Abstract
In the context of organ shortage, the opioid epidemic, and effective direct-acting antiviral (DAA) therapy for hepatitis C (HCV), more HCV-infected donor organs may be used for liver transplantation. Current data regarding outcomes after donor-derived HCV in previously non-viremic liver transplant recipients are limited. Clinical data for adult liver transplant recipients with donor-derived HCV infection from March 2017 to January 2018 at our institution were extracted from the medical record. Ten patients received livers from donors known to be infected with HCV based on positive nucleic acid testing (NAT). Seven had a prior diagnosis of HCV and were treated before liver transplantation. All recipients were non-viremic at the time of transplantation. All 10 recipients derived hepatitis C infection from their donor and achieved sustained virologic response at 12 weeks post-treatment (SVR-12) with DAA-based regimens, with a median time from transplant to treatment initiation of 43 days (IQR 20–59). There have been no instances of graft loss or death, with median follow-up of 380 days (IQR 263–434) post-transplant. Transplantation of HCV-viremic livers into non-viremic recipients results in acceptable short-term outcomes. Such strategies may be used to expand the donor pool and increase access to liver transplantation.
1. Introduction
The demand for liver transplantation continues to outpace the supply of donor organs in the United States.(1) In the context of this organ shortage, it is essential to make the best use of all transplantable organs. Previously, donor livers infected with hepatitis C virus (HCV) typically have been either discarded or used in patients already infected with HCV.(2–4) Donor livers infected with HCV are still not widely accepted for non-viremic recipients, due to concerns regarding HCV transmission, the natural history of untreated HCV infection after liver transplantation, and limited evidence in this specific population.(5) Highly effective and well-tolerated direct-acting antiviral (DAA) therapies are now available for the treatment of HCV infection and can eliminate HCV in the allograft in greater than 95% of cases.(6)
In the setting of the ongoing organ shortage, the opioid epidemic, and the advent of DAA therapy, more HCV-infected donor organs are being utilized to expand the donor pool, and non-viremic patients are being given the option to accept these grafts in some centers, including ours.(4, 7, 8) The aim of our study was to summarize the post-transplant outcomes after donor-derived HCV infection in non-viremic liver transplant recipients at our institution.
2. Materials and Methods
Clinical and laboratory data for adult liver transplant recipients with donor-derived HCV infection at our institution from March 2017 to January 2018 were extracted from the electronic medical record. Donor data were obtained from the Organ Procurement and Transplantation Network (OPTN) database and UNet, which is maintained by the United Network for Organ Sharing and stores all OPTN data pertaining to waitlist, organ matching, and transplants.
Donor-derived HCV infection was defined as post-transplant HCV viremia in a previously non-viremic liver transplant recipient, who received a donor liver with HCV infection, determined by positive nucleic acid testing (NAT). We recorded recipient demographics and clinical data at the time of transplantation, as well as pre-transplant HCV viral load, HCV genotype, and HCV treatment history.
Prior to transplantation, patients had consented to potentially receive an organ infected with HCV. The option of accepting livers from Public Health Service (PHS) increased-risk or extended criteria donors was discussed in detail with the patient by the transplant hepatologist and transplant surgeon at initial listing or during the evaluation process. This included not only HCV-infected livers but also organs from donors with positive hepatitis B core Ab, PHS increased-risk, older donors, donation after cardiac death (DCD), and split livers. Patients could decided whether they would be willing to be offered organs that were HCV antibody or NAT positive.
If the patient was offered an HCV NAT positive organ, the surgeon then discussed the specific donor risk based on the information and evidence available at the time and consented for transplantation with the HCV NAT positive liver. The discussion addressed the fact that the patient would acquire HCV from the donor and therefore require HCV treatment after transplantation, which would involve checking genotype and viral load, consulting with hepatology, and obtaining insurance approval. There was a possibility that HCV or its treatment could affect the post-transplant course, or that the HCV could not be treated. The alternative option was to remain on the liver transplant waitlist for another organ offer.
HCV NAT positive organs were offered to patients with a high estimated risk of waitlist dropout while awaiting liver transplantation, either from complications of decompensated liver disease or HCC growth. With a high median MELD for deceased donor transplant in our donor service area, these patients may not otherwise receive many viable organ offers.
