Abstract
Breast white adipose tissue inflammation (BWATi) is associated with obesity and higher breast cancer (BC) risk among non-Hispanic white women. Obesity is prevalent in Hispanic/Latina BC patients, and the occurrence of BWATi in this population is not well-characterized. The association between BWATi and body mass index (BMI) was evaluated in Hispanic/Latina BC patients who underwent mastectomy. BWATi was defined as the presence of crown-like structures of the breast (CLS-B), detected by CD68 immunohistochemistry in non-tumor breast tissue. BWATi severity was quantified as number of CLS-B/cm2. Adipocyte diameter was measured using hematoxylin and eosin (H&E) stained breast tissue sections. Preoperative BMI (within 1 week prior to mastectomy) was categorized as normal (18.5 to <25.0 kg/m2), overweight (25.0 to <30.0 kg/m2), class I obesity (30.0 to <35.0 kg/m2), and class II-III obesity (35.0 kg/m2 or above). Patient charts were abstracted to record clinicopathologic features and liver function tests <90 days before mastectomy. The study included 91 women (mean age 69 years; range 36–96 years). Prevalence of BWATi increased with BMI (24% in normal weight, 34% in overweight, 57% in class I obesity, and 65% in class II-III obesity; P for trend <0.01). Severe BWATi (>0.27 CLS-B/cm2) was associated with higher BMI (P for trend=0.046) and greater adipocyte diameter (P=0.04). Adjusting for BMI, neoadjuvant chemotherapy and elevated alanine aminotransferase were associated with severe BWATi, and current smoking was associated with mild BWATi (all P<0.05). BWATi was associated with higher BMI in Hispanic/Latina BC patients, consistent with previously described associations in other populations.
Keywords: White adipose tissue inflammation, Crown-like structures, Obesity, Breast cancer, Hispanic population
INTRODUCTION
Obesity is a risk factor for both hormone receptor-positive breast cancer (BC) in postmenopausal women and triple negative BC (1–4). In addition to its impact on the risk of BC, obesity is associated with poorer prognosis among BC survivors (5,6). Obesity-related effects on adipokines, hormones and inflammation have all been suggested to play a role in breast carcinogenesis (7–10). As obesity is a modifiable risk factor for BC risk and recurrence, biomarkers are needed to elucidate the pathways by which obesity exerts its effects on BC.
Obesity causes subclinical inflammation in adipose tissue (11,12). Both animal models and human studies of overweight and obesity have shown that macrophages infiltrate visceral and subcutaneous adipose tissue and form characteristic crown-like structures (CLS) around dead or dying adipocytes (12–15). In a population of predominantly non-Hispanic white BC patients, we previously reported that the presence of CLS in the breast (CLS-B) is associated with activation of the NF-κB transcription factor in breast tissue and higher expression of aromatase, the rate-limiting enzyme for estrogen biosynthesis (16). Consistent with these findings, breast white adipose tissue inflammation (BWATi), as defined by the presence of CLS-B, has also been associated with an increased ratio of estrogens to androgens in both breast tissue and blood (17). Additionally, BWATi is associated with altered systemic levels of adipokines, proinflammatory mediators and insulin (18) and the postmenopausal state (19). Based on both the local and systemic changes that correlate with inflamed breast adipose tissue, BWATi may be involved in both an increased risk of BC and worse prognosis of obese BC patients. This hypothesis is supported by recent case-control studies showing that higher CLS-B density is associated with elevated risk of BC in women with benign breast disease (20,21). Moreover, our group has reported that BWATi is associated with worse distant recurrence-free survival and the metabolic syndrome in BC patients (18). In another recent study, BWATi was associated with reduced overall survival among BC survivors (22).
There are high rates of obesity and overweight among Hispanic/Latina BC patient populations, which is reflective of the general US Hispanic/Latina population (23). Compared with non-Hispanic white women, Hispanics/Latinas are less likely to be diagnosed with localized BC, and are more likely to be diagnosed with larger, hormone receptor negative tumors, which have poorer survival rates (24–26). Previous studies have reported conflicting results on whether Hispanic/Latinas have worse BC survival after accounting for differences in age, stage at diagnosis and tumor characteristics (25,27). These disparities may be partly due to the high rates of overweight and obesity in this population. Studies are needed to elucidate mechanisms by which obesity may be related to BC risk and recurrence in Hispanics/Latinas. In this pilot study, we extended our prior investigations of the association between BWATi and body mass index (BMI) to examine whether similar patterns previously reported in non-Hispanic white women are also observed in Hispanic/Latina BC patients.
METHODS
Study participants
Hispanic/Latina patients with stage 0-III BC who underwent mastectomy at Columbia University Medical Center (CUMC) between February 2007 and November 2012 were identified via the Herbert Irving Comprehensive Cancer Center (HICCC) breast oncology database and the CUMC Pathology database. All patients had provided written, informed consent for future use of their tissue for research. Patients who met the following eligibility criteria were included: 1) self-identified Hispanic/Latina, 2) stage 0 to III BC, 3) BMI ≥18.5 kg/m2 at the time of surgery, 4) previously provided written informed consent to be included in the breast oncology database and that their pathology specimens could be used for future research, and 5) available archived formalin-fixed paraffin embedded sample of normal adjacent breast tissue from a quadrant uninvolved by tumor obtained via mastectomy. This study was approved by the Institutional Review Boards at Columbia University Medical Center and Memorial Sloan Kettering Cancer Center (MSKCC).
Anthropometric measures
BMI was calculated using preoperative body weight and height measured by anesthesiologists and recorded in the patient chart within one week prior to mastectomy. BMI was categorized as normal weight (18.5 to <25.0 kg/m2), overweight (25.0 to <30.0 kg/m2), class I obesity (30.0 to <35.0 kg/m2), and class II-III obesity (≥35.0 kg/m2).
