Abstract
Resistant maltodextrin (RMD) is a soluble dietary fibre that exerts several physiological functions as a result of its microbial degradation and changes in the intestinal environment. It has been reported that RMD enhanced immunoglobulin A (IgA) secretion, which protects the mucosa from foreign substances. However, the effect of RMD on excessive immunity has yet to be investigated. In this study, we aimed to investigate the effect of RMD on excessive immune responses such as food allergy. OVA23-3 mice were fed an AIN-76-based diet containing 20% egg-white protein with or without RMD. While RMD was shown to contribute to an increase in goblet cells, RMD did not change the overall inflammatory status when ingested with the egg-white diet. RMD suppressed IL-4 and IL-10 production from splenocytes but not cells from mesenteric lymph nodes. RMD also downregulated the serum levels of OVA-specific Th1- and Th2-related antibodies, which were elevated in the food-allergic condition. RMD significantly increased the total amount of short-chain fatty acids, especially acetate and propionate, in the caecum of OVA23-3 mice fed the egg-white diet. Our study demonstrated that dietary RMD modulates systemic rather than intestinal antigen-specific immune responses in the food-allergic condition of OVA23-3 mice. Although the relevant mechanism has yet to be investigated, RMD shows potential for alleviating food allergy through adjustment of systemic immunity.
Keywords: resistant maltodextrin, systemic immunity, food allergy, short-chain fatty acid
INTRODUCTION
Resistant maltodextrin (RMD) is a soluble dietary fibre that is used in many types of food and beverages available in the market. Dietary RMD is known to regulate bowel movement [1] and suppress postprandial blood glucose elevation [2] and triglyceride (TG) elevation [3]. Degradation of RMD by intestinal microorganisms causes changes in the intestinal environment, such as the amount and composition of short-chain fatty acids and intestinal flora, thereby contributing to physiological functions [4, 5]. In addition to the promotion of glucagon-like peptide 1 (GLP-1) secretion [6] and enhancement of mineral absorption [7], dietary RMD has been reported to enhance immunoglobulin A (IgA) secretion in the intestine of mice through changes of the intestinal environment [5]. Increased IgA protects the intestinal mucosa from foreign substances, such as viruses and bacteria. However, the effect of RMD on excessive immune responses, such as allergies, has yet to be investigated.
Among allergies, food allergies are increasingly serious problems both in developed and developing countries [8], especially in children. Food allergies significantly affect the quality of life (QOL) of those who have them. Children who suffer from food allergies tend to avoid food items that contain allergens, possibly affecting their growth because of nutrient deficiency [9]. Since the decay of QOL and economic loss of allergic patients are recognised as social issues, governments have developed policies for mandatory labelling of allergens and for therapeutic strategies.
In this study, we aimed to investigate the effect of RMD on excessive immune responses. The OVA23-3 mouse model of food allergy expresses the ovalbumin-specific T cell receptor [10], develops intestinal inflammation followed by weight loss, induces Th2-skewed cytokine responses, and produces OVA-specific IgE and IgG subclass antibodies in sera after feeding on an egg-white-based diet [11, 12]. Therefore, we used this experimental system to investigate the immunomodulatory effect of dietary RMD on the systemic and intestinal immune response.
MATERIALS AND METHODS
Animals and diets
OVA23-3 (+/−) mice were obtained by backcrossing OVA23-3 (+/+) mice with BALB/c mice (CLEA Japan, Tokyo, Japan). Mice were maintained at a room temperature between 23–25°C and 40–60% relative humidity with a 12-hr light-dark cycle. Mice were grouped into plastic cages and given free access to an experimental diet and sterile deionised water. All experiments were approved by the Nihon University Animal Care and Use Committee (approval numbers: AP14B028-1, AP15B067, and AP16B037) and carried out in accordance with their guidelines.
Male OVA23-3 (+/−) mice, 8–10 weeks of age (24.26 ± 1.89 g), were divided into two groups, an egg white group (E) and egg white-RMD group (ER), such that the average body weights of the groups were comparable. Each group of mice (n=5 or 6) was housed in a separate cage and fed an AIN-76-based diet containing 20% egg-white protein (as a substitute for casein) with or without 7.5% RMD (Table 1). Experimental diets were solidified in pellets and sterilised with gamma irradiation at Funabashi Farm Co., Ltd. (Chiba, Japan). RMD was manufactured by Matsutani Chemical Industry Co., Ltd. (Hyogo, Japan). The number of animals in each group was set according to previous studies [11].