The Institutional Review Board at Stanford University approved a prospective study to monitor post-transplant outcomes that included this population. Notably, our protocol was established prior to the release of the American Society of Transplantation (AST) consensus statement and thus deviated from the recommendation that the transplantation of HCV-viremic organs into HCV-naïve recipients should be done only under an IRB-approved research protocol. There was no separate consent form for these donors, and the physicians were ultimately responsible for the informed consent based on our institutional experience and the evidence available at the time.
Specialized transplant pharmacists assisted with insurance authorization and monitoring for potential drug-drug interactions. Our team was committed to obtain appropriate DAA therapy for these patients and worked with insurers to do so, but there was no institutional mechanism in place to guarantee payment for or access to the drug in case it was ultimately not approved. Hepatitis C RNA viral level was followed in a standardized fashion during the post-transplant period. HCV RNA and genotype was checked within 4 days after liver transplantation. Treatment was started once other transplant-related issues were stable, and insurance authorization was obtained. Patients were closely monitored for potential complications related to HCV infection or HCV therapy.
In post-transplant follow-up, we noted the selected HCV treatment regimen and duration, any obstacles to starting HCV therapy, exposure to immunosuppressive agents, HCV RNA viral kinetics, liver chemistry profile and renal function, changes to immunosuppression, and any potential complications including rejection, hepatitis related to HCV infection, acute kidney injury, graft failure, and death. Standard post-transplant immunosuppression at our institution includes induction with anti-thymocyte globulin or basiliximab, followed by maintenance calcineurin inhibitor and mycophenolate mofetil. Clinical data were recorded until July 31, 2018.
3. Results
During the study period, 10 liver transplant recipients received organs from HCV NAT positive donors (Table 1). The median age was 61 years (IQR 54–65), and 40% were female. Seven (70%) recipients had a prior diagnosis of HCV and had achieved sustained virologic response (SVR) with DAA-based regimens. All had completed therapy and were non-viremic at the time of transplantation; five of the seven had documented SVR at 12 weeks post-treatment (SVR-12).
Table 1.
Recipient characteristics. GT = genotype, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, LT = liver transplantation, VL = viral load.
| ID | Age | Sex | LT Native MELD |
LT Allocation MELD |
HCC exception |
Pre-LT HCV VL (IU/mL) |
Pre-LT HCV GT |
SVR- 12 |
Blood type |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 42 | M | 11 | 31 | Y | 0 | 1A | N | A |
| 2 | 53 | M | 16 | 33 | Y | 0 | 1A | N | O |
| 3 | 64 | F | 11 | 31 | Y | 0 | 1A | Y | O |
| 4 | 51 | M | 11 | 29 | Y | 0 | 3 | Y | O |
| 5 | 57 | F | 16 | 34 | Y | 0 | 4 | Y | O |
| 6 | 70 | M | 8 | 33 | Y | 0 | 1B | Y | A |
| 7 | 57 | F | 31 | 31 | N | - | - | - | A |
| 8 | 64 | M | 15 | 33 | Y | 0 | 1A | Y | O |
| 9 | 70 | M | 38 | 38 | N | - | - | - | O |
| 10 | 65 | F | 34 | 34 | N | - | - | - | O |
Among recipients, the median biochemical MELD was 16 (IQR 11–24) at the time of transplant, and the median allocation MELD was 33 (31–34). Seven had exception points for HCC, and seven were blood type O. The median time on the liver transplant waitlist was 812 days (IQR 388–1487).
Table 2 outlines the characteristics of the HCV-infected donors. All 10 donors tested positive for HCV by NAT at the time of organ procurement. One donor was HCV NAT-positive and Ab-negative. Nine were considered PHS increased-risk, and two also had positive hepatitis B core antibody, for which recipients received appropriate antiviral prophylaxis. The median distance from our transplant center to the donor recovery center was 1153 miles (IQR 308–1728). The median donor age was 33 years (IQR 30–38), terminal AST 43 U/mL (IQR 29–71), terminal ALT 65 U/mL (IQR 39–132), and terminal bilirubin 0.5 mg/dL (IQR 0.2–0.6). Three donors died of drug overdose deaths, 3 from intracranial hemorrhage or stroke, 2 from trauma, and the remaining 2 from other causes leading to anoxia. Three of the nine donor livers with pre-operative liver biopsy had macrovesicular steatosis (5–10%), and none had documented fibrosis.
Table 2.
