Abstract
Introduction
We evaluated the performance of multiparametric prostate MRI (mp-MRI) and MRI/TRUS fusion-guided biopsy (FB) for monitoring prostate cancer (PCa) patients on active surveillance (AS).
Materials and Methods
Patients undergoing mp-MRI and FB of target lesions identified on mp-MRI between August 2007-August 2014 were reviewed. Patients meeting AS criteria (Clinical Stage T1c, Gleason grade ≤6, PSA density ≤0.15, tumor involving ≤2 cores, and ≤50% involvement of any single core) based on extended-sextant, 12 core TRUS biopsy (SB) were included. They were followed with subsequent 12-core biopsy as well as mp-MRI and MRI/TRUS-fusion biopsy at follow-up visits until Gleason score progression (Gleason ≥7 in either 12-core or MRI/TRUS-fusion biopsy). We evaluated whether progression on mp-MRI (defined as an increase in suspicion level, largest lesion diameter, or number of lesions) was predictive of Gleason score progression.
Results
Of 152 patients meeting AS criteria on initial SB (mean age of 61.4 years and mean PSA of 5.26 ng/ml), 34(22.4%) had Gleason ≥7 on confirmatory SB/FB. Of the 118 remaining patients, 58 chose AS and had at least one subsequent mp-MRI with SB/FB (median follow-up; 16.1months). Gleason progression was subsequently documented in 17(29%) of these men, in all cases to Gleason 3+4. The PPV and NPV of mp-MRI for Gleason progression was 53% (95%CI 28-77%) and 80% (95%CI 65-91%), respectively. The sensitivity and specificity of mp-MRI for increase in Gleason were also 53% and 80%, respectively. The number needed to biopsy to detect one Gleason progression was 8.74 for SB versus 2.9 for FB.
Conclusions
Stable findings on mp-MRI are associated with Gleason score stability. mp-MRI appears promising as a useful aid for reducing the number of biopsies in the management of patients on active surveillance. A prospective evaluation of mp-MRI as a screen to reduce biopsies in the follow-up of men on AS appears warranted.
Keywords: Image guided biopsy, prostatic adenocarcinoma, diagnosis, management, PSA
1.INTRODUCTION
It is estimated that 233,000 new cases PCa. will be diagnosed in the United States in 2014, the majority of which present as localized disease, nearly half of which are low grade cancer(1). Thus, active surveillance (AS) has emerged as a management strategy to defer treatment and spare men with presumably indolent disease the adverse effects of definitive therapy. The aim of AS is to offer patients with low-risk disease the possibility of avoiding the side effects associated with radical treatments without missing the window of opportunity for definitive therapy, if needed.
Currently, patients on AS are followed with serial PSA and repeat biopsy although other tests such as PCA3 and quantitative gene expression profiles are being evaluated to detect disease progression(2). Recent intermediate-term series suggest that AS may safely defer intervention in a low-risk population, but because up to 35% of patients ultimately receive definitive treatment, improved strategies are needed to better stratify risk, characterize and follow disease progression(3-5).
Repeat prostate biopsy is an integral component in the follow-up of AS candidates because it identifies pathological progression of PCa. The current standard-of-care consists of a systematic, extended-sextant, 12-core TRUS-guided biopsy (SB), which does not target specific lesions and therefore, may under-sample clinically significant lesions. Multiparametric magnetic resonance imaging (mp-MRI) can potentially improve the selection of men for AS and as an adjunct in the surveillance of men with PCa on AS(6,7). PSA alone has its limitations, and the addition of mp-MRI and MRI-TRUS guided fusion biopsy (FB) in surveillance can potentially improve the detection of clinically significant disease or can be used as a filter for progression at follow-up visits to limit the number of biopsy sessions(8, 9). Prostate mp-MRI can accurately identify lesions suspicious for PCa.. It has been reported that levels of suspicion assigned to lesions on prostate mp-MRI significantly correlate with adverse pathology on confirmatory biopsy and prostatectomy as well as with the clinical risk stratification of an AS population(10-12). Furthermore, when mp-MRI is fused with real-time transrectal ultrasound, suspicious lesions can be accurately targeted for biopsy, thus improving the detection of PCa in patients with prior negative biopsy, lesions in occult locations, enlarged prostates, and those with potentially high-risk disease(13-15). Additionally, with serial FB, prior areas of concern may be reassigned as targets to ensure adequate sampling at each biopsy session(16). In the setting of AS, mp-MRI and FB may validate appropriate eligibility for AS(6). In this study, we assess the performance of mp-MRI and FB for Gleason progression in men on AS.
