Abstract
Purpose:
To describe the imaging response and survival after radioembolization for metastatic breast cancer, and to delineate genetic predictors of imaging responses and outcomes.
Materials and Methods:
This IRB-approved, HIPAA-compliant retrospective study included 31 women (average age 52 years), with liver metastasis from invasive ductal carcinoma, who underwent resin and glass radioembolization (average cumulative dose 54±48 mCi), from January 2011—September 2017, after receiving ≥3 lines of chemotherapy. Twenty-four underwent genetic profiling with MSK-IMPACT or Sequenom; 26 had PET/CT imaging before and after treatment. Survival after the first radioembolization and 2—4-month PET/CT imaging response was assessed. Laboratory and imaging features were assessed to determine variables predictive of outcomes. Unpaired Student’s t tests and Fisher’s exact tests were used to compare responders and non-responders categorized by changes in FDG avidity. Kaplan-Meier survival analysis was used to determine the impact of predictors on survival after radioembolization.
Results:
Median survival after radioembolization was 11 (range: 1—49) months. Most (18/26; 69%) patients had complete or partial response, determined by changes in FDG avidity. Imaging response was associated with longer survival (p=0.005). Whereas 100% of patients with PI3K pathway mutations had an imaging response, only 45% of wildtype patients had a response (p=0.01). Median survival did not differ between PI3K pathway wildtype (10.9 months) and mutant (undefined) patients (p=0.50).
Conclusion:
These preliminary data suggest that genomic profiling may predict which metastatic breast cancer patients benefit most from radioembolization. PI3K pathway mutations are associated with improved imaging response, which is associated with longer survival.
Introduction
Metastatic breast cancer is the second leading cause of cancer death for women [1]. Though only 5% of metastatic breast cancer patients have metastatic disease confined to the liver, half of patients develop liver metastases, and progression of liver disease is considered the cause of death in nearly a fifth of patients [2]. Liver-directed therapy can palliate symptoms such as abdominal pain and has the potential to prolong life.
For multifocal hepatic metastasis, transarterial therapy with chemoembolization and yttium 90 radioembolization have both been used with an acceptable safety profiles, imaging response, and post-procedure survival [3–13]. To date, no randomized trial comparing these treatments as adjuvant to systemic therapy has been undertaken, emphasizing the importance of identifying biomarkers to aid clinicians in distinguishing patients who might benefit from one over another. Metastatic breast cancer patients often progress despite multiple chemotherapies including those administered during chemoembolization, and will progress regardless of type of therapy. Furthermore, in the context of primary liver malignancy, radioembolization is associated with better quality of life scores compared to chemoembolization [14]. Retrospective studies have not yielded reproducible pre-procedure predictors of response to radioembolization in metastatic breast cancer patients, with some suggesting pre-existing liver dysfunction as an indicator of poor survival [5, 9]. Large-scale profiling studies after radiation demonstrate that in breast cancer, several pathways, including HER2, ER, PI3K and JAK/STAT3, and androgen receptor (AR) expression impact radiation resistance and sensitivity [15]. MAPK/ERK pathway mutations have been suggested to impact response to radioembolization for colorectal cancer [16], and TP53 mutations are associated with poor response to radiotherapy in breast cancer [17]. Hypothetically, mutations in pathways involving radiosensitivity may impact response to radioembolization. The purpose of this study was to describe the imaging response and survival after radioembolization for metastatic breast cancer, and to delineate predictors of image response and outcomes, with a focus on potential genetic prognosticators.
Materials and Methods
Patient Population
This retrospective, single-center study included all consecutive metastatic breast cancer patients who underwent radioembolization (January 2011—September 2017) prior to this study at this academic university hospital. In this Health Insurance Portability and Accountability Act-compliant, institutional review board-approved study, informed consent was waived. An institutional database search identified 31 metastatic breast cancer patients treated with radioembolization. Eligibility criteria for radioembolization included: age of 18 years or older, any racial/ethnic group, with measurable liver metastasis involving >10% of the liver parenchyma; Eastern Cooperative Oncology Group Performance Status 0—1; serum creatinine ≤ 2.0 mg/dL; total bilirubin < 1.2 x upper limit of normal; albumin ≥ 2.0 g/dL. Exclusion criteria included tumor replacement > 70% of liver; absolute contraindications to angiography and visceral catheterization (e.g., uncorrectable coagulopathy, or anaphylactic allergy to contrast agent); pulmonary insufficiency or clinically evident chronic obstructive pulmonary disease; cirrhosis, portal hypertension, or history of hepatic encephalopathy; ascites (trace ascites was acceptable); life-threatening or comorbid disease (e.g., on dialysis, severe infection) that would put patient at undue risk during radioembolization treatment; progressive extrahepatic metastasis thought to immediately threaten survival; and, breastfeeding. Patients were selected for treatment based on a multidisciplinary discussion with breast oncologists. Breast cancer patients with multiple liver lesions, or oligometastatic liver metastases that were not considered safe or amenable to percutaneous ablation, were offered radioembolization. Transarterial chemoembolization was not offered at our institution.
All 31 patients were female with metastatic invasive ductal carcinoma, with an average age of 52.2 ± 11.4 years (Table 1). One patient had liver-only metastasis; all others had other sites of metastasis. No patient underwent external radiation to the liver or prior transarterial therapy. All but two patients had multifocal liver disease. All patients had received at least 3 lines of systemic therapy, and were therefore considered heavily pretreated. On average, patients received 11.0 ± 4.5 systemic therapy agents prior to radioembolization. Whereas approximately three-quarters of patients were ER-positive, one fourth of patients were HER2-positive. HER2 status was assessed in tumoral tissue from both the breast and liver in 25 patients, and was discordant in only 2 of these patients.
