Abstract
We present 2 patients born in Argentina who were newly diagnosed with advanced HIV disease and Chagas disease reactivation with central nervous system involvement. The patients received concurrent benznidazole treatment and antiretroviral therapy, showing good response. Improvement in morbidity and mortality due to early treatment makes this treatment appropriate for coinfected patients.
Keywords: benznidazole, Chagas disease, coinfection, HIV, treatment
Chagas disease (CD) is one of the most important zoonotic infectious diseases and a is public health concern in Latin America, where it is endemic. It is caused by the flagellate protozoa Trypanosoma cruzi (T. cruzi), and about 6–7 million people worldwide are estimated to be infected [1]. T. cruzi is mainly transmitted by Triatomine species (called “vinchuca”) but can also be spread by transfusion of infected blood, vertical transmission, organ transplantation, and consumption of contaminated food. It presents 2 phases: acute phase, diagnosed by identification of the parasite in the bloodstream, where meningoencephalitis and/or myocarditis can occur, albeit rarely (<1%) [2], and symptoms usually resolve spontaneously; and chronic phase, where parasitemia is intermittent and usually of low magnitude. To confirm diagnosis, it is necessary to have 2 positive serologic methods to detect IgG antibodies to T. cruzi [2]. Twenty percent to 30% of patients develop target organ involvement, mainly of the heart, and less frequently of the gastrointestinal tract [3].
Chagas disease reactivation has been reported in immunocompromised hosts, HIV-infected patients among them. The first case of coinfection with HIV was reported in our country by Del Castillo and colleagues [4]; since then, many cases have been published showing high mortality rates [3, 5]. To the best of our knowledge, little has been published regarding the optimal timing for initiation of antiretroviral therapy (HAART) in these patients.
We identified 2 patients born in Argentina who were newly diagnosed with HIV concomitantly presenting CD reactivation with central nervous system (CNS) involvement, presenting as a space-occupying mass, with good progress following combination of antiparasitic drugs with HAART.
CASE REPORTS
Patient 1
A 52-year-old woman born in Chaco Province, Argentina; she was admitted on January 2011 because of asthenia, fever, and headache for 3 months. No distinctive features were found on physical examination.
She had a previously confirmed diagnosis of nontreated Chagas infection. She reported no blood transfusions or intravenous drug use. She had had sex without a condom.
A brain computed tomography scan showed a hypodense white matter mass in the temporo-parietal region.
Laboratory Findings
The enzyme-linked immunosorbent assay (ELISA) test for HIV1 was positive. Her viral load (VL) was 4700 copies/log 3.7. Her absolute CD4 count was 5 cells. The rest of the results were within normal limits (Table 1). Cerebrospinal fluid (CSF) showed trypomastigotes on direct microscopic observation.
Table 1.
Laboratory Results at Diagnosis and After Treatment
| CSF Findings | T.cruzi Observed on Direct Smear | Chagas PCR | Other CNS Infections | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient | CD4 Count | HIV Viral Load | WBC | Protein | Glucose | CSF | Blood | CSF | Blood | Test/Result |
| 1 | 5 | 4700 | 0 | 25 | 51 | Yes | Yes | NP | NP | Crypto/Toxo negative |
| 2 | 53 | 1 380 000 | 1 | 30 | 47 | No | Yes | + | + | Crypto/Toxo negative |
| Post-treatment (8 and 12 mo after initiated treatment, respectively) | ||||||||||
| 1 | 131 | ND | ||||||||
| 2 | 364 | ND | ||||||||
| Post-treatment (7 and 2 y after initiated treatment, respectively) | ||||||||||
| 1 | 554 | ND | ||||||||
| 2 | 450 | ND | ||||||||
Abbreviations: CNS, central nervous system; CSF, cerebrospinal fluid; ND, nondetectable; NP, not performed; PCR, polymerase chain reaction.
