Abstract
Sirolimus is an important immunosuppressive drug in renal transplantation but contains numerous side effects. In this study, we describe a case of renal transplant recipient treated with sirolimus who developed pericardial effusion associated with interstitial pneumonia. An extensive search for alternative causes were all negative, and all symptoms disappeared after sirolimus interruption. Therefore, this case demonstrates that sirolimus can cause pericardial effusion possibly through a proinflammatory mechanism.
Keywords: pericardial disease, cardiovascular system, immunological products and vaccines, renal system
Background
Sirolimus, an immunosuppressive agent, is used to prevent acute rejection in solid-organ transplantation. Also, it has antiproliferative properties that can be used to treat proliferative diseases such as tuberous sclerosis (TB), psoriasis and malignancy.1 These functions are attributed to inhibition of mechanistic target of rapamycin (mTOR) by sirolimus. Following entry into the cytoplasm, sirolimus binds to the FK-binding protein 12 and then inhibits the activity of the mTOR, which regulates cellular metabolism, growth and proliferation. Specifically, inhibition of mTOR decreases expression of interleukin (IL)-2, which is important for activation of T cells and B cells.1 2 Therefore, sirolimus has immunosuppressant functions by reducing expression of IL-2 through mTOR inhibition.
However, sirolimus has numerous side effects, including hypersensitivity reactions, angioedema, delayed wound healing, proteinuria, interstitial lung disease and increased risk of developing lymphoma and other malignancy.3 4 Recently, there are three reports showing sirolimus may cause pericardial effusion in renal transplant patients but the mechanism remains unknown.5–7
In this study, we describe a case of renal transplant recipient treated with sirolimus who developed pleural and pericardial effusions. The potential mechanism is discussed as well.
Case presentation
This is a 61-year-old woman with a history of glomerulonephritis and received a kidney transplant in 2008. The immunosuppressive regimen included sirolimus and prednisone. The patient claimed that her symptom had been well controlled, and she was in compliance with medication without significant side effects during the past 10 years.
She presented to the hospital with complaints of fever and rapid progressive exertional dyspnoea that progressed rapidly to acute respiratory hypoxia respiratory failure within 1–2 days. The patient became dependent on bilevel positive airway pressure, with hypotension (85/58) and tachycardia (130–150). Physical exam revealed that bilateral crackles were heard on the base of the lung fields. Cardiac exam identified a jugular venous distension and distant heart sound.
Investigations
The patient’s chest X-ray showed evidence of a pulmonary oedema with bilateral pleural effusions (figure 1). A chest CT scanner showed bilateral interstitial pneumonia and an abundant circumferential pericardial effusion (figure 2). Transthoracic echocardiogram (TTE) also confirmed large pericardial effusion as shown in figure 3.
Figure 1.
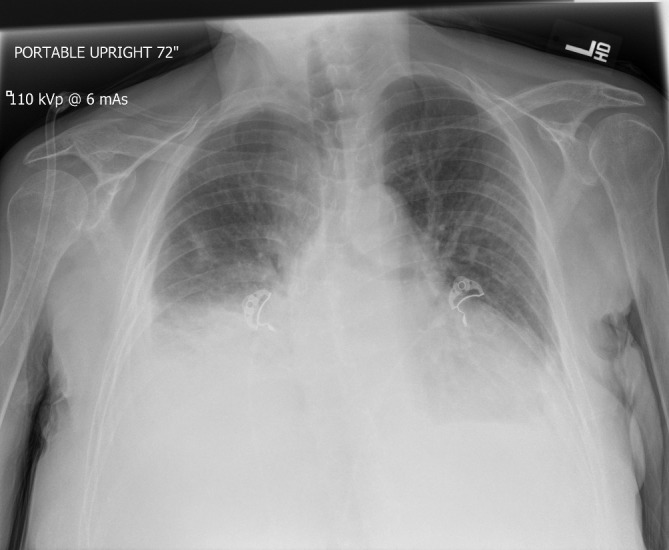
Chest X-ray showed bilateral pleural effusions.
Figure 2.

Chest CT showed bilateral pleural effusions (arrowhead) and a large circumferential pericardial effusion (arrowhead).
Figure 3.

TTE showed a large pericardial effusion (arrowhead). TTE, transthoracic echocardiogram.