Table 1.
Patient characteristics.
| Total number (n) | 31 |
| Age (years) | 52.2 ± 11.4 |
| Post-menopausal at diagnosis | 13/31 (42%) |
| Pre-procedure total bilirubin (mg/dL) | 0.7 ± 0.6 |
| Pre-procedure NLR | 3.9 ± 2.3 |
| Number of other sites of metastasis | 2.5 ± 1.1 |
| Sites of metastasis | |
| Bone | 26/31 (84%) |
| Lung | 11/31 (35%) |
| Lymph node | 26/31 (84%) |
| Brain | 7/31 (23%) |
| Other | 9/31 (29%) |
| Hormonal markers (breast) | |
| HER2-positive | 7/29 (24%) |
| ER-positive | 24/31 (77%) |
| PR-positive | 20/29 (69%) |
| Hormonal markers (liver) | |
| HER2-positive | 7/27 (26%) |
| ER-positive | 21/28 (75%) |
| PR-positive | 9/26 (35%) |
| Extent of liver disease | |
| Unilobar | 3/31 (10%) |
| Bilobar | 28/31 (90%) |
| Percent of tumor involvement (%) | 22.4 ± 21.2 |
| Mean pre-procedure SUVmax | 11.6 ± 7.5 |
| Mean post-procedure SUVmax | 5.8 ± 4.2 |
| Mean lung shunt fraction (%) | 3.1 ± 1.9 |
| Prior partial hepatectomy | 4/31 (13%) |
| Prior breast surgery | 27/31 (87%) |
| Mastectomy | 21/31 (68%) |
| Lumpectomy | 6/31 (19%) |
| Number of prior systemic therapy agents | 11.0 ± 4.5 |
| Time from diagnosis of breast cancer to liver metastasis (years) | 6.5 ± 7.2 |
| Time from diagnosis of liver metastasis to radioembolization (years) | 2.6 ± 1.8 |
Genetic Profiling
Twenty-four patients underwent genetic profiling before radioembolization, with tumor specimens obtained from liver (21/24), lung (2/24), breast (2/24), femur (1/24), and lymph node (1/24). All liver specimens were obtained from lesions subsequently treated with radioembolization. Biopsy specimens were formalin-fixed and paraffin-embedded. After microscopic examination confirmed the diagnosis of adenocarcinoma, tissue was sent to a molecular diagnostic laboratory in the Department of Pathology for extraction of genomic DNA. Each area reviewed by pathologist to select a sample most suitable for molecular testing. Extraction of genomic DNA was performed within 9.8±8.4 months of RE in the molecular diagnostic laboratory in the Department of Pathology, using the DNeasy Tissue kit (Qiagen, Valencia, CA). Genotyping was performed using the Sequenom Mass Array system (Agena Bioscience, San Diego, CA) or MSK-IMPACT.
In the Sequenom assay, samples are tested in duplicate using multiplexed assays to interrogate mutations in hotspots of genes of the PI3K pathway (AKT1, PIK3CA), the MAPK/ERK pathway (EGFR, KRAS, BRAF, NRAS, MAP2K1), and ERBB2 [18]. In total, 92 non-synonymous mutations were tested in 6 multiplex reactions. Using specific primers designed with the Sequenom Assay Designer v3.1 software, genomic DNA amplification and single base pair extension steps were performed. Then, allele-specific single base extension products were quantitated using the Sequenom Mass Array Spectrometer’s matrix-assisted laser desorption/ionization-time of flight mass spectrometry. Sequenom does not detect copy number aberrations.
MSK-IMPACT is a hybridization capture-based next generation sequencing assay for targeted deep sequencing of all exons and selected introns of key cancer genes [19]. Briefly, DNA from tumor and matched normal blood samples from each patient were extracted (Qiagen DNeasy and EZ1 Advanced XL, respectively). Libraries were prepared via the KAPA HTP protocol (Kapa Biosystems, Wilmington, MA) and the Biomek FX system (Beckman Coulter, Brea, CA). The NimbleGen SeqCap EZ library custom oligo system was used to synthesize custom DNA probes, targeting exons and selected introns of several hundred genes. Paired-end read sequencing was performed on the illumina HiSeq 2500 system. Single nucleotide variant calling on tumor samples and their respective matched normal samples was performed with MuTect (version 1.1.4). Small indel events (<30 bp in length) were detected using SomaticIndelDetector. Copy number aberrations were identified by comparing sequence coverage of targeted regions in tumor specimens relative to standard diploid normal samples. If the fold change was greater than 2, it was considered amplification. If the fold change was −2 or below, it was considered a deletion. Full details regarding sample preparation and bioinformatic pipeline have been published elsewhere [19].
For the PI3K pathway, in addition to genes assessed by Sequenom, MSK-IMPACT also assessed AKT2, mTOR, PTEN, PIK3CB, PIK3R1—3. Of the 24 patients, 13 had MSK-IMPACT only, 5 had Sequenom only, and 6 had both MSK-IMPACT and Sequenom genotyping. For the MAPK/ERK pathway, in addition to genes assessed by Sequenom, MSK-IMPACT also assessed MAP2K2—7 and MAPK. If a patient had multiple specimens genotyped, all mutations from all specimens were recorded. Patients were considered PI3K pathway mutants if mutations or copy number aberrations were described involving AKT1, AKT2, mTOR, PTEN, PIK3CA, PIK3CB, or PIK3R1—3 [20]; or MAPK/ERK pathway mutants if mutations or copy number aberrations were described involving ARAF, BRAF, CRAF, EGFR, MAPK, MAP2K1—7, HRAS, KRAS or NRAS (Figure 1). While there may be substantial crosstalk between these two pathways, they are considered distinct as they can be activated in a mutually exclusive manner [21].
