Description
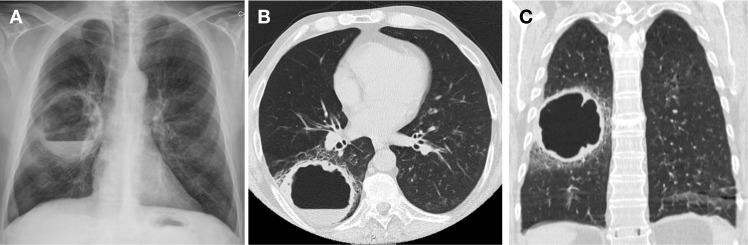
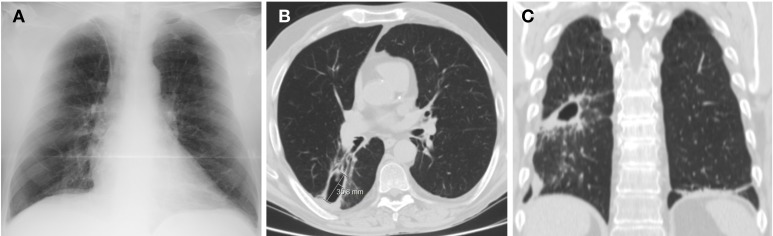
A 58-year-old man presented to the hospital with a 1-week history of dyspnea, cough, purulent sputum, fever and malaise. Patient had known HIV−1 infection for 10 years, with nadir CD4+ count of 31/µL and history of pneumocystosis as single opportunistic infection. He was under antiretroviral therapy (lamivudine and darunavir/ritonavir). Comorbidities included emphysema and history of tobacco, alcohol, cocaine and heroin abuse, now abstinent for several years. He presented with type 2 respiratory failure, neutrophilic leukocytosis and increased C-reactive protein (350 mg/L); CD4+ count was 212/µL and HIV viral load was undetectable. Chest radiograph revealed a large right-sided pulmonary cavity with dependent air-fluid level (figure 1A). CT confirmed the presence of a thick-wall cavity in the right inferior lobe with a major axis of 8.6 cm (figure 1B,C). Findings were consistent with diagnosis of a large pulmonary abscess and patient was started on empirical therapy with meropenem. Sputum cultures revealed growth of an extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli and Pseudomonas aeruginosa. Fluorescent microscopy and cultures of sputum were negative for mycobacteria. Meropenem was kept for 3 weeks with resolution of symptoms, respiratory failure and laboratory anomalies. A 3-week control CT scan showed downsizing of abscess dimensions while sputum cultures were now consistently negative. At this time, due to infection risk associated with maintenance of a central line in a patient without peripheral venous access, and the interest in switching to an outpatient regimen, treatment was changed to intramuscular ertapenem. Patient was under carbapenem therapy for a total of 8 weeks. Chest radiograph and CT scan at the end of treatment showed a smaller lesion with thin walls and without liquid component (figure 2A–C).
Figure 1.
Chest X-ray (A) and CT scan (B and C) at admission, showing a large right-sided pulmonary cavity with dependent air-fluid level.
Figure 2.
Chest X-ray (A) and CT scan (B and C) after 8 weeks of antibiotic therapy, showing a smaller lesion with thin walls and without liquid component.
Lung abscesses in patients with cell-mediated immune defects have a particular range of microbial flora, including bacteria, mycobacteria and fungi.1 The most common bacterial agents include P.aeruginosa, Enterobacteriaceae and anaerobes. Lung abscesses are often polymicrobial infections and anaerobes are difficult to isolate in sputum cultures. Empirical antibiotic options include clindamycin, β-lactam/β-lactamase-inhibitor combinations, carbapenems and quinolones with anaerobic activity.2 Meropenem was chosen because of its good penetration in lung parenchyma, and to target both anaerobes and multidrug-resistant aerobes (as patient had structural lung disease). Treatment duration is controversial; some authors advocate a standard regimen of 3–6 weeks.3 4 In this case, after 3 weeks of meropenem therapy with general improvement, it was considered safe to switch to intramuscular ertapenem, allowing an outpatient regimen. It was chosen instead of oral antimicrobials because of the interest in prolonging antibiotic coverage for ESBL-producing E.coli, in addition to anaerobes. However, ertapenem presents limited activity against Pseudomonas; patient was kept under close surveillance and, as outcome was favourable, previous 3-week treatment was considered adequate for this agent. Pharmacokinetics of intramuscular ertapenem are similar to intravenous administration in terms of area under the concentration curve and duration over which plasma levels exceed the susceptibility breakpoint.5 Total duration of treatment was determined by clinical and imaging response, and it was kept until CT scan showed a small, stable and residual lesion.
Learning points.
Lung abscess is characterised by necrosis of pulmonary parenchyma caused by microbial infection. In immunocompromised hosts, differential diagnosis is broad and includes a range of bacterial, mycobacterial and fungal agents. Pseudomonas aeruginosa, Enterobacteriaceae and anaerobes are frequent bacterial pathogens in this group.
Duration of antibiotic treatment should be based on clinical and radiological response, maintaining intravenous therapy until fever, putrid sputum and abscess fluid have resolved. Treatment can then be switched to a route that facilitates an outpatient regimen.
The pharmacokinetics of intramuscular ertapenem is similar to intravenous administration. Its single daily dosage makes it a convenient alternative for patients who can be discharged for home parenteral therapy.
Footnotes
Contributors: IME is the first author and contributed to the case study and conception of the manuscript. RC, DVC and CA contributed to the case study and critical review. CA supervised the writing critically. All authors have made significant contributions for this manuscript. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Kuhajda I, Zarogoulidis K, Tsirgogianni K, et al. . Lung abscess-etiology, diagnostic and treatment options. Ann Transl Med 2015;3:183 10.3978/j.issn.2305-5839.2015.07.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mustafa M, Iftikhar H, Muniandy R, et al. . Lung Abscess: Diagnosis, Treatment and Mortality. Int J Pharm Sci Invent 2015;4:37–41. [Google Scholar]
- 3. Loukeri A, Kampolis C, Tomos P, et al. . Diagnosis, treatment and prognosis of lung abscess. Pneumon 2015;28:54–60. [Google Scholar]
- 4. Levison ME, Mangura CT, Lorber B, et al. . Clindamycin compared with penicillin for the treatment of anaerobic lung abscess. Ann Intern Med 1983;98:466–71. 10.7326/0003-4819-98-4-466 [DOI] [PubMed] [Google Scholar]
- 5. Musson DG, Majumdar A, Birk K, et al. . Pharmacokinetics of intramuscularly administered ertapenem. Antimicrob Agents Chemother 2003;47:1732–5. 10.1128/AAC.47.5.1732-1735.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]