Donor characteristics. DCD = donation after cardiac death; GT = genotype, HCV = hepatitis C; Ab = antibody, NAT = nucleic acid testing, HBcAb = hepatitis B core antibody, PHS = Public Health Service.
| ID | Age | PHS increased risk |
HCV Ab+ |
HCV NAT+ |
Donor HCV GT |
HBcAb+ | Liver biopsy |
Miles from center |
Region | DCD | Cold ischemia time (h) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27 | Y | + | + | 3 | N | Y | 1772 | 10 | N | 9 |
| 2 | 33 | Y | + | + | 1A | N | Y | 1542 | 7 | N | 9 |
| 3 | 29 | Y | + | + | 3 | Y | Y | 1758 | 11 | N | 12 |
| 4 | 48 | Y | + | + | 3 | N | N | 23 | 5 | Y | 5 |
| 5 | 53 | N | + | + | 1A | Y | Y | 296 | 6 | N | 8 |
| 6 | 30 | Y | + | + | 3 | N | Y | 764 | 5 | N | 7 |
| 7 | 37 | Y | + | + | 1B | N | Y | 1714 | 10 | N | 9 |
| 8 | 33 | Y | + | + | 2 | N | Y | 58 | 5 | N | 5 |
| 9 | 38 | Y | − | + | 2B | N | Y | 1732 | 3 | N | 11 |
| 10 | 29 | Y | + | + | 2B | N | Y | 343 | 5 | N | 8 |
All recipients had documented HCV viremia within 4 days of liver transplant. Table 3 describes the specific treatment regimen and outcomes for each of the transplant recipients. Six patients received sofosbuvir/velpatasvir-based therapy, 3 patients received ledipasvir/sofosbuvir-based therapy, and 1 patient received sofosbuvir/daclatasvir-based therapy. Seven patients received a 12-week duration of therapy, 3 received a 24-week course of therapy either due to clinician or insurer preference. Use of ribavirin was based on clinical characteristics and clinician judgment. All recipients began treatment post-transplant with DAA-based regimens, with a median time from transplant to treatment of 43 days (IQR 20–59). Three patients had been switched to cyclosporine-based immunosuppression during their index hospitalization, due to concern for tacrolimus-related side effects. Potential interactions with cyclosporine were considered when selecting the antiviral regimen. In two cases, the first choice of antiviral therapy was not authorized by the recipient’s insurance; however, the second proposed option was approved within 1 day of re-submission. In the first case, the insurance declined glecaprevir/pibrentasvir and suggested a sofosbuvir/daclatasvir-based regimen. In the second case, insurance denied glecaprevir/pibrentasvir and requested use of sofosbuvir/velpatasvir, which was on their formulary.
Table 3.
Treatment and follow-up data. ACR = biopsy-proven acute cellular rejection; AMR = biopsy-proven antibody-mediated rejection, CsA = cyclosporine; FK = tacrolimus; LDV = ledipasvir; LOS = length of stay; LT = liver transplantation; IMS = immunosuppresion; MMF = mycophenolate mofetil; RBV = ribavirin; SOF = sofosbuvir; VEL = velpatasvir.
| ID | LOS POST- LT |
HCV genotype |
Treatment x weeks |
Days to treatment |
IMS exposure* |
Comments |
|---|---|---|---|---|---|---|
| 1 | 11 | 3 | sof/dcv/rbv x 24 | 84 | FK | None |
| 2 | 15 | 1A | sof/ldv/rbv x 24 | 38 | FK, MMF | None |
| 3 | 10 | 3 | sof/vel x 12 | 47 | FK, MMF | None |
| 4 | 13 | 3 | sof/vel/rbv x 12 | 75 | FK, MMF | None |
| 5 | 24 | 1A | sof/ldv x 12 | 48 | CsA, MMF | AMR treated with IVIG, rituximab† |
| 6 | 7 | 3 | sof/vel/rbv x 12 | 30 | FK, MMF | None |
| 7 | 18 | 1B | sof/vel x 24 | 62 | FK, CsA, MMF, sirolimus |
ACR treated with prednisone‡, AMR treated with IVIG, rituximab, thymoglobulin‡, ACR treated with prednisone |
