Abstract
Nonalcoholic fatty liver disease (NAFLD) is a hepatic manifestation of metabolic syndrome. Its prevalence increases with increasing rates of obesity, insulin resistance, and diabetes mellitus. The pathogenesis of NAFLD involves many factors, including the gastrointestinal microbiota. However, there is still debate about the impact of gut dysbiosis in the NAFLD disease progression. Therefore, this paper aims to review the relationship between gut microbiota and other risk factors for NAFLD and how gut dysbiosis plays a role in the pathogenesis of NAFLD. Hopefully, this paper can make an appropriate contribution to the development of NAFLD research in the future.
Keywords: gut microbiota, dysbiosis, NAFLD, inflammatory pathway, metabolic pathway
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a hepatic manifestation of metabolic syndrome [1, 2]. It can be classified as nonalcoholic fatty liver (NAFL) or nonalcoholic steatohepatitis (NASH). The spectrum of NAFLD disease itself includes simple hepatic steatosis, NASH, fibrosis, cirrhosis, and subsequent complications such as hepatocellular carcinoma (HCC) [1]. Clinically NAFLD does not have symptoms, and it is often diagnosed inceidentally when liver enzyme abnormalities are found or imaging id performed [2]. In most patients, NAFLD is associated with comorbidities such as obesity, insulin resistance, diabetes mellitus, and dyslipidemia [1]. Nonalcoholic fatty liver disease has also been found to be a risk factor for extrahepatic diseases such as cardiovascular disease and chronic kidney disease [3].
A meta-analysis found that the global prevalence of NAFLD, based on diagnostic imaging, is 25.24% (95% CI: 22.10–28.65%), with the highest prevalence in the Middle East and South America and the lowest prevalence in Africa. NAFLD is the leading chronic liver disease in the Asian region. The prevalence of NAFLD in Asia is lower than that in the West, but it has shown a tendency to increase due to increasing cases of obesity, diabetes mellitus, and metabolic syndrome resulting from changes in lifestyles. The prevalence of NAFLD in Asia varies between 15 and 20% and is affected by location (urban versus rural area), gender, ethnicity, and age [4].
Theories regarding the pathogenesis of NAFLD have been developed, from the emergence of the two-hits hypothesis to the latest multiple-hits hypothesis. The two-hits hypothesis explains that initially there was a first hit in the form of triglycerides accumulation in the liver (hepatic steatosis) due to sedentary lifestyles, a high-fat diet, insulin resistance, and obesity. This triglyceride accumulation then increases the liver susceptibility to a second hit second-hit, namely lipotoxicity from free fatty acids (FFA) influx, which then activates the pro-inflammatory cytokine cascade, promotes oxidative stress, and triggers the fibrogenesis pathway that leads to its severe phenotypes. The two-hits hypothesis is no longer considered valid because it cannot explain the role of nutrition, gut microbiota, hormones of adipose tissue, and insulin resistance that runs parallel in individuals who have a genetic predisposition to develop NAFLD disease. In the multiple-hits theory, it is postulated that genetic factors, diet, and environment can cause dysbiosis of the gut microbiota, insulin resistance, and obesity, which simultaneously promotes the occurrence of NAFLD [5, 6]. These risk factors could be reciprocally related in some manner.
The mechanisms of NAFLD have been widely researched and it involved various pathways. The gut microbiota is a part of one of the pathways and is now being targeted for NAFLD therapeutical strategies. The gut microbiota is interconnected with diet, obesity, and insulin resistance. Disruption of the gastrointestinal microbiota composition plays a role in increasing the production of FFA in the intestine and increasing intestinal permeability [5, 7]. Studies in animals have shown that germ-free mice given a diet high in fat and sugar have lower body weights than conventional mice. Germ-free mice have higher insulin sensitivity, and after undergoing microbiota transplantation from conventional mice, they exhibit an increased adipose tissue mass. In mice that received the obesogenic diet, administration of antibiotics can improve insulin resistance independently of food intake and levels of adipose tissue. In studies with other mice, administration of antibiotics correlated with lower lipogenesis and hepatic steatosis [8]. Meanwhile, studies in humans have found that transfer of gut microbiota from thin donors to recipients with metabolic syndrome results in increased insulin sensitivity [7].
DYSBIOSIS OF THE GUT MICROBIOTA IN NAFLD
The liver gets most of its blood flow (70%) from the intestinal vascularization, so it is constantly exposed to nutrients, toxins, and antigens from food; to the products of the gut microbiota; and to the microbiota itself [6, 9]. Meanwhile, the gastrointestinal tract receives a liver product in the form of bile acid, which is channeled through the biliary duct [10]. This functional bidirectional relationship between the liver and gastrointestinal tract is known as the gut-liver axis (GLA) [6, 9]. The GLA is composed of complex components. Because of this close anatomical and functional relationship, changes in one component such as the gut barrier or gut microbiota can affect the condition of the liver [10]. The relationship between the gut microbiota and liver disease has long been known. Older studies on the rat model of