Abstract
A 75-year-old woman presented with new onset of confusion, intense episodic dizziness and formed visual hallucinations. Herpes simplex encephalitis and non-convulsive temporal lobe seizures were confirmed with cerebrospinal fluid (CSF) and electroencephalography testing. In addition, her hospital course was complicated by syndrome of inappropriate antidiuretic hormone secretion and atonic bladder contributing to an episode of urinary tract infection. After completing 3 weeks of acyclovir treatment, the patient became obtunded with right arm choreiform movements and persistent inflammatory CSF findings not attributable to persistent herpes simplex virus infection or other confounding factors. The patient responded to steroid treatment. Repeated autoimmune and paraneoplastic evaluations were negative. Both clinical (cognitive testing and atonic bladder) and CSF inflammatory finding improved in the follow-up period.
Keywords: neurology, clinical neurophysiology, epilepsy and seizures, immunology
Background
Encephalitis refers to an inflammatory condition of the brain with many causes, including both infectious and non-infectious causes, including autoimmune and paraneoplastic aetiologies. Herpes simplex encephalitis (HSE) is one of the most common infectious causes of encephalitis, accounting for approximately 14% of encephalitis hospital admissions and 22% of inpatient encephalitis deaths in the USA between 2000 and 2010.1 In approximately 5%–26% of HSE cases,2 the development of neurological relapses or worsening of deficits after the completion of acyclovir treatment is a difficult complication. There are number of possibilities for such a presentation, including autoimmune aetiologies. We describe a case of postinfectious steroid-responsive encephalitis after HSE. In addition to providing an approach to evaluate such patients, we also describe neurogenic bladder as a rare and less recognised complication of HSE.
Case presentation
A 75-year-old woman presented to the emergency room with a 3-day history of confusion and episodic intermittent intense isolated dizziness. During this period, the patient also reportedly experienced a formed visual hallucination of seeing her daughter, a sight not confirmed by her husband. Aside from one episode of self-limiting, moderate headache 1 day prior to presentation, no other significant focal neurological or constitutional symptoms were elicited, including fever, numbness/tingling, weakness or sensitivity to light or sound. Two months prior to presentation, she had a diagnosis of shingles on her face. This was treated with antivirals without any recurrence of her rash since that time.
Her medical history was notable for hypertension, atrioventricular block status post pacemaker placement, osteoarthritis, scoliosis and bilateral total hip replacement. Her medications included perindopril and as needed ibuprofen. Her father died from Parkinson disease and her mother died from an unknown malignancy. Patient was a former smoker and consumed alcohol socially. Patient denied any history of Illicit drug use. Her vitals, general and neurological examination were unremarkable except for deficits identified on higher mental function testing involving memory, insight, orientation, reasoning as well as mild difficulty with gait.
Investigations
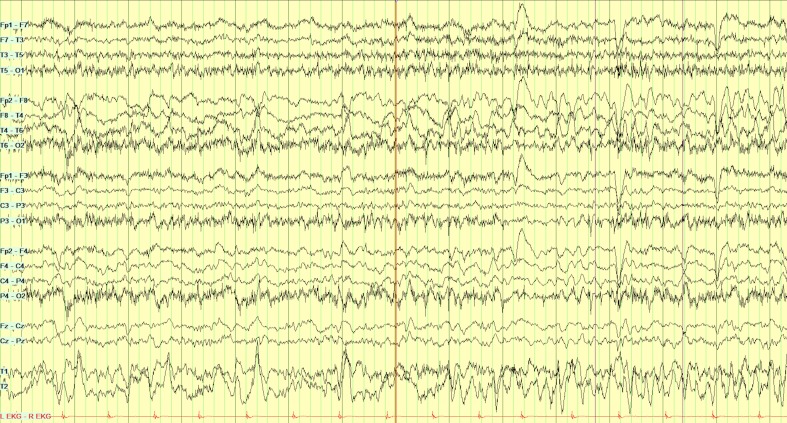
Her complete blood count, comprehensive metabolic profile, thyroid-stimulating hormone and erythrocyte sedimentation count were normal except for a sodium of 135 (normal 136–145 mEq/L). A CT scan of the brain and chest X-ray (CXR) were unremarkable. MRI brain could not be obtained due to the incompatible implanted pacemaker. Cerebrospinal fluid (CSF) analysis showed pleocytosis of 51 (normal 0–5/mm3) with lymphocytic predominance (44%), 23 red blood cells and positive PCR for herpes simplex virus 1 (HSV-1); the remaining CSF studies including cytology were negative. Video electroencephalography (EEG) showed moderate right-sided focal frontal temporal slowing with frequent moderate amplitude sharp waves. A right temporal electroencephalographic seizure was also identified on Electroencephalography (figure 1).
Figure 1.