| 8 | 8 | 1A | sof/vel x 12 | 11 | FK, MMF | None |
| 9 | 16 | 1A | sof/ldv x 12 | 17 | CsA, MMF | None |
| 10 | 26 | 2B | sof/vel x 12 | 15 | CsA, MMF | Mild ACR, no change in IMS ‡ |
Exposure during HCV treatment
Occurred prior to initiation of HCV therapy
Occurred after completion of HCV therapy
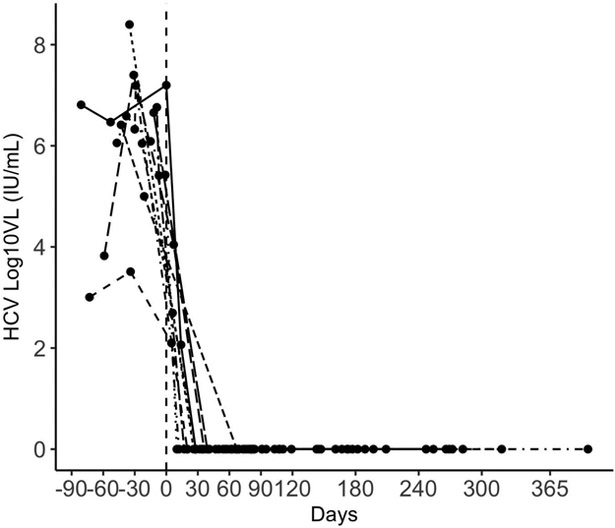
Figure 1 displays the individual trends in HCV viral load in relation to the start of therapy, and Figure 2 the trends in AST, ALT, bilirubin, and alkaline phosphatase. All patients have completed the full course of HCV therapy with end-of-treatment response and all have achieved SVR-12. No patients in our cohort have experienced graft loss or death, with a median follow-up of 380 days (IQR 263–434) post-transplant.
Figure 1.
Trends in HCV viral load; reference line represents the start date of antiviral therapy.
Figure 2.
Biochemical parameters during post-transplant follow-up; reference line represents start date of antiviral therapy.
One patient (ID 2) was hospitalized with gastrointestinal symptoms, anemia, and neutropenia 3 months after transplant and 6 weeks after starting sofosbuvir and ledipasvir with ribavirin. His hospitalization and symptoms were ultimately attributed to his post-transplant medications, including his hepatitis C treatment regimen, and thus both sulfamethoxazole-trimethoprim and ribavirin were discontinued, and the dose of mycophenolate mofetil was reduced. He then completed 24 weeks of therapy with sofosbuvir and ledipasvir and achieved SVR-12 without further incident.
A second patient (ID 7) was treated 1 month after liver transplantation for biopsy-proven acute cellular rejection (ACR) and antibody-mediated rejection (AMR) with ATG, IVIG, rituximab, and steroid taper prior to starting DAA therapy. On liver biopsy, there were no features suggestive of recurrent HCV as the cause of the elevated liver tests. She was then admitted the following month with renal failure complicated by volume overload. A repeat liver biopsy showed changes suggestive of venous outflow obstruction and large-caliber venous organizing thrombus, and the circulating donor specific antibodies were no longer detected. During this hospitalization, she was started on sofosbuvir and velpatasvir due to concern for hepatorenal physiology, although the liver biopsy did not show histologic evidence of chronic hepatitis C. Tacrolimus trough levels ranged from 5.0 to 14.3 over the next 2 weeks, but due to worsening renal function requiring temporary hemodialysis and evidence of calcineurin toxicity on kidney biopsy, she was ultimately switched from tacrolimus to sirolimus, while also continuing mycophenolate mofetil and low-dose prednisone for immunosuppression. She was hospitalized again 3 months later (at week 12 of DAA treatment) for biopsy-proven ACR, again without features suggestive of recurrent HCV, which was treated with methylprednisolone bolus and subsequent corticosteroid taper. Sirolimus trough levels had ranged from 5.5 to 16.6 mcg/L in the 2 months prior to the diagnosis of ACR, with one dose adjustment for high levels; the sirolimus trough level at the time of ACR was 6.1 mcg/L. She completed the 24-week course of sofosbuvir and velpatasvir without further events and has achieved SVR-12.