2.METHODS
2.1 Study population
A retrospective analysis was performed of patients enrolled in an Institutional Review Board-approved protocol, with written informed consent, between August 2007 and August 2014. Patients included in this analysis met the JHU criteria for AS (Clinical Stage T1c, Gleason grade (GG) ≤ 6, PSA density ≤ 0.15, tumor involving ≤ 2 cores, and ≤ 50% involvement of any single core) on a SB before referral to our institution(17). All patients had at least one serum PSA measurement, digital rectal examination, and underwent a standardized mp-MRI. After enrollment in the study each patient underwent another SB along with a FB (termed a confirmatory biopsy) using the mp-MRI. During follow up visits, they underwent subsequent SB and FB until Gleason score progression. Confirmatory SB and FB of suspicious lesions identified on mp-MRI, was performed using a free hand image fusion platform (UroNav, InVivo Corp). Patients confirmed to have GG ≤ 6 were followed with serial mp-MRI and SB/FB.
2.2 MRI acquisition and interpretation
All images were acquired using a 3.0 T MRI scanner (Achieva, Philips Healthcare, Cleveland, OH) with a 6 or 16-channel body coil (SENSE, Philips Healthcare, Cleveland, OH) using an endorectal coil (BPX-30), distended to a volume of approximately 45 cc with perfluorocarbon (3 mol/L-Fluorinert, 3M, St. Paul, MN), Medrad, Pittsburgh, PA.(18) The mp-MRI sequence parameters included T1W imaging, triplanar (coronal, sagittal and axial), T2 with diffusion-guided-weighted imaging and apparent diffusion coefficient mapping, multi-voxel 3-dimensional localized spectroscopy, and axial 3-D fast field echo dynamic contrast-enhanced MRI sequences(6). All mp-MRIs were blindly evaluated by two experienced genitourinary radiologists (BT and PLC with prostate MRI accumulated experiences of 7 and 14 years respectively). Those patients with at least one suspicious lesion subsequently underwent SB/FB with two cores sampled for each lesion (axial and sagittal)(12). All pathological specimens were reviewed by a single genitourinary pathologist (MJM).
2.3 Study design
For each lesion identified on mp-MRI, a non-weighted suspicion score categorized as low, moderate or high was assigned based in part on the number of positive sequences.(6) The diameter of each lesion was manually determined using a picture archiving and communication system workstation (Carestream, Inc, Rochester, NY). The highest suspicion score for any lesion, diameter of each lesion, and number of lesions were recorded prior to each biopsy session, as well as patient demographics and pathology data. MRI progression was defined as an increase in highest mp-MRI suspicion score, lesion diameter, or number of lesions between biopsy sessions for each patient during follow-up. Pathologic progression was defined as an increase to GG ≥3+4 in any core in either the SB or FB. When pathologic progression occurred, patients were not followed further for the purposes of this analysis. Some sought definitive therapy while others with low-volume GG 7 disease continued on AS.