Electroencephalography at first admission showing a right temporal electroencephalographic seizure. Subclinical electrographic seizure, lasting for 34 s, consists of a run of initial rhythmic theta-delta discharge over the right temporal region that then increases in amplitude and then slows down to delta waves with 1 Hz spike waves before abruptly ending.
Treatment
Our patient was started on empiric acyclovir, ceftriaxone, vancomycin and ampicillin. Antibiotics were discontinued after the positive HSV PCR result. Levetiracetam was started for electroencephalographic seizures.
Her hospital course was complicated by syndrome of inappropriate antidiuretic hormone secretion (SIADH), with a sodium nadir of 124 on the second day of admission. Treatment initially required hypertonic saline, followed by tolvaptan, which resulted in gradual normalisation of serum sodium.
In addition, she developed urinary retention with postvoid residual (PVR) urine volume of more than 600 mL. Urological consultation was obtained, and the impression was that her symptoms were multifactorial in the setting of cognitive dysfunction, hyponatraemia and likely an underlying atonic bladder. Intermittent catheterisation was started with a plan for urodynamic studies should urinary retention not improve. After 4 days of hospitalisation, patient was discharged to a rehabilitation facility on tolvaptan and levetiracetam with a plan to complete 3-week course of intravenous acyclovir.
Outcome and follow-up
Two weeks after her initial presentation and while receiving intravenous acyclovir, the patient was readmitted to our inpatient service due to both persistent and worsening symptoms. In addition to an ongoing fever (maximum temperature of 39.2οC), she also developed worsening cognitive impairment, somnolence, generalised weakness and difficulty walking. She had no symptoms of headache, photophobia, dysuria, diarrhoea, cough or rash.
Her initial investigations were significant for abnormal urine analysis with >50 white blood cells. She was initially treated at rehabilitation facility with cefexime and vancomycin which were changed to ampicillin after cultures and returned positive for Escherichia coli and Enterococcus. A CT scan of the abdomen and pelvis with contrast was negative for a perinephric or other occult abscess. Her tolvaptan dose was increased from 10 mg to 15 mg/day after her sodium declined to 132 mq/L, resulted in its normalisation. On presentation, a lumbar puncture (LP) was not performed for several reasons. First, it was thought that it would be unlikely that her HSV-1 encephalitis had worsened while receiving active treatment with acyclovir. In addition, HSV-1 DNA can remain detectable in the CSF for several weeks after treatment, which reduced the importance of HSV PCR testing. Finally, acyclovir resistance was thought to be atypical or unlikely in this setting as patient is immunocompetent and had a good initial response to acyclovir. A drug fever from acyclovir was of theoretical concern, occurring in <1% of cases. As this would not explain her remaining symptoms, and because the benefits outweighed the risks of discontinuation, acyclovir was continued. A urinary tract infection (UTI) was thought to be the most likely cause of her fevers and encephalopathy. After her symptoms improved, though not completely to baseline, she was transferred back to the rehabilitation centre. She was maintained on acyclovir, ampicillin and tolvaptan.
Four weeks from initial presentation, after finishing the acyclovir course, the patient was readmitted with worsening encephalopathy and the development of new generalised choreiform movements, worse in right arm. Her vital signs were normal. Her examination revealed an obtunded state with response limited to intermittent eye opening to verbal command. She also demonstrated diffuse paratonia, withdrawal to painful stimuli, and choreiform movements as described previously, but otherwise lacked lateralisation.
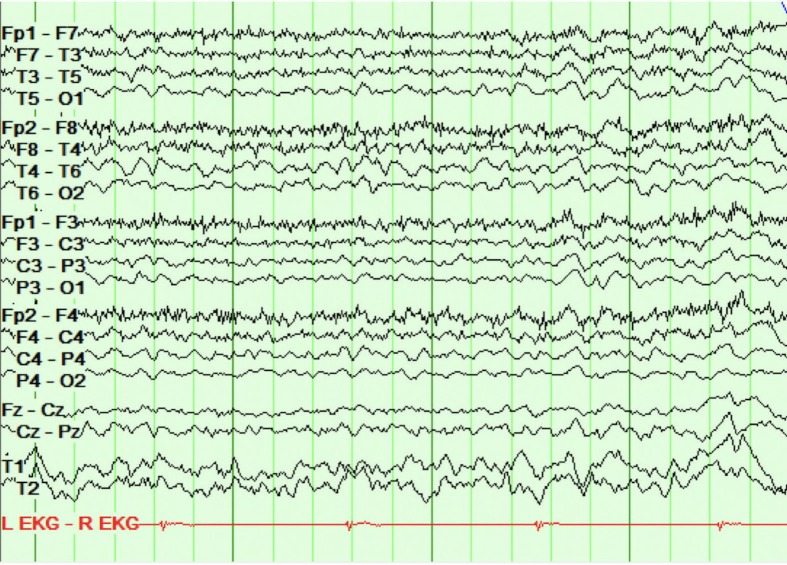
Repeat complete blood count, complete metabolic profile, blood and urine cultures, CXR and head CT were negative. Repeat LP demonstrated significant protein elevation (186 mg/dL, normal 12–60), lymphocytic pleocytosis (44%, 44 white blood cells, 90% lymphocytic), normal glucose, no red blood cells and negative HSV PCR. EEG (figure 2) showed generalised mild to moderate, 5–7 Hz, generalised slowing; previously seen focal slowing and electrographic seizures were not identified. A CT angiogram of the head and neck, as well as inflammatory and connective tissue markers (erythrocyte sedimentation rate, antinuclear antibody, antineutrophil cytoplasmic antibodies, Anti-Sjögren’s-syndrome-related antigen A (anti-SSA) antibodies and Anti-Sjögren’s-syndrome-related antigen B (anti-SSB antibodies), was negative and not suggestive of vasculitis. Malignancy workup with CT abdomen, pelvis and chest was negative. An autoimmune encephalopathy panel, including anti-NMDA antibodies, was obtained (table 1). Pending the results, despite a negative HSV PCR, considering lack of a clear alternate diagnosis and relative safety of acyclovir, acyclovir was initiated for presumed HSV reactivation and presumed false-negative PCR test.