Two other patients have been diagnosed with rejection: one with ACR within 1 month of transplant (ID 10) prior to initiation of HCV therapy, and one with AMR 5 months after transplant (ID 5), after completion of HCV therapy. On liver biopsy, neither of these patients had histologic changes characteristic of HCV infection in the allograft. For the latter patient, cyclosporine trough levels had ranged from 172–339 ng/mL while on sofosbuvir and ledipasvir, with several dose adjustments. She had been on cyclosporine-based immunosuppression since post-operative day 13 due to hyperglycemia and acute kidney injury. She was known to be highly sensitized, with cPRA of 100% prior to liver transplant, and had new donor-specific antibodies and a positive C4d immunostain on liver biopsy, consistent with AMR. She was treated again for AMR 11 months after transplant with IVIG and rituximab and has also had complications related to an anastomotic stricture requiring percutaneous biliary drainage and ultimately Roux-en-Y choledochojejunostomy. Figure 3 and Table S1 show the biochemical parameters and immunosuppression levels during and after DAA treatment for each of these patients. One recipient of an HCV-infected DCD liver (ID 4) was successfully treated for HCV and achieved SVR12, but has since experienced complications related to ischemic cholangiopathy.
Figure 3.
Immunosuppression levels with relation to HCV therapy in liver transplant recipients with rejection. ACR = acute cellular rejection; AMR = antibody-mediated rejection; HCV = hepatitis C, VL = viral load, ALT = alanine aminotransferase, ALKP = alkaline phosphatase, SOF = sofosbuvir, LDV = ledipasvir, VEL = velpatasvir.
4. Discussion
Transplantation of HCV-viremic livers into non-viremic recipients results in acceptable short-term outcomes. All patients with donor-derived HCV began treatment for HCV within 3 months of transplantation, with no significant adverse events directly related to HCV, including fibrosing cholestatic hepatitis, graft failure, or death. All of our patients completed treatment and have achieved SVR-12.
Three of the 10 patients were treated for acute rejection post-transplant, with two occurring during or after the course of HCV therapy. Liver biopsies did not show features of HCV infection in the allograft. Drug interactions between DAA and post-transplant immunosuppression are well-recognized, and though drug levels were closely monitored during the course of HCV therapy, DAA therapy may have affected immunosuppression levels.(9, 10) The rate of biopsy-proven acute rejection that we observed exceeds the range of what is reported in the general post-liver transplant population (15.6–26.9%), though our sample size is small.(11) Notably, recipients with HCV have been observed to have an increased risk of acute rejection.(11) In particular, HCV treatment post-transplant has been associated with rejection and immune graft dysfunction, possibly related to declining immunosuppression levels or changes in the immune profile after HCV elimination.(12, 13) The potential added risk of rejection and/or graft dysfunction with donor-derived HCV infection deserves attention and further study, and both providers and patients accepting HCV-viremic organs should be fully aware of this possibility.
Historically, it has been difficult at times to differentiate and manage the contributions of acute cellular rejection and recurrent HCV in the allograft. In the DAA era, the diagnosis and treatment of rejection is more straightforward, given that effective therapy with DAAs can prevent the HCV flares previously observed during treatment for rejection. None of the patients in our cohort who developed ACR or AMR had confounding features of concomitant hepatitis C infection on liver biopsy. Though the episodes of rejection in our study sample were not clearly related to HCV or HCV therapy, they highlight the need for continued vigilance for post-transplant complications and potential drug interactions in the post-transplant period in this population.
Conventionally, organs either known to be or potentially infected with HCV have been declined for non-viremic recipients, due to concerns regarding transmission and the ability to treat HCV in the allograft. Without effective treatment for HCV, liver transplant recipients with HCV-infected grafts are susceptible to accelerated fibrosis and increased risk of graft loss.(14, 15) The advent of highly effective DAA therapy has now made it possible to consistently treat HCV after liver transplantation, with rates of SVR that exceed 95%.(6, 16–20) Moreover, ribavirin-free pan-genotypic options are now available, which should allow earlier treatment in anemic post-transplant in patients who cannot tolerate ribavirin and reduce the overall time that the transplanted organ is exposed to HCV.(21, 22)
In the context of the opioid epidemic and the advent of DAAs, more HCV-antibody positive organs are being used for transplantation, though this appears to be largely into patients already infected with HCV.(4, 7) Despite the availability of DAA therapy for post-transplant HCV, a positive HCV antibody continues to be a risk factor for organ discard.(4) For both viremic and non-viremic patients, the usage of HCV-infected livers can improve access to transplantation and reduce waitlist mortality, particularly in regions of organ scarcity. In simulation, HCV-negative recipients with MELD ≥ 20 who were willing to accept an HCV-infected donor had increased life expectancy, compared to those willing to accept only HCV-negative livers — even when the Markov model attributed a higher risk of graft failure to these livers.(23) Further evidence regarding the absolute risk of graft failure with these livers will likely drive this MELD threshold lower.