2.4 Data Analysis
Patient demographic data was reported using descriptive statistics. The performance of mp-MRI was assessed by determining sensitivity, specificity, positive (PPV) and negative predictive value (NPV) relating mp-MRI progression (positivity), defined as an increase in suspicion level, largest lesion diameter, or number of lesions to Gleason progression on either 12-core or FB at the same follow-up session. Exact binomial confidence limits were used for sensitivity, specificity, PPV and NPV. Categorical variables were compared with a Pearson chi-square test. Association of quantitative and ordered variables were evaluated using the Spearman non-parametric correlation coefficient. A p-value < 0.05 was considered statistically significant.
3.RESULTS
3.1 First mp-MRI and biopsy session
One hundred and fifty-two patients met the JHU criteria for AS on their initial outside SB (Figure 1). The mean age of this cohort was 61.4±7.1 (range 40-79) years, and mean pre-biopsy PSA was 5.2±3.2 (range 0.2-23.3) ng/mL (Table 1). Thirty-two of the patients (21.1% - 32/152) had low suspicion lesions by mp-MRI, and only 1 of these 32 (3.2%) was found to have GG 7 disease. Seventy-one percent of the entire cohort (109/152) were classified as moderately suspicious lesions on mp-MRI. Of these 109 patients - 29.4% (32/109) were found to harbor GG 7 disease on confirmatory biopsy. Finally, eleven (11/152) patients were classified as highly suspicious lesions, of which 6/11 (54.5%) were found to have acute chronic inflammation or high grade prostatic intraepithelial neoplasia (HGPIN), 4/11 were found to have GG 6 or 7 cancer and 1 was found to have GG 4+5 disease. A total of 34 patients (22.4%; 34/152) had GG ≥7 at their first confirmatory SB/FB biopsy session on either SB cores or FB (targeted) cores - 35.3% (12/34) detected by FB alone and 29% (10/34) by 12-core biopsy alone. Hence, both mp-MRI/FB and 12- core biopsy contributed to re-assessing eligibility for AS. Gleason upgrading was significantly more likely to occur in patients harboring more focal mp-MRI lesions; 33.3% for patients having ≥3 lesions on mp-MRI versus 16.1% for those with ≤ 2 lesions (p = 0.01). There was also a positive correlation between suspicion level and number of lesions on mp-MRI (Spearman correlation p = 0.048)
Figure 1.
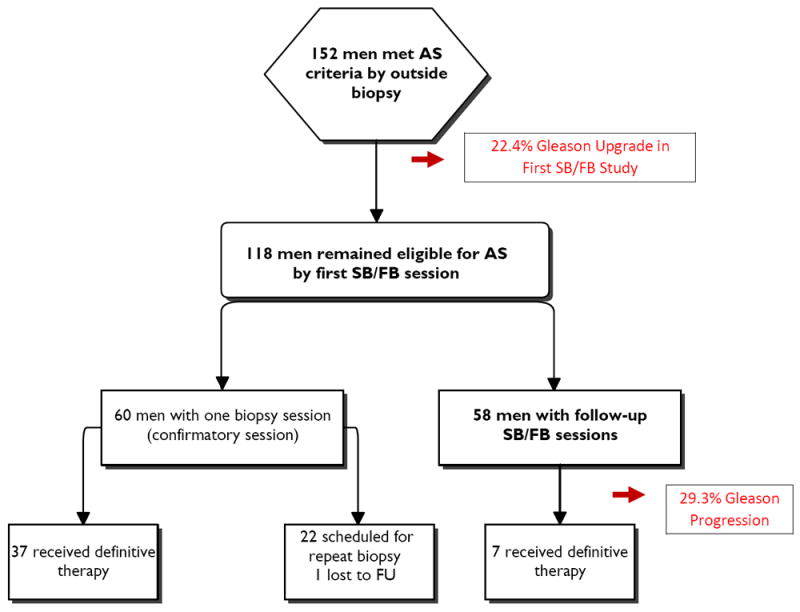
Flowchart of study results.
Table 1.