Figure 2.
Electroencephalography (EEG) at third admission showing mild-to-moderate generalised slowing with no seizure. Repeat EEG showed generalised mild-to-moderate, 5–7 Hz, generalised slowing; previously seen focal slowing and electrographic seizures were not identified.
Table 1.
Autoimmune encephalopathy panel during first admission
| Result name | Result |
| NMDA-R Ab, CSF | Negative |
| VGKC-complex Ab, CSF | 0.00 |
| LGI1-IgG, CSF | Negative |
| CASPR2-IgG, CSF | Negative |
| GAD65 Ab Assay, CSF | 0.00 |
| GABA-B-R Ab, CSF | Negative |
| AMPA-R Ab, CSF | Negative |
| Antineuronal nuclear Ab, type 1 | Negative |
| Antineuronal nuclear Ab, type 2 | Negative |
| Antineuronal nuclear Ab, type 3 | Negative |
| Antiglial nuclear Ab, type 1 | Negative |
| Purkinje cell cytoplasmic Ab type 1 | Negative |
| Purkinje cell cytoplasmic Ab type 2 | Negative |
| Purkinje cell cytoplasmc Ab type Tr | Negative |
| Amphiphysin Ab, CSF | Negative |
| CRMP-5-IgG, CSF | Negative |
Ab, antibody; AMPAR, alpha-amino-3-hydroxy-5-methyl-4 isoxazolepropionic acid receptor; Caspr2, contactin-associated protein-like 2; CSF, cerebrospinal fluid; GAD65, glutamic acid decarboxylase; LGI1, leucine-rich glioma inactivated protein-1; NMDA-R, anti-N-methyl-d-aspartate receptor antibodies; VGKC, voltage gated potassium channel.
The patient’s condition continued to deteriorate despite treatment, such that her family had considered a comfort-oriented plan. A trial of intravenous methylprednisolone (1 g daily) was started on the second day of repeated acyclovir treatment, at this time treating for presumed autoimmune post herpes encephalitis. Within 24–48 hours of steroid treatment, patient had significant improvement in her cognition with the resolution of her abnormal movements. Acyclovir was replaced with oral valacycovir to prevent HSV relapse. Patient received 5 days of intravenous methylprednisolone which then was changed to oral prednisone 60 mg/day. CSF and serum autoimmune panel subsequently came back normal. Patient was discharged on tapering prednisone schedule.
Three months post discharge, she showed progressive improvement at follow-up. A Montreal Cognitive Assessment Test Score improved to 26/30 compared with 14/30 in the rehabilitation unit. Her urinary retention persisted for up to 10 weeks, after which she was able to void independently with repeat PVR of 0 mL. Paralleling her clinical improvement, a repeat CSF testing at 6 months showed significant decrease in white cell count from 0.044 to 0.0090×109/L and a significant decrease in protein level from 186 to 95, prompting the discontinuation of levetiracetam. To expand the search for autoimmune aetiologies, a repeat CSF autoimmune encephalopathy panel sent to an international research laboratory returned negative (table 2).
Table 2.