Table 1. Composition of the test diet (g/kg diet).
| Ingredient | E | ER |
|---|---|---|
| Egg white | 200 | 200 |
| Corn starch | 484.7 | 409.7 |
| α-Starch | 90 | 90 |
| Sucrose | 50 | 50 |
| Cellulose | 50 | 50 |
| Soybean oil | 60 | 60 |
| Vitamin mixture* | 13 | 13 |
| Mineral mixture* | 50 | 50 |
| Cholin chloride | 2.3 | 2.3 |
| RMD | - | 75 |
* Vitamin and mineral mixtures were prepared according to AIN-76 formulation. E: egg white group; ER: egg white-RMD group; RMD: resistant maltodextrin.
Mice were fed experimental diets for eight weeks, and their weights were recorded. Blood samples were collected from the tail vein every week to analyse the immunoglobulin titre in the serum. In another experiment, mice were fed the experimental diet for nine days. The mesenteric lymph nodes (MLN), the spleen (SPL), the caecum, and a segment from the jejunum were removed from the mice for further analysis. Mice were anaesthetised by isoflurane inhalation before dissection to reduce suffering.
Histologic analysis
Three-centimetre segments of the jejunum (just next to the duodenum) were excised, opened longitudinally, fixed with 10% formalin, and embedded in paraffin. Sections were prepared and stained with haematoxylin and eosin (HE) for morphological evaluation. The extent of morphological alterations in the hyperplasia of goblet cells, elongation of crypts, cell infiltration of the lamina propria, and inflammation of crypts was evaluated on a scale of 0 to 4: 0, normal or insignificant lesion; 1, minor alteration in a limited area; 2, multiple alterations in a limited area; 3, lesions in a broad area; 4, lesions in the whole area.
Cell preparation
Single-cell suspensions were prepared from the MLNs and the SPLs. Briefly, the SPLs were physically dissociated with glass slides in RPMI 1640 medium supplemented with 50 U/ml penicillin, 50 µg/ml streptomycin, 2 mM L-glutamine, and 50 μM 2-mercaptoethanol and filtered through a 40-μm cell strainer. The cell suspensions were treated with BD Pharm LyseTM Lysing Buffer (BD Biosciences, Franklin Lakes, NJ, USA) to lyse erythrocytes and then resuspended in RPMI medium containing 10% fetal calf serum (FCS). The MLNs were washed in RPMI 1640 medium, minced into small pieces, and incubated for 5 min in RPMI 1640 containing 10% FCS, 1 mg/ml collagenase D (Roche Diagnostics, Indianapolis, IN, USA), and 20 µg/ml DNase I (Roche Diagnostics). The cell suspensions were filtered through a 70-μm cell strainer and resuspended in RPMI 1640 medium containing 10% FCS.
Cell culture
Whole cells (2.5 × 106 cells) from the SPLs and the MLNs of the experimental mice were cultured in 48-well flat-bottom plates in the presence of 5 mg/ml ovalbumin (OVA) (Seikagaku Corporation, Tokyo, Japan) for 72 hr. Culture supernatants were harvested for subsequent analysis by enzyme-linked immunosorbent assay (ELISA).
ELISA
Immunoglobulin concentration in serum and cytokine concentration in cell culture supernatant were assayed by sandwich ELISA.
Purified rat anti-mouse IFN-γ (AN-18), IL-2 (JES6-1A12), IL-4 (BJD4-1D11), and IgE (R35-92) monoclonal antibodies or OVA were used as capture reagents; biotinylated rat anti-mouse IFN-γ (XMG1.2), IL-2 (JES6-5H4), IL-4 (BJD6-34G2), IgE (LO-ME-2, Serotec, Oxford, UK), or biotinylated goat anti-mouse IgG1 and IgG2a monoclonal antibodies (SouthernBiotech, Birmingham, AL, USA) were used for detection. All antibodies were purchased from BD Biosciences unless otherwise specified. IL-10 level was measured using the BD OptEIA Mouse ELISA set, following the manufacturer’s instructions.
Measurement of short-chain fatty acids (SCFA)
Caecal content was removed and stored at −20°C until analysed. A portion of the content was extracted with ethanol, and SCFA content was measured by HPLC using a YMC-Pack FA assay kit (YMC Co., Ltd., Kyoto, Japan).