Figure 1. Pathways that may impact radiosensitivity.
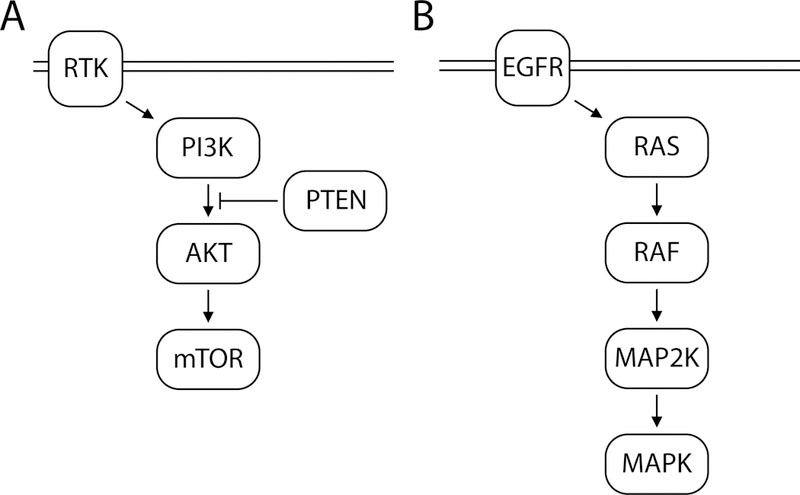
A. The PI3K pathway is activated by signaling through receptor tyrosine kinases. PI3K is composed of a p85 regulatory subunit and a p110 catalytic subunit. Regulatory subunits are expressed by variants of the PIK3R1—3 genes. Catalytic subunits are expressed by PIK3CA, PIK3CB, and PIK2CD genes. PI3K phosphorylates and actives AKT. AKT1 and AKT2 are isoforms of AKT, which activates mTOR, a protein kinase that regulates many cell functions such as cell growth, proliferation, motility, and survival. PTEN is a tumor suppressor gene that negatively regulates the PI3K pathway. B. The MAPK/ERK pathway is activated by the cell surface receptor, EGFR. EGFR activates the small GTPase, RAS. The RAS family includes HRAS, KRAS and NRAS. RAS in turn activates RAF kinases, including ARAF, BRAF, and CRAF, which activate MAP2K, a kinase enzyme encoded by seven genes, MAP2K1—7. MAP2K phosphorylates MAPK, a protein kinase involved in many signaling cascades.
HER2 status was determined in all patients using fluorescence in situ hybridization by the HER2 IQFISH pharmDx™ assay (Agilent, Santa Clara, CA). When available, HER2 results from liver biopsies were documented; in 4 patients, liver specimens were not assessed, so results from breast tumor tissue were used to categorize patients as HER2-positive or negative. Estrogen receptor (ER) and progesterone receptor (PR) nuclear staining was assayed by immunohistochemistry performed on formalin-fixed cell block tissue; any staining 1% or greater was considered positive [22].
Radioembolization Technique
Radioembolization was performed with moderate sedation and fluoroscopic guidance by four fellowship-trained interventional radiologists (AD, HY, CS, LB) with 4, 5, 18 and 21 years of experience respectively, with an indication of progression of liver disease despite systemic therapy. Radioembolization was performed using a microcatheter placed into the targeted vessel; for 22 patients, resin spheres (SirSpheres, Sirtex SIR-Spheres Pty Ltd, Lane Cove, Australia) were used; the remaining 9 were treated with glass spheres (Theraspheres, MDS Nordion, Ottawa, Ontario, Canada). Dosimetry for resin treatments was based on body surface area and the volume of tumor to be treated. Glass sphere treatment dosage was calculated with a target dose of 120 Gy. Among all patients, the mean cumulative decay-corrected dose administered was 54 ± 48 mCi. Mean cumulative dose was 31 ± 13 mCi in patients who received resin microspheres and 102 ± 61 mCi in patients who received glass microspheres (p=0.02). Mean lung shunt fraction was 3% (range: 1—9%). Systemic therapy was held for at least one week before and after each radioembolization treatment. Fourteen patients had both hepatic lobes treated, each treatment separated in time by at least one month; the remaining patients underwent unilobar or segmental treatments. Four patients had repeated radioembolization treatment to the same target regions; survival was calculated from the first treatment and radiographic response was determined only from the first treatment.
Data Collection and Imaging Review
Chart review was used to record labs (complete blood count, total bilirubin) before and after radioembolization, information on pathology (hormone receptor status, next generation sequencing data), procedural variables (type of microsphere used, lung shunt fraction, total dose administered), and post-procedure adverse events, categorized per Society of Interventional Radiology guidelines [23]. The neutrophil-to-lymphocyte ratio was calculated by dividing the number of neutrophils by number of lymphocytes, assessed by pre- and post-procedure peripheral complete blood count. Liver tumor burden was assessed on pre-procedural CT imaging during the calculation of yttrium-90 doses using TeraRecon (TeraRecon, Foster City, CA), performed by three board-certified body imaging fellowship-trained attending radiologists with 6, 8, and 14 years of experience. Twelve patients underwent triple phase CT of the liver, obtained prior to radioembolization with a standard institutional protocol [24]. Elliptical regions of interest (ROIs) were placed on the abdominal aorta at the level of the celiac artery, the portal vein, and the largest target tumor, on all three phases. The mean attenuation value in Hounsfield units was recorded for every ROI, allowing calculation of the arterial enhancement fraction. Remaining patients had either non-contrast or only venous phase CT available, such that the arterial enhancement fraction could not be calculated.
