Abstract
Oral anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitors (TKI) have shown significant benefit in the management of ALK-rearranged non-small cell lung cancer (NSCLC). However, almost all patients will experience disease progression after front-line ALK-TKIs such as crizotinib. Treatment with third generation ALK-TKI lorlatinib can have a significant clinical impact following disease progression, even in patients with a very poor performance status. Here, we review two clinical cases with metastatic ALK-rearranged NSCLC who had pulmonary disease control with first-generation ALK inhibitor. However, disease progressed rapidly in the central nervous system with severe neurological symptoms. Treatment with lorlatinib, a third-generation ALK-TKI, led to a rapid radiological and clinical cerebral response in both patients. Lorlatinib can overcome ALK resistance to crizotinib, and the presented cases suggest a potential role for lorlatinib in patients with rapidly progressive cerebral and leptomeningeal metastases.
Keywords: cancer intervention, lung cancer (oncology), pharmacokinetics, tyrosine kinase inhibitor
Background
A small subset (3%–13%) of patients with non-small cell lung cancer (NSCLC) develop rearrangements in the anaplastic lymphoma kinase(ALK) gene. Over the last few years, small molecule tyrosine kinase inhibitors (TKIs) have shown significant benefit in the management of ALK-rearranged NSCLC.1 Crizotinib, a first-generation ALK inhibitor has a significant activity in ALK-rearranged NSCLC. Progression-free survival (PFS) for crizotinib group was 10.9 months and for platinum-based chemotherapy group was 7.0 months. The overall response rate (ORR) was 74% (95% CI 67% to 81%) for crizotinib versus 45% (95% CI 37% to 53%) for chemotherapy.1
However, most patients with NSCLC experience disease progression of the central nervous system during treatment with crizotinib in first-line setting. Treatment with more potent and selective next-generation ALK-TKIs demonstrated outstanding activity in both crizotinib-naïve and crizotinib-resistant ALK-rearranged NSCLC, with superior penetration of the blood–brain barrier (BBB).1
Ceritinib, brigatinib and alectinib are a selective second-generation ALK-TKIs and are more potent than crizotinib against native ALK protein and are able to inhibit gatekeeper mutations.1
Objective response rates for these ALK inhibitors in postcrizotinib setting seem similar across most trials.
The ASCEND -1 trial showed an intracranial ORR of 56% (95% CI 45% to 67%) for ceritinib in patients who progressed under crizotinib, and a progression free survival of 6.9 months (95% CI 5.6 to 9.5).2
According to a phase I/II trial, the ORR of brigatinib was 71% (95% CI 59% to 82%) in patients pretreated with crizotinib, with an overall median PFS of 13.4 months.3
Just recently as presented at American Society of Clinical Oncology 2018, the ALEX trial, comparing alectinib with crizotinib in treatment-naive ALK-rearranged NSCLC, showed a PFS of 34.8 months (95% CI 17.7 to not estimable) for alectinib compared with crizotinib 10.4 months (95% CI 9.1 to 12.9), making alectinib now a drug of choice for the front-line therapy of ALK-rearranged NSCLC.4
Currently, third-generation TKIs are being developed and assessed. Lorlatinib is a third-generation ALK/ROS1 TKI for patients who are refractory to both crizotinib and ceritinib.5 The physiochemical properties of lorlatinib were specifically created to have a high potency in inhibiting ALK phosphorylation and to maximise central nervous system (CNS) availability by controlling molecular structure.