Statistical analysis
Data are expressed as the mean ± SD. Statistical differences were calculated using SPSS software (Ver. 13.0 J, SPSS Japan). Data were analysed using one-way analysis of variance (ANOVA) followed by the Mann-Whitney U test. Differences were considered statistically significant at p-values <0.05.
RESULTS
Body weight changes
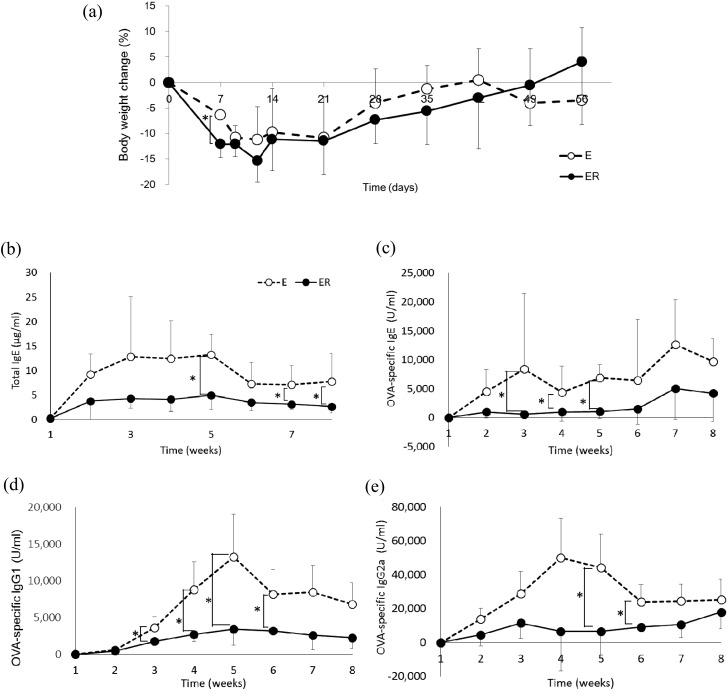
Changes in body weight after the eight-week feeding period are shown in Fig. 1a. The body weights of both the E and ER groups decreased after receiving the experimental diets, were lowest on day 12, and recovered thereafter. Body weight was significantly lower in the ER group than in the E group on day 7 (p<0.05); a significant difference in body weight between the groups was not observed at any other time point.
Fig. 1.
Body weight (a) and serum immunoglobulin levels (b, c, d, e) in OVA23-3 mice fed ovalbumin with or without RMD. (b) Total IgE, (c) OVA-specific IgE, (d) OVA specific IgG1, (e) OVA-specific IgG2a. Open circles indicate group E (egg white) and filled circles indicate group ER (egg white + RMD). Values are means ± SD (n=5–6). An asterisk indicates a significant difference between the two groups (p<0.05).
Serum immunoglobulin levels
Serum immunoglobulin levels are shown in Fig. 1b–e. The levels of total and OVA-specific IgE in the E group increased after administration of the experimental diet and was highly maintained during the experimental period. These antibodies were, however, maintained at lower levels in the ER group (Fig. 1b, c). Compared with the E group, total IgE levels of the ER group were significantly lower at weeks 5, 7, and 8, and OVA-specific IgE levels of the ER group were significantly lower from weeks 3 to 5. The titre of OVA-specific IgG1 and IgG2a in the ER group was also maintained at a lower level compared with that in the E group (Fig. 1d, e).
Histological analysis
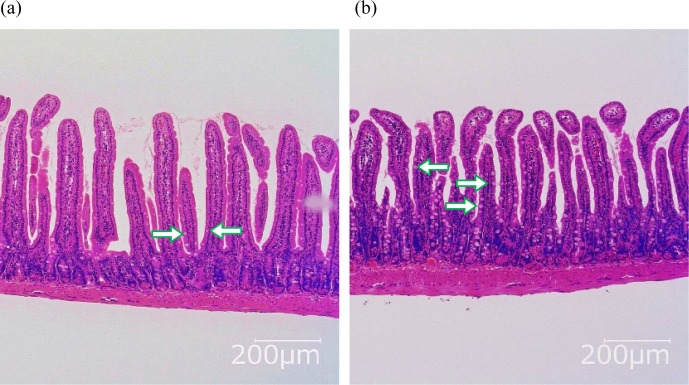
Representative images of HE-stained intestinal segments from each group of mice are shown in Fig. 2, and average evaluation scores of inflammatory condition are shown in Table 2. Villous atrophy was not observed in either group. Scores regarding hyperplasia of goblet cells were significantly higher in the ER group compared with those in the E group. Elongation and inflammation of crypts were mild, and the extent of cell infiltration in the lamina propria was mild to moderate; there was no significant difference between the groups.