Adipose tissue collection
Within each BMI group, 18–54 patients with banked tissue samples were selected, which were provided to the CUMC Pathology laboratory. Pathology staff pulled FFPE blocks from stored mastectomy specimens and blocks from nontumorous quadrants were examined. Blocks were excluded if the representative section showed biopsy site change or traumatic fat necrosis. In this manner, one white adipose tissue-enriched block was selected per patient. Useable samples in each BMI category (n=29 for BMI 18.5 to <25.0 kg/m2, n=51 for BMI 25.0 to <30.0 kg/m2, n=33 for BMI 30.0 to <35.0 kg/m2, n=18 for BMI ≥ 35.0 kg/m2) were sent to the Herbert Irving Comprehensive Cancer Center (HICCC) Molecular Pathology Shared Resource for pathology examination and microtomy. For each case, tissue samples were cut into seven sections that were approximately 50 μm apart. Slides were 5 mm thin and approximately 2 cm in diameter. Sections were cut at 50 μm intervals to avoid duplicate quantification of CLS-B.
Measurement of breast white adipose tissue inflammation
The MSKCC Pathology Core Facility stained 2 sections from each block with hematoxylin and eosin (H&E) and 5 sections for cluster of differentiation 68 (CD68), a macrophage marker (mouse monoclonal KP1 antibody; Dako; dilution 1:4,000). CD68-stained sections were examined under light microscopy by the study pathologist (DG) to detect CLS-B. When present, the number of CLS-B in each section were counted and recorded. BWATi was defined by the presence of CLS-B. Consistent with previously reported methods (16), the density of CLS-B by surface area was determined as follows: each CD68-stained slide was digitally archived by gross photography. Images were stored in the tagged image file format (TIFF), and the white adipose tissue area on each slide was measured using Image J Software (NIH, Bethesda, MD) (28). Epithelial tissue area and areas of fibrosis were excluded. The severity of BWATi was quantified as CLS-B per square centimeter of white adipose tissue (CLS-B/cm2), with the median CLS-B/cm2 as the cutoff to differentiate between severe and mild inflammation. Previously using this definition, BWATi severity was shown to be positively associated with aromatase expression in breast tissue (29), providing useful biological information beyond presence or absence of CLS-B.
Measurement of adipocyte size
Following previously reported methods (16), we measured adipocyte diameters using H&E stained FFPE breast tissue sections. Representative H&E sections with preserved adipocyte architecture were manually selected by a histopathologist (DJF) and were photographed at 20× using an Olympus BX50 microscope and MicroFire digital camera (Optronics, Goleta, CA). Mean adipocyte diameters were calculated using the average diameters of 30 randomly selected individual adipocytes for each patient using the linear dimensional tool in the Canvas 11 Software (ACD Systems International, Inc., Victoria, Canada).
Demographic and clinical data
Demographic and clinical characteristics were collected from the electronic medical record, including age at date of surgery, and tumor characteristics. The HICCC breast oncology database provided data on chemotherapy and endocrine therapy received as well as menopausal status. Women were defined as postmenopausal if they had undergone bilateral oophorectomy or reported permanent cessation of menses for ≥12 months in the absence of chemotherapy or endocrine therapy. For patients whose menopausal status was unknown, women >51 years old were defined as postmenopausal. When available, liver function tests (i.e., alanine aminotransferase and aspartate aminotransferase) measured up to 90 days prior to date of mastectomy were abstracted from CUMC electronic medical records; values were averaged for each patient if more than one liver function test was recorded.
Statistical analysis
We tested the linear trend in BWATi from low to high BMI levels using the trend test of proportions. We examined the correlates of BWATi using Fisher’s exact test for categorical variables and F-test for continuous variables. For factors associated with the presence or severity of BWATi, we further examined their associations by adjusting for BMI in multivariable logistic regression and multinomial logistic regressions. All statistical tests were two-sided with α=0.05. Statistical analyses were performed in R (version 3.4.3).
RESULTS
Participant characteristics
A total of 137 eligible Hispanic/Latina BC patients with breast white adipose tissue samples were identified, of which 99 patients with useable tissue were selected according to BMI category. Upon examination by the study pathologist, 8 samples with traumatic fat necrosis were excluded, leaving a total of 91 participants in the final analyses (Supplemental Figure 1). The median age of the 91 patients was 69 years (range: 36–96 years) (Table 1). The median BMI was 29.9 kg/m2 (range: 19.9–50.5 kg/m2). The majority of participants were of Dominican Republic descent (63%), followed by Puerto Rican (16%) and other Hispanics (16%). Approximately, 79% of women were postmenopausal, and 21% were premenopausal. The majority of patients had tumors that were estrogen receptor positive (86%) and progesterone receptor positive (70%), and 16% received neoadjuvant chemotherapy (Table 1).
Table 1.