The selectivity of lorlatinib is enhanced by targeting of a specific residue in the ALK tyrosine kinase domain—leucine at position 1198 (L1198)—which is detected in only approximately 25% of kinases.5 Lorlatinib is ∼10-fold more potent against wild-type EML4–ALK and ∼40-fold more potent against EML4–ALK L1196M as compared with crizotinib.6 Lorlatinib was designed to be a low-efflux substrate from cell lines overexpressing P-glycoprotein to increase its potential CNS penetration.6 The cerebral response rate varies between 50% and 80%.5
Lorlatinib was recently Food and Drug Administration approved based on a non-randomised, dose-ranging and activity-estimating, multicohort, multicentre phase I/II study, evaluating lorlatinib for the treatment of patients with ALK-positive metastatic NSCLC, who were previously treated with one or more ALK-TKIs. A total of 215 patients with ALK-positive metastatic NSCLC were enrolled across various subgroups based on prior treatment. Among these patients, ORR was 48% (95% CI 42% to 55%), and importantly, 57% had previous treatment with more than one ALK-TKI. In the trial, 69% of patients had a history of brain metastases, and intracranial ORR was 60% (95% CI 49% to 70%). Responses were rapid. The median time to first tumour response was just 1–4 months. Lorlatinib even maintains its clinical activity regardless of number of previous administrated TKIs.5–7
Here, we describe two cases with metastatic ALK-rearranged NSCLC who had pulmonary disease control with first-generation ALK inhibitor. However, treatment with crizotinib had no effect on the CNS metastases. Treatment with lorlatinib led to a remarkable and rapid radiological and clinical response of the CNS metastases.
Case presentation
Case 1
A 48-year-old woman was diagnosed with stage IV NSCLC of the left lower lobe. Molecular analysis on a cervical lymph node revealed an ALK rearrangement, confirmed with fluorescent in situ hybridisation and immunohistochemistry. Treatment with crizotinib 250 mg two times per day was initiated. After 1 month, a follow-up CT scan showed regression of the lung tumour and extrapulmonary metastases. However, tumour markers increased, suggesting incomplete disease control. Since the patient had a clear clinical benefit, treatment with crizotinib was continued.
Case 2
A 54-year-old man with ALK-rearranged stage IV NSCLC of the right lower lobe started with crizotinib. After 4 months, a CT scan showed radiological partial intrathoracic response.
Outcome and follow-up
Case 1
An MRI of the brain was planned despite the fact that she had no symptoms suggesting brain involvement. The MRI of the brain showed multiple metastases on the right parietal and occipital lobe, without evident hydrocephalus (figure 1). At the same time, a thoracoabdominal CT scan showed further regression of the lung tumour and the extrapulmonary metastases. Ceritinib was the only available second-line drug and treatment was started at a dose of 750 mg/day. After 11 days of treatment, the patient, however, developed a progressive paresis of the right arm and reduced consciousness, with a Glascow Coma Scale of 13. The new neurological symptoms were based on a new obstructive hydrocephalus although high doses of dexamethasone (16 mg/day). We placed a ventriculoperitoneal drain to reduce the intracranial pressure. However, several days after start of ceritinib, the patient’s condition continued to deteriorate. At that time, the patient became comatose. Ceritinib was switched to lorlatinib 100 mg/day through a nasogastric tube. Within 2 days, the patient showed neurological improvement with a gradual decline of tumour markers (figure 2). One month after the start of lorlatinib, the patient had a complete recovery of consciousness and a clear improvement of the paresis of the right arm with only minimal neurological sequelae. After 1 year, the patient still has minimal neurological symptoms and a MRI scan of the brain shows no sign of metastases. The intrathoracic disease still continued to decrease in size.
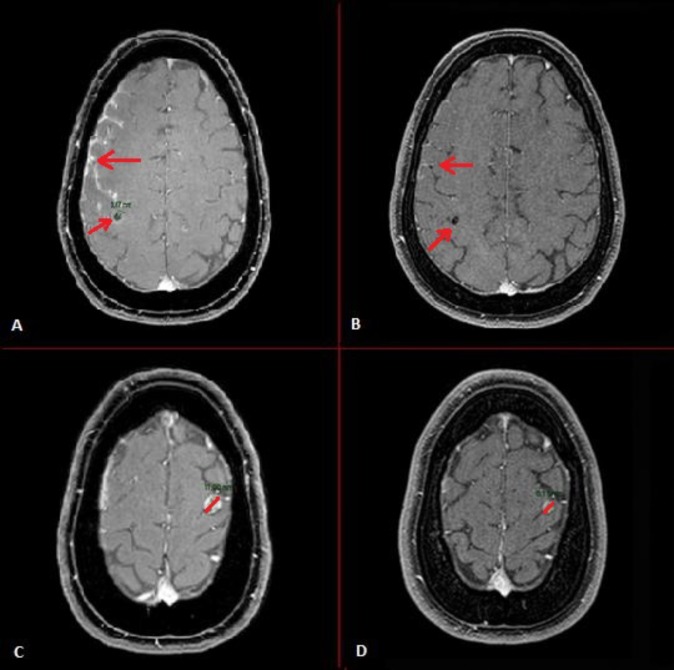
Figure 1.