Fig. 2.
Jejunal section of OVA23-3 mice fed ovalbumin with or without RMD for 9 days. (a) E, (b) ER.
The images are representative of each group (n=5–6). Arrows indicate goblet cells, and more goblet cells were observed in the villus of ER-fed mice.
Table 2. Morphological evaluation of jejunum in OVA23-3 mice fed ovalbumine with or without RMD for 9 days.
| E | ER | p-value | ||
|---|---|---|---|---|
| Findings | ||||
| Hyperplasia, goblet cell | 2.00 ± 0.00 | 2.83 ± 0.41* | 0.017 | |
| Elongation, crypt | 1.80 ± 0.45 | 1.83 ± 0.41 | 0.931 | |
| Inflammation, crypt | 1.60 ± 0.55 | 1.83 ± 0.41 | 0.537 | |
| Cell infiltration, lamina propria | 2.80 ± 0.45 | 3.00 ± 0.00 | 0.662 | |
| Total score | 8.20 ± 0.84 | 9.50 ± 1.22 | 0.052 | |
Evaluation scores for each item: 0, normal or no significant lesion; 1, minor alteration in a limited area; 2, multiple alteration in a limited area; 3, lesions in a broad area; 4, lesions in the whole area. Values are means ± SD (n=5–6). An asterisk indicates a significant difference between the two groups (p<0.05). E: egg white group; ER: egg white-RMD group.
Cytokine production
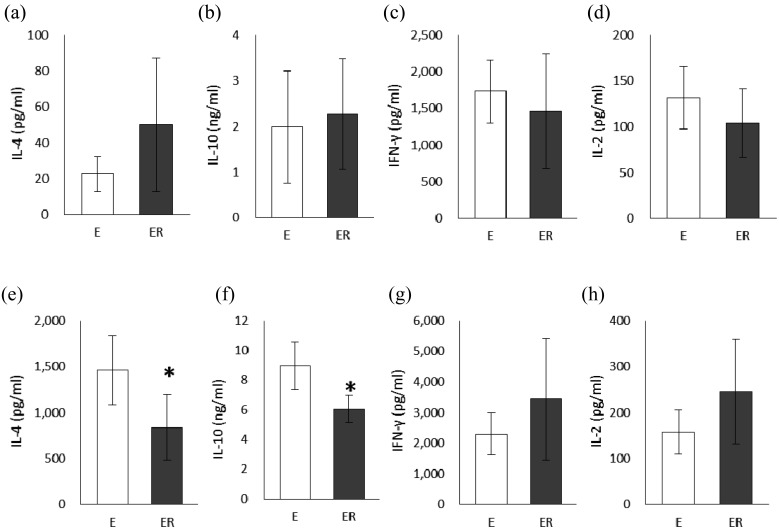
Cytokine levels in the supernatants from the culture of MLN cells or splenocyte are shown in Fig. 3, respectively. There was no significant difference between the E and ER groups in IL-4, IL-10, IFN-γ, or IL-2 production by MLN cells. On the other hand, IL-4 and IL-10 production by SPL cells from the ER group was significantly lower than that from the E group (Fig. 3e, f), although there was no significant difference between the groups in IFN-γ and IL-2 production (Fig. 3g, f).
Fig. 3.
Cytokine production from mesenteric lymph node cells (a–d) and splenocyte (e–h) in OVA23-3 mice fed ovalbumin with or without RMD for 9 days. (a, e) IL-4, (b, f) IL-10, (c, g) IFN-γ, (d, h) IL-2.
White bars indicate group E (egg white) and gray bars indicate group ER (egg white + RMD). Values are means ± SD (n=5–6). An asterisk indicates a significant difference between the two groups (p<0.05).
