Abstract
Pyoderma gangrenosum is a neutrophilic skin disease that leads to extensive, painful, necrotic ulcerations, particularly at surgical sites. As obstetric cases with pyoderma gangrenosum are rare and, therefore, often misdiagnosed initially, it is important to raise awareness about this rare complication. Here, we describe a patient who presented with pyoderma gangrenosum at the surgical site 4 days after undergoing a caesarean section. The erythema was initially misdiagnosed as wound infection, and the patient, who was experiencing pain, underwent antibiotic treatment and surgical wound debridement. When the wound was unresponsive to these treatments, a dermatologist was consulted who suspected pyoderma gangrenosum and began a high-dose corticosteroids therapy, which led to a fulminant improvement of the local wound. In conclusion, the rare diagnosis of pyoderma gangrenosum should be considered in the differential diagnosis of a suspected surgical wound infection. Early interdisciplinary treatment is essential to avoid further complications.
Keywords: obstetrics and gynaecology, dermatology, surgery
Background
Pyoderma gangrenosum is a rare neutrophilic skin disease that leads to extensive, painful, necrotic ulcerations.1 Postsurgical pyoderma gangrenosum can occur at the surgery site after minor traumas or surgical procedures. It is characterised by its rapid progression and often misdiagnosed as wound infection.2 3 This misdiagnosis is particularly problematic, since surgical debridement, which is a standard treatment of wound infections, exacerbates pyoderma gangrenosum because of the skin’s pathergy. In addition, antibiotics are not effective in healing wounds affected by pyoderma gangrenosum.3
The pathophysiology of this rare disease is still poorly understood. Pyoderma gangrenosum is known to be associated with systematic autoimmune diseases like vasculitis, inflammatory bowel disease and arthritis.1 The absence of specific serologic or histological markers for pyoderma gangrenosum further complicates its diagnosis, which is therefore based on the clinical manifestation and the exclusion of other causes for skin disease.1 For patients with pyoderma gangrenosum, treatment includes high-dose corticosteroids, and cyclosporine can be used as a second-line treatment. In addition, wound dressing is an essential part of the treatment.2
To date, there are only a few documented cases of pyoderma gangrenosum after gynaecological and obstetric procedures, and even fewer cases reported after caesarean sections.4 Herein, we report a case of fulminant pyoderma gangrenosum after a caesarean section, review the existing literature on this topic and postulate a recommendation in the clinical management of pyoderma gangrenosum.
Case presentation
A 34-year-old primipara with an unremarkable family history was admitted to the Department of Obstetrics and Gynaecology at the Medical University of Vienna because of a preterm premature rupture of membranes at 30 gestational weeks after experiencing an uneventful pregnancy. After admission, the patient received treatment for fetal lung maturation with corticosteroids and tocolysis with an oxytocin-receptor antagonist, as well as prophylactic antibiotic treatment with intravenous ampicillin. After completing the fetal lung maturation treatment, magnesium was administered for 24 hours for fetal neuroprotection. After completing the neuroprotection, the patient delivered a healthy, male newborn with a birth weight of 1500 g at 30+2 gestational weeks by scheduled caesarean section without any intraoperative complications. The newborn was transferred to the neonatal intensive care unit because of his preterm birth. The following three postoperative days (POD) were uneventful with adequate postsurgical pain. On POD 4, the patient reported increasing pain at the surgical site, and the section wound started to show erythema; the patient was afebrile and lochial discharge was normal.