Association of CLS-B with demographic and clinical characteristics
| Total | CLS-B + (N=41) | CLS-B - (N=50) | Pa | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | N | % | N | % | N | % | ||
| Age | Median (range) | 69 (36, 96) | 72 (36, 91) | 65.5 (39, 96) | 0.19 | |||
| BMI (kg/m2) | Median (range) | 29.9 (19.9, 50.5) | 31.6 (23, 50.5) | 28 (19.9, 43.2) | <0.01 | |||
| Country of origin | Dominican Republic | 57 | 63% | 23 | 56% | 34 | 68% | 0.10 |
| Puerto Rican | 15 | 16% | 10 | 24% | 5 | 10% | ||
| South American | 2 | 2% | 0 | 0% | 2 | 4% | ||
| Mexican | 2 | 2% | 2 | 5% | 0 | 0% | ||
| Cuban | 0 | 0% | 0 | 0% | 0 | 0% | ||
| Other Hispanic | 15 | 16% | 6 | 15% | 9 | 18% | ||
| Education | High School | 31 | 34% | 14 | 34% | 17 | 34% | 0.48 |
| College | 22 | 24% | 7 | 17% | 15 | 30% | ||
| Graduate School | 24 | 26% | 12 | 29% | 12 | 24% | ||
| Employment | Employed | 18 | 20% | 7 | 17% | 11 | 22% | 0.43 |
| Unemployed | 25 | 27% | 10 | 24% | 15 | 30% | ||
| Retired | 27 | 30% | 16 | 39% | 11 | 22% | ||
| Homemaker | 8 | 9% | 3 | 7% | 5 | 10% | ||
| Income | 0-$15,000 | 49 | 54% | 27 | 66% | 22 | 44% | 0.30 |
| $15,000+ | 21 | 23% | 8 | 20% | 13 | 26% | ||
| Post menopause | Yes | 72 | 79% | 33 | 80% | 39 | 78% | 0.80 |
| No | 19 | 21% | 8 | 20% | 11 | 22% | ||
| Current smoking | Yes | 8 | 9% | 6 | 15% | 2 | 4% | 0.13 |
| No | 71 | 78% | 30 | 73% | 41 | 82% | ||
| History of smoking | Current | 7 | 8% | 5 | 12% | 2 | 4% | 0.38 |
| Ever | 11 | 12% | 5 | 12% | 6 | 12% | ||
| Never | 52 | 57% | 22 | 54% | 30 | 60% | ||
| Current alcohol use | Yes | 25 | 27% | 12 | 29% | 13 | 26% | 1.00 |
| No | 55 | 60% | 25 | 61% | 30 | 60% | ||
| Stage | 0 | 9 | 10% | 5 | 12% | 4 | 8% | 0.48 |
| I | 36 | 40% | 15 | 37% | 21 | 42% | ||
| II | 35 | 38% | 14 | 34% | 21 | 42% | ||
| III | 11 | 12% | 7 | 17% | 4 | 8% | ||
| ER | Positive | 78 | 86% | 34 | 83% | 44 | 88% | 0.53 |
| Negative | 11 | 12% | 6 | 15% | 5 | 10% | ||
| PR | Positive | 64 | 70% | 29 | 71% | 35 | 70% | 1.00 |
| Negative | 25 | 27% | 11 | 27% | 14 | 28% | ||
| HER2b | Positive | 7 | 8% | 5 | 12% | 2 | 4% | 0.53 |
| Negative | 42 | 46% | 17 | 41% | 25 | 50% | ||
| Indeterminate | 29 | 32% | 13 | 32% | 16 | 32% | ||
| Not applicable | 7 | 8% | 3 | 7% | 4 | 8% | ||
| Neoadjuvant chemotherapy | Yes | 15 | 16% | 11 | 27% | 4 | 8% | 0.02 |
| No | 75 | 82% | 29 | 71% | 46 | 92% | ||
| Biological markers | N | N | Mean (SD) | N | Mean (SD) | P | ||
| Liver function tests | ||||||||
| ALT (U/L) | 86 | 39 | 20.5 (9.8) | 47 | 17.5 (9.1) | 0.14 | ||
| AST (U/L) | 86 | 39 | 20.6 (6.4) | 47 | 19.5 (6.0) | 0.42 | ||
| Adipocyte diameter (μm) | ||||||||
| Mean (SD) | 89 | 40 | 105.6 (14.1) | 49 | 99.3 (16.9) | 0.06 | ||
| Range | 59.7–139.8 | 59.7–135.2 | ||||||
Note: For all variables, the number of participants who did not respond to the question were not displayed in this table, therefore the percentages may not add up to 100%
Abbreviation: SD, standard deviation; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; ALT, alanine aminotransferase; AST, aspartate aminotransferase
Fisher’s exact test excluded the “Unknown” category
Patients with stage 0 breast cancer were considered not applicable for immunohistochemistry test
Association between presence of CLS-B and BMI
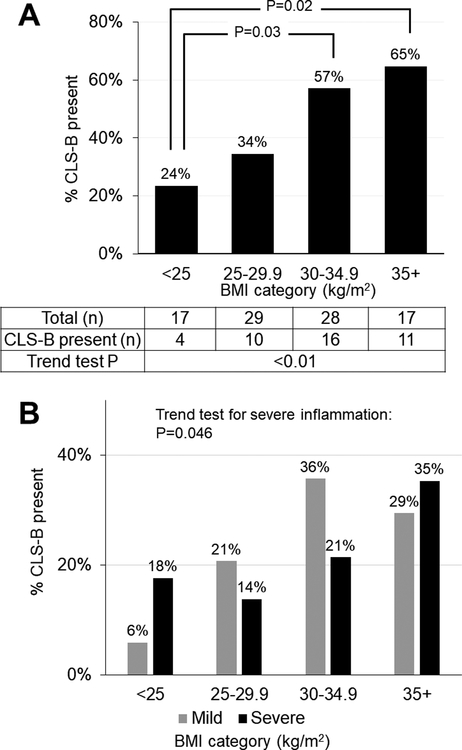
CLS-B were present in 45% of Hispanic/Latina BC patients. The prevalence of CLS-B increased with BMI, with CLS-B present in 24% of normal weight patients, 34% of overweight patients, 57% of patients with class I obesity, and 65% of patients with class II-III obesity (P for trend <0.01) (Figure 1A). Compared to the normal weight group, the presence of CLS-B was statistically significantly more common in patients with class I obesity (P=0.03) and class II-III obesity (P=0.02).
Figure 1. Breast white adipose tissue inflammation by BMI category (n=91).
Figure 1A shows the prevalence of CLS-B by BMI category, and Figure 1B shows the distribution of mild and severe inflammation by BMI category. In Figure 1B, inflammation severity is defined categorically as follows and using median number of CLS-B/cm2 (0.27) as the cutoff: no inflammation (CLS-B absent), mild inflammation (≤0.27 CLS-B/cm2), and severe inflammation (>0.27 CLS-B/cm2).
Association between severity of breast white adipose tissue inflammation and BMI
Using the median as a cutoff, the severity of BWATi was defined as mild (≤0.27 CLS-B/cm2) in 22 of 91 (24%) patients and severe (>0.27 CLS-B/cm2) in 19 of 91 (21%) patients. The prevalence of severe BWATi was positively associated with BMI among patients with CLS-B: 18% in normal weight, 14% in overweight, 21% in Class I obesity, and 35% in Class II-III obesity; P for trend = 0.046) (Figure 1B).