Expanded autoimmune antibodies testing at international research laboratory
| Result name | Result |
| NMDA-R Abs, CSF | Negative |
| AMPA Abs, CSF | Negative |
| GABA (A) Abs, CSF | Negative |
| GABA (B) Abs, CSF | Negative |
| mGLuR1 abs, CSF | Negative |
| mGluR5 abs | Negative |
| LGI1 | Negative |
| Caspr2 | Negative |
| DPPX | Negative |
| Neurexin3 | Negative |
| Iglon5 | Negative |
CSF, cerebrospinal fluid; DPPX, dipeptidyl-peptidase-like protein-6; GABA, gamma-aminobutyric acid; NMDA-R, anti-N-methyl-d-aspartate receptor antibodies.
Discussion
Our case highlights multiple features associated with the diagnosis, management and complications of herpes encephalitis.
Suspected HSE without MRI of brain
It is important to consider the diagnosis of HSE in an elderly patient when the presenting symptoms are non-specific, especially in the absence of signs of infection (no fever or systemic leucocytosis) and when contraindication existed for the MRI of brain. Temporal lobes are preferentially involved in HSE through either olfactory or trigeminal nerves, which provide viral access into the central nervous system (CNS).3 In the absence of MRI, which demonstrates diagnostic abnormalities in approximately 90% of patients with PCR-proven HSE, additional clues confirming temporal lobe involvement were present in history and ancillary investigations of our case.4
For example, while our patient had non-specific symptoms including headache and changes in mental status, she was also experiencing visual hallucinations. Visual hallucinations can be divided into simple (or non-formed) versus complex hallucinations. Simple hallucinations are often distortions of size, shape and colour, such as seen with migraine, and localise to occipitoparietal regions of the cortex. Complex hallucinations can take the form of people, animals or other familiar objects, and localise to the occipitotemporal region and the temporal lobe.5 In our case, her formed hallucinations represented complex hallucinations, suggestive of temporal lobe involvement.
In addition, intense periodic dizziness, seen in our case, was best explained on the basis of electroencephalographic seizures emanating from right temporal region. Isolated episodic dizziness/vertigo has rarely been reported due to temporal lobe seizures, with a right-hemisphere dominance, postulated due to the right hemisphere’s predominant role in spatial perception.6 7 Focal right temporal slowing and electrographic seizures, seen on EEG in our case, was in keeping with the previous studies, where diffuse slowing, focal abnormalities in the temporal regions or periodic lateralising epileptiform discharges (PLEDs) were seen in approximately >80% of cases with PCR-proven HSE.8
Furthermore, the diagnosis in our case was confirmed by the detection of HSV DNA in the CSF by PCR, a gold standard test with high sensitivity (98%) and specificity (94–100%).9 While PCR is positive early in the course of the illness (within the first 24 hours of onset of symptoms), it could be negative in hyperacute presentation, suboptimal assay, or in the presence of substances in the CSF that inhibit amplification (eg, blood), necessitating clinical correlation and treatment for HSE with acyclovir.
Considerations for worsening of neurological deficits after treatment of HSE
It is important to remember the variety of potential diagnoses in the development of neurological relapses or worsening of deficits after the diagnosis and treatment of HSE. A broad differential diagnosis looking for toxic-metabolic, viral, drug-induced, inflammatory and autoimmune aetiologies (figure 3) should be entertained.
Figure 3.
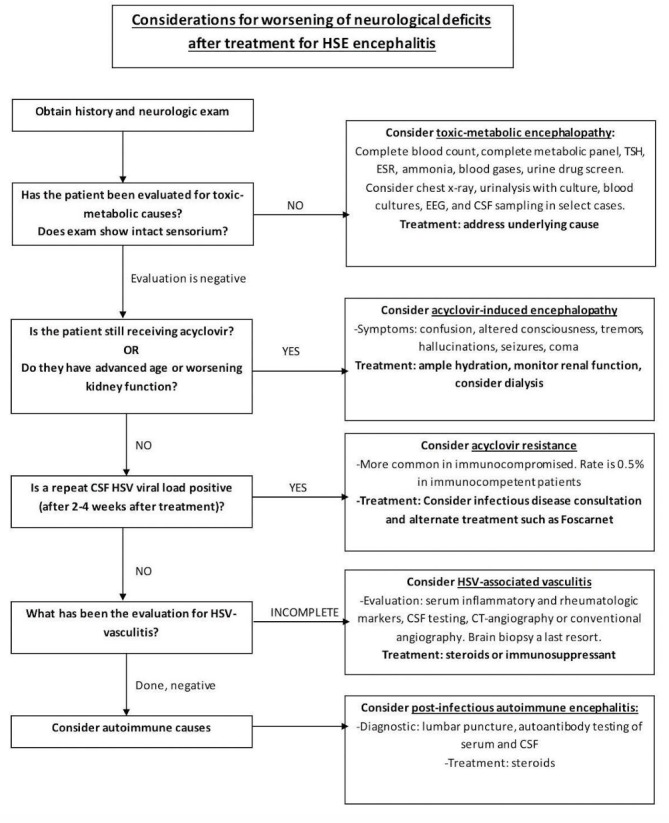
Considerations for worsening of neurological deficits after the treatment of herpes simplex encephalitis (HSE). In patients with recent HSE with recurrence or worsening of deficits, one needs to consider common toxic, metabolic, drug-induced or infectious causes. If these are evaluated or treated, and the symptoms cannot be explained on the basis of aforementioned aetiologies (acyclovir-induced encephalopathy, acyclovir resistance leading to treatment failure and herpes simplex virus (HSV)-associated CNS vasculitis or non-convulsive seizures), repeated cerebrospinal fluid (CSF) testing should be considered. If reactivation or persistence of the HSV is excluded by the absence of detection of viral DNA in CSF, one should consider the possibility of inflammatory or autoimmune aetiologies. Therefore, the consideration of autoantibody testing should be considered in these circumstances to help establish a diagnosis. EEG, electroencephalography; ESR, erythrocyte sedimentation rate; TSH, thyroid-stimulating hormone.