Twenty-six patients had PET/CTs available to document imaging response, performed on average 38±35 before and 80±33 days after radioembolization. Imaging response was assessed on FDG-PET/CT imaging [25, 26] for each hepatic area targeted by radioembolization, the SUVmax was measured on pre- and post-procedure imaging, by a nuclear medicine fellowship-trained attending diagnostic radiologist with 8 years of experience (CR). The SUVmax was normalized by substracting the average SUV of uninvolved liver parenchyma. Normalized SUVmax was compared before and after radioembolization. Response was categorized as complete response when the normalized SUVmax decreased by >80%, partial response with normalized SUVmax decreased by 30—80%, stable disease when a change in by normalized SUVmax between −30% and 30%, and progressive disease when normalized SUVmax increased by more than 30% [27]. Imaging response was defined as complete or partial response. Five patients did not have imaging available for analysis: three had PET/CT before radioembolization, but were either deceased or lost to follow up before a post-treatment PET/CT was performed; two patients had neither pre- or post-radioembolization PET/CTs performed, and had been evaluated with contrast-enhanced CT instead.
Statistical Analysis
Outcomes assessed included imaging response (complete or partial) by PET/CT and survival, calculated from the date of the first radioembolization treatment. Statistical analysis was performed with GraphPad Prism 7.0 (GraphPad Software, Inc., La Jolla, CA). Continuous data is presented as mean ± standard deviation unless otherwise specified. Unpaired Student’s t tests were used to compare normally distributed data. Fisher’s exact tests were used to compare frequencies of events between groups. Log rank (Mantel-Cox) Kaplan-Meier survival analysis was used to determine the impact of specific predictors on survival after radioembolization, and presented with the hazard ratio (HR) and 95% confidence interval (CI). For continuous variables (e.g., bilirubin levels), the median was calculated and used to divide the cohort into two groups. A p<0.05 was considered significant.
Results
Thirty-one patients underwent radioembolization for liver metastasis from metastatic breast cancer. Fourteen of the 31 patients were still alive at the time of this study resulting in a median survival from first radioembolization to death of 10.9 (CI 9.5—31.8) months (Figure 2). Of the 26 patients with imaging follow up, 18/26 (69%) had an imaging response, including 7 with complete response and 11 with partial response. Six patients had stable disease, and 2 had progressive disease. Mean post-procedure SUVmax was 5.8±4.2, compared to 11.6±7.5 before the procedure (p=0.001). The mean neutrophil-to-lymphocyte ratio, measured 10.3±10.1 days after the treatment, was 9.2±7.3, compared to 3.9±2.3 before the procedure (p=0.0001).
Figure 2. Survival after radioembolization.

Kaplan-Meier survival analysis of all patients in the cohort demonstrates a median survival of 10.9 months.
Univariate Analysis of Factors Impacting Imaging Response
A univariate analysis was performed to assess factors that demonstrated a correlation with imaging response (Table 2). Among the 26 patients with PET/CT before and after radioembolization, 18 had an imaging response (6 complete and 13 partial responses), and 8 were non-responders (5 with stable and 2 with progressive disease). Genetic profiling was available in 24 patients with results shown in Figure 3. Twenty-one patients had both genetic profiling and imaging available for analysis, 15 of whom had an imaging response (complete or partial response) and 6 who were non-responders (stable or progressive disease). Radiographic response was associated with PI3K mutations (p=0.01), but not MAPK/ERK pathway (p>0.99) or TP53 mutations (p>0.99), or HER2 receptor status (p>0.99) (Table 3). Whereas only 5/11 (45%) of PI3K wildtype patients had an imaging response, 10/10 (100%) of PI3K mutants had a response (p=0.01) (Figure 4). Of the 24 patients with next generation sequencing, 21 had specimens take from the liver. Subgroup analysis of the impact of PI3K pathway mutations on imaging response among these patients showed that 7/7 (100%) PI3K pathway mutants had an imaging response, compared with only 5/11 (45%) of wildtype patients (p=0.04). No other pre-procedure variable significantly differed between responders and non-responders. The median number of mutations among patients was 6. There was no significant difference in the rate of imaging response between patients with high number of mutations (>6), compared to those with a lower number of mutations (≤ 6) by Fisher’s exact test (p=0.15).
Table 2. Peri-procedural variables in patients with and without PET/CT imaging response.