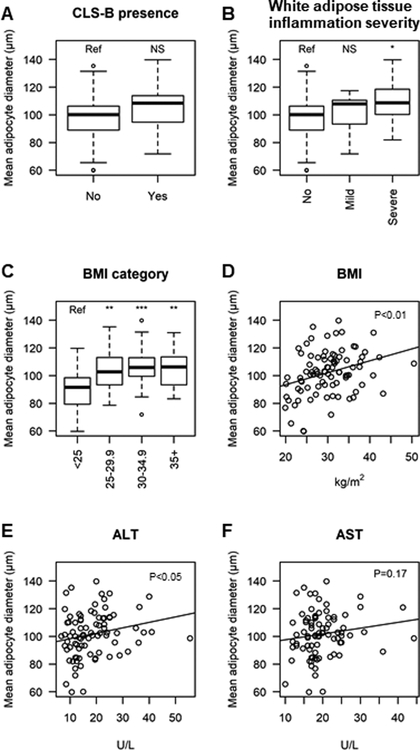
Association between breast white adipose tissue inflammation and adipocyte diameter
Patients with CLS-B had a trend toward larger adipocyte diameters (mean=105.6 μm, SD=14.1 μm) compared to patients without CLS-B (mean=99.3 μm, SD=16.9 μm, P=0.06; Table 1 and Figure 2A). The adipocyte diameter was significantly larger in patients with severe BWATi (mean=108.3 μm, SD=16.6 μm) compared to those without CLS-B (mean=99.3 μm, SD=16.9 μm, P=0.04; Table 2 and Figure 2B). Larger adipocyte size was also associated with overweight and obesity (all P<0.05; Figures 2C and 2D). In addition, larger adipocyte size was associated with higher serum alanine aminotransferease (ALT) (P<0.05, Figure 2E) but not serum alanine aminotransferease (ALT) (Figure 2F).
Figure 2. Association between average adipocyte diameter and biomarkers of liver function.
The box plots compare the mean adipocyte diameters between groups, and the scatter plots and fitted linear regression lines show the correlation between mean adipocyte diameter and each biomarker. Patients with higher BMI and more severe breast WAT inflammation had larger adipocyte diameter (all P<0.05). For box plots, statistically significant differences between groups were denoted by *: P<0.05, **: P<0.01, ***: P<0.001, and NS: not significant. Abbreviations: BMI, body mass index; CLS-B, crown-like structure of the breast; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Table 2.
Association of breast white adipose tissue inflammation severity with demographic and clinical characteristics
| Characteristics | Total | Severea (N=19) | Milda (N=22) | None (N=50) | Pb | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | N | % | N | % | N | % | ||||
| Age | Median (range) | 91 | 74 (53, 91) | 69 (36, 89) | 66 (39, 96) | 0.15 | ||||
| Country of origin | Dominican Republic | 57 | 8 | 42% | 15 | 68% | 34 | 68% | 0.03 | |
| Puerto Rican | 15 | 8 | 42% | 2 | 9% | 5 | 10% | |||
| South American | 2 | 0 | 0% | 0 | 0% | 2 | 4% | |||
| Mexican | 2 | 0 | 0% | 2 | 9% | 0 | 0% | |||
| Cuban | 0 | 0 | 0% | 0 | 0% | 0 | 0% | |||
| Other Hispanic | 15 | 3 | 16% | 3 | 14% | 9 | 18% | |||
| Education | High School | 31 | 7 | 37% | 7 | 32% | 17 | 34% | 0.40 | |
| College | 22 | 5 | 26% | 2 | 9% | 15 | 30% | |||
| Graduate School | 24 | 4 | 21% | 8 | 36% | 12 | 24% | |||
| Employment | Employed | 18 | 4 | 21% | 3 | 14% | 11 | 22% | 0.26 | |
| Unemployed | 25 | 2 | 11% | 8 | 36% | 15 | 30% | |||
| Retired | 27 | 10 | 53% | 6 | 27% | 11 | 22% | |||
| Homemaker | 8 | 1 | 5% | 2 | 9% | 5 | 10% | |||
| Income | 0-$15,000 | 49 | 11 | 58% | 16 | 73% | 22 | 44% | 0.41 | |
| $15,000+ | 21 | 4 | 21% | 4 | 18% | 13 | 26% | |||
| Post menopause | Yes | 72 | 17 | 89% | 16 | 73% | 39 | 78% | 0.45 | |
| No | 19 | 2 | 11% | 6 | 27% | 11 | 22% | |||
| Current smoking | Yes | 8 | 1 | 5% | 5 | 23% | 2 | 4% | 0.04 | |
| No | 71 | 16 | 84% | 14 | 64% | 41 | 82% | |||
| History of smoking | Current | 7 | 0 | 0% | 5 | 23% | 2 | 4% | 0.10 | |
| Ever | 11 | 3 | 16% | 2 | 9% | 6 | 12% | |||
| Never | 52 | 11 | 58% | 11 | 50% | 30 | 60% | |||
| Current alcohol use | Yes | 25 | 6 | 32% | 6 | 27% | 13 | 26% | 0.95 | |
| No | 55 | 11 | 58% | 14 | 64% | 30 | 60% | |||
| Stage | 0 | 9 | 0 | 0% | 5 | 23% | 4 | 8% | 0.18 | |
| I | 36 | 9 | 47% | 6 | 27% | 21 | 42% | |||
| II | 35 | 6 | 32% | 8 | 36% | 21 | 42% | |||
| III | 11 | 4 | 21% | 3 | 14% | 4 | 8% | |||
| ER | Positive | 78 | 16 | 84% | 18 | 82% | 44 | 88% | 0.62 | |
| Negative | 11 | 2 | 11% | 4 | 18% | 5 | 10% | |||
| PR | Positive | 64 | 13 | 68% | 16 | 73% | 35 | 70% | 1.00 | |
| Negative | 25 | 5 | 26% | 6 | 27% | 14 | 28% | |||
| HER2c | Positive | 7 | 1 | 5% | 4 | 18% | 2 | 4% | 0.24 | |
| Negative | 42 | 10 | 53% | 7 | 32% | 25 | 50% | |||
| Indeterminate | 29 | 7 | 37% | 6 | 27% | 16 | 32% | |||
| Not applicable | 7 | 0 | 0% | 3 | 14% | 4 | 8% | |||
| Neoadjuvant chemotherapy | Yes | 15 | 5 | 26% | 6 | 27% | 4 | 8% | 0.04 | |
| No | 75 | 13 | 68% | 16 | 73% | 46 | 92% | |||
| Biological markers | N | N | Mean (SD) | N | Mean (SD) | P | N | Mean (SD) | P | |
| Liver function tests | ||||||||||