Investigations
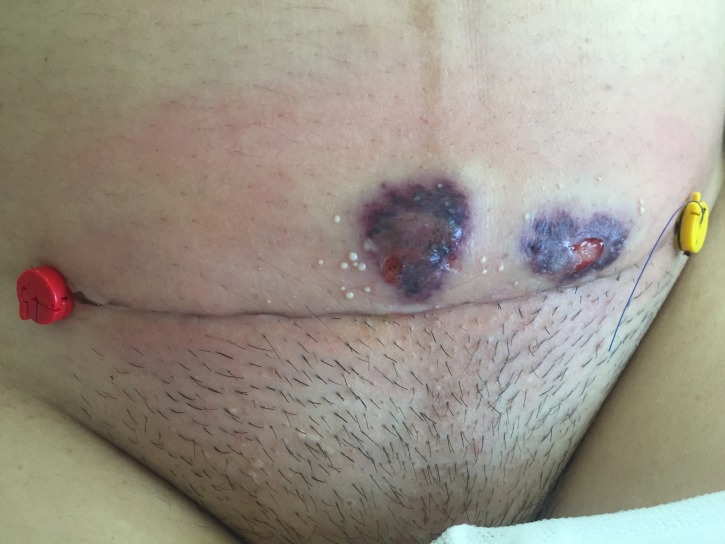
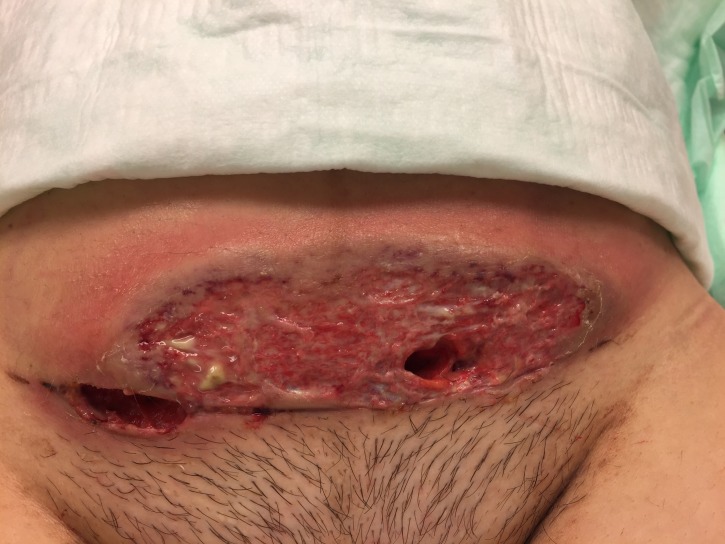
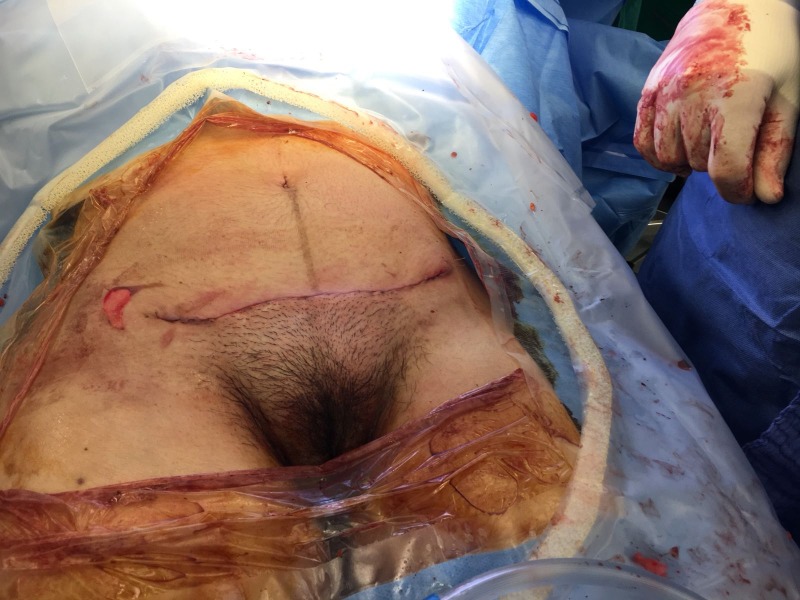
Laboratory results on POD 4 showed a leucocytosis of 13.49×109/L and an elevated C reactive protein (CRP) of 24.45 mg/dL. The elevated inflammatory parameters and the clinical signs of infection (erythema of the wound and pain experienced by the patient) led to the initiation of an antibiotic treatment (amoxicillin 875 mg and clavulanic acid 125 mg three times per day) and an increase in the analgesic therapy. On POD 6, erythema of the wound increased and formed stipples. The antibiotic regimen was broadened to include clindamycin 600 mg three times per day orally, which was changed to intravenous administration on POD 7. At this time, a wound swab was taken and sent to the microbiology laboratory. The report showed scattered staphylococcus, but no other bacteria were found. On POD 8, the skin lesion formed two separate bubbles; the patient was afebrile but still in pain (figure 1). Amoxicillin and clavulanic acid were changed to piperacillin 4 g and tazobactam 0.5 g three times per day, and clindamycin was increased to 1.2 g three times per day. The leucocytosis decreased to 12.73×109/L and CRP to 8.91 mg/dL. On POD 10, the bubbles at the wound site started to join each other, the wound secretion increased, and the margins were still erythematous. Despite declining inflammatory laboratory parameters and consequent treatment with sterile wound dressing and silver coverings, the local wound deteriorated (figure 2). On POD 15, a plastic surgeon initiated surgical wound debridement, followed by the installation of negative pressure wound therapy (VAC-system; KCI, Vienna, Austria). The intraoperative microbiological report of the wound debridement showed no bacterial growth, the histological report revealed inflamed granulated tissue. On POD 19, the VAC-system was removed, and the wound was secondarily closed down to the fascia of the muscle (figure 3). After this second surgical debridement, the inflammatory laboratory parameters became negative, the patient was still afebrile, and the antibiotic therapy was further continued. On POD 22 (ie, day 3 after the second wound debridement), the wound became erythematous again. As antibiotic therapy and wound debridement did not show any effect, a dermatologist was consulted, who suspected pyoderma gangrenosum on POD 23.