Student’s t test was used to compare groups.
| Peri-Treatment Variable | Response | No Response | p value |
|---|---|---|---|
| Laboratory Values | |||
| Pre-treatment NLR | 3.7 ± 2.3 | 4.6 ± 2.8 | 0.37 |
| Post-treatment NLR | 8.9 ± 5.8 | 10.7 ± 10.9 | 0.59 |
| Total bilirubin (gm/dL) | 0.8 ± 0.7 | 0.6 ± 0.3 | 0.46 |
| Clinical Characteristics | |||
| Number of other metastatic sites | 2.6 ± 0.9 | 2.3 ± 1.5 | 0.53 |
| Number of lines of systemic therapy | 11.2 ± 4.5 | 11.4 ± 4.0 | 0.91 |
| Age (years) | 52 ± 11 | 51 ± 11 | 0.81 |
| Time from diagnosis of liver metastasis to radioembolization (years) | 2.8 ± 1.8 | 2.1 ± 1.5 | 0.41 |
| Imaging and Procedure Features | |||
| Tumor volume (% of liver) | 18 ± 18 | 19 ± 21 | 0.93 |
| Arterial enhancement fraction (%) | 56 ± 18 | 38 ± 17 | 0.13 |
| Lung shunt fraction | 3.3 ± 2.4 | 2.9 ± 0.6 | 0.93 |
| Pre-treatment SUVmax | 12.6 ± 9.1 | 9.2 ± 3.5 | 0.33 |
| Use of glass microspheres | 6/18 (33%) | 1/8 (13%) | 0.27 |
| Dose of administration (mCi) | 32.9 ± 20.5 | 33.0 ± 26.1 | 0.99 |
| Number of treatment cycles | 1.2 ± 0.4 | 1.0 ± 0 | 0.24 |
Figure 3. OncoPrint of cohort.
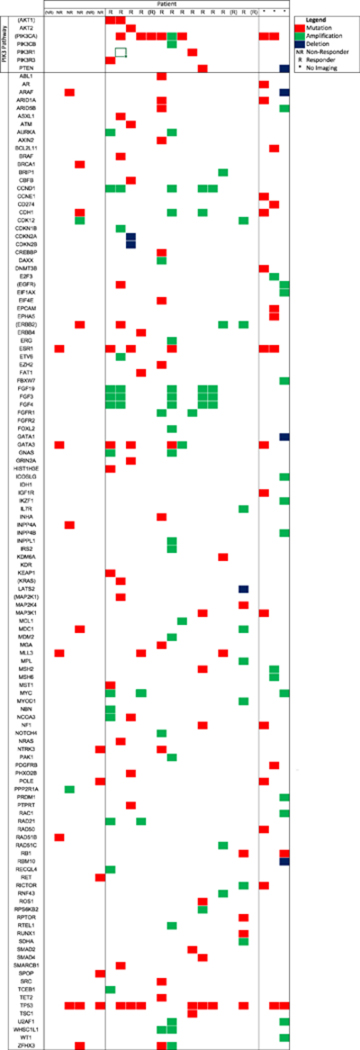
This heat map presents genes with mutations and copy number aberrations identified in the 24 patients who were evaluated with next-generation sequencing. R denotes responders and NR denotes non-responders, by PET/CT. Parentheses indicate patients who had Sequenom but not MSK-IMPACT performed. Genes in parentheses indicate those assessed by Sequenom, all of which were also assessed by MSK-IMPACT.
Table 3. PET/CT response rate in patients with and without mutations and hormonal markers.
Fisher’s exact tests were used to compare groups.
| Pathway Mutations | Mutant | Wildtype | |
| PI3K pathway | 10/10 (100%) | 5/11 (45%) | 0.01 |
| MAPK/ERK pathway | 3/4 (75%) | 12/17 (71%) | >0.99 |
| TP53 | 7/10 (70%) | 8/11 (73%) | >0.99 |
| Hormone Receptor | Positive | Negative | |
| ER | 15/21 (71%) | 3/5 (60%) | 0.63 |
| PR | |||
| HER2 | 4/5 (80%) | 12/17 (71%) | >0.99 |
| Total mutation count | > 6 Mutations | ≤ 6 Mutations | |
| 9/10 (90%) | 6/11 (55%) | 0.15 | |
Figure 4. PI3K mutation status and radiographic response at 2—4 months by PET/CT.

All patients with PI3K pathway mutations had an imaging response, whereas fewer than half of wildtype patients had a response.
Of the 24 patients who had next generation sequencing, 13 (54%) had PI3K pathway mutations. The mutations are presented in Table 4.
Table 4.
PI3K pathway mutations and copy number aberrations present in cohort. Thirteen patients had PI3K pathway mutations or copy number aberrations. AMP indicates amplification and DEL indicates deletion.
| Patient | AKT1 | AKT2 | PI3KCA | PI3KCB | PI3KR1 | PI3KR3 | PTEN |
|---|---|---|---|---|---|---|---|
| 1 | N345K | ||||||
| 2 | H1047R | ||||||
| 3 | H1047R | ||||||
| 4 | H1047R | ||||||
| 5 | E542K | ||||||
| 6 | E542K | ||||||
| 7 | E17K | E542K | |||||
| 8 | E17K | E194D | |||||
| 9 | C60_I75dup | ||||||
| 10 | AMP | AMP | |||||
| 11 | V402_L413del | ||||||
| 12 | DEL | ||||||
| 13 | A151K |
Survival Analysis
Complete or partial response by PET/CT at 2—4 months was associated with significantly longer median survival (967 days) compared to patients without imaging response (291 days) (p=0.005; HR 3.3; CI 1.1—9.9) (Figure 5). No other variables significantly predicted survival, including PI3K mutation status (p=0.58), HER2 status (p=0.36), lung shunt fraction (p=0.68), type of microsphere used (p=0.43), post-procedure neutrophil-to-lymphocyte ratio (p=0.64), pre-procedure SUVmax (p=0.31), age (p=0.15), or the number of other extrahepatic metastatic sites (p=0.80). Lower pre-procedure bilirubin levels (p=0.15; HR 1.6; CI 0.6—4.3), neutrophil-to-lymphocyte ratio (p=0.06; HR 4.5; CI 1.6—12.3), and tumor burden (p=0.06; HR 1.5; CI 0.6—4.1) were associated with improved survival, but trends did not reach significance.