| ALT (U/L) | 86 | 19 | 24.4 (8.8) | 20 | 16.9 (9.6) | 0.01 | 47 | 17.5 (9.1) | 0.01 | |
| AST (U/L) | 86 | 19 | 22.8 (6.6) | 20 | 18.5 (5.4) | 0.03 | 47 | 19.5 (6.0) | 0.05 | |
| Adipocyte diameter (μm) | ||||||||||
| Mean (SD) | 89 | 19 | 108.3 (16.6) | 21 | 103.2 (11.4) | 0.31 | 49 | 99.3 (16.9) | 0.04 | |
| Range | 81.9–139.8 | 71.8–117.5 | 59.7–135.2 | |||||||
Note: For all variables, the number of participants who did not respond to the question were not displayed in this table, therefore the percentages may not add up to 100%
Abbreviation: SD, standard deviation; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; ALT, alanine aminotransferase; AST, aspartate aminotransferase
Inflammation severity is defined categorically as follows and using the value of 0.27 CLS-B/cm2 as the cutoff: no inflammation (absent CLS-B), mild inflammation (≤0.27 CLS-B/cm2), and severe inflammation (>0.27 CLS-B/cm2)
Fisher’s exact test excluded the “Unknown” category
Patients with stage 0 breast cancer were considered not applicable for immunohistochemistry test
Associations between breast white adipose tissue inflammation and clinical and demographic characteristics
Both the incidence (P=0.02, Table 1) and severity (P=0.04, Table 2) of BWATi were greater in patients who received neoadjuvant chemotherapy. In multivariable logistic regression models adjusting for BMI, neoadjuvant chemotherapy was associated with higher odds of CLS-B (adjusted OR= 4.3, 95% CI: 1.2, 18.3) and higher odds of severe BWATi (adjusted OR= 5.2, 95% CI: 1.1, 25.7) independent of BMI (Table 3).
Table 3.
Association of breast white adipose tissue inflammation with chemotherapy, smoking, ALT and AST, adjusting for BMI
| Unadjusted | Adjusted for BMIc | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | ||
| Presence of CLS-B1 | CLS-B present vs. absent | CLS-B present vs. absent | |||||||
| Neoadjuvant chemotherapy received | |||||||||
| Yes vs. no | 4.4 (1.4, 16.9) | 0.02 | 4.3 (1.2, 18.3) | 0.03 | |||||
| Severity of breast white adipose tissue inflammation2 | Severe vs. no inflammation | Mild vs. no inflammation | Severe vs. no inflammation | Mild vs. no inflammation | |||||
| Country of origin | |||||||||
| Dominican Republic vs. other Hispanic | 0.9 (0.2, 3.8) | 0.85 | 1.0 (0.3, 3.3) | 0.96 | 0.6 (0.1, 3.1) | 0.57 | 0.8 (0.2, 3.0) | 0.74 | |
| Puerto Rican vs. other Hispanic | 5.9 (1.1, 32.0) | 0.04 | 0.9 (0.1, 6.2) | 0.90 | 5.0 (0.9, 28.8) | 0.07 | 0.8 (0.1, 6.1) | 0.83 | |
| Current smoking | |||||||||
| Smoker vs. non-smoker | 1.3 (0.1, 15.1) | 0.84 | 7.3 (1.3, 42.1) | 0.03 | 1.3 (0.1, 16.4) | 0.84 | 6.4 (1.0, 39.2) | 0.04 | |
| Neoadjuvant chemotherapy received | |||||||||
| Yes vs. no | 4.4 (1.0, 18.9) | 0.04 | 4.3 (1.1, 17.3) | 0.04 | 5.2 (1.1, 25.7) | 0.04 | 3.8 (0.9, 16.2) | 0.07 | |
| ALT | |||||||||
| Per 1 interquartile range increase | 2.1 (1.1, 4.0) | 0.02 | 0.9 (0.4, 1.9) | 0.78 | 2.0 (1.1, 3.9) | 0.03 | 0.8 (0.3, 1.7) | 0.52 | |
| AST | |||||||||
| Per 1 interquartile range increase | 1.6 (1.0, 2.8) | 0.07 | 0.8 (0.4, 1.6) | 0.47 | 1.5 (0.9, 2.7) | 0.12 | 0.7 (0.3, 1.5) | 0.39 | |
Note
Abbreviation: CLS-B, crown-like structure of the breast; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index.
Presence of CLS-B was analyzed using logistic regression with “CLS-B absent” as the referent.
Severity of breast white adipose tissue inflammation was a 3-level categorical variable, and was analyzed using multinomial logistic regression with “no inflammation” as the referent group.
BMI was analyzed as a categorical variable (<25 kg/m2, 25–29.9 kg/m2, 30–34.9 kg/m2, 35+ kg/m2)
In unadjusted analyses, patients with severe BWATi were more likely to be Puerto Ricans compared to patients with mild or no inflammation (P=0.03) (Table 2). However, there was no significant association between country of origin and BWATi after adjusting for BMI. In unadjusted analyses, patients with mild BWATi were also more likely to be current smokers compared to patients with severe or no inflammation (P=0.04) (Table 2). The association between smoking and mild inflammation remained after adjusting for BMI (adjusted OR=6.4, 95% CI: 1.0, 39.2) (Table 3).