Patient demographics for the initial and follow-up cohorts.
| Patient Characteristic | Initial | Follow-Up |
|---|---|---|
|
| ||
| Number of men | 152 | 58 |
| Age, mean ± SD (range) | 61.4 ± 7.1 years (40-79) | 61.3 ± 7.02 years (45-77) |
| PSA, ng/mL, mean ± SD (range) | 5.2 ± 3.2 ng/mL (0.2-23.3) | 5.0 ± 3.1 ng/mL (0.2-20.0) |
| PSA density, mean ± SD (range) | 0.09 ± 0.03 ng/mL2 (0.01-0.15) | 0.09 ± 0.04 ng/mL2 (0.01-0.15) |
| Clinical stage | ||
| T1c | 152 | 58 |
| Race (%) | ||
| White | 127 (83.6%) | 51 (88%) |
| African-American | 21 (13.8%) | 7 (12%) |
| Other | 4 (2.6%) | 0 |
|
| ||
| Prostate volume, mean ± SD (range) | 58 ± 28 cm3 (23-161) | 56 ± 25 cm3 (22-146) |
| Number of lesions, mean ± SD (range) | 2.3 ± 1.2 (1-9) | 2.3 ± 1.1 (1-5) |
| Highest suspicion score, (%) | ||
| Low | 32 (21.1%) | 18 (31%) |
| Moderate | 109 (71.7%) | 36 (62%) |
| High | 11 (7.2%) | 4 (7%) |
Abbreviations: PSA, prostate-specific antigen; SD, standard deviation.
3.2 Follow-up cohort
One hundred and eighteen men remained eligible for the analysis after their confirmatory SB/FB at the NIH (Figure 1). However, to date, 60 of these patients have not been re-staged: 37 underwent immediate definitive whole gland therapy, 22 are too early for repeat imaging and biopsy and one patient was lost to follow-up.
Thus, Fifty-eight men underwent at least two mp-MRI and SB/FB sessions with median follow-up of 16.1 months (mean 22.1) (range 12-56) (Table 1). Seventeen of these 58 patients had mp-MRI progression. Nine of these 17 had increased GG on SB/FB. This data implies that the use of MRI/FB during follow-up more than doubled the detection of pathological progression. Forty-one of these 58 patients had stable mp-MRIs, 33 of whom had stable GG on SB/FB. Overall, seventeen of the fifty-eight patients (29%) evaluable for this analysis had GG progression during the follow-up interval. All cases demonstrated progression from GG 3+3 to 3+4. The median time to progression was 16.1 months. Nine of these pathological progressions were detected on FB (Table 2). Of the 17 patients with mp-MRI progression, 7 had increased MRI suspicion level, 2 had an increase in lesion diameter, 3 had new lesions and the remainder with a combination of the above parameters. The sensitivity and specificity of mp-MRI for pathologic progression were 53% CI (0.28, 0.77) and 80% CI (0.65, 0.91) respectively. The NPV and PPV for mp-MRI in predicting pathologic progression for AS patients in this cohort were 80% CI (0.65, 0.91) and 53% CI (0.28, 0.77) respectively. Table 2 describes the performance of mp-MRI in assessing GG progression. It demonstrates that 20% (8/41) of men with stable mp-MRIs had pathologic progression, in contrast to 53% (9/17) of men with mp-MRI progression. Of the 17 patients with pathologic progression, 9 (53%) were detected on FB only versus 6 (35.3%) by SB only.
Table 2.
Expanded 2 × 2 table relating patients who progressed by mp-MRI or Gleason grade.
| Gleason score increase | No MRI progression | MRI progression | Total |
|---|---|---|---|
|
| |||
| No | 33 | 8 | 41 |
|
| |||
| Yes, detected by: | 8 | 9 | 17 |
| Targeted biopsy only | 4 | 5 | 9 |
| SB only (12-core) | 4 | 2 | 6 |
| Both | 0 | 2 | 2 |
| Total | 41 | 17 | 58 |
All Gleason grade increases were to 3+4=7. 9/17 of these increases were detected by targeted biopsy alone.