Figure 1.
Wound condition on day 8 after caesarean section.
Figure 2.
Wound condition on day 10 after caesarean section.
Figure 3.
Wound condition on day 19 after caesarean section.
Differential diagnosis
In patients developing an erythema and pain at a wound after surgery, the first suspected diagnosis is usually a wound infection. In this case, since the wound was unresponsive to the various antibiotic treatments and two wound debridement procedures, other options had to be considered. Therefore, a dermatologist was consulted who suspected pyoderma gangrenosum because the wound swab showed a negative result and antibiotic treatment proved ineffective. Furthermore, the histology of the wound debridement revealed diffuse inflammation with neutrophilic infiltrates. As pyoderma gangrenosum is a diagnosis of exclusion, the challenge in the present case was to rule out infectious diseases and bacterial wound infections and consider other causes for the presented pathology.
Treatment
After the diagnosis of pyoderma gangrenosum in this patient, she underwent intravenous treatment with prednisolone 500 mg, and the antibiotic regimen was changed to meropenem 2 g three times per day. After initiation of the corticosteroid treatment, healing of the wound began immediately, and the pain resolved. By POD 26 (day 4 after initiation of the corticosteroid treatment), the wound had healed completely, the patient was pain free, and the corticosteroid dose could be decreased. On POD 29, the patient was discharged from the hospital, without any signs of infection at the wound site. Antibiotic treatment was discontinued as the inflammatory parameters returned to normal, and corticosteroid treatment was adapted to methylprednisolone (20 mg orally two times per day).
Outcome and follow-up
Throughout the follow-up period of 6 months, the patient visited the dermatologist’s clinic on a regular basis. Treatment with oral corticosteroids was continued until POD 50 with tapered dosage. By then, the wound had completely healed and showed no sign of reoccurrence. The topical steroid cream, which was started on POD 33 (10 days after the initial diagnosis), was continued for an additional week until POD 57. Two weeks after the weaning of the oral steroids (POD 64), the patient began to develop erythematous lesions in two places on the scar from the caesarean section, indicating recurrence of pyoderma gangrenosum. Methylprednisolone was initiated again at POD 64 and finally tapered over 12 weeks. Since then, no sign of any activity of the pyoderma gangrenosum has been noted.
Discussion
Pyoderma gangrenosum is a rare neutrophilic skin disease that can easily be misdiagnosed. This is of particular importance in a postoperative setting as patients are more likely to develop wound infection than pyoderma gangrenosum.1 3 The pathophysiology of pyoderma gangrenosum remains unclear, although it is well known that the mechanism of this autoimmune disease involves a loss of congenital immune regulation and altered neutrophil chemotaxis. Furthermore, a genetic predisposition is also suspected,1 in addition to an increased risk during pregnancy and postpartum due to progressive neutrophilia.5 Approximately 50% of the cases of pyoderma gangrenosum are associated with an underlying autoimmune disease, such as inflammatory bowel disease, arthritis or haematological disorders.1 It is an autoimmune disease that can occur in all age groups, peak at 20–50 years of age and affect both sexes equally. The disease can be categorised into several variants: classic, bullous, pustular, vegetative, drug induced, postsurgical and peristomal.2 Postsurgical pyoderma gangrenosum presents at the surgical site most commonly after breast, chest, cardiothoracic or orthopaedic surgery.2 6 However, reports of occurrence of pyoderma gangrenosum after caesarean sections are very sparse. The associated symptoms include: fever, pain and a rapidly progressive erythematous skin lesion; raised edges and a non-specific purulent necrosis at the centre of the wound. The first-line treatment for pyoderma gangrenosum is high-dosed corticosteroids; second and third-line treatments include cyclosporine and tumour necrosis factor alpha inhibitors. Apart from this, avoidance of the trigger, adequate wound care and pain therapy are essential.2
Currently, there are no published studies available in the literature about pyoderma gangrenosum after caesarean section, except for a few case reports. In most of these cases, patients were initially treated with antibiotics. In the oldest available report by Shands et al7 in 1987, ampicillin and gentamicin were used for treatment. Corticosteroids were initiated because of the negative wound swab results and leucocytosis shown in the laboratory tests. This diagnostic error also occurred in the present case. Antibiotic treatment regimens were changed multiple times before the diagnosis of pyoderma gangrenosum was finally made and corticosteroid treatment was initiated.4 8–10 The lack of bacteria in wound swabs and leucocytosis in the white cell count should therefore be findings that lead to the re-evaluation of a suspected wound infection, thus broadening the horizon for possible neutrophilic diseases.