Treatment with benznidazole was started 7 days after admission: 5 mg/kg daily for 75 days. She concomitantly started HAART with lamivudine/tenofovir plus nevirapina. Her symptoms improved 5 days later.
Patient 2
A 38-year-old woman born in Buenos Aires, Argentina, was admitted in May 2016 because she had a seizure after a fall, with traumatic brain injury and loss of consciousness. There was no history of intravenous drugs use or blood transfusions. Her husband had a negative HIV test; she used to have sex without a condom with other people. She traveled frequently to Chaco and also had a confirmed diagnosis of Chagas infection (it was made by a control of serology, the patient said) during her first pregnancy 15 years ago. She never received treatment. The patient described having right-sided headache for 4 months, associated with recurrent episodes of fever. No distinctive features were found on physical examination. Magnetic resonance showed a hypointense mass in the right-frontal lobe, with perilesional edema.
Laboratory Findings
The fourth-generation ELISA test was HIV-positive, confirmed by VL test (1 380 000 copies/mL, log 6.4), and her absolute CD4 count was 53 cells (7%). The ELISA test and indirect hemagglutination for Chagas were positive. Strout’s concentration method and polymerase chain reaction (PCR) were positive in the blood [6]. PCR for CD was conducted at the National Institute of Parasitological “Doctor Mario Fatala Chaben,” which is a regional center that collaborates with the World Health Organization.
Lumbar puncture was performed: CSF was within normal values. PCR for CD was positive (Table 1).
She initiated treatment with benznidazole; 5 mg/kg daily was given for 60 days, and HAART with lamivudine/tenofovir plus atazanavir/ritonavir was administered concomitantly. Her symptoms improved after 10 days of treatment.
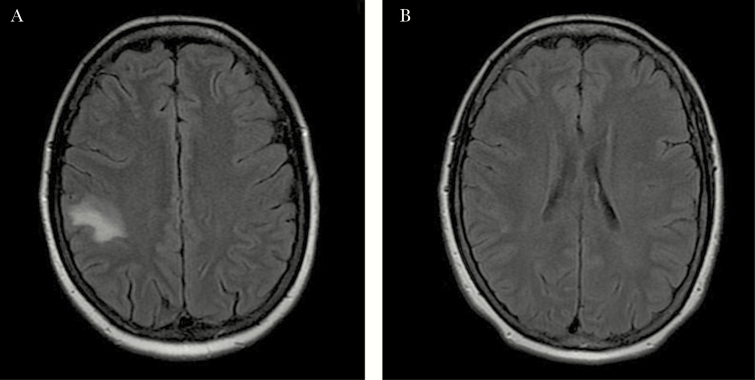
A follow-up scan showed no lesions (Figure 1).
Figure 1.
Patient 2: A, Gadolinium-based magnetic resonance image (MRI) of T1. Hypointense image in Right frontal lobe is heterogeneous with focal perilesional edema and contrast enhancement. Patient 2: B, MRI 6 months after finishing treatment shows disappearance of lesions.
Both patients continued with secondary prophylactic antitrypanosomal therapy at 5 mg/kg/d, 3 days a week, until achieving 2 CD4 counts >200 copies and undetectable VL. They achieved an undetectable viral load 8 and 12 months after treatment initiation, respectively.
Up to the time of writing this article, there has been no relapse of CD, and they have had undetectable HIV VLs. (Table 1).
The lack of prospective controlled studies of treatment for CNS Chagas and HIV coinfections [7], unlike other opportunistic infections such as tuberculosis disease where the timing to start HAART has been established in guidelines [8], limits the ability to recommend an ideal time for antiparasitic treatment and HAART. The HIV epidemic has shown relevant changes in recent years. However, there is still much work to be done to achieve the UNAIDS 90-90-90 diagnostic, therapeutic, and viral load suppression targets by 2020 [9]. It is estimated that 122 000 people are HIV-positive in Argentina [9]. According to data published during 2017, there were 2500 new diagnoses; 35% of cases were diagnosed at an advanced stage of infection. The same year, 1500 people died from AIDS-related illnesses [9].