In this cohort, fifty-eight patients had one or more follow-up SB/FB after their initial biopsy confirming GG < 7. We performed seventy follow-up SBs in fifty-eight men in order to detect 8 GG progressions. Had we only performed follow-up biopsies when there was an mp-MRI progression, we would have performed 26 biopsies (SBs+FBs) to detect 9 GG progressions. The number needed to biopsy (NNB) to detect one Gleason progression was 8.74 (70/8) for SB versus 2.9 (26/9) for FB. This difference in the number of biopsy sessions per Gleason progression detected (70/8 for SB vs 26/9 for FB) is statistically significant (p<0.02).
4. DISCUSSION
With the advent of widespread screening, there has been an acknowledged shift in the demographics of PCa towards younger men with low-volume and low-risk disease, and in turn, possible overdiagnosis and overtreatment(19, 20). Active surveillance has been proposed for men diagnosed with PCa deemed to be indolent and safe to monitor over time. This management strategy allows patients with favorable risk disease to defer treatment while they are actively followed by serial PSA and periodic repeat biopsy(5). The aim of AS is to offer patients with low-risk disease the possibility of avoiding or delaying the potential side effects associated with radical treatment without missing the window of opportunity for curative therapy. By more accurately characterizing disease status, mp-MRI and FB represent a promising method of selecting and monitoring AS patients.
A crucial element for successful AS is accurately selecting patients with low-grade, low-volume disease. However, prior series have reported that approximately 35% of AS candidates are found to have higher grade disease on their confirmatory biopsy (3). It is in this context that mp-MRI is able to enhance the identification of higher grade or higher volume cancers that would be more appropriately treated with immediate definitive therapy. In this study approximately 22.4% of men meeting the stringent JHU AS criteria on SB were discovered to have GG 3+4 tumors after mp-MRI and FB plus SB. Of the 34 patients upgraded by the re-biopsy, 35.4% were upgraded based on MRI/TRUS fusion targets alone. Hence, mp-MRI/FB was of value, in addition to SB, for selecting patients for AS. In those patients who were not upgraded on confirmatory biopsy, there is increased confidence in the diagnosis of low-grade, low-volume disease and in the decision to safely pursue AS.
In an attempt to better identify and inform the most appropriate candidates for AS, our group generated a nomogram that used the number of lesions, lesion suspicion level, and lesion density on mp-MRI to determine the probability of confirming AS candidacy(6). The ultimate goal is to develop a reliable way of classifying patients who are truly low-risk, confidently follow them, and identify progression in a timely manner if and when it occurs. This may ultimately result in fewer repeat biopsies.
Moore, et al reported on 91 men on AS who underwent at least two MRI scans. Thirty-seven percent (34/91) of their patients had a lesion present at baseline. Defining radiological progression as an increase in size, intensity or number of lesions, they reported that the presence of a measurable lesion increased the likelihood of progression (OR 2.55)(21).
In our series, we found that in 152 referred as eligible for AS, 22.4% harbored Gleason 7 disease on confirmatory SB/FB, which is now a well-known phenomenon(22). The number of visible lesions was associated with increased Gleason upgrading in the confirmatory biopsies. We report on sequential mp-MRI and MRI/TRUS fusion-guided biopsy in the follow-up of 58 patients defined as low-risk by strict criteria who chose to undergo AS and were confirmed to have Gleason 6 disease on their first SB/FB biopsy. During AS follow-up using standard 12-core biopsy as well as mp-MRI/FB, we found that the use of mp-MRI/FB increased the rate of detection of Gleason progression from 8/58 (13.8%) with SB alone to 17/58 (29.3%). We also found that of the 17 pathologic progressions, 9 were predicted by an increase in the suspicion level of mp-MRI. Consequently, if mp-MRI were used as a filter for when to perform follow-up SB/FB, the number of biopsy sessions per pathologic progression detected would have decreased from 8.75 (70/8) to 2.89 (26/9).