Resolution of intracranial metastases of patient in case 1. MRI scans of (A) and (C) show multiple intracranial metastases during crizotinib use, whereas, MRI scans of (B) and (D) show regression of the intracranial metastases after 1 week therapy with lorlatinib.
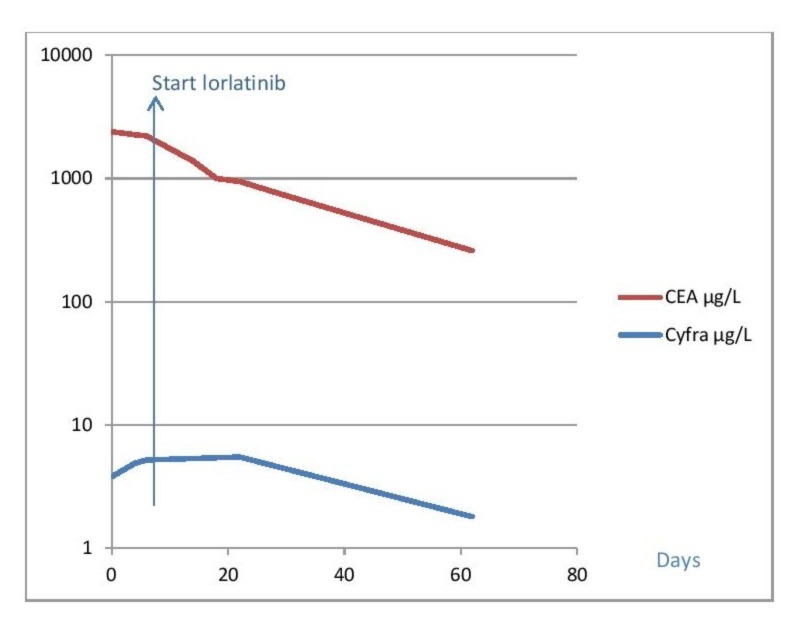
Figure 2.
Tumour markers activity after starting ceritinib and lorlatinib. At baseline, (day 0) the patient in case 1 was using ceritinib. When the neurological symptoms progressed, lorlatinib was started at day 7. Tumour markers declined rapidly, corresponding with the clinical response to lorlatinib.
Case 2
After more than 1 year, the disease slowly progressed, therefore crizotinib was switched to brigatinib.
Unfortunately, after 2 months of treatment with brigatinib, the patient developed headaches, nausea, vomiting and paraesthesia of the right hand. An MRI scan showed multiple new cerebral and leptomeningeal metastases (figure 3), while intrathoracic disease was still stable. Lorlatinib was started 100 mg/day, and the patient recovered fully within days. However, the tumour markers did not decline at a same pace as seen in case 1.
