother Infect Dis. 1988;2:171–82. doi: 10.1016/0888-0786(88)90052-2. [DOI] [Google Scholar]
- 25.Raqib R, Mia SM, Qadri F, Alam TI, Alam NH, Chowdhury AK, Mathan MM, Andersson J. Innate immune responses in children and adults with Shigellosis. Infect Immun. 2000;68:3620–29. http://www.ncbi.nlm.nih.gov/pubmed/10816520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raqib R, Moly PK, Sarker P, Qadri F, Alam NH, Mathan M, Andersson J. Persistence of mucosal mast cells and eosinophils in Shigella-infected children. Infect Immun. 2003;71:2684–92. http://www.ncbi.nlm.nih.gov/pubmed/12704143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raqib R, Qadri F, SarkEr P, Mia SM, Sansonnetti PJ, Albert MJ, Andersson J. Delayed and reduced adaptive humoral immune responses in children with shigellosis compared with in adults. Scand J Immunol. 2002;55:414–23. http://www.ncbi.nlm.nih.gov/pubmed/11967124. [DOI] [PubMed] [Google Scholar]
- 28.Simon JK, Wahid R, Maciel M Jr., Picking WL, Kotloff KL, Levine MM, Sztein MB. Antigen-specific B memory cell responses to lipopolysaccharide (LPS) and invasion plasmid antigen (Ipa) B elicited in volunteers vaccinated with live-attenuated Shigella flexneri 2a vaccine candidates. Vaccine. 2009;27:565–72. doi: 10.1016/j.vaccine.2008.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mel D, Gangarosa EJ, Radovanovic ML, Arsic BL, Litvinjenko S. Studies on vaccination against bacillary dysentery. 6. Protection of children by oral immunization with streptomycin-dependent Shigella strains. Bull World Health Organ. 1971;45:457–64. http://www.ncbi.nlm.nih.gov/pubmed/4948417. [PMC free article] [PubMed] [Google Scholar]
- 30.Li A, Cam PD, Islam D, Minh NB, Huan PT, Rong ZC, Karlsson K, Lindberg G, Lindberg AA. Immune responses in Vietnamese children after a single dose of the auxotrophic, live Shigella flexneri Y vaccine strain SFL124. J Infect. 1994;28:11–23. http://www.ncbi.nlm.nih.gov/pubmed/8163828. [DOI] [PubMed] [Google Scholar]
- 31.Passwell JH, Ashkenzi S, Banet-Levi Y, Ramon-Saraf R, Farzam N, Lerner-Geva L, Even-Nir H, Yerushalmi B, Chu C, Shiloach J, et al. Age-related efficacy of Shigella O-specific polysaccharide conjugates in 1-4-year-old Israeli children. Vaccine. 2010;28:2231–35. doi: 10.1016/j.vaccine.2009.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakraborty S, Harro C, DeNearing B, Ram M, Feller A, Cage A, Bauers N, Bourgeois AL, Walker R, Sack DA. Characterization of mucosal immune responses to enterotoxigenic escherichia coli vaccine antigens in a human challenge model: response profiles after primary infection and homologous rechallenge with strain H10407. Clin Vaccine Immunol. 2016;23:55–64. doi: 10.1128/CVI.00617-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qadri F, Wenneras C, Ahmed F, Asaduzzaman M, Saha D, Albert MJ, Sack RB, Svennerholm A. Safety and immunogenicity of an oral, inactivated enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Bangladeshi adults and children. Vaccine. 2000;18:2704–12. http://www.ncbi.nlm.nih.gov/pubmed/10781858. [DOI] [PubMed] [Google Scholar]
- 34.Levine MM, Kotloff KL, Barry EM, Pasetti MF, Sztein MB. Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nat Rev Microbiol. 2007;5:540–53. doi: 10.1038/nrmicro1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, Ngwira B, Victor JC, Gillard PH, Cheuvart BB, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289–98. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 36.Patriarca PA, Wright PF, John TJ. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev Infect Dis. 1991;13:926–39. http://www.ncbi.nlm.nih.gov/pubmed/1660184. [DOI] [PubMed] [Google Scholar]