In patients confirmed to have GG 6 disease, the sensitivity and specificity of mp-MRI for pathological progression after a median follow-up of 16 months was 53% and 80%, respectively. Similarly, the NPV and PPV for mp-MRI progression was 80% and 53%, respectively. This compares with the results of Mullin and colleagues who described mp-MRI as having a sensitivity of only 19% and a PPV of 46% for the detection of lesions >10mm or >2 lesions, however with excellent specificity (97%) and negative predictive values (90%). Our sensitivity and PPV are higher than these reported values, however different criteria were applied, and fusion-guided biopsy was added to our follow-up algorithm(23). Ultimately, mp-MRI may be used to avoid repeat biopsies on certain intervals when mp-MRI is completely stable as the NPV was high at both time points. Currently, the standard-of-care is repeat extended sextant TRUS-guided biopsy, which is not only costly, but also puts patients at risk for hemorrhage and sepsis(24). In this setting mp-MRI could be used to identify suspicious lesions suitable for MRI/FB instead of simply repeating the SB. Previous studies of mp-MRI have shown that on a diagnosis per core basis, mp-MRI/FB detects more cancer per core than the 12-core TRUS-guided biopsy(13). For instance, to achieve similar detection rates, the 12-core TRUS approach required 12 needles while the mp-MRI/FB required only 5.7. Additionally, mp-MRI/FB detected more clinically-significant (GG ≤ 4+3) prostate cancer(25). Thus mp-MRI/FB is more efficient and also more specific for higher risk cancers. Specifically in the setting of AS, mp-MRI/FB also allows for resampling of previously biopsied lesions during AS.
This study was performed on a highly selected group of patients meeting JHU criteria for AS with very low-grade and low-volume PCa, confirmed by repeat SB/FB. These criteria are considered conservative and many physicians have extended AS to those with low volume GG 3+4=7 disease(5). We do not address this cohort in the current study and acknowledge that MRI is generally optimized for detection of higher grade and higher volume disease. Additionally, the median follow-up was only 16 months (range 12-55). While FB monitoring led to a relatively high, 29% rate of GG progression, the short duration of follow-up warrants a note of caution regarding the interpretation of our NPV data. It is possible that the NPV of mp-MRI might diminish over time. However, the observation that the pathological increase was in all cases limited to GG 3+4 is reassuring. Perhaps most importantly, the use of mp-MRI with FB to follow patients on AS allowed us to detect GG progression with fewer biopsies. The NNB to detect one GG progression was 8.74 for SB versus 2.9 for MRI screened SB/FB. Thus, mp-MRI could prove to be very useful in reducing the number of biopsies, thereby decreasing the complications and costs associated with repeated biopsies in this patient population. Using MRI in follow-up to determine whether to perform another SB/FB biopsy session permits a major reduction in the number of biopsy sessions with about the same yield of GG progressions. To maximize the number of GG progressions, however, the data from this series suggests that SB/FB should be repeated regardless of the results of the follow-up MRI.
This study has several limitations. In addition to its retrospective nature and limited number of patients, the study focused on grade migration and growth of incident cancers but did not address percent core involvement as criteria for progression. Since FB is inherently a guided biopsy whereas traditional biopsies are random, it is likely that FB will yield higher percent core involvement as has previously been reported by our group and corroborated by others(26, 27). However, the significance, if any, of percent involvement on FB specimens is still unknown and might require different criteria to define eligibility for AS. Another limitation of the study is that small increases (2-3 mm) in diameter was considered as MRI progression, even though this does not have a precedent in the literature and others have used the presence of lesions > 10mm diameter(23). Ultimately the optimal time between serial MRI studies is currently not defined but is an area of great interest that requires ongoing investigation(28).