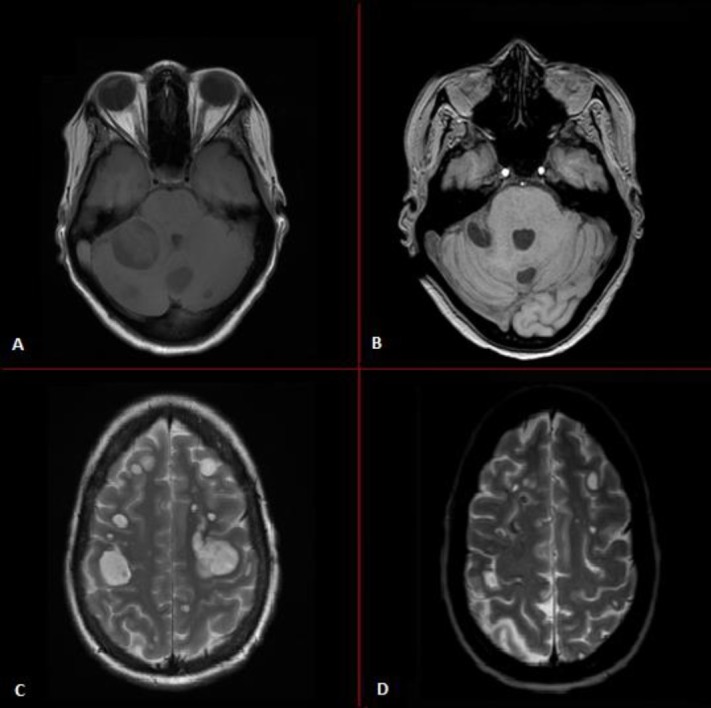
Figure 3.
Resolution of intracranial and leptomeningeal metastases of patient in case 2. MRI scans of (A) and (C) show multiple intracranial and leptomeningeal metastases during brigatinib use, whereas MRI scans of (B) and (D) show regression of the intracranial and leptomeningeal metastases after 1 month therapy with lorlatinib.
After 1 year the patient has no symptoms related to his tumour. The MRI scan shows regression of the cerebral and leptomeningeal metastases, whereas the intrathoracic disease slightly progressed, however without symptoms, and therefore lorlatinib is being continued at the time of reporting.
Discussion
In these two cases, a second-generation ALK-TKI appeared to be less efficacious than lorlatinib in targeting the brain metastases as both patients showed neurological deterioration during treatment with a second-generation ALK-TKI and a rapid clinical and radiological improvement after switch to lorlatinib. There are several reasons for the development of intracranial disease progression in patients treated with a second-generation ALK-TKI.
First, different mechanisms of acquired resistance to first and second-generation TKIs, including secondary ALK mutations, ALK fusion gene amplification and activation of alternative signalling pathways have been identified.5
Lorlatinib is 30-fold more potent than crizotinib in suppressing ALK-dependent signalling and inhibitory cell growth and inducing apoptosis, therefore, making it the most potent inhibitor against all clinical relevant crizotinib, ceritinib and alectinib-resistant ALK mutation, especially against G1202R, which is the most common cause of resistance against first and second-generation TKIs.7–9
In these cases, it is not known whether a mutation had developed resulting in an acquired resistance to crizotinib, brigatinib and ceritinib. Due to the rapid course of the disease and site of progression, a biopsy for molecular analysis could not be obtained.
A second reason for developing intracranial disease progression is the disability to cross the BBB. It is known that the first-generation oral TKIs, such as crizotinib, have a low penetration of the BBB, while the majority of second and third-generation ALK inhibitors efficiently penetrates the BBB, reaching high concentrations in the cerebral spinal fluid. Actually, the ratio of the CNS to serum concentration of crizotinib seems to be in the range of 0.0006–0.001, as established by individual case reports.1 Whereas, in vivo studies demonstrated that CSF concentrations of lorlatinib achieved 75% of unbound plasma concentrations. In addition, BBB penetration by lorlatinib was confirmed by Collier et al using carbon11-labelled and fluorine18-labelled lorlatinib and initial positron emission tomography imaging in a non-human primate model.5
Zou et al demonstrated that a more potent ALK inhibitor with 1.5-fold greater potency than lorlatinib but with a low CNS availability (6%) failed to sustain tumour growth inhibition in the same intracranial tumour model, suggesting penetration into the brain metastasis plays a major role in the activity of lorlatinib.9
Overall, these results suggest that lorlatinib can effectively cross the BBB to induce significant and durable CNS responses.7 8
In our first case, ceritinib was used briefly, therefore the actual effect of ceritinib on the brain metastases remains largely unknown, but a treatment switch was thought to be adamant.