To date, the outcomes of intentional donor-derived HCV infection in non-viremic recipients are limited — successful treatment has been described in select recipients of HCV-infected organs, including 1 lung, 10 kidneys, 3 livers, and 10 hearts.(24–28) In reported cases of unintentional transmission in non-viremic liver transplant recipients, outcomes have been excellent in the DAA era.(29, 30) Most recently, Bari et al. described HCV transmission in 4 of 25 non-viremic liver transplant recipients from HCV Ab+/NAT- donor livers; 3 of the 4 have achieved at least end-of-treatment response, and none experienced complications related to HCV.(31)
Hepatitis C antibody positive donors, when used, have largely been allocated for HCV-viremic recipients, with good outcomes.(4) The question of whether HCV antibody or NAT positive donors can be accepted for non-viremic patients becomes more relevant as fewer patients on the waitlist remain viremic over time — a growing proportion of HCV patients on the liver transplant waitlist will be treated successfully prior to listing or transplant, especially those with MELD scores <20, for whom the potential to delist is higher.(32) In addition, waitlist registrations and transplants for HCV continue to decrease sharply, while the proportion of candidates with alcoholic liver disease and other indications increase.(1) Acceptance of donor livers known to be infected with HCV may be a preferable option in regions of relative organ scarcity, where the median MELD score needed to receive organ offers is high — particularly for patients who may never achieve the highest priority in match runs, including those with HCC exception points encountering the HCC MELD cap, those with common blood types, and those with low MELD scores despite poor quality of life. This strategy may parallel the trajectory of donors with positive anti-hepatitis B core antibody that has occurred with the advent of potent polymerase inhibitors, which is now routine practice.
Limitations of this study include its small sample size at a single institution and limited follow-up time. However, given what we currently know regarding the efficacy of DAA therapy after liver transplantation, we expect that these findings can be extrapolated to the general post-transplant population and will be maintained over the long-term. Outcomes for these patients should continue to be closely monitored in controlled, prospective settings to ensure long-term safety, tolerability, and efficacy, as recommended by the AST consensus conference.(33) These data can help clarify which transplant candidates will benefit from acceptance of an HCV-infected organ. In addition, there will need to be uniform agreement among payers who provide reimbursement for the DAA therapy that these transplant recipients will have unimpaired access to therapy post-transplant. Given the diverse donor HCV genotypes we noted in our cohort, a protocolized pan-genotypic post-transplant treatment strategy may reduce logistical hurdles associated with initiation of treatment. Even with the added cost of DAA therapy, accepting an HCV-infected liver may not only be life-saving but also cost-effective, compared to remaining on the liver transplant waitlist.(34)
In our study, all liver transplant recipients with donor-derived HCV infection initiated DAA therapy within 3 months of transplantation, have completed therapy, and have achieved SVR-12. Larger prospective studies with longer follow-up may further clarify if the risk of rejection is truly increased for recipients of HCV-viremic donors in the modern era — whether from HCV itself or HCV therapy. For select patients, acceptance of HCV-infected organs may be an option to increase the probability of transplantation and reduce waitlist mortality.