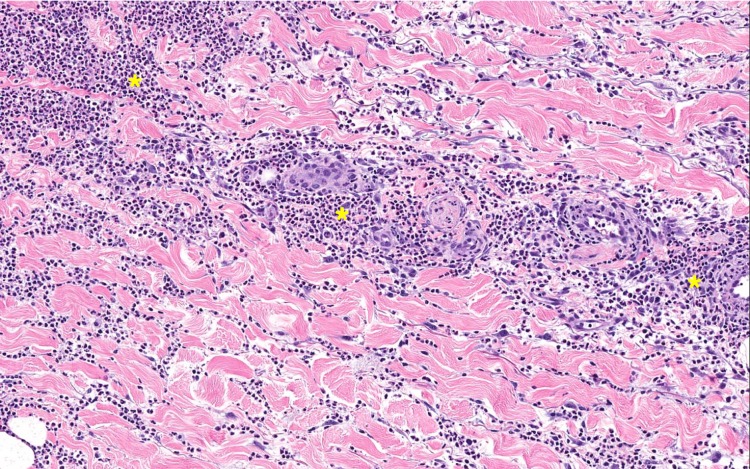
Contrary to that of a conventional postoperative wound infection, the local condition of skin affected by pyoderma gangrenosum does not show improvement after surgical debridement. The skin exhibits pathergy, the phenomenon of exacerbation of a skin lesion after a minor trauma or surgery (figure 4).2 Wound debridement after antibiotic therapy also did not lead to an improvement in the local skin condition. However, it caused fulminant deterioration of the wound over the following 2 days.4 Ronnau et al11 reported a case of an ulcerative wound dehiscence with worsening after wound debridement following an appendectomy and subsequent caesarean section. Pyoderma gangrenosum was finally diagnosed after the caesarean section, and corticosteroid therapy was initiated. In the present case, wound debridement was performed twice before the final diagnosis of pyoderma gangrenosum was made. This resulted in prolonged hospitalisation and a worse cosmetic result of the surgical scar. Worsening of the wound dehiscence after debridement should be a red flag for a physician involved in the treatment of a patient with prolonged and inflammatory wound healing.
Figure 4.
Histopathological specimen of pyoderma gangrenosum after surgery with diffuse inflammation from the dermis to the subcutis and neutrophilic infiltrates between collagen fibres (*indicates diffuse dermal neutrophilic infiltrates).
In the diagnostic process of pyoderma gangrenosum, evaluation of the associated diseases is of paramount importance (figure 5). In most of the available case reports, the dermatologists made the final diagnosis of pyoderma gangrenosum after gynaecologists attempted different antibiotic and surgical treatments that caused a significant delay in the appropriate corticosteroid therapy.4 8 9 This delay in treatment occurred in the present case as well. Therefore, we recommend early multidisciplinary care for patients with suspected postoperative wound infections if they are unresponsive to antibiotic treatment, and their microbiology reports show normal results. This could help to ensure early and adequate treatment without further complications, shorter hospital stays and better aesthetic outcomes.
Figure 5.
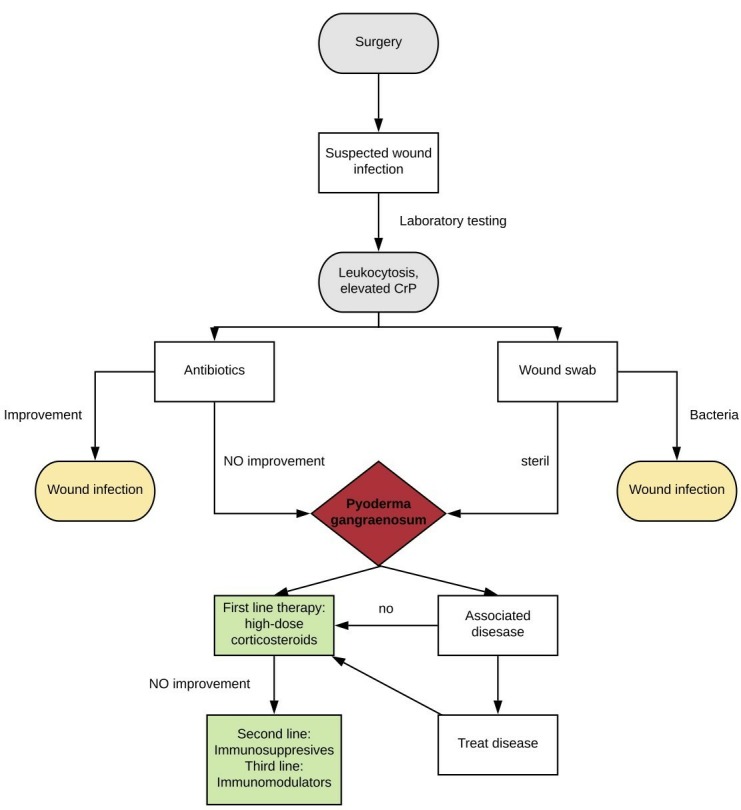
Diagnosis and treatment recommendations for pyoderma gangrenosum after surgery.