Figure 5. Impacting of imaging response on survival after radioembolization.

Kaplan-Meier survival analysis showed that response on 2—4 month PET/CT was associated with improved survival.
Adverse Events
There were three major adverse events after resin microspheres radioembolization. One patient developed liver failure shortly after the first lobar radioembolization and died 26 days after treatment. Pre-procedure total bilirubin was 0.9 mg/dL. On pre-treatment imaging, the portal vein was occluded in the treated lobe due to extensive tumor burden (53% tumor involvement). The two other patients had gastric ulcers that presented with anorexia and abdominal pain; both cases were confirmed with endoscopic biopsy which demonstrated microspheres in gastric tissue and treated medically without further sequelae. No major adverse events were encountered after glass microsphere radioembolization.
Discussion
Radioembolization is an evolving approach for control of hepatic disease in patients with metastatic breast cancer, with a potential to prolong survival [3–5, 7–9, 11, 12]. Theoretically, elucidating variables that predict oncologic outcomes of radioembolization would optimize patient selection. This study achieved a median 10.9-month survival after radioembolization in heavily pretreated patients who all received at least three lines of systemic therapy. PET/CT imaging response at 2—4 months was associated with longer survival. In this exploratory cohort, PI3K pathway mutations were associated with radiographic response.
The finding that imaging response at 2—4 months predicted survival corroborates prior retrospective studies in metastatic breast cancer [5, 8, 9, 12] and other populations including colorectal cancer [13, 28, 29]. Similarly, early imaging follow-up after chemoembolization predicts survival [30]. Together, results point to the prognostic value of early follow-up imaging after liver-directed therapy. Factors that improve imaging response could be expected to translate into survival benefit.
Univariate analysis showed that mutations or copy number aberrations in PI3K pathway genes (AKT1, AKT2, PIK3CA, PIK3CB, PIK3R1, PIK3R3, and PTEN) were associated with better imaging outcomes after radioembolization in metastatic breast cancer. Early imaging response after radioembolization was associated with improved survival. These results resonate with a prior report of increased time to local progression among PI3K pathway mutant colorectal cancer patients after radioembolization [31]. The PI3K pathway plays important roles in cell death mechanisms after radiation [32]. Interestingly, PI3K pathway mutations increase radioresistance in breast cancer cell lines [15]. Constitutive activation of AKT or HRAS in breast cancer cell lines cause radioresistance, and treatment with PI3K pathway inhibitors induce radiosensitivity [33], as in colorectal cancer in vitro studies [34]. Similarly, overexpression of integrin α6 in breast cancer cell culture is associated with radioresistance, an effect mediated by the PI3K pathway [35]. Most PI3K pathway mutations in breast cancer are activating [36], and might therefore be expected to increase radioresistance based on in vitro studies.
It is not clear why PI3K pathway mutations were instead associated with better response to radioembolization in human patients. The preliminary findings warrant further prospective investigation. It is also interesting that the improved imaging response among patients with PI3K mutations did not translate into a survival benefit. This may be due to the small sample size; among the 13 patients with mutations, only 3 were deceased at the time of the study, prohibiting calculation of the median survival among these patients. It is possible that with longer term follow up, a survival benefit may be realized.
No other peri-procedural factors were associated with response to radioembolization. Lower pre-procedural bilirubin levels, neutrophil-to-lymphocyte ratio, and tumor burden were associated with longer survival; though trends did not reach significance, these findings are similar to prior reports showing that these are associated with longer survival after radioembolization in metastatic breast cancer [4, 5, 7, 37] and other diseases including colorectal cancer [38]. In the context of colorectal cancer, hepatocellular carcinoma, and neuroendocrine tumor, intratumoral vascular shunting has been associated with poor survival after radioembolization (e.g., [39]). This was not evident in the population of metastatic breast cancer patients studied here, perhaps because of the very low lung shunt fractions noted among these patients, with a low median of 3%. Furthermore, neither pre-procedure SUVmax nor arterial enhancement fraction predicted survival or imaging response, contrasting prior reports in breast and colorectal cancer [8, 24]. Though, our cohort may have been too small to show this.
The cohort size is too small to allow meaningful comparison between glass and resin microspheres. All three adverse events occurred with resin microspheres, with two non-target embolizations. This corroborates prior studies showing a higher rate of non-target embolization using resin spheres, approximately 10%, compared with glass spheres, approximately 5% [3–5, 7–9]. Heavily pre-treated patients who have undergone multiple lines of chemotherapy for hypovascular tumors such as colon and breast metastases may have arterial changes predisposing to a higher risk of reflux; for example, nearly 40% of metastatic breast cancer patients undergoing radioembolization with resin microspheres have reflux during administration [11], not unlike colorectal cancer patients treated in the salvage setting [40]. Future prospective studies are warranted to assess the relative risk and benefit of using glass versus resin microspheres in metastatic breast cancer patients.