5. CONCLUSION
MRI and FB is an important complement to SB in selecting candidates for AS. Both FB and SB have utility in the continued follow-up of patients on active surveillance and mp-MRI/FB substantially increased the number of pathologic progressions that would not have been detected by SB alone. Stable findings on mp-MRI were strongly associated with Gleason score stability in patients with Gleason 6 PCa on AS and can potentially substantially reduce the number of unnecessary biopsies and delay definitive treatment. With further validation, MRI/FB may permit fewer repeat biopsies in men undergoing active surveillance. As the technology of mp-MRI and MRI/TRUS fusion-guided biopsy becomes more widely adopted multicenter studies will define its role in active surveillance.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, the Center for Cancer Research, and the Center for Interventional Oncology. NIH and Philips Healthcare have a cooperative research and development agreement (CRADA). NIH and Philips share intellectual property in the field.
This research was also made possible through the National Institutes of Health Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc., The Doris Duke Charitable Foundation, The Alexandria Real Estate Equities, Inc. and Mr. and Mrs. Joel S. Marcus, and the Howard Hughes Medical Institute, as well as other private donors. For a complete list, please visit the Foundation website at: http://fnih.org/work/education-training-0/medical-research-scholars-program
We also thank the administrative support staff of the Urologic Oncology Branch and the Center for Cancer Research for assisting with the manuscript review and submission process.
Funding: No specific funding
Bradford J. Wood and Peter A Pinto: Philips Healthcare - In-Vivo Corp: Research related support: CRADA-NIH- Philips. Cooperative research and development agreement Philips Healthcare and In Vivo Corp (CRADA).
Footnotes
Financial Disclosures: Not financial
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014 Jan-Feb;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Crawford ED, Ventii K, Shore ND. New biomarkers in prostate cancer. Oncology (Williston Park) 2014 Feb;28(2):135–42. [PubMed] [Google Scholar]
- 3.Dall’Era MA, Albertsen PC, Bangma C, et al. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol. 2012 Dec;62(6):976–83. doi: 10.1016/j.eururo.2012.05.072. [DOI] [PubMed] [Google Scholar]
- 4.Tosoian JJ, JohnBull E, Trock BJ, et al. Pathological outcomes in men with low risk and very low risk prostate cancer: implications on the practice of active surveillance. J Urol. 2013 Oct;190(4):1218–22. doi: 10.1016/j.juro.2013.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klotz L, Zhang L, Lam A, et al. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010 Jan 1;28(1):126–31. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 6.Stamatakis L, Siddiqui MM, Nix JW, et al. Accuracy of multiparametric magnetic resonance imaging in confirming eligibility for active surveillance for men with prostate cancer. Cancer. 2013 Sep 15;119(18):3359–66. doi: 10.1002/cncr.28216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turkbey B, Mani H, Aras O, et al. Prostate cancer: can multiparametric MR imaging help identify patients who are candidates for active surveillance? Radiology. 2013 Jul;268(1):144–52. doi: 10.1148/radiol.13121325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shakir NA, George AK, Siddiqui MM, et al. Identification of threshold prostate specific antigen levels to optimize the detection of clinically significant prostate cancer by magnetic resonance imaging/ultrasound fusion guided biopsy. J Urol. 2014 Dec;192(6):1642–9. doi: 10.1016/j.juro.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George AK, Pinto PA, Rais-Bahrami S. Multiparametric MRI in the PSA screening era. Biomed Res Int. 2014;2014:465816. doi: 10.1155/2014/465816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rais-Bahrami S, Siddiqui MM, Turkbey B, et al. Utility of multiparametric magnetic resonance imaging suspicion levels for detecting prostate cancer. J Urol. 2013 Nov;190(5):1721–7. doi: 10.1016/j.juro.