In our patient, the evaluation for toxic-metabolic aetiologies was negative except for a UTI, which could be an explanation of her fluctuating neurological deficits. However, persistent worsening despite antibiotic and antiviral treatment suggested additional causes for her ongoing encephalopathy.
The differential for worsening symptoms should include causes related to resistance or toxicity of treatment for HSE. While acyclovir is well tolerated, even at high doses, a syndrome of acyclovir-induced encephalopathy has been reported. This typically occurs in older patients with pre-existing renal dysfunction, hypothesised be due to preferential accumulation of acyclovir metabolite, 9-carboxymethoxymethylguanine.10 11 While establishing a temporal relationship between drug intake and onset of symptoms is important, differentiation between the HSE and acyclovir-induced encephalopathy can be challenging due to overlapping clinical features. Signs of meningismus, abnormal CSF and imaging are helpful. However, CSF abnormalities have been reported in acyclovir neurotoxicity due to the presence of neurovascular inflammation.11 Renal elimination accounts for up to 90% of acyclovir clearance, emphasising the importance of ample hydration to avoid the potential of acyclovir-induced nephrotoxicity and dose adjustment in patients with creatinine clearance <50 mL/min. Some patients may recover with acyclovir discontinuation or dose reduction. Others may require dialysis. Since a 6-hour dialysis session has been shown to remove 60% of acyclovir from the circulation, an extended session (or more frequent sessions) of dialysis is suggested to facilitate drug removal.10 11 While our patient did not develop acyclovir-induced nephrotoxicity, she had SIADH, which typically would have required fluid restriction. Since this intervention had the potential of aggravating acyclovir-induced crystal-induced acute kidney injury, we instead used tolvaptan, a selective vasopressin receptor 2antagonist, allowing us to liberalise the fluids and prevent this potential complication.
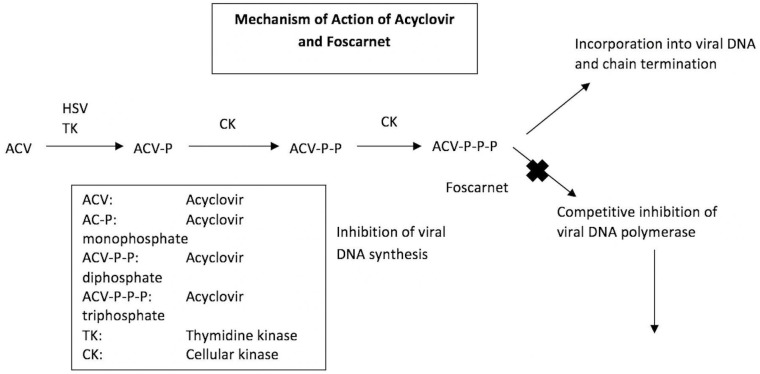
Acyclovir resistance has rarely been described in HSE, more common in immunocompromised patients and with prolonged use. The prevalence of acyclovir resistance can vary between 0.5% in immunocompetent patients versus 3.5%–10% in immunocompromised patients.12 Acyclovir resistance in HSE had been documented to occur either via a mutation of the viral thymidine-kinase gene, mediating approximately 95% of cases of acyclovir resistance, or via mutation of the DNA polymerase gene (figure 4).13 Resistance to acyclovir can be established by clinical observation (deterioration despite antiviral therapy and persistent virus load in the CSF), phenotyping (testing the susceptibility of a viral isolate to different drug doses) or genotyping (sequencing of thymidine kinase and DNA polymerase genes to identify specific mutations linked with acyclovir resistance).14 15 Before diagnosing acyclovir resistance, one should be mindful that HSV DNA can be detectable via PCR analysis of the CSF for at least 2 weeks and up to 1 month after the onset of clinical disease. Foscarnet, a direct inhibitor of the virus DNA polymerases can be used as an alternative drug for the treatment of HSV-resistant patients. The possibility of acyclovir resistance in our case was ruled out due to negative PCR in the repeat CSF study.
