Abstract
We present an elderly diabetic man with left hallux pain and drainage who was initially diagnosed with acute gouty arthritis using the diagnostic rule for acute gout and monosodium urate crystals presented on synovial fluid analysis. Further investigation with surgical debridement, plain X-ray, MRI and wound culture revealed concomitant Citrobacter koseri septic arthritis with osteomyelitis. C. koseri is considered an opportunistic infection that rarely causes musculoskeletal infections. Acute gouty arthritis and septic arthritis are rarely seen occurring concomitantly in the same joint and are often difficult to differentiate due to similar findings on exam and imaging. The present case illustrates that osteomyelitis with an opportunistic organism can present concomitantly with acute gouty arthritis, and the diagnosis of one should not exclude the other.
Keywords: infections, musculoskeletal and joint disorders, diabetes, pain
Background
Citrobacter koseri is a motile gram-negative bacillus that is part of the normal flora found in mammalian gastrointestinal and urinary tracts.1 It is considered an opportunistic infection and is well known for its involvement in paediatric central nervous system infections like neonatal brain abscesses and meningitis.2 It is very rarely seen in musculoskeletal infections, and currently, there have only been 12 reported cases of C. koseri osteomyelitis in humans with only three of those found in a diabetic foot.3 Concomitant gout has not been present with any case of C. koseri osteomyelitis reported. A study done by Ngonda4 demonstrated that 35.5% of male toilets were contaminated in a large hospital and the prevalence of Citrobacter spp was 9.4%, making bathrooms/toilets a likely significant source of contamination.
Here, we report a case of C. koseri osteomyelitis in an elderly diabetic man with acute gouty arthritis successfully treated with ertapenem.
Case presentation
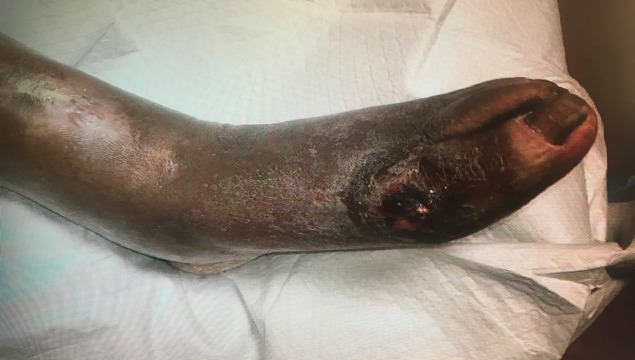
An 81-year-old African American man presented to the emergency department at Advocate Illinois Masonic Medical Center in Chicago, Illinois, complaining of a painful wound on his left foot with a white discharge. The patient had a previous medical history significant for diastolic heart failure, hypertension, chronic kidney disease stage III, peripheral vascular disease, gout, hypothyroidism and a 10-year history of diabetes mellitus type II controlled with oral glimepiride. The patient stated that his wife was taking off his compression stocking the day prior and noticed a white discharge oozing out of a wound on his left foot near the great toe. The patient denied any trauma to the foot, fevers, chills or night sweats. He described the pain as sharp, non-radiating, worse while standing on it and rated it 5/10. On exam, the patient had a 2×2 cm ulceration of the dorsomedial aspect of the metatarsal phalangeal joint of the left hallux open down to the level of the joint with a foul-smelling serous drainage containing irregularly shaped white tophi-like sediment (figure 1). There was erythema, warmth and fluctuance surrounding the wound with non-pitting oedema extending up to the knee. Dorsalis pedis and posterior tibialis arteries were non-palpable in both legs. Motor function was normal in his ankle, foot and toes. Sensation to light touch was severely diminished in both feet.
Figure 1.
Ulceration (2×2 cm) of the dorsomedial aspect of the metatarsal phalangeal joint of the left hallux.