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borofsky MS, Rosenkrantz AB, Abraham N, et al. Does suspicion of prostate cancer on integrated T2 and diffusion-weighted MRI predict more adverse pathology on radical prostatectomy? Urology. 2013 Jun;81(6):1279–83. doi: 10.1016/j.urology.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Rastinehad AR, Baccala AA, Jr, Chung PH, et al. D’Amico risk stratification correlates with degree of suspicion of prostate cancer on multiparametric magnetic resonance imaging. J Urol. 2011 Mar;185(3):815–20. doi: 10.1016/j.juro.2010.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonn GA, Natarajan S, Margolis DJ, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol. 2013 Jan;189(1):86–91. doi: 10.1016/j.juro.2012.08.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vourganti S, Rastinehad A, Yerram NK, et al. Multiparametric magnetic resonance imaging and ultrasound fusion biopsy detect prostate cancer in patients with prior negative transrectal ultrasound biopsies. J Urol. 2012 Dec;188(6):2152–7. doi: 10.1016/j.juro.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walton Diaz A, Hoang AN, Turkbey B, et al. Can magnetic resonance-ultrasound fusion biopsy improve cancer detection in enlarged prostates? J Urol. 2013 Dec;190(6):2020–5. doi: 10.1016/j.juro.2013.05.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raskolnikov D, Rais-Bahrami S, George AK, et al. The Role of Image Guided Biopsy Targeting in Patients with Atypical Small Acinar Proliferation. J Urol. 2014 Aug 20; doi: 10.1016/j.juro.2014.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epstein JI, Walsh PC, Carmichael M, et al. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994 Feb 2;271(5):368–74. [PubMed] [Google Scholar]
- 18.Turkbey B, Mani H, Shah V, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol. 2011 Nov;186(5):1818–24. doi: 10.1016/j.juro.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klotz L. Prostate cancer overdiagnosis and overtreatment. Curr Opin Endocrinol Diabetes Obes. 2013 Jun;20(3):204–9. doi: 10.1097/MED.0b013e328360332a. [DOI] [PubMed] [Google Scholar]
- 20.Cooperberg MR, Carroll PR, Klotz L. Active surveillance for prostate cancer: progress and promise. J Clin Oncol. 2011 Sep 20;29(27):3669–76. doi: 10.1200/JCO.2011.34.9738. [DOI] [PubMed] [Google Scholar]
- 21.Moore CM, Ridout A, Emberton M. The role of MRI in active surveillance of prostate cancer. Curr Opin Urol. 2013 May;23(3):261–7. doi: 10.1097/MOU.0b013e32835f899f. [DOI] [PubMed] [Google Scholar]
- 22.Hu JC, Chang E, Natarajan S, et al. Targeted prostate biopsy in select men for active surveillance: do the Epstein criteria still apply? J Urol. 2014 Aug;192(2):385–90. doi: 10.1016/j.juro.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullins JK, Bonekamp D, Landis P, et al. Multiparametric magnetic resonance imaging findings in men with low-risk prostate cancer followed using active surveillance. BJU Int. 2013 Jun;111(7):1037–45. doi: 10.1111/j.1464-410X.2012.11641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjurlin MA, Wysock JS, Taneja SS. Optimization of prostate biopsy: review of technique and complications. Urol Clin North Am. 2014 May;41(2):299–313. doi: 10.1016/j.ucl.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siddiqui MM, Rais-Bahrami S, Truong H, et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol. 2013 Nov;64(5):713–9. doi: 10.1016/j.eururo.2013.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011 Oct;186(4):1281–5. doi: 10.1016/j.juro.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rastinehad AR, Turkbey B, Salami SS, et al. Improving detection of clinically significant prostate cancer: magnetic resonance imaging/transrectal ultrasound fusion guided prostate biopsy. J Urol. 2014 Jun;191(6):1749–54. doi: 10.1016/j.juro.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rais-Bahrami S, Turkbey B, Rastinehad AR, et al. Natural history of small index lesions suspicious for prostate cancer on multiparametric MRI: recommendations for interval imaging follow-up. Diagn Interv Radiol. 2014 Jul-Aug;20(4):293–8. doi: 10.5152/dir.2014.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]