Figure 4.
Mechanism of action of acyclovir and foscarnet. Acyclovir is converted by viral thymidine kinase (TK) to acyclovir monophosphate encoded by the human herpesvirus 1 UL23 gene. Further phosphorylation steps occur through cellular TK lead to the formation of activated acyclovir triphosphate. The active form inhibits herpes simplex virus (HSV) replication by selective inhibition of viral DNA polymerase and by the termination of growing viral DNA strands. Foscarnet, a direct inhibitor of the virus DNA polymerases can be used as an alternative drug for the treatment of HSV-resistant patients.
Another consideration for deterioration in this patient was HSV-associated CNS vasculitis. While this complication is more commonly reported with varicella zoster virus, there are rare reports of HSV-associated vasculitis, particularly in neonates.16 This complication has been hypothesised due to transaxonal spread of reactivated virus to the arterial adventitia followed by transmural spread within the vessels. This leads to their destruction due to either direct invasion by the virus or from the immune response related to the invasion. Proposed mechanisms for such immune-mediated vasculopathies include either a direct attack by antiendothelial antibodies or immune complex-associated inflammation and generation of membrane attack complexes.16 The diagnosis of HSV-associated vasculitis is established by combination of imaging studies, CSF profile, angiogram and if required brain biopsy. In our case, both imaging and angiogram studies were not supportive of vasculitis.
Once above-mentioned possibilities are excluded, one should consider the possibility of inflammatory or autoimmune aetiologies. While a strong initial immune response is required to suppress viral replication early in the course of infection, an exaggerated sustained immune activation may contribute to secondary inflammatory changes and cerebral oedema.17 As a result, the pathogenesis of HSE includes both initial direct virus-mediated cell lysis as well as immune-mediated pathological changes.17 18 Recent studies have reported that within 2 months, 27% of the patient with HSE who were originally negative for anti-N-methyl-d-aspartate receptor antibodies (NMDAR) showed the presence of these antibodies in the setting of relapsing symptoms not attributable to HSE relapse. In young children (aged ≤4 years), choreoathetosis was the prominent phenotypic feature while older children (aged >4 years) and adults exhibited predominantly behavioural symptoms.18 Postulated mechanisms include molecular mimicry such as similarity between NMDAR and HSV proteins or via release of antigenic proteins from HSV-induced neuronal injury that become autoimmune targets. In our case, repeated testing for autoimmune and paraneoplastic aetiologies related to known antigens was negative. Therefore, our case most probably represented steroid-responsive post HSE related to exaggerated immune activation and cerebral oedema. While waiting for prospective studies, based on the anti-inflammatory properties of corticosteroids documented in animal models as well as case reports and retrospective analyses in humans, prophylactic use of corticosteroids, without increasing the risk of enhanced viral replication and dissemination, could be considered during the viral phase of the disease to prevent the development of autoimmune/inflammatory complications.19–21
Atonic bladder in the setting of HSE
Another finding in our case was of urinary retention due to atonic bladder suggested by PVR of 600 mL. HSE has rarely been reported to have associated urinary retention, with onset from 5 days to 2 months. In these reports, HSE-associated urinary retention occurred in the setting of concomitant myeloradiculitis, encephalomyeloradiculitis, acute disseminated encephalomyelitis or herpetic brainstem encephalitis.22–25 With lack of brainstem deficits, back pain, long track signs, sensorimotor or reflex findings to suggest pontine micturition centre, spinal cord, cauda equina or peripheral nerve involvement, our case is suggestive of a previous hypothesis of preferential parasympathetic pelvic nerve involvement in HSE. Like previous reports,22 resolution was documented in our case as well. Since most patients in the early phases of HSE require bladder catheterisation due to disturbances in consciousness, bladder involvement in HSE may be under-reported, requiring larger scale studies to confirm and characterise such an association.
In summary, our case emphasised the importance of an organised approach in the evaluation of patients with persistent encephalopathy after treatment with acyclovir for HSE. Moreover, a less recognised complication of neurogenical bladder in HSE is highlighted. Finally, adjunctive corticosteroids with concomitant acyclovir is currently under study, but may have a theoretical role in reducing potential autoimmune complications after HSE.
Patient perspective’s.
I have little recollection of my illness and rely on my husband and family for details.
My illness came on very suddenly. I woke up one morning feeling very lethargic with a pressure in my head and a tremendous sense of doom, but no other symptoms. When I hallucinated about my children who weren’ t there my family took me to the ER where I was diagnosed with viral encephalitis. After a week on antiviral drugs I went to a rehab center where I continued to improve at first. But then my condition deteriorated, and I eventually ended up semi-conscious, clutching the bedrails and shaking uncontrollably. I was sent back to hospital where my condition deteriorated into a coma. I was put on high doses of prednisone, but the situation seemed hopeless and my family prepared themselves for the worst. My husband arrived at the hospital in the early morning prepared to say goodbye to me. But I had regained consciousness in the night and asked, ‘Where have you been?’ when he came in. It was so great, like a scene from a movie.