However, the clinical deterioration together with the serum tumour marker pattern suggested progression. Whereas, the use of ventriculoperitoneal drain showed no effect on patient’s clinic, possibly due the already existing hydrocephalus. Measurement of carcinoembryonic antigen (CEA) over time was shown to be useful as a predictive marker for the disease course. Serum CEA levels might reflect the extent of disease and therapeutic response in some ALK-rearranged NSCLC patients.
In case 2, disease progressed despite using brigatinib for 2 months.
The subsequent rapid decline of the tumour markers in case 1 and swift clinical improvement in both patients indicate a rapid antitumour activity of lorlatinib in the brain. This was confirmed both clinically and radiologically in both cases. The good cerebral response on lorlatinib in our patients suggests that lorlatinib can be an alternative to brain radiotherapy in patients with cerebral progression during treatment with a second-generation ALK-TKI.
Learning points.
Lorlatinib seems to be able to overcome cerebral anaplastic lymphoma kinase (ALK) resistance to first or second-generation ALK inhibitor such as crizotinib, ceritinib and brigatinib.
Lorlatinib seems to be able to penetrate the blood–brain barrier and shows a remarkable rapid cerebral response in patients with cerebral progression and a very poor performance status despite treatment with second-generation ALK tyrosine kinase inhibitor (TKI).
Treatment with lorlatinib can therefore be considered as an alternative to brain radiotherapy in patients with rapid neurological deterioration during treatment with a second-generation ALK-TKI.
Footnotes
Contributors: The first two authors HG and QDW were involved in the care of the patient at the ward, with supervision of MVDH. We consulted AC for neurological advise. All authors contributed to the final manuscript with regard to interpreting the outcome and conclusion. HG searched on Pubmed for relevant studies and wrote the manuscript with supervision of QDW, MVDH and AC.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Wu J, Savooji J, Liu D. Second- and third-generation ALK inhibitors for non-small cell lung cancer. J Hematol Oncol 2016;9:19 10.1186/s13045-016-0251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shaw AT, Kim DW, Mehra R, et al. . Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:1189–97. 10.1056/NEJMoa1311107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosell R, Gettinger SN, Bazhenova LA, et al. . Brigatinib efficacy and safety in patients (Pts) with anaplastic lymphoma kinase (ALK)-positive (ALK+) non-small cell lung cancer (NSCLC) in a phase 1/2 trial. J Thorac Oncol 2016;4S:S113–42. [Google Scholar]
- 4. Peters S, Camidge DR, Shaw AT, et al. . Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829–38. 10.1056/NEJMoa1704795 [DOI] [PubMed] [Google Scholar]
- 5. Akamine T, Toyokawa G, Tagawa T, et al. . Spotlight on lorlatinib and its potential in the treatment of NSCLC: the evidence to date. Onco Targets Ther 2018;11:5093–101. 10.2147/OTT.S165511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shaw AT, Bauer TM, Felip E, et al. . Clinical activity and safety of PF-06463922 from a dose escalation study in patients with advanced ALK+ or ROS1+ NSCLC. J Clin Oncol 2015;33:8018 10.1200/jco.2015.33.15_suppl.8018 [DOI] [Google Scholar]
- 7. Solomon BJ, Besse B, Bauer TM, et al. . Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol 2018;19:1654–67. 10.1016/S1470-2045(18)30649-1 [DOI] [PubMed] [Google Scholar]
- 8. Shaw AT, Felip E, Bauer TM, et al. . Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol 2017;18:1590–9. 10.1016/S1470-2045(17)30680-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zou HY, Friboulet L, Kodack DP, et al. . PF-06463922, an ALK/ROS1 inhibitor, overcomes resistance to first and second generation alk inhibitors in preclinical models. Cancer Cell 2015;28:70–81. 10.1016/j.ccell.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]