An extensive workup was performed to figure out the reason causing pericardial and pleural effusions, including viral and bacterial infection, neoplastic, autoimmune, uraemia and cardiovascular origins. According to the results, the white blood cell count was 10.6×109/L, with 78% neutrophils and 11% lymphocytes. Haemoglobinaemia was 142 g/L and the platelet count was 194×109/L. The serum creatinine was 0.87 mg/dL, albumin was 3.7 g/dL and the urine protein to creatinine ratio was 0.11. Respiratory viral PCR was negative, and TB was negative. Thyroid-stimulating hormone (TSH) was 6.2 IU/mL. Rheumatology panel showed that erythrocyte sedimentation rate was 12 mm/hour, and C reactive protein was 102 mg/L. Antinuclear antibody, antineutrophil cytoplasmic antibody and rheumatoid factor were all negative.
Due to haemodynamic instability, a surgical pericardial window was performed. The pericardial fluid was exudative with a protein concentration of 3.6 g/dL and did not contain white blood cells. Glucose, protein and cell count in pericardial fluid were all within normal range. Acid-fast bacilli, fungal, bacterial cultures were all negative. Biopsy showed non-specific change of pericardium, ruling out malignancy.
Differential diagnosis
Because of interstitial pneumonia, the patient was given empiric treatment with Zosyn. However, fever persisted, and the effusion symptoms were not alleviated. After pericardial window was performed, the patient’s condition was stabilised immediately. An extensive search for infections turned out to be all negative, ruling out infectious complications. Other causes were ruled out as well, such as neoplastic, autoimmune, uraemia and cardiovascular origins. One exception was that sirolimus had a supratherapeutic level of 18.5 ng/mL (normal range: 4–12 ng/mL). Therefore, we suspected that the pericardial and pleural effusions were likely caused by side effects of sirolimus.
Treatment
Sirolimus was replaced with tacrolimus and prednisone.
Outcome and follow-up
Patient did not have any more episodes of pericardial effusion and was discharged home. Two months after discontinuation of sirolimus, pleural effusion disappeared as well. During the follow-up in October 2018, she had no recurrence of pericardial effusion.
Discussion
Sirolimus remains an important immunosuppressive agent in solid-organ transplantation. As with all immunosuppressants, the risks of sirolimus must be carefully considered before choosing it for transplant patients. Common side effects of sirolimus include diarrhoea, constipation, nausea, skin rash, headache, etc.3 Some of the more unusual toxicities include interstitial pneumonia, proteinuria, microcytic anaemia, lymphocytic meningitidis and pericardial effusion.3 Among them, pericardial effusion is commonly observed in cardiac transplant recipients with a prevalence of 28.6% but it is extremely rare in other transplant patients.8 There are only three reports of pericardial effusion coincident with sirolimus in renal transplant patients and the aetiologies remain unknown.5–7 In this case, we report a renal transplant patient for 10 years with pleural and pericardial effusions, which were possibly attributed to sirolimus.
In order to identify sirolimus as the cause of pericardial effusion, the followed criteria should be presented: exposition to sirolimus precedes the symptoms; no alternative cause is associated with the symptoms; withdrawal or replacement of sirolimus leads to resolution of the symptoms. In our case, an extensive workup was performed to rule out other alternative cause, including viral and bacterial infection, neoplastic, autoimmune, uraemia and cardiovascular origins. After replacement of sirolimus with tacrolimus, both pericardial and pleural effusion disappeared and no recurrence has been observed. Therefore, this case strongly suggests that sirolimus can cause pericardial effusion in renal transplant patients even 10 years after the introduction of the therapy.
However, little is known about the potential mechanisms relating sirolimus with pericardial effusion. A report suggested that lymphocele associated with sirolimus may predispose to development of pericardial effusion.7 It is also reported that adenovirus-induced viraemia may lead to pericardial effusion.5 Nevertheless, neither lymphocele nor virus was detected in our case, but interestingly non-infectious interstitial pneumonia was observed in our study and in another report.6 This finding indicated that patients had a sirolimus-associated inflammatory syndrome, which may induce pericardial effusion. As previous studies revealed, inhibition of mTOR with sirolimus can activate the nuclear factor (NF)-kB which is the master of regulating inflammatory responses.9 Therefore, sirolimus plays a proinflammatory role and may lead to serosal inflammation, interstitial pneumonia or pericarditis, all of which can contribute to pericardial effusion.