My improvement after this episode was much slower than the first time, but I finally made it out of rehab and back home. Once at home my physical condition improved rapidly but I had no memory of my hospitalization, and memory and cognitive issues remained. I had to relearn basic household and cooking procedures, even recipes I had done hundreds of times.
Now, a year later, I’m probably 95% back to where I was before my illness. I hike and snowshoe for several miles but have a bit more trouble with balance. Cognitively, I have some memory and orientation issues but it’s hard to tell what is disease-related and what just age is.
I feel that I am incredibly lucky and extend my heartfelt thanks to the entire UVM team and especially to you.
Learning points.
An algorithmic approach with variety of possibilities should be considered in patients with herpes simplex encephalitis who relapse after initial acyclovir. Such possibilities include toxic-metabolic aetiologies, acyclovir-induced encephalopathy and acyclovir resistance leading to treatment failure, herpes simplex virus (HSV)-associated central nervous system vasculitis, non-convulsive seizures and inflammatory or autoimmune aetiologies.
An exaggerated sustained immune activation with or without identifiable antineuronal antibodies contributing to secondary inflammatory changes and cerebral oedema should be suspected in any patient who comes with neurocognitive changes or abnormal movement few weeks after HSV encephalitis.
Immunotherapy should be considered for post herpetic immune-mediated encephalitis while waiting for the results of cerebrospinal fluid and serum neuronal cell surface antibodies. Atonic bladder presenting as urinary retention is a rare complication of HSV encephalitis.
Footnotes
Contributors: LM: collected the date including the history of present illness, workup, treatment and follow-up, collected the EEG images and write the description, obtained patient’s consent, wrote the paper, wrote the abstract and submitted the paper. RF: reviewed and edited the discussion and introduction parts of paper, write the mechanism of action of acyclovir and discussion of neurological deficits in post HSE patient (images 3 and 4), helped with final edition of abstract and paper. AM: reviewed and edited the description of EEG images, reviewed and edited the discussion part of paper and helped with final edition of abstract and paper. WW: reviewed and edited the paper, reviewed and edited the data including history of present illness, workup and follow-up, reviewed and edited images 3 and 4, reviewed and edited the abstract, provide guidance to writing and organising the paper.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. George BP, Schneider EB, Venkatesan A. Encephalitis Hospitalization Rates and Inpatient Mortality in the United States, 2000-2010. PLoS One 2014;9:e104169 10.1371/journal.pone.0104169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Tiège X, Rozenberg F, Des Portes V, et al. Herpes simplex encephalitis relapses in children: differentiation of two neurologic entities. Neurology 2003;61:241–3. 10.1212/01.WNL.0000073985.71759.7C [DOI] [PubMed] [Google Scholar]
- 3. Menasria R, Canivet C, Piret J, et al. Infiltration Pattern of Blood Monocytes into the Central Nervous System during Experimental Herpes Simplex Virus Encephalitis. PLoS One 2015;10:e0145773 10.1371/journal.pone.0145773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hatipoglu HG, Sakman B, Yuksel E. Magnetic resonance and diffusion-weighted imaging findings of herpes simplex encephalitis. Herpes 2008;15:13. [PubMed] [Google Scholar]
- 5. Bien CG, Benninger FO, Urbach H, et al. Localizing value of epileptic visual auras. Brain 2000;123(Pt 2):244–53. 10.1093/brain/123.2.244 [DOI] [PubMed] [Google Scholar]
- 6. Tarnutzer AA, Lee SH, Robinson KA, et al. Clinical and electrographic findings in epileptic vertigo and dizziness: a systematic review. Neurology 2015;84:1595–604. 10.1212/WNL.0000000000001474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hewett R, Guye M, Gavaret M, et al. Benign temporo-parieto-occipital junction epilepsy with vestibular disturbance: an underrecognized form of epilepsy? Epilepsy Behav 2011;21:412–6. 10.1016/j.yebeh.2011.05.017 [DOI] [PubMed] [Google Scholar]