Concentration of caecal SCFA
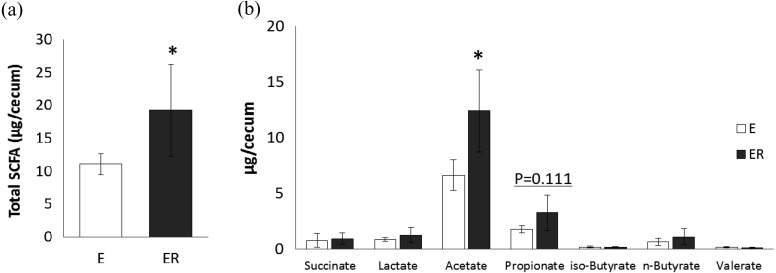
Figure 4 shows the SCFA content in the caecum of mice fed egg white, with or without RMD. Total SCFA content was significantly higher in the ER group than in the E group (Fig. 4a). Regardless of the experimental group, the most abundant SCFA in the caecum was acetate, followed by propionate and butyrate. The amount of acetate was significantly higher in the ER group than in the E group (Fig. 4b). Small amounts of succinate and lactate were found in both groups, with no significant difference between them.
Fig. 4.
SCFA contents in the cecum in OVA23-3 mice fed ovalbumin with or without RMD for 9 days. (a) Total SCFAs; (b) individual SCFAs. White bars indicate group E (egg white) and gray bars indicate group ER (egg white + RMD). Values are means ± SD (n=5–6). An asterisk indicates a significant difference between the two groups (p<0.05).
DISCUSSION
In the current study, the body weights of egg-white diet-fed OVA23-3 mice decreased until day 12 and recovered thereafter (Fig. 1a). There was no significant difference in body weight between the groups during the 8-week-long experimental period, except on day 7. Some dietary fibre caused transient diarrhea that led to weight loss. However, RMD did not cause diarrhea in mice when supplemented at a proportion of 7.5% in the diet. Although the reason for the temporal difference in body weight remains unclear, it is inferred that supplementation with RMD did not have a considerable effect on body weight during continuous feeding of egg white to OVA23-3 mice.
Since weight loss in OVA23-3 mice is a result of intestinal inflammation, we evaluated and compared the inflammatory status in the animals. In OVA23-3 mice, an egg-white diet for 7 to 10 days has been reported to cause hyperplasia of goblet cells, elongation of crypts, and inflammatory cell infiltration in the lamina propria, especially in the jejunum [11, 13]. Similar histological changes were observed in this study as well. Although the overall state of intestinal inflammation in both groups was not severe, more goblet cells were observed in ER-group mice compared with in E-group mice (Table 2). Goblet cells secrete mucin to protect the mucosa against foreign substances. Both allergens and soluble dietary fibre contribute to an increase in the number of goblet cells. It has been reported that the administration of soluble dietary fibre increased the number of goblet cells and mucin production, thereby enhancing the protective effect on the mucosa [14]. In the present study, RMD was shown to contribute to the increase in goblet cells, as a soluble dietary fibre, but RMD did not change the overall inflammatory status when ingested with egg white.
Adachi et al. proved that intestinal, but not systemic, immune responses to a dietary antigen are responsible for the induction of intestinal inflammation in OVA23-3 mice [11]. In this study, cytokine production from MLN cells was not changed by RMD in egg-white-fed OVA23-3 mice (Fig. 3a–d). As described above, RMD did not influence overall intestinal inflammatory status (Table 2). These results suggested that RMD does not modulate intestinal antigen-specific immune responses in the food allergy induced by feeding of egg white.
On the other hand, IL-4 and IL-10 production from splenocytes were significantly decreased by feeding of RMD in egg-white-fed OVA23-3 mice (Fig. 3e–h), indicating the suppression of systemic Th2 cytokine responses by RMD. It has been reported that the increase in serum levels of total and OVA-specific IgE and OVA-specific IgG subclass antibodies is elevated in OVA23-3 mice by feeding of an OVA-containing diet [12]. In this study, dietary RMD suppressed the high levels of total IgE and OVA-specific IgE, IgG1, and IgG2a titres in the serum that were increased by egg-white ingestion (Fig. 1b–e). The decrease in the serum levels of IgE and IgG1 caused by feeding of RMD might be due to modulation of splenic cytokine responses, since the Th2 cytokine IL-4 can induce IgE and IgG1 class-switching of B cells. However, the mechanisms underlying the downregulation of the serum level of OVA-specific IgG2a, a Th1-related IgG subclass, caused by feeding of RMD remain unclear, as splenic Th1 cytokine responses were not significantly changed (Fig. 3e–h). At minimum, our data suggested that dietary RMD has the potential to suppress excessive systemic production of antigen-specific antibodies during food allergy.