It is worth mentioning that tacrolimus may also cause pericardial effusion as recently reported.10 Though tacrolimus and sirolimus bind to the same FK12 protein, the binding sites and downstream pathways are different.11 Tacrolimus inhibits T-cell proliferation by blocking the Ca2+/calcineurin-dependent transcriptional activation of genes required for T-cell growth.11 Different from our case, no significant inflammation was observed in that study and the mechanism by which tacrolimus uses to induce pericardial effusion remains obscure. Because tacrolimus may also cause pericardial effusion, we should keep a close eye on the patients for the recurrence of symptoms. Otherwise, pericardial effusion may eventually develop to a more emergent and life-threatening condition— cardiac tamponade.12
Learning points.
In conclusion, after exclusion of other causes, sirolimus may be a potential cause of pericardial effusions.
Proinflammatory role of sirolimus is the potential mechanism for this association.
Withdrawal or replacement of sirolimus should be immediately initiated due to the potential risk of developing cardiac tamponade.
It is imperative to keep a close eye for the recurrence of symptoms since other replacement therapy may also cause pericardial effusion.
Footnotes
Contributors: Supervised by DG. Patient was under the care of LM and DG. Report was written by LM, BD and BL.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc 2003;35:S7–S14. 10.1016/S0041-1345(03)00211-2 [DOI] [PubMed] [Google Scholar]
- 2. Young DA, Nickerson-Nutter CL. mTOR--beyond transplantation. Curr Opin Pharmacol 2005;5:418–23. 10.1016/j.coph.2005.03.004 [DOI] [PubMed] [Google Scholar]
- 3. Stallone G, Infante B, Grandaliano G, et al. Management of side effects of sirolimus therapy. Transplantation 2009;87:S23–S26. 10.1097/TP.0b013e3181a05b7a [DOI] [PubMed] [Google Scholar]
- 4. Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant 2004;18:446–9. 10.1111/j.1399-0012.2004.00188.x [DOI] [PubMed] [Google Scholar]
- 5. Lobach NE, Pollock-Barziv SM, West LJ, et al. Sirolimus immunosuppression in pediatric heart transplant recipients: a single-center experience. J Heart Lung Transplant 2005;24:184–9. 10.1016/j.healun.2004.11.005 [DOI] [PubMed] [Google Scholar]
- 6. Bertrand D, Desbuissons G, Pallet N, et al. Sirolimus therapy may cause cardiac tamponade. Transpl Int 2013;26:e4–e7. 10.1111/tri.12025 [DOI] [PubMed] [Google Scholar]
- 7. Rocha S, Pedroso S, Almeida M, et al. Pericardial and pleural effusions associated with sirolimus and discussion of possible mechanisms. Portuguese Journal of Nephrology & Hypertension 2012;26:165–9. [Google Scholar]
- 8. Steele GH, Adamkovic AB, Demopoulos LA, et al. Pericardial effusion coincident with sirolimus therapy: a review of Wyeth’s safety database. Transplantation 2008;85:645–7. 10.1097/TP.0b013e3181636061 [DOI] [PubMed] [Google Scholar]
- 9. Weichhart T, Costantino G, Poglitsch M, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity 2008;29:565–77. 10.1016/j.immuni.2008.08.012 [DOI] [PubMed] [Google Scholar]
- 10. Prashar R, Stewart D, Moza A. Tacrolimus as a rare cause of pericardial effusion in a renal transplant recipient. Heart Views 2017;18:145 10.4103/HEARTVIEWS.HEARTVIEWS_6_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kiem H-P, Anasetti C. Sirolimus and tacrolimus binding proteins: double-edged swords for GVHD prophylaxis. Blood 2003;102:1562–62. 10.1182/blood-2003-06-2178 [DOI] [Google Scholar]
- 12. Appleton CP, Hatle LK, Popp RL. Cardiac tamponade and pericardial effusion: respiratory variation in transvalvular flow velocities studied by Doppler echocardiography. J Am Coll Cardiol 1988;11:1020–30. 10.1016/S0735-1097(98)90060-2 [DOI] [PubMed] [Google Scholar]