- 8. Rose JW, Stroop WG, Matsuo F, et al. Atypical herpes simplex encephalitis: clinical, virologic, and neuropathologic evaluation. Neurology 1809;42:1809 10.1212/WNL.42.9.1809 [DOI] [PubMed] [Google Scholar]
- 9. Wildemann B, Ehrhart K, Storch-Hagenlocher B, et al. Quantitation of herpes simplex virus type 1 DNA in cells of cerebrospinal fluid of patients with herpes simplex virus encephalitis. Neurology 1997;48:1341–1346. 10.1212/WNL.48.5.1341 [DOI] [PubMed] [Google Scholar]
- 10. Helldén A, Lycke J, Vander T, et al. The aciclovir metabolite CMMG is detectable in the CSF of subjects with neuropsychiatric symptoms during aciclovir and valaciclovir treatment. J Antimicrob Chemother 2006;57:945–9. 10.1093/jac/dkl067 [DOI] [PubMed] [Google Scholar]
- 11. Thind GS, Roach R. A case of acyclovir neurotoxicity presenting with atypical cerebrospinal fluid findings. BMJ Case Rep 2017;2017:bcr-2017-220372 10.1136/bcr-2017-220372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stránská R, Schuurman R, Nienhuis E, et al. Survey of acyclovir-resistant herpes simplex virus in the Netherlands: prevalence and characterization. J Clin Virol 2005;32:7–18. 10.1016/j.jcv.2004.04.002 [DOI] [PubMed] [Google Scholar]
- 13. Andrei G, Snoeck R. Herpes simplex virus drug-resistance: new mutations and insights. Curr Opin Infect Dis 2013;26:551–60. 10.1097/QCO.0000000000000015 [DOI] [PubMed] [Google Scholar]
- 14. Sauerbrei A. Acyclovir resistance in herpes simplex virus type I encephalitis: a case report. J Neurovirol 2017;23:638–9. 10.1007/s13365-017-0537-9 [DOI] [PubMed] [Google Scholar]
- 15. Ogura H, Fukae J, Kimura S, et al. Acyclovir resistant acute herpes simplex encephalitis associated with acute retinal necrosis: A case report and review of the literature. Rinsho Shinkeigaku 2017;57:230–3. 10.5692/clinicalneurol.cn-000959 [DOI] [PubMed] [Google Scholar]
- 16. Joshi P. Multiple strokes associated with herpes simplex virus type-2 infection: case report. J Neurovirol 2016;22:251–3. 10.1007/s13365-015-0385-4 [DOI] [PubMed] [Google Scholar]
- 17. Piret J, Boivin G. Innate immune response during herpes simplex virus encephalitis and development of immunomodulatory strategies. Rev Med Virol 2015;25:300–19. 10.1002/rmv.1848 [DOI] [PubMed] [Google Scholar]
- 18. Armangue T, Spatola M, Vlagea A, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol 2018;17:760–72. 10.1016/S1474-4422(18)30244-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meyding-Lamadé UK, Oberlinner C, Rau PR, et al. Experimental herpes simplex virus encephalitis: a combination therapy of acyclovir and glucocorticoids reduces long-term magnetic resonance imaging abnormalities. J Neurovirol 2003;9:118–25. 10.1080/13550280390173373 [DOI] [PubMed] [Google Scholar]
- 20. Ramos-Estebanez C, Lizarraga KJ, Merenda A. A systematic review on the role of adjunctive corticosteroids in herpes simplex virus encephalitis: is timing critical for safety and efficacy? Antivir Ther 2014;19:133–9. 10.3851/IMP2683 [DOI] [PubMed] [Google Scholar]
- 21. Martinez-Torres F, Menon S, Pritsch M, et al. Protocol for German trial of Acyclovir and corticosteroids in Herpes-simplex-virus-encephalitis (GACHE): a multicenter, multinational, randomized, double-blind, placebo-controlled German, Austrian and Dutch trial [ISRCTN45122933]. BMC Neurol 2008;8:40 10.1186/1471-2377-8-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fukuoka T, Nakazato Y, Miyake A, et al. A case of urinary retention in the early stages of herpes simplex virus type-1 encephalitis. Clin Neurol Neurosurg 2017;157:17–18. 10.1016/j.clineuro.2017.03.011 [DOI] [PubMed] [Google Scholar]
- 23. Naito K, Hashimoto T, Ikeda S. Herpes simplex virus type-1 meningoencephalitis showing disseminated cortical lesions. Intern Med 2007;46:761–4. 10.2169/internalmedicine.46.6276 [DOI] [PubMed] [Google Scholar]
- 24. Kusuhara T, Nakajima M, Inoue H, et al. Parainfectious encephalomyeloradiculitis associated with herpes simplex virus 1 DNA in cerebrospinal fluid. Clin Infect Dis 2002;34:1199–205. 10.1086/339811 [DOI] [PubMed] [Google Scholar]
- 25. Sakakibara R, Hattori T, Fukutake T, et al. Micturitional disturbance in herpetic brainstem encephalitis; contribution of the pontine micturition centre. J Neurol Neurosurg Psychiatry 1998;64:269–72. 10.1136/jnnp.64.2.269 [DOI] [PMC free article] [PubMed] [Google Scholar]