Dietary RMD causes an increase in SCFAs in the lower intestinal tract of animals and humans. RMD has been reported to significantly increase acetate and propionate in the faeces of healthy human subjects [4] and acetate, propionate, and butyrate in the caecum of rats [7, 15]. SCFAs are produced as metabolites of dietary fibre by intestinal bacteria. Various kinds of bacteria co-inhabit the intestine and form the intestinal flora, which contributes to SCFA production. SCFAs are reported to have immunomodulatory effects and to contribute to the maintenance of intestinal health. Bifidobacteria produce acetate that enhances the anti-inflammatory effect on intestinal epithelia and protects against Escherichia coli infection [16]. Clostridia produce butyrate that promotes regulatory T cells and suppresses colitis [17]. Trompette et al. reported that propionate is involved in the suppression of allergic airway disease onset [18]. SCFAs produced in the caecum penetrate the blood; the penetrated propionate inhibits Th2-type immune response through GPR41 signalling. Dietary RMD significantly increased the total amount of SCFA, especially acetate (p=0.032) and propionate (p=0.111), in the caecum of OVA23-3 mice fed egg white (Fig. 4). The results for SCFA production suggested that the suppressive effect of RMD on systemic Th2-type immune response might be attributed to GPR41 signalling, since the amount of propionate tended to be increased by RMD supplementation. Our study demonstrated that dietary RMD modulates systemic rather than intestinal antigen-specific immune responses in the food-allergic condition of OVA23-3 mice, which might result from increased production of SCFAs in the caecum.
There are many reports that explain the immunomodulatory properties of prebiotics through changes in the intestinal environment. Short-term administration of FOS has been reported to change intestinal immune response [19]. Genda et al. suggested that immunomodulatory effects of FOS result from increased gut permeability and mucosal inflammation due to the accumulation of lactate and succinate with a rapid decrease of pH in the caecum. On the other hand, RMD is known to be fermented relatively slower than FOS [20], indicating that fermentation properties of RMD are different from those of prebiotic oligosaccharides. In our experiments, we observed increased acetate and propionate levels, but not increased lactate and succinate levels, in the caecal content of mice fed RMD. Each prebiotics has different metabolites and a different rate of fermentation, which may lead to different immunomodulatory effects and mechanisms. Although this was an animal study and the relevant mechanism has yet to be investigated, this study suggested that RMD is favourable for alleviating food allergy through adjustment of systemic immunity.
Acknowledgments
We are grateful to Dr. Shuichi Kaminogawa for the insightful suggestions and encouragement. We are also grateful to Dr. Haruyo Nakajima-Adachi and Dr. Satoshi Hachimura for the support and advice at the early stage of this work.
References
- 1.Watanabe N, Suzuki M, Yamaguchi Y, Egashira Y. 2018. Effects of resistant maltodextrin on bowel movements: a systematic review and meta-analysis. Clin Exp Gastroenterol 11: 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Livesey G, Tagami H. 2009. Interventions to lower the glycemic response to carbohydrate foods with a low-viscosity fiber (resistant maltodextrin): meta-analysis of randomized controlled trials. Am J Clin Nutr 89: 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kishimoto Y, Oga H, Tagami H, Okuma K, Gordon DT. 2007. Suppressive effect of resistant maltodextrin on postprandial blood triacylglycerol elevation. Eur J Nutr 46: 133–138. [DOI] [PubMed] [Google Scholar]
- 4.Fastinger ND, Karr-Lilienthal LK, Spears JK, Swanson KS, Zinn KE, Nava GM, Ohkuma K, Kanahori S, Gordon DT, Fahey GC., Jr.2008. A novel resistant maltodextrin alters gastrointestinal tolerance factors, fecal characteristics, and fecal microbiota in healthy adult humans. J Am Coll Nutr 27: 356–366. [DOI] [PubMed] [Google Scholar]
- 5.Miyazato S, Kishimoto Y, Takahashi K, Kaminogawa S, Hosono A. 2016. Continuous intake of resistant maltodextrin enhanced intestinal immune response through changes in the intestinal environment in mice. Biosci Microbiota Food Health 35: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hira T, Ikee A, Kishimoto Y, Kanahori S, Hara H. 2015. Resistant maltodextrin promotes fasting glucagon-like peptide-1 secretion and production together with glucose tolerance in rats. Br J Nutr 114: 34–42. [DOI] [PubMed] [Google Scholar]
- 7.Miyazato S, Nakagawa C, Kishimoto Y, Tagami H, Hara H. 2010. Promotive effects of resistant maltodextrin on apparent absorption of calcium, magnesium, iron and zinc in rats. Eur J Nutr 49: 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Allergy Organization.2013. WAO White Book on Allergy 2013 Update. http://www.worldallergy.org/UserFiles/file/WhiteBook2-2013-v8.pdf (accessed 2018-02-23).