Supplementary Material
Acknowledgments
This work was supported in part by a grant from the National Institute of Diabetes, Digestive and Kidney Disease (T32 DK-007056) and a KL2 Mentored Career Development Award of the Stanford Clinical and Translational Science Award to Spectrum (NIH KL2 TR-001083). The funding organization played no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Abbreviations:
- (DAA)
Direct-acting antiviral
- (HCV)
hepatitis C virus
- (MELD)
Model for End-Stage Liver Disease
- (OPTN)
Organ Procurement and Transplantation Network
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- 1.Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Harper AM et al. OPTN/SRTR 2016 Annual Data Report: Liver. Am J Transplant 2018;18 Suppl 1:172–253. [DOI] [PubMed] [Google Scholar]
- 2.Northup PG, Argo CK, Nguyen DT, McBride MA, Kumer SC, Schmitt TM et al. Liver allografts from hepatitis C positive donors can offer good outcomes in hepatitis C positive recipients: a US National Transplant Registry analysis. Transpl Int 2010;23(10):1038–1044. [DOI] [PubMed] [Google Scholar]
- 3.Saab S, Ghobrial RM, Ibrahim AB, Kunder G, Durazo F, Han S et al. Hepatitis C positive grafts may be used in orthotopic liver transplantation: a matched analysis. Am J Transplant 2003;3(9):1167–1172. [DOI] [PubMed] [Google Scholar]
- 4.Bowring MG, Kucirka LM, Massie AB, Luo X, Cameron A, Sulkowski M et al. Changes in Utilization and Discard of Hepatitis C-Infected Donor Livers in the Recent Era. Am J Transplant 2017;17(2):519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai JC, O’Leary JG, Trotter JF, Verna EC, Brown RS, Stravitz RT et al. Risk of advanced fibrosis with grafts from hepatitis C antibody-positive donors: a multicenter cohort study. Liver Transpl 2012;18(5):532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwo PY, Mantry PS, Coakley E, Te HS, Vargas HE, Brown R et al. An interferon-free antiviral regimen for HCV after liver transplantation. N Engl J Med 2014;371(25):2375–2382. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez SA, Trotter JF. The Rise of the Opioid Epidemic and Hepatitis C Positive Organs: A New Era in Liver Transplantation. Hepatology 2017. [DOI] [PubMed] [Google Scholar]
- 8.Shaffer AA, Thomas AG, Bowring MG, Van Pilsum Rasmussen SE, Cash A, Kucirka L et al. Changes in Practice and Perception of Hepatitis C and Liver Transplantation: Results of a National Survey. Transpl Infect Dis 2018:e12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gentil MA, González-Corvillo C, Perelló M, Zarraga S, Jiménez-Martín C, Lauzurica LR et al. Hepatitis C Treatment With Direct-Acting Antivirals in Kidney Transplant: Preliminary Results From a Multicenter Study. Transplant Proc 2016;48(9):2944–2946. [DOI] [PubMed] [Google Scholar]
- 10.Kwo PY, Badshah MB. New hepatitis C virus therapies: drug classes and metabolism, drug interactions relevant in the transplant settings, drug options in decompensated cirrhosis, and drug options in end-stage renal disease. Curr Opin Organ Transplant 2015;20(3):235–241. [DOI] [PubMed] [Google Scholar]
- 11.Levitsky J, Goldberg D, Smith AR, Mansfield SA, Gillespie BW, Merion RM et al. Acute Rejection Increases Risk of Graft Failure and Death in Recent Liver Transplant Recipients. Clin Gastroenterol Hepatol 2017;15(4):584–593.e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan C, Schiano T, Agudelo E, Paul Haydek J, Hoteit M, Laurito MP et al. Immune-mediated graft dysfunction in liver transplant recipients with hepatitis C virus treated with direct-acting antiviral therapy. Am J Transplant 2018. [DOI] [PubMed] [Google Scholar]
- 13.Terrault NA, Berenguer M, Strasser SI, Gadano A, Lilly L, Samuel D et al. International Liver Transplantation Society Consensus Statement on Hepatitis C Management in Liver Transplant Recipients. Transplantation 2017;101(5):956–967. [DOI] [PubMed] [Google Scholar]
- 14.Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology 2002;122(4):889–896. [DOI] [PubMed] [Google Scholar]
- 15.Sánchez-Fueyo A, Restrepo JC, Quintó L, Bruguera M, Grande L, Sánchez-Tapias JM et al. Impact of the recurrence of hepatitis C virus infection after liver transplantation on the long-term viability of the graft. Transplantation 2002;73(1):56–63. [DOI] [PubMed] [Google Scholar]