- 37.Qadri F, Chowdhury MI, Faruque SM, Salam MA, Ahmed T, Begum YA, Saha A, Al Tarique A, Seidlein LV, Park E, et al. Peru-15, a live attenuated oral cholera vaccine, is safe and immunogenic in Bangladeshi toddlers and infants. Vaccine. 2007;25:231–38. doi: 10.1016/j.vaccine.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 38.Qadri F, Chowdhury MI, Faruque SM, Salam MA, Ahmed T, Begum YA, Saha A, Alam MS, Zaman K, Seidlein LV, et al. Randomized, controlled study of the safety and immunogenicity of Peru-15, a live attenuated oral vaccine candidate for cholera, in adult volunteers in Bangladesh. J Infect Dis. 2005;192:573–79. doi: 10.1086/432074. [DOI] [PubMed] [Google Scholar]
- 39.Parker EP, Ramani S, Lopman BA, Church JA, Iturriza-Gomara M, Prendergast AJ, Grassly NC. Causes of impaired oral vaccine efficacy in developing countries. Future Microbiol. 2018;13:97–118. doi: 10.2217/fmb-2017-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kosek MN, Lee GO, Guerrant RL, Haque R, Kang G, Ahmed T, Bessong P, Ali A, Mduma E, Penataro Yori P, et al. Age and sex normalization of intestinal permeability measures for the improved assessment of enteropathy in infancy and early childhood: results from the MAL-ED study. J Pediatr Gastroenterol Nutr. 2017;65:31–39. doi: 10.1097/MPG.0000000000001610. [DOI] [PubMed] [Google Scholar]
- 41.Lee GO, McCormick BJJ, Seidman JC, Kosek MN, Haque R, Olortegui MP, Lima AAM, Bhutta ZA, Kang G, Samie A, et al. Infant nutritional status, feeding practices, enteropathogen exposure, socioeconomic status, and illness are associated with gut barrier function as assessed by the lactulose mannitol test in the MAL-ED birth cohort. Am J Trop Med Hyg. 2017;97:281–90. doi: 10.4269/ajtmh.16-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pasetti MF, Simon JK, Sztein MB, Levine MM. Immunology of gut mucosal vaccines. Immunol Rev. 2011;239:125–48. doi: 10.1111/j.1600-065X.2010.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harris V, Ali A, Fuentes S, Korpela K, Kazi M, Tate J, Parashar U, Wiersinga WJ, Giaquinto C, de Weerth C, et al. Rotavirus vaccine response correlates with the infant gut microbiota composition in Pakistan. Gut Microbes. 2017:1–9. doi: 10.1080/19490976.2017.1376162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris VC, Armah G, Fuentes S, Korpela KE, Parashar U, Victor JC, Tate J, de Weerth C, Giaquinto C, Wiersinga WJ, et al. Significant correlation between the infant gut microbiome and rotavirus vaccine response in rural Ghana. J Infect Dis. 2017;215:34–41. doi: 10.1093/infdis/jiw518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huda MN, Lewis Z, Kalanetra KM, Rashid M, Ahmad SM, Raqib R, Qadri F, Underwood MA, Mills DA, Stephensen CB. Stool microbiota and vaccine responses of infants. Pediatrics. 2014;134:e362–72. doi: 10.1542/peds.2013-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jamieson AM. Influence of the microbiome on response to vaccination. Hum Vaccin Immunother. 2015;11:2329–31. doi: 10.1080/21645515.2015.1022699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chandrasekaran L, Lal M, Van De Verg LL, Venkatesan MM. A study of different buffers to maximize viability of an oral Shigella vaccine. Vaccine. 2015;33:6156–60. doi: 10.1016/j.vaccine.2015.09.063. [DOI] [PubMed] [Google Scholar]
- 48.Tennant SM, Steele AD, Pasetti MF. Highlights of the 8th international conference on vaccines for enteric diseases: the Scottish encounter to defeat diarrheal diseases. Clin Vaccine Immunol. 2016;23:272–81. doi: 10.1128/CVI.00082-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon request.