Investigations
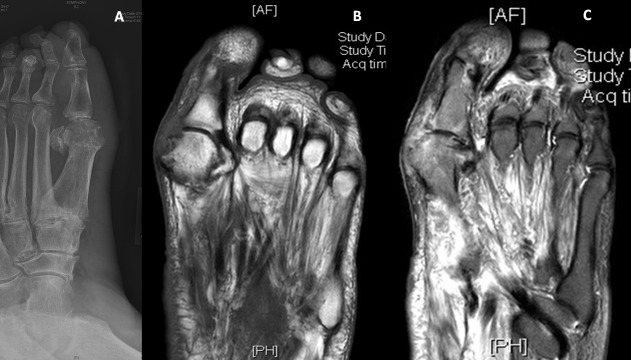
The patient was afebrile on admission, and his initial lab results were remarkable for leucocytes 11.2x109/L, erythrocyte sedimentation rate (ESR) 77 mm/hour and C reactive protein (CRP) 10.5 mg/dL. X-ray of the left foot showed a hallux valgus deformity with irregular bone erosion along the first metatarsal head consistent with osteomyelitis (figure 2A). Podiatry, vascular surgery and infectious disease were consulted from the emergency department. The patient was started on piperacillin–tazobactam 3.375 g intravenously every 8 hours and vancomycin 1500 mg intravenously once for empiric treatment of suspected osteomyelitis. Infectious disease specialist recommended discontinuing the vancomycin after the first dose. Podiatrist performed a bedside debridement and sent a synovial fluid sample for analysis as well as a wound biopsy for gram smear and both anaerobic and aerobic cultures. Initial analysis of synovial fluid showed uric acid crystals, which prompted initiation of a 3-day course of prednisone for a suspected gouty flare. The patient stated that he frequently has gouty attacks of his left great toe even though he is compliant with both colchicine 0.6 mg and allopurinol 100 mg daily. Initial gram smear showed gram-negative bacilli with polymorphonuclear cells. Culture then elucidated pan-susceptible C. koseri. MRI without contrast confirmed osteomyelitis of the medial aspects of the distal first metatarsal and proximal first phalanx of the left foot (figure 2B,C).
Figure 2.
(A) X-ray of the left foot showing a hallux valgus deformity with irregular bone erosion along the first metatarsal head consistent with osteomyelitis. (B) and (C) MRI of the left foot without contrasts: (B) showing T1 image with hypoattenuation and (C) showing T2 image with hyperattenuation in the distal first metatarsal and proximal first phalanx.
Differential diagnosis
The podiatrist suspected that the patient initially had a gouty abscess that drained leading to a secondary bacterial infection of the joint. The vascular surgeon ordered arterial Doppler of both of his lower extremities, which showed moderate to severe peripheral artery disease with monophasic waveforms in the below-the-knee segment in his left lower extremity. The patient previously had a CO2 angiogram done 2 years ago with angioplasty done on the left popliteal, left posterior tibial and left peroneal arteries.
Treatment
Infectious disease specialist recommended ertapenem 1 g intravenously daily for 5 weeks after receiving 4 days of piperacillin–tazobactam and one dose of Vancomycin in the hospital. The minimum inhibitory concentration of piperacillin–tazobactam was ≤4 and given the pan-susceptibility to all tested antibiotics, ertapenem was assumed to be susceptible as well. Although both antibiotics provided adequate coverage, the decision to switch to ertapenem was due to the intravenous dosing regimen. Ertapenem is dosed only once daily while piperacillin–tazobactam is dosed three times daily. The patient was able to have his wife easily administer ertapenem at home once daily through a pre-placed PICC line for the entire 6 weeks of treatment with guidance from a home health nurse. The patient was discharged on day 4 with topical collagenase to be applied to his wound daily, a CO2 angiogram to be done after discharge followed by further debridement by podiatry after the CO2 angiogram.
Outcome and follow-up
On follow-up, labs were drawn at 4 weeks and faxed to the infectious disease office showing resolution of leucocytosis (8.8x109/L) and normal range ESR (20 mm/hour) and CRP (2.2 mg/L). The patient’s wound was evaluated at 7 weeks and showed healthy granulation tissue without evidence of infection (pus, eschar, erythema and so on). This provided sufficient evidence to support complete resolution of the patient’s osteomyelitis.
Discussion
Osteomyelitis caused by C. koseri is very rare, and there have been very few cases of documented musculoskeletal infections in humans.4 Of the reported cases of musculoskeletal infections involving C. koseri, newborns and elderly persons are at the highest risk of infection.4 Diabetes was associated with all three cases of C. koseri osteomyelitis infections involving the foot with peripheral vascular disease being present in two.4–6 Diabetes and peripheral vascular disease are well-known risk factors for skin ulcers and osteomyelitis developing in the feet.7 Certain features can predict the diagnosis of osteomyelitis in diabetic foot ulcers. Typically, the size of the ulcer (>2 cm²), nonspecific serum inflammatory markers like ESR (>70 mm/hour) and imaging studies can significantly increase the likelihood of osteomyelitis.8 The gold standard for diagnosing osteomyelitis is a bone biopsy with culture and tissue analysis but the accuracy of this test is significantly reduced if antibiotics are given prior to biopsy.9 MRI is the recommended imaging modality for diagnosing osteomyelitis and has an accuracy of 89% when the MRI is abnormal.9 In the case presented, empiric vancomycin and piperacillin–tazobactam were initiated prior to bedside debridement; therefore, only wound drainage was collected for analysis and culture. It is recommended that broad-spectrum antibiotics be started as soon as possible because bone biopsies from diabetic foot osteomyelitis frequently yield polymicrobial cultures.10 All of the cases reported of C. koseri osteomyelitis in a diabetic foot have been successfully treated with either monotherapy or a combination of a penicillin, macrolide and/or fluoroquinolone.3 5 6 Our case was the first to report its successful treatment with a carbapenem.