- 16.Charlton M, Gane E, Manns MP, Brown RS, Curry MP, Kwo PY et al. Sofosbuvir and ribavirin for treatment of compensated recurrent hepatitis C virus infection after liver transplantation. Gastroenterology 2015;148(1):108–117. [DOI] [PubMed] [Google Scholar]
- 17.Kwo PY. Direct acting antiviral therapy after liver transplantation. Curr Opin Gastroenterol 2016;32(3):152–158. [DOI] [PubMed] [Google Scholar]
- 18.Pillai AA, Maheshwari R, Vora R, Norvell JP, Ford R, Parekh S et al. Treatment of HCV infection in liver transplant recipients with ledipasvir and sofosbuvir without ribavirin. Aliment Pharmacol Ther 2017;45(11):1427–1432. [DOI] [PubMed] [Google Scholar]
- 19.Kwok RM, Ahn J, Schiano TD, Te HS, Potosky DR, Tierney A et al. Sofosbuvir plus ledispasvir for recurrent hepatitis C in liver transplant recipients. Liver Transpl 2016;22(11):1536–1543. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal K, Castells L, Müllhaupt B, Rosenberg WMC, McNabb B, Arterburn S et al. Sofosbuvir/velpatasvir for 12 weeks in genotype 1–4 HCV-infected liver transplant recipients. J Hepatol 2018;69(3):603–607. [DOI] [PubMed] [Google Scholar]
- 21.Reau N, Kwo PY, Rhee S, Brown RS, Agarwal K, Angus P et al. Glecaprevir/Pibrentasvir Treatment in Liver or Kidney Transplant Patients With Hepatitis C Virus Infection. Hepatology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agarwal K, Castells L, Mullhaupt B, Rosenberg WM, McNabb BL, Arterburn S et al. Sofosbuvir/Velpatasvir for 12 Weeks in Genotype 1–4 HCV-Infected Liver Transplant Recipients. Hepatology 2017;66(1):571A. [DOI] [PubMed] [Google Scholar]
- 23.Chhatwal J, Samur S, Bethea ED, Ayer T, Kanwal F, Hur C et al. Transplanting HCV-positive livers into HCV-negative patients with preemptive antiviral treatment: A modeling study. Hepatology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theodoropoulos N, Whitson BA, Martin SI, Pouch S, Pope-Harman A. Successful treatment of donor-derived hepatitis C infection in a lung transplant recipient. Transpl Infect Dis 2017;19(2). [DOI] [PubMed] [Google Scholar]
- 25.Martins PN, Movahedi B, Bozorgzadeh A. Transplanting HCV-Infected Kidneys into Uninfected Recipients. N Engl J Med 2017;377(11):1104–1105. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg DS, Abt PL, Reese PP, Investigators TT. Transplanting HCV-Infected Kidneys into Uninfected Recipients. N Engl J Med 2017;377(11):1105. [DOI] [PubMed] [Google Scholar]
- 27.Schlendorf KH, Zalawadiya S, Shah AS, Wigger M, Chung CY, Smith S et al. Early outcomes using hepatitis C-positive donors for cardiac transplantation in the era of effective direct-acting anti-viral therapies. J Heart Lung Transplant 2018. [DOI] [PubMed] [Google Scholar]
- 28.Moayedi Y, Gulamhusein AF, Ross HJ, Teuteberg JJ, Khush KK. Accepting hepatitis C virus-infected donor hearts for transplantation: Multistep consent, unrealized opportunity, and the Stanford experience. Clin Transplant 2018;32(7):e13308. [DOI] [PubMed] [Google Scholar]
- 29.Shah AP, Cameron A, Singh P, Frank AM, Fenkel JM. Successful treatment of donor-derived hepatitis C viral infection in three transplant recipients from a donor at increased risk for bloodborne pathogens. Transpl Infect Dis 2017;19(2). [DOI] [PubMed] [Google Scholar]
- 30.Suryaprasad A, Basavaraju SV, Hocevar SN, Theodoropoulos N, Zuckerman RA, Hayden T et al. Transmission of Hepatitis C Virus From Organ Donors Despite Nucleic Acid Test Screening. Am J Transplant 2015;15(7):1827–1835. [DOI] [PubMed] [Google Scholar]
- 31.Bari K, Luckett K, Kaiser T, Diwan T, Cuffy M, Schoech M et al. Hepatitis C Transmission from Seropositive, Non-Viremic Donors to Non-Hepatitis C Liver Transplant Recipients. Hepatology 2017. [DOI] [PubMed] [Google Scholar]
- 32.Terrault NA, McCaughan GW, Curry MP, Gane E, Fagiuoli S, Fung JYY et al. International Liver Transplantation Society Consensus Statement on Hepatitis C Management in Liver Transplant Candidates. Transplantation 2017;101(5):945–955. [DOI] [PubMed] [Google Scholar]
- 33.Levitsky J, Formica RN, Bloom RD, Charlton M, Curry M, Friedewald J et al. The American Society of Transplantation Consensus Conference on the Use of Hepatitis C Viremic Donors in Solid Organ Transplantation. Am J Transplant 2017;17(11):2790–2802. [DOI] [PubMed] [Google Scholar]
- 34.Bethea ED, Samur S, Kanwal F, Ayer T, Hur C, Roberts MS et al. Cost-effectiveness of Transplanting HCV-Infected Livers into Uninfected Recipients with Preemptive Antiviral Therapy. Clin Gastroenterol Hepatol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.