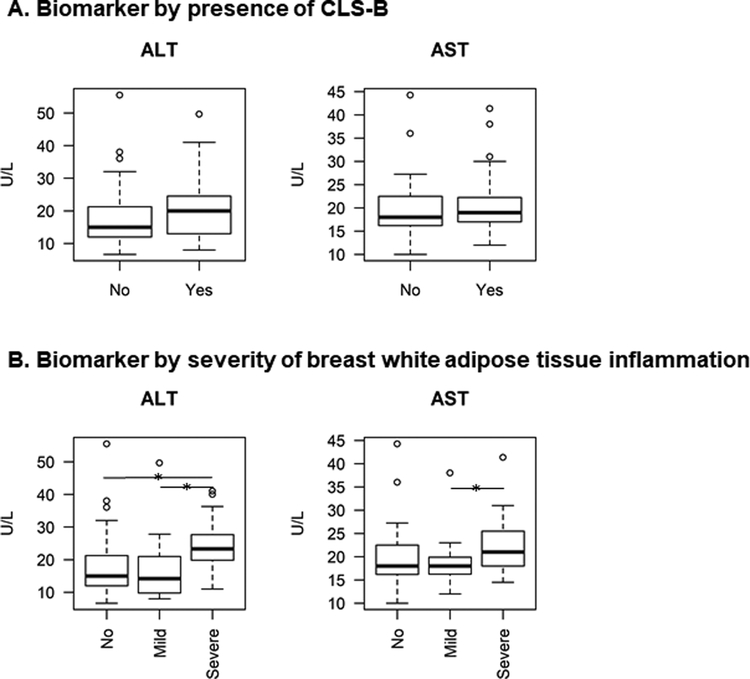
On average, patients had 2.2 liver function tests recorded in the electronic medical record in the 90 days before mastectomy, with a median of 2 measurements and a range of 1–8 measurements. In unadjusted analyses, patients with severe BWATi had higher serum ALT compared to patients with mild (P=0.01) or no BWATi (P=0.01) (Table 2, Figure 3A). Similarly, in unadjusted analyses, patients with severe BWATi had higher AST compared to patients with mild BWATi (P=0.03) (Table 2, Figure 3B). After adjusting for BMI, severe BWATi remained associated with elevated ALT when compared with no BWATi (per interquartile range increase: OR=2.0, 95% CI: 1.1, 3.9) (Table 3).
Figure 3. Biomarkers of liver function by presence of CLS-B (Figure 3A) and severity of breast white adipose tissue inflammation (Figure 3B).
The boxplot shows the median (dark horizontal line inside box), the 25% percentile (lower bound of box), and the 75% percentile (upper bound of box) of each biomarker by inflammation status. The horizontal bar with a star denotes statistically significant differences between groups. Patients with severe breast WAT inflammation had higher ALT compared to patients with mild (P=0.02) or no breast WAT inflammation (P=0.01). Patients with severe breast WAT inflammation had higher AST compared to patients with mild breast WAT inflammation (P=0.03). Abbreviations: CLS-B, crown-like structure of the breast; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
DISCUSSION
In this pilot study, BWATi was observed in nearly half of Hispanic/Latina BC survivors who underwent mastectomy. The prevalence and severity of BWATi were strongly associated with higher BMI in Hispanic/Latina BC patients. Patients with severe BWATi had significantly larger adipocytes. These findings are consistent with prior studies in predominantly non-Hispanic white and Asian populations (16,18,19,30).
The prevalence of BWATi in this cohort of Hispanic/Latina BC patients (45%) is comparable with previous reports in non-Hispanic white patients, in which 36–59% BC patients had BWATi (16,18,19,30–32). The positive association between presence and severity of BWATi and BMI category is also consistent with prior publications (16,30,32,33). However, BWATi was present in 24% of Hispanic/Latina BC patients who had normal weight (BMI<25 kg/m2), but was absent in 35% of patients with Class II and III obesity. The fact that some obese patients are not inflamed and a subset of patients with normal BMI are inflamed suggests that BMI alone does not accurately predict BWATi.
Consistent with our hypothesis and previous studies (16,19), patients who were overweight or obese had larger adipocytes, and adipocyte diameter was positively associated with BWATi severity. These findings support the hypothesis that obesity-associated adipocyte hypertrophy leads to immune cell recruitment and BWATi (34). However, adipocyte size was not significantly associated with BWATi when evaluated as a categorical variable (CLS-B absent/present). The relatively higher BMI and larger adipocyte diameters in our sample of Hispanic/Latina BC survivors vs. non-Hispanic white patients in our previous studies may explain the marginal differences in adipocyte diameters between patients with and without CLS-B (16,19). For example, in one of our previous studies of 237 predominantly non-Hispanic white women with a median BMI of 25.3 kg/m2 (19), the average adipocyte diameter was 92 μm (SD=16 μm) in women without CLS-B, compared to 107 μm (SD=12 μm) in women with CLS-B. In comparison, in the current study, median BMI was 29.9 kg/m2, which is higher than the median BMI in our previous population of non-Hispanic white women and may explain the narrower difference in adipocyte diameter between women without CLS-B (mean=99 μm, SD=17 μm) and with CLS-B (mean=106 μm, SD=14 μm). It is also possible that the normal range of adipocyte diameter varies by racial/ethnic groups, and that specific racial/ethnic groups may have a greater propensity to develop CLS-B due to having larger adipocytes. Future studies can test this hypothesis by examining the interaction between race/ethnicity and adipocyte diameter in relation to CLS-B.
Several novel associations were observed in this study. To our knowledge, this is the first report indicating that smoking history and preoperative neoadjuvant chemotherapy were associated with CLS-B. Smoking is known to induce inflammation, however an association between smoking and adipose inflammation has not been previously described. Although there were only 8 current smokers in our sample, contributing to the large variance of the odds ratio for smoking, the association between smoking and BWATi is biologically plausible. Smoking causes atherosclerosis (35), and cigarettes contain vasoactive compounds e.g., nicotine, which can lead to tissue hypoxia (36). Adipocyte death in hypoxic fat pads could lead to inflammation and the formation of CLS-B. Thus, smoking may contribute to BWATi by inducing a hypoxic state. However, mechanistic studies are needed to better understand the association between smoking and BWATi.
Of particular interest and novelty, we found an association between abnormal liver function tests (LFTs) and severity of BWATi in Hispanic/Latina BC patients. Elevated transaminases are indicative of underlying hepatic dysfunction which is most commonly due to nonalcoholic fatty liver disease (NAFLD) in obese individuals. The association between elevated ALT and BWATi is likely to be indicative of underlying NAFLD. While the finding of NAFLD in individuals with elevated BMI is not surprising, the association between elevated ALT and BWATi was independent of BMI. This independent association is consistent with our prior studies reporting BWATi in a subset of normal BMI individuals who are likely to have metabolic obesity (37). Furthermore, our findings suggest that measurement of LFTs, which are routinely followed in clinical practice, could be developed as a noninvasive method to diagnose BWATi. These novel findings warrant future studies with larger sample sizes to better understand the prevalence and correlates of BWATi among diverse populations of BC patients.