There are several study limitations. As a retrospective study with a small number of patients, our data is exploratory and warrants prospective validation in a larger cohort. The small study size dictates that this research has limited power to detect differences in many of the variables considered, and precludes multivariate analysis. While the observed prolonged patient survival is promising, it may partly reflect bias in patient selection as well as the effect of additional therapies that the patients received before and after radioembolization. Our dataset may be too small to detect the impact of certain variables. Several of the patients were profiled with Sequenom, which assays significantly fewer genes and only a select number of mutations within chosen genes, compared with MSK-IMPACT. It is likely that several PI3K pathway mutations were not detected among these patients, and that some patients classified as wildtype may actually have had PI3K pathway mutations, partly accounting for many of the wildtype patients having an imaging response. Prospective profiling with a broader assay such as MSK-IMPACT is necessary to validate findings. Sampling from only one site also fails to represent intrinsic intra- and inter-tumoral mutational heterogeneity. Also, 3 of the 24 patients with genetic profiling did not have specimens from the liver, but rather other sites of disease, which may have confounded results due to inter-tumoral mutational heterogeneity. Furthermore, radioembolization was performed by multiple operators using both resin and glass microspheres, and was followed at variable time points after treatment with imaging, there was inherent variability that likely decreased the study’s power to detect significant associations between variables studied and outcomes. Finally, no breast cancer patients underwent chemoembolization at our institution to enable comparisons of outcomes in regard to sequencing data. Therefore, this data cannot be used at this time to guide therapy.
In summary, PI3K pathway mutations are associated with early (2—4 month) PET/CT imaging response after radioembolization of liver metastases in metastatic breast cancer patients, and imaging response is associated with prolonged survival. Therefore, mutations in the PI3K pathway may be associated with improved response to radioembolization in breast cancer.
Footnotes
The authors declare no potential conflicts of interest.
Reference
- [1].Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64:9–29. [DOI] [PubMed] [Google Scholar]
- [2].Hagemeister FB Jr., Buzdar AU, Luna MA, Blumenschein GR. Causes of death in breast cancer: a clinicopathologic study. Cancer 1980; 46:162–7. [DOI] [PubMed] [Google Scholar]
- [3].Bangash AK, Atassi B, Kaklamani V, et al. 90Y radioembolization of metastatic breast cancer to the liver: toxicity, imaging response, survival. J Vasc Interv Radiol 2007; 18:621–8. [DOI] [PubMed] [Google Scholar]
- [4].Cianni R, Pelle G, Notarianni E, et al. Radioembolisation with (90)Y-labelled resin microspheres in the treatment of liver metastasis from breast cancer. Eur Radiol 2013; 23:182–9. [DOI] [PubMed] [Google Scholar]
- [5].Fendler WP, Lechner H, Todica A, et al. Safety, Efficacy, and Prognostic Factors After Radioembolization of Hepatic Metastases from Breast Cancer: A Large Single-Center Experience in 81 Patients. J Nucl Med 2016; 57:517–23. [DOI] [PubMed] [Google Scholar]
- [6].Giroux MF, Baum RA, Soulen MC. Chemoembolization of liver metastasis from breast carcinoma. J Vasc Interv Radiol 2004; 15:289–91. [DOI] [PubMed] [Google Scholar]
- [7].Gordon AC, Gradishar WJ, Kaklamani VG, et al. Yttrium-90 radioembolization stops progression of targeted breast cancer liver metastases after failed chemotherapy. J Vasc Interv Radiol 2014; 25:1523–32, 32 e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Haug AR, Tiega Donfack BP, Trumm C, et al. 18F-FDG PET/CT predicts survival after radioembolization of hepatic metastases from breast cancer. J Nucl Med 2012; 53:371–7. [DOI] [PubMed] [Google Scholar]
- [9].Jakobs TF, Hoffmann RT, Fischer T, et al. Radioembolization in patients with hepatic metastases from breast cancer. J Vasc Interv Radiol 2008; 19:683–90. [DOI] [PubMed] [Google Scholar]
- [10].Martin RC, Robbins K, Fages JF, et al. Optimal outcomes for liver-dominant metastatic breast cancer with transarterial chemoembolization with drug-eluting beads loaded with doxorubicin. Breast Cancer Res Treat 2012; 132:753–63. [DOI] [PubMed] [Google Scholar]
- [11].Pieper CC, Meyer C, Wilhelm KE, et al. Yttrium-90 Radioembolization of Advanced, Unresectable Breast Cancer Liver Metastases-A Single-Center Experience. J Vasc Interv Radiol 2016; 27:1305–15. [DOI] [PubMed] [Google Scholar]
- [12].Saxena A, Kapoor J, Meteling B, Morris DL, Bester L. Yttrium-90 radioembolization for unresectable, chemoresistant breast cancer liver metastases: a large single-center experience of 40 patients. Ann Surg Oncol 2014; 21:1296–303. [DOI] [PubMed] [Google Scholar]
- [13].Seyal AR, Parekh K, Velichko YS, Salem R, Yaghmai V. Tumor growth kinetics versus RECIST to assess response to locoregional therapy in breast cancer liver metastases. Acad Radiol 2014; 21:950–7. [DOI] [PubMed] [Google Scholar]
- [14].Salem R, Gilbertsen M, Butt Z, et al. Increased quality of life among hepatocellular carcinoma patients treated with radioembolization, compared with chemoembolization. Clin Gastroenterol Hepatol 2013; 11:1358–65 e1. [DOI] [PubMed] [Google Scholar]