Patient’s perspective.
It was a terrible experience to suffer from this rare complication. The doctors and nurses were clearly making an effort. However, they were in the dark for a long time until the dermatologist finally diagnosed pyoderma gangrenosum. I hope that this article leads to an earlier consideration of pyoderma gangrenosum among medical staff.
Learning points.
Pyoderma gangrenosum is a rare complication after a caesarean section.
It is a neutrophilic skin disease that can easily be misdiagnosed as wound infection.
Antibiotic resistance and sterile wound swabs are indications to consider the disease.
Wound debridement induces a fulminant deterioration of the wound.
Awareness about this rare disease is important, and early multidisciplinary care is essential to avoid complications.
Footnotes
Contributors: This case report was written by PF, UJ, HK and AF. PF and AF were responsible for the overall content as guarantors, conceived of the idea for this article and performed the literature research. HK gave input on obstetrical treatment and outcome. UJ was responsible for writing the dermatological section and provided information of the patient’s follow-up.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol 2012;13:191–211. 10.2165/11595240-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 2. Alavi A, French LE, Davis MD, et al. Pyoderma Gangrenosum: An Update on Pathophysiology, Diagnosis and Treatment. Am J Clin Dermatol 2017;18:355–72. 10.1007/s40257-017-0251-7 [DOI] [PubMed] [Google Scholar]
- 3. Tolkachjov SN, Fahy AS, Cerci FB, et al. Postoperative Pyoderma Gangrenosum: A Clinical Review of Published Cases. Mayo Clin Proc 2016;91:1267–79. 10.1016/j.mayocp.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 4. Radhika AG, Singal A, Radhakrishnan G, et al. Pyoderma gangrenosum following a routine caesarean section: Pseudo-infection in a caesarean wound. Qatar Med J 2015;2015(1):1 10.5339/qmj.2015.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Steele RB, Nugent WH, Braswell SF, et al. Pyoderma gangrenosum and pregnancy: an example of abnormal inflammation and challenging treatment. Br J Dermatol 2016;174:77–87. 10.1111/bjd.14230 [DOI] [PubMed] [Google Scholar]
- 6. Ebrad S, Severyns M, Benzakour A, et al. Pyoderma gangrenosum after orthopaedic or traumatologic surgery: a systematic revue of the literature. Int Orthop 2018;42:239–45. 10.1007/s00264-017-3672-2 [DOI] [PubMed] [Google Scholar]
- 7. Shands JW, Flowers FP, Hill HM, et al. Pyoderma gangrenosum in a kindred. Precipitation by surgery or mild physical trauma. J Am Acad Dermatol 1987;16:931–4. [PubMed] [Google Scholar]
- 8. Amin SV, Bajapai N, Pai A, et al. Pyoderma gangrenosum in two successive pregnancies complicating caesarean wound. Case Rep Obstet Gynecol 2014;2014:1–3. 10.1155/2014/654843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Park JY, Lee J, Park JS, et al. Successful vaginal birth after prior cesarean section in a patient with pyoderma gangrenosum. Obstet Gynecol Sci 2016;59:62–5. 10.5468/ogs.2016.59.1.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Banga F, Schuitemaker N, Meijer P. Pyoderma gangrenosum after caesarean section: a case report. Reprod Health 2006;3:9 10.1186/1742-4755-3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rönnau AC, von Schmiedeberg S, Bielfeld P, et al. Pyoderma gangrenosum after cesarean delivery. Am J Obstet Gynecol 2000;183:502–4. 10.1067/mob.2000.105898 [DOI] [PubMed] [Google Scholar]