- 9.Mehta H, Ramesh M, Feuille E, Groetch M, Wang J. 2014. Growth comparison in children with and without food allergies in 2 different demographic populations. J Pediatr 165: 842–848. [DOI] [PubMed] [Google Scholar]
- 10.Sato T, Sasahara T, Nakamura Y, Osaki T, Hasegawa T, Tadakuma T, Arata Y, Kumagai Y, Katsuki M, Habu S. 1994. Naive T cells can mediate delayed-type hypersensitivity response in T cell receptor transgenic mice. Eur J Immunol 24: 1512–1516. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima-Adachi H, Kikuchi A, Fujimura Y, Shibahara K, Makino T, Goseki-Sone M, Kihara-Fujioka M, Nochi T, Kurashima Y, Igarashi O, Yamamoto M, Kunisawa J, Toda M, Kaminogawa S, Sato R, Kiyono H, Hachimura S. 2014. Peyer’s patches and mesenteric lymph nodes cooperatively promote enteropathy in a mouse model of food allergy. PLoS One 9: e107492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shida K, Hachimura S, Ametani A, Ishimori M, Ling M, Hashiguchi M, Ueda Y, Sato T, Kumagai Y, Takamizawa K, Habu S, Kaminogawa S. 2000. Serum IgE response to orally ingested antigen: a novel IgE response model with allergen-specific T-cell receptor transgenic mice. J Allergy Clin Immunol 105: 788–795. [DOI] [PubMed] [Google Scholar]
- 13.Nakajima-Adachi H, Ebihara A, Kikuchi A, Ishida T, Sasaki K, Hirano K, Watanabe H, Asai K, Takahashi Y, Kanamori Y, Shimojo N, Matsuda H, Kohno Y, Hachimura S, Kaminogawa S. 2006. Food antigen causes TH2-dependent enteropathy followed by tissue repair in T-cell receptor transgenic mice. J Allergy Clin Immunol 117: 1125–1132. [DOI] [PubMed] [Google Scholar]
- 14.Hino S, Takemura N, Sonoyama K, Morita A, Kawagishi H, Aoe S, Morita T. 2012. Small intestinal goblet cell proliferation induced by ingestion of soluble and insoluble dietary fiber is characterized by an increase in sialylated mucins in rats. J Nutr 142: 1429–1436. [DOI] [PubMed] [Google Scholar]
- 15.Kishimoto Y, Wakabayashi S, Takeda H. 1995. Hypocholesterolemic effect of dietary fiber: relation to intestinal fermentation and bile acid excretion. J Nutr Sci Vitaminol (Tokyo) 41: 151–161. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, Taylor TD, Itoh K, Kikuchi J, Morita H, Hattori M, Ohno H. 2011. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469: 543–547. [DOI] [PubMed] [Google Scholar]
- 17.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. 2013. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504: 446–450. [DOI] [PubMed] [Google Scholar]
- 18.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ. 2014. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20: 159–166. [DOI] [PubMed] [Google Scholar]
- 19.Genda T, Sasaki Y, Kondo T, Hino S, Nishimura N, Tsukahara T, Sonoyama K, Morita T. 2017. Fructo-oligosaccharide-induced transient increases in cecal immunoglobulin A concentrations in rats are associated with mucosal inflammation in response to increased gut permeability. J Nutr 147: 1900–1908. [DOI] [PubMed] [Google Scholar]
- 20.Flickinger EA, Wolf BW, Garleb KA, Chow J, Leyer GJ, Johns PW, Fahey GC., Jr2000. Glucose-based oligosaccharides exhibit different in vitro fermentation patterns and affect in vivo apparent nutrient digestibility and microbial populations in dogs. J Nutr 130: 1267–1273. [DOI] [PubMed] [Google Scholar]