- [15].Yard BD, Adams DJ, Chie EK, et al. A genetic basis for the variation in the vulnerability of cancer to DNA damage. Nat Commun 2016; 7:11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lahti SJ, Xing M, Zhang D, Lee JJ, Magnetta MJ, Kim HS. KRAS status as an independent prognostic factor for survival after yttrium-90 radioembolization therapy for unresectable colorectal cancer liver metastases. Journal of vascular and interventional radiology 2015; 26:1102–11. [DOI] [PubMed] [Google Scholar]
- [17].Bergh J, Norberg T, Sjogren S, Lindgren A, Holmberg L. Complete sequencing of the p53 gene provides prognostic information in breast cancer patients, particularly in relation to adjuvant systemic therapy and radiotherapy. Nat Med 1995; 1:1029–34. [DOI] [PubMed] [Google Scholar]
- [18].Rekhtman N, Paik PK, Arcila ME, et al. Clarifying the spectrum of driver oncogene mutations in biomarker-verified squamous carcinoma of lung: lack of EGFR/KRAS and presence of PIK3CA/AKT1 mutations. Clin Cancer Res 2012; 18:1167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn 2015; 17:251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Holmes D PI3K pathway inhibitors approach junction. Nature Publishing Group, 2011. [DOI] [PubMed] [Google Scholar]
- [21].De Luca A, Maiello MR, D’Alessio A, Pergameno M, Normanno N. The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in cancer pathogenesis and implications for therapeutic approaches. Expert opinion on therapeutic targets 2012; 16:S17–S27. [DOI] [PubMed] [Google Scholar]
- [22].Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010; 28:2784–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Omary RA, Bettmann MA, Cardella JF, et al. Quality improvement guidelines for the reporting and archiving of interventional radiology procedures. J Vasc Interv Radiol 2003; 14:S293–5. [DOI] [PubMed] [Google Scholar]
- [24].Boas FE, Brody LA, Erinjeri JP, et al. Quantitative Measurements of Enhancement on Preprocedure Triphasic CT Can Predict Response of Colorectal Liver Metastases to Radioembolization. AJR Am J Roentgenol 2016; 207:671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].O JH, Lodge MA, Wahl RL. Practical PERCIST: A Simplified Guide to PET Response Criteria in Solid Tumors 1.0. Radiology 2016; 280:576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zerizer I, Al-Nahhas A, Towey D, et al. The role of early (1)(8)F-FDG PET/CT in prediction of progression-free survival after (9)(0)Y radioembolization: comparison with RECIST and tumour density criteria. Eur J Nucl Med Mol Imaging 2012; 39:1391–9. [DOI] [PubMed] [Google Scholar]
- [27].Fendler WP, Tiega DBP, Ilhan H, et al. Validation of several SUV-based parameters derived from 18F-FDG PET for prediction of survival after SIRT of hepatic metastases from colorectal cancer. Journal of Nuclear Medicine 2013; 54:1202–8. [DOI] [PubMed] [Google Scholar]
- [28].Shady W, Sotirchos VS, Do RK, et al. Surrogate Imaging Biomarkers of Response of Colorectal Liver Metastases After Salvage Radioembolization Using 90Y-Loaded Resin Microspheres. AJR Am J Roentgenol 2016; 207:661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Soydal C, Kucuk ON, Gecim EI, Bilgic S, Elhan AH. The prognostic value of quantitative parameters of 18F-FDG PET/CT in the evaluation of response to internal radiation therapy with yttrium-90 in patients with liver metastases of colorectal cancer. Nucl Med Commun 2013; 34:501–6. [DOI] [PubMed] [Google Scholar]
- [30].Shim JH, Lee HC, Kim SO, et al. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology 2012; 262:708–18. [DOI] [PubMed] [Google Scholar]
- [31].Ziv E, Bergen M, Yarmohammadi H, et al. PI3K pathway mutations are associated with longer time to local progression after radioembolization of colorectal liver metastases. Oncotarget 2017; 8:23529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Golden EB, Pellicciotta I, Demaria S, Barcellos-Hoff MH, Formenti SC. The convergence of radiation and immunogenic cell death signaling pathways. Front Oncol 2012; 2:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Liang K, Jin W, Knuefermann C, et al. Targeting the phosphatidylinositol 3-kinase/Akt pathway for enhancing breast cancer cells to radiotherapy. Mol Cancer Ther 2003; 2:353–60. [PubMed] [Google Scholar]
- [34].Chen YH, Wei MF, Wang CW, et al. Dual phosphoinositide 3-kinase/mammalian target of rapamycin inhibitor is an effective radiosensitizer for colorectal cancer. Cancer Lett 2015; 357:582–90. [DOI] [PubMed] [Google Scholar]
- [35].Hu T, Zhou R, Zhao Y, Wu G. Integrin α6/Akt/Erk signaling is essential for human breast cancer resistance to radiotherapy. Scientific reports 2016; 6:33376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Miller TW, Rexer BN, Garrett JT, Arteaga CL. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res 2011; 13:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Azab B, Bhatt VR, Phookan J, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol 2012; 19:217–24. [DOI] [PubMed] [Google Scholar]
- [38].Sukato DC, Tohme S, Chalhoub D, et al. The Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Patients with Unresectable Hepatocellular Carcinoma Treated with Radioembolization. J Vasc Interv Radiol 2015; 26:816–24 e1. [DOI] [PubMed] [Google Scholar]
- [39].Deipolyi AR, Iafrate AJ, Zhu AX, Ergul EA, Ganguli S, Oklu R. High lung shunt fraction in colorectal liver tumors is associated with distant metastasis and decreased survival. J Vasc Interv Radiol 2014; 25:1604–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sofocleous CT, Garcia AR, Pandit-Taskar N, et al. Phase I trial of selective internal radiation therapy for chemorefractory colorectal cancer liver metastases progressing after hepatic arterial pump and systemic chemotherapy. Clin Colorectal Cancer 2014; 13:27–36. [DOI] [PubMed] [Google Scholar]