Our case is also the first case reported to have C. koseri osteomyelitis and concomitant acute gouty arthritis affecting the same joint. Lytic lesions caused by osteomyelitis and intra-osseous gout may look similar making it difficult to differentiate between the two.11 Rousseau et al reported five cases where osteomyelitis was initially suspected and treated accordingly based on imaging. After treatment failure in all five cases, further investigation with either needle aspiration or digit amputation revealed gouty arthritis with no evidence of infection.12 Four out of the five cases had a negative smear and negative cultures.12 The last one had a white discharge that grew Staphylococcus and Streptococcus species but it was suspected that the sample may just have abundant monosodium urate (MSU) crystals contaminated with skin-flora; however, the sample was never specifically tested for MSU crystals.12 It is very important to make the distinction between gouty arthritis and osteomyelitis early with bone biopsy or needle aspiration if imaging or clinical presentation is questionable, so there is no delay in appropriate treatment. Dual-energy computed tomography (DECT) is an imaging technique with a sensitivity of up to 85% and specificity of 93% for MSU crystals, and in the right clinical setting, can help make this differentiation early on.11 DECT may be unhelpful in a case like ours where both conditions were present simultaneously.
It is very rare to see both acute gouty arthritis and septic arthritis co-exist in the same joint, and only a few cases have been reported in the first metatarsal phalangeal joint.13 One theory is that this is because gout is episodic in nature rather than being a long-standing chronic condition.14 Acute gouty arthritis and septic arthritis have similar features on physical exam and share similar laboratory derangements (table 1).
Table 1.
Similar features of acute gouty arthritis and septic arthritis
| Physical exam findings | Laboratory values | Radiographic findings |
| Erythema Swelling Tenderness to palpation Pain with movement |
Elevated ESR Elevated CRP Elevated WBC count |
Erosion/lytic lesions Overlying soft tissue swelling Common to have NO signs |
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; WBC, white blood cell.
The gold standard for diagnosing acute gouty arthritis is synovial fluid analysis and the presence of MSU crystals.15 Based on the diagnostic rule proposed by Janssens et al,16 points are allocated to certain variables that are highly predictive of acute gouty arthritis without the need for synovial fluid analysis (table 2).
Table 2.
The diagnostic rule variables are each allocated a score and help predict the likelihood of a diagnosis of acute gouty arthritis
| Diagnostic rule variables | Patient’s variables | Patient’s score |
| Male=2 points | Male | 2 |
| Previous attack=2 points | Yes | 2 |
| Onset within 1 day=0.5 points | Yes | 0.5 |
| Joint redness=1 point | Yes | 1 |
| MTP involvement=2.5 points | Yes | 2.5 |
| HTN or at least one CVD=1.5 points | Yes | 1.5 |
| Uric acid (serum) >5.88 = 3.5 points | No | 0 |
| Total = 9.5 (High probability*) |
*High probability is a score >8, intermediate probability is a score 4–8 and low risk probability is a score <4.
Total, 9.5.
CVD, cardiovascular disease; HTN, hypertension; MTP, metatarsophalangeal.
Lee et al applied the diagnostic rule to a group of 136 patients, 82 of which had acute gout, and 54 had septic arthritis and found it very predictive when comparing the two. They found that the high-risk group had a positive predictive value of 95.3% making it a very useful tool for diagnosing gout.17 They also found that the low-risk group had a negative predictive value of 94.8% making it a useful tool for ruling out gout as well.17
In the case presented, podiatrist initially suspected acute gouty arthritis due to the presence of MSU crystals in the synovial fluid after analysis and the clinical presentation. Our patient’s score was calculated using the diagnostic rule, and with a score of 9.5, he was placed in the high probability group making acute gouty arthritis very likely. After further investigation with MRI and wound cultures, it was found that the patient also had septic arthritis with osteomyelitis. It is important for physicians to be aware that a diagnosis of acute gouty arthritis does not rule out septic arthritis and vice versa. If appropriate treatment for septic arthritis had been delayed, there could have been a devastating outcome given a reported 7%–15% mortality rate for in-hospital septic arthritis.18
To conclude, C. koseri is a rare cause of osteomyelitis in the diabetic foot and, although not common, acute gouty arthritis may co-exist at the same time complicating the diagnosis and potentially delaying lifesaving treatment.
Learning points.
Citrobacter koseri is a rare cause of osteomyelitis in the foot, and risk factors may include diabetes and peripheral vascular disease.
Acute gouty arthritis and septic arthritis may co-exist, and a diagnosis of one should not exclude the other.