Our study contributes to a growing body of literature examining the association between preoperative neoadjuvant chemotherapy and the presence of BWATi. In our study, Hispanic/Latina BC patients who received neoadjuvant chemotherapy had five-time higher odds of having CLS-B compared to patients who received adjuvant or no chemotherapy. Another study of 237 BC patients reported that BWATi was observed in 19 of 34 (56%) patients who received preoperative chemotherapy, which was numerically but not statistically significantly higher compared with patients who did not receive preoperative chemotherapy (51%, P=0.71) (33). It is possible that the types of neoadjuvant chemotherapy varied in these 2 studies accounting for the different findings. Although the mechanisms linking chemotherapy use to BWATi are unclear, it is possible that cytotoxic chemotherapy could induce breast adipocyte death, leading to the formation of CLS-B. Dead and dying adipocytes are known to recruit macrophages, which leads to the formation of crown-like structures (12,14). Future studies need to examine associations between receipt of neoadjuvant chemotherapy, subsequent development of BWATi, and measurable clinical outcomes.
Although our study identified new and potentially clinically relevant correlates of BWATi, we did not observe an association between BWATi and postmenopausal status as in previous studies (18,19). Notably, the median age of our study population was higher than in prior studies, thereby reducing the power of this study to detect differences in menopausal status by white adipose tissue inflammation.
Our study is limited by its retrospective design. Specifically, detailed information on diet, physical activity and alcohol drinking were not collected prospectively using standard questionnaires, making it difficult to examine the causal relations between lifestyle factors and BWATi. Furthermore, the current study relied on clinically measured LFTs collected retrospectively from medical records rather than single-batch measurement of markers in prospectively collected blood from fasting women. Prospectively designed studies in ethnically diverse populations are needed. The relatively small sample size also limited our power to analyze multiple factors simultaneously. Finally, the cross-sectional association between CLS-B and obesity does not imply that chronic inflammation as measured by CLS-B mediates the obesity-breast cancer association. Nonetheless, this is the first study to evaluate BWATi in a cohort of exclusively Hispanic/Latina women with BC. Our findings establish the rationale for further studies of BWATi in this population.
In conclusion, BWATi is associated with higher BMI in Hispanic/Latina BC patients. Smoking history, preoperative chemotherapy, and abnormal liver function tests were associated with BWATi in this cohort, independent of BMI. However, a causal association between these factors would require replication in a prospective cohort study. Understanding the mechanisms contributing to worse BC outcomes in obese individuals, such as BWATi, will provide key insights into selecting intervention strategies that are likely to be effective. Moreover, elucidating the intersections among race/ethnicity and BWATi is a novel and biologically-informed approach to combating the obesity epidemic which has disproportionately affected minority populations. As BWATi has been associated with increased risk of breast cancer and worse breast cancer prognosis, the findings here support the need to develop non-invasive strategies to identify women with BWATi and to identify effective interventions to reduce BWATi and reduce breast cancer risk and/or improve breast cancer prognosis. Efforts are underway to develop non-invasive biomarkers to identify women with CLS-B. Furthermore, it is possible that lifestyle interventions (e.g., diet, physical activity and/or weight loss) could reduce BWATi and reduce risk. Similarly, for women who are found to have BWATi at the time of mastectomy, anti-inflammatory interventions may improve prognosis. These findings are provocative and require future prospectively designed studies with larger sample sizes to better understand the prevalence and correlates of BWATi among diverse populations of BC patients.
Supplementary Material
ACKNOWLEDGMENTS
We thank study participants for donating their tissue samples for research. We also thank Saiful Lughmani for providing preoperative weight and height data, and Kathleene Ulanday for her work in abstracting clinical data. This study was supported by the Herbert Irving Comprehensive Cancer Center Core/Support Grant (P30 CA013696), Breast Cancer Research Foundation, the Conquer Cancer Foundation of the American Society of Clinical Oncology, Myrna and Bernard Posner, the Fishman Family Foundation, Kat’s Ribbon of Hope, the Botwinick-Wolfensohn Foundation (in memory of Mr. and Mrs. Benjamin Botwinick), NIH/NCI U54 CA210184-01, the Transdisciplinary Research on Energetics and Cancer Training Workshop (R25CA203650), and the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Footnotes
PRIOR PRESENTATION: This study was presented as a poster presentation at the 2018 AACR Special Conference on Obesity and Cancer.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Ahn J, Schatzkin A, Lacey JV Jr., Albanes D, Ballard-Barbash R, Adams KF, et al. Adiposity, adult weight change, and postmenopausal breast cancer risk. Arch Intern Med 2007;167(19):2091–102 doi 10.1001/archinte.167.19.2091. [DOI] [PubMed] [Google Scholar]
- 2.Morimoto LM, White E, Chen Z, Chlebowski RT, Hays J, Kuller L, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women’s Health Initiative (United States). Cancer Causes Control 2002;13(8):741–51. [DOI] [PubMed] [Google Scholar]
- 3.Pierobon M, Frankenfeld CL. Obesity as a risk factor for triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat 2013;137(1):307–14 doi 10.1007/s10549-012-2339-3. [DOI] [PubMed] [Google Scholar]