Chagas disease still represents one of the most important endemic diseases of the American continent. With an annual incidence of 28 000 cases in the region, CD causes about 12 000 deaths per year [1].
Argentina is one of the 21 endemic countries for this zoonosis, and it is still considered an important socioeconomic problem. Currently, around 1 505 235 Argentinians are infected [1].
The first cases of coinfection were reported between 1989 and 1992, mainly in Brazil and Argentina [5], in the pre HAART era. Based on these cases, it has been established that risk of reactivation depends on the severity of immunosuppression, which is more likely in patients with a CD4 count <200/mm3. Currently, CD is considered an opportunistic disease in those countries [10].
The number of HIV-positive patients coinfected with CD in Argentina is unknown. A study published in 2008 found a prevalence of 4.2% [11], whereas a more recent publication that studied 280 HIV-positive patients found a coinfection rate of 2.9% [12]. In a study by our group in 2016 that included 1225 HIV+ patients (958 men, 267 women), 8 were coinfected (median age, 42 years), representing 0.65% of the studied population (median CD4 count [range], 170 [16–423] cells/mm3). In a reference center from Brazil, a 1.3% rate was reported in 2010 [13]. This means that the prevalence in South American countries could be significant and should be taken into account.
Current guidelines in Argentina recommend treatment with trypanocidal drugs such as benznidazole (5–8 mg daily) or nifurtimox (5–10 mg daily) for 60 days [5]. However, clinical evidence is not enough to recommend definite treatment duration and post-treatment prophylaxis, so each case must be considered individually, taking into account response to treatment and adverse effects. As mentioned above, there are insufficient data to determine adequate timing to start HAART in relation to the start date of parasiticidal treatment. A major concern, particularly in HIV-infected patients with CNS lesions on HAART, is the potential development of immune reconstitution inflammatory syndrome (IRIS), which can cause complications. However, to our knowledge, IRIS cases related to Chagas CNS disease and HAART have been rarely reported.
An infectious diseases service in Buenos Aires studied 235 HIV+ patients: 4 had positive serology for Chagas, and during the start of therapy with HAART, 1 developed Chagoma in the context of IRIS [14]. However, in our 2 patients, we observed good response with joint treatments, and the patients did not develop IRIS. Currently, there are no data to support the indication of primary or secondary prophylaxis with parasiticidal drugs. As to primary prophylaxis, serological studies and evaluation of organ lesions in those individuals coming from endemic areas are necessary, as indicated by Argentina’s guidelines [15]. In AIDS patients with brain lesions, the diagnosis of CD must be considered, looking for direct parasite detection methods in the blood and CSF (85% sensitivity) [10], also using PCR. Regarding secondary prophylaxis, the current guidelines in Argentina recommend administration of benznidazole 3 times a week (2.5–5 mg/kg daily) until immunological improvement (CD4 >200/mm3) is achieved [10]. In reactivation of CD, the most frequently affected organ is the CNS, clinically manifested as acute meningoencephalitis or, generally, as a mass-occupying lesion (chagoma) in 75%–80% [10] of coinfected patients. This event has a high mortality ratio (79%–100% [16]. The prognosis after the HAART era has improved, but the mortality remains high [3, 5, 16]. In our experience, the early start of combined treatment after confirming the diagnosis had very good results, with disease-free survival.
CONCLUSIONS
Although the literature is scarce regarding adequate timing of initiation of HAART, based on our limited experience, we believe that an early diagnosis and initiation of joint therapy considerably improve survival without high risk for development of IRIS, which seems to be an infrequent event. This practice could change the prognosis of the disease. Prospective and controlled studies are needed to determine the timing and efficacy of early treatment intervention and the role of prophylaxis with parasiticidal drugs.
Acknowledgments
We would like to thank our infectious diseases colleagues at the Department of Sanatorio Trinidad Mitre and Dra. Allende M. for helpful collaboration.