Osteomyelitis and intra-osseous gout may look similar on imaging studies.
Footnotes
Contributors: DT took care of the patient and wrote the manuscript. NNK contributed to the writing and reviewing of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Lin SY, Ho MW, Yang YF, et al. Abscess caused by Citrobacter koseri infection: three case reports and a literature review. Intern Med 2011;50:1333–7. 10.2169/internalmedicine.50.4962 [DOI] [PubMed] [Google Scholar]
- 2. Doran TI. The role of Citrobacter in clinical disease of children: review. Clin Infect Dis 1999;28:384–94. 10.1086/515106 [DOI] [PubMed] [Google Scholar]
- 3. Kwaees TA, Hakim Z, Weerasinghe C, et al. Musculoskeletal infections associated with Citrobacter koseri. Ann R Coll Surg Engl 2016;98:446–9. 10.1308/rcsann.2016.0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ngonda F. Assessment of bacterial contamination of toilets and bathroom doors handle/knobs at Daeyang Luke hospital. Pharmaceutical and Biological Evaluations 2017;4:193 10.26510/2394-0859.pbe.2017.31 [DOI] [Google Scholar]
- 5. Lipsky BA, Hook EW, Smith AA, et al. Citrobacter infections in humans: experience at the Seattle Veterans Administration Medical Center and a review of the literature. Rev Infect Dis 1980;2:746–60. 10.1093/clinids/2.5.746 [DOI] [PubMed] [Google Scholar]
- 6. Arslan K, Tufan Z. Osteomyelitis Due to Citrobacter koseri Infection in a Diabetic Patient. J Endocrinol Metab 2011;1:146–8. 10.4021/jem36e [DOI] [Google Scholar]
- 7. Oliver TI, Mutluoglu M. Diabetic Foot Ulcer StatPearls. Treasure Island (FL): StatPearls Publishing StatPearls Publishing LLC, 2019. [Google Scholar]
- 8. Lipsky BA, Aragón-Sánchez J, Diggle M, et al. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev 2016;32(Suppl 1):45–74. 10.1002/dmrr.2699 [DOI] [PubMed] [Google Scholar]
- 9. La Fontaine J, Bhavan K, Lam K, et al. Comparison Between Tc-99m WBC SPECT/CT and MRI for the Diagnosis of Biopsy-proven Diabetic Foot Osteomyelitis. Wounds 2016;28:271–8. [PubMed] [Google Scholar]
- 10. Aragón-Sánchez J, Lipsky BA. Modern management of diabetic foot osteomyelitis. The when, how and why of conservative approaches. Expert Rev Anti Infect Ther 2018;16:35–50. 10.1080/14787210.2018.1417037 [DOI] [PubMed] [Google Scholar]
- 11. An JW, Troster SM. Lytic bone lesions: osteomyelitis or intraosseous gout? J Rheumatol 2017;44:1410–1. 10.3899/jrheum.170125 [DOI] [PubMed] [Google Scholar]
- 12. Rousseau I, Cardinal E E, Raymond-Tremblay D, et al. Gout: radiographic findings mimicking infection. Skeletal Radiol 2001;30:565–9. 10.1007/s002560100392 [DOI] [PubMed] [Google Scholar]
- 13. Prior-Espa˜nol A, García-Mira Y, Mínguez S, et al. Coexistencia de artritis séptica y microcristalina: un reto diagnostic. A propósito de 25 casos. Reumatol Clin 2017. [DOI] [PubMed] [Google Scholar]
- 14. O’Connell PG, Milburn BM, Nashel DJ. Coexistent gout and septic arthritis: a report of two cases and literature review. Clin Exp Rheumatol 1985;3:265–7. [PubMed] [Google Scholar]
- 15. Vaidya B, Bhochhibhoya M, Nakarmi S. Synovial fluid uric acid level aids diagnosis of gout. Biomed Rep 2018;9:60–4. 10.3892/br.2018.1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Janssens HJ, Fransen J, van de Lisdonk EH, et al. A diagnostic rule for acute gouty arthritis in primary care without joint fluid analysis. Arch Intern Med 2010;170:1120–6. 10.1001/archinternmed.2010.196 [DOI] [PubMed] [Google Scholar]
- 17. Lee KH, Choi ST, Lee SK, et al. Application of a novel diagnostic rule in the differential diagnosis between acute gouty arthritis and septic arthritis. J Korean Med Sci 2015;30:700–4. 10.3346/jkms.2015.30.6.700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Margaretten ME, Kohlwes J, Moore D, et al. Does this adult patient have septic arthritis? JAMA 2007;297:1478–88. 10.1001/jama.297.13.1478 [DOI] [PubMed] [Google Scholar]