- 4.Neuhouser ML, Aragaki AK, Prentice RL, Manson JE, Chlebowski R, Carty CL, et al. Overweight, Obesity, and Postmenopausal Invasive Breast Cancer Risk: A Secondary Analysis of the Women’s Health Initiative Randomized Clinical Trials. JAMA Oncol 2015;1(5):611–21 doi 10.1001/jamaoncol.2015.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol 2005;23(7):1370–8 doi 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 6.Kwan ML, John EM, Caan BJ, Lee VS, Bernstein L, Cheng I, et al. Obesity and mortality after breast cancer by race/ethnicity: The California Breast Cancer Survivorship Consortium. Am J Epidemiol 2014;179(1):95–111 doi 10.1093/aje/kwt233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes 2013;2013:291546 doi 10.1155/2013/291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorincz AM, Sukumar S. Molecular links between obesity and breast cancer. Endocr Relat Cancer 2006;13(2):279–92 doi 10.1677/erc.1.00729. [DOI] [PubMed] [Google Scholar]
- 9.Cleary MP, Grossmann ME. Minireview: Obesity and breast cancer: the estrogen connection. Endocrinology 2009;150(6):2537–42 doi 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and inflammation: new insights into breast cancer development and progression. Am Soc Clin Oncol Educ Book 2013:46–51 doi 10.1200/EdBook_AM.2013.33.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev 2009;18(10):2569–78 doi 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- 12.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 2010;72:219–46 doi 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 13.Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4(3):329–46 doi 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 2005;46(11):2347–55 doi 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112(12):1796–808 doi 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4(7):1021–9 doi 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kloting N, Bluher M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev Endocr Metab Disord 2014;15(4):277–87 doi 10.1007/s11154-014-9301-0. [DOI] [PubMed] [Google Scholar]
- 18.Iyengar NM, Zhou XK, Gucalp A, Morris PG, Howe LR, Giri DD, et al. Systemic Correlates of White Adipose Tissue Inflammation in Early-Stage Breast Cancer. Clin Cancer Res 2016;22(9):2283–9 doi 10.1158/1078-0432.CCR-15-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iyengar NM, Morris PG, Zhou XK, Gucalp A, Giri D, Harbus MD, et al. Menopause is a determinant of breast adipose inflammation. Cancer Prev Res (Phila) 2015;8(5):349–58 doi 10.1158/1940-6207.CAPR-14-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carter JM, Hoskin TL, Pena MA, Brahmbhatt R, Winham SJ, Frost MH, et al. Macrophagic “Crown-like Structures” Are Associated with an Increased Risk of Breast Cancer in Benign Breast Disease. Cancer Prev Res (Phila) 2018;11(2):113–9 doi 10.1158/1940-6207.CAPR-17-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaik A, Kiavash K, Stark K, Boerner J, Ruterbusch J, Ali-Fehmi R, et al. Adipose inflammation and the risk of benign and malignant breast disease in African American women. AACR Annual Meeting. Chicago, IL. [Google Scholar]
- 22.Koru-Sengul T, Santander AM, Miao F, Sanchez LG, Jorda M, Gluck S, et al. Breast cancers from black women exhibit higher numbers of immunosuppressive macrophages with proliferative activity and of crown-like structures associated with lower survival compared to non-black Latinas and Caucasians. Breast Cancer Res Treat 2016;158(1):113–26 doi 10.1007/s10549-016-3847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014;311(8):806–14 doi 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boone SD, Baumgartner KB, Joste NE, Pinkston CM, Yang D, Baumgartner RN. The joint contribution of tumor phenotype and education to breast cancer survival disparity between Hispanic and non-Hispanic white women. Cancer Causes Control 2014;25(3):273–82 doi 10.1007/s10552-013-0329-3. [DOI] [PubMed] [Google Scholar]
- 25.Ooi SL, Martinez ME, Li CI. Disparities in breast cancer characteristics and outcomes by race/ethnicity. Breast Cancer Res Treat 2011;127(3):729–38 doi 10.1007/s10549-010-1191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Cancer Society. Cancer Facts & Figures for Hispanics/Latinos 2015–2017. Atlanta: American Cancer Society; 2015. [Google Scholar]
- 27.Warner ET, Tamimi RM, Hughes ME, Ottesen RA, Wong YN, Edge SB, et al. Racial and Ethnic Differences in Breast Cancer Survival: Mediating Effect of Tumor Characteristics and Sociodemographic and Treatment Factors. J Clin Oncol 2015;33(20):2254–61 doi 10.1200/JCO.2014.57.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9(7):671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown KA, Iyengar NM, Zhou XK, Gucalp A, Subbaramaiah K, Wang H, et al. Menopause Is a Determinant of Breast Aromatase Expression and Its Associations With BMI, Inflammation, and Systemic Markers. J Clin Endocrinol Metab 2017;102(5):1692–701 doi 10.1210/jc.2016-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iyengar NM, Chen IC, Zhou XK, Giri DD, Falcone DJ, Winston LA, et al. Adiposity, Inflammation, and Breast Cancer Pathogenesis in Asian Women. Cancer Prev Res (Phila) 2018;11(4):227–36 doi 10.1158/1940-6207.CAPR-17-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mullooly M, Yang HP, Falk RT, Nyante SJ, Cora R, Pfeiffer RM, et al. Relationship between crown-like structures and sex-steroid hormones in breast adipose tissue and serum among postmenopausal breast cancer patients. Breast Cancer Res 2017;19(1):8 doi 10.1186/s13058-016-0791-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaysse C, Lomo J, Garred O, Fjeldheim F, Lofteroed T, Schlichting E, et al. Inflammation of mammary adipose tissue occurs in overweight and obese patients exhibiting early-stage breast cancer. NPJ Breast Cancer 2017;3:19 doi 10.1038/s41523-017-0015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and cancer: local and systemic mechanisms. Annu Rev Med 2015;66:297–309 doi 10.1146/annurev-med-050913-022228. [DOI] [PubMed] [Google Scholar]
- 34.Wensveen FM, Valentic S, Sestan M, Turk Wensveen T, Polic B. The “Big Bang” in obese fat: Events initiating obesity-induced adipose tissue inflammation. Eur J Immunol 2015;45(9):2446–56 doi 10.1002/eji.201545502. [DOI] [PubMed] [Google Scholar]
- 35.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol 2004;43(10):1731–7 doi 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 36.Jensen JA, Goodson WH, Hopf HW, Hunt TK. Cigarette smoking decreases tissue oxygen. Arch Surg 1991;126(9):1131–4. [DOI] [PubMed] [Google Scholar]
- 37.Iyengar NM, Brown KA, Zhou XK, Gucalp A, Subbaramaiah K, Giri DD, et al. Metabolic Obesity, Adipose Inflammation and Elevated Breast Aromatase in Women with Normal Body Mass Index. Cancer Prev Res (Phila) 2017;10(4):235–43 doi 10.1158/1940-6207.CAPR-16-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.