Potential conflicts of interest. The authors have nothing to disclose. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Chagas disease (American trypanosomiasis). 2018. Available at: https://www.who.int. Accessed 1 September 2018.
- 2. Bern C. Chagas’ disease. N Engl J Med 2015; 373:456–66. [DOI] [PubMed] [Google Scholar]
- 3. Cordova E, Boschi A, Ambrosioni J, et al. Reactivation of Chagas disease with central nervous system involvement in HIV-infected patients in Argentina, 1992–2007. Int Soc Infect Dis 2008; 12:587–92. [DOI] [PubMed] [Google Scholar]
- 4. Del Castillo M, Mendoza G, Oviedo J, et al. AIDS and Chagas’ disease with central nervous system tumor-like lesion. Am J Med 1990; 88:693–4. [DOI] [PubMed] [Google Scholar]
- 5. Lattes R, Lasala MB. Chagas disease in the immunosuppressed patient. Clin Microbiol Infect 2014; 20:300–9. [DOI] [PubMed] [Google Scholar]
- 6. Ramirez J, Cura C, Moreira O, et al. Analytical validation of quantitative real-time PCR methods for quantification of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. Am Soc Invest Path Assoc Mol Path 2015; 17:605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Almeida EA, Ramos AN Jr, Correia D, Shikanai-Yasuda MA. Co-infection Trypanosoma cruzi/HIV: systematic review (1980-2010). Rev Soc Bras Med Trop 2011; 44:62–770. [DOI] [PubMed] [Google Scholar]
- 8. AIDSinfo. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV Mycobacterium tuberculosis disease with HIV coinfection. 2009. Available at: https:// aidsinfo.nih.gov. Accessed 20 January 2019.
- 9. Dirección de Sida y ETS, Ministerio de Salud de la Nación Argentina. Boletín sobre el VIH, SIDA e ITS en la Argentina. 2017. Available at: http://www.msal.gob.ar. Accessed 21 September 2018.
- 10. SADI. Comisión de SIDA y ETS Recomendaciones sobre el manejo de infecciones oportunistas en pacientes con infección por HIV. 2016. Available at: https://www.sadi.org.ar. Accessed 10 November 2018.
- 11. Dolcini G, Ambrosioni J, Andreani G, Pando MA, Martinez Peralta L, Benetucci J. Prevalencia de la coinfección virus de la inmunodeficiencia humana (VIH)-Trypanosoma cruzi e impacto del abuso de drogas inyectables en un centro de salud de la ciudad de Buenos Aires. Rev Argent Microbiol 2008; 40:164–6. [PubMed] [Google Scholar]
- 12. Benchetrit A, Andreani G, Avila MM, et al. High HIV-Trypanosoma cruzi coinfection levels in vulnerable populations in Buenos Aires, Argentina. AIDS Res Hum Retroviruses 2017; 33:330–1. [DOI] [PubMed] [Google Scholar]
- 13. Stauffert D, da Silveiraa MF, Mesenburg MA, et al. Prevalence of Trypanosoma cruzi/HIV coinfection in southern Brazil. Braz J Infect Dis 2017; 21:180–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Warley E, Antabak N, Desse J, et al. Desarrollo de neoplasias e infecciones definitorias de SIDA después de iniciar la terapia antirretroviral de alta eficacia. Development of neoplasia and AIDS defining-infections after initiation of highly efficacy antirretroviral therapy. Rev Med 2010; 70:49–52. [PubMed] [Google Scholar]
- 15. Ministerio de Salud. Argentina. Enfermedades infecciosas, Chagas. Atención al paciente infectado con Trypanosoma Cruzi. Guía para el equipo de Salud. 2012. Available at: http://www.msal.gob.ar. Accessed 20 February 2017.
- 16. Bern C. Chagas disease in the immunosuppressed host. Curr Opin Infect Dis 2012; 25:450–7. [DOI] [PubMed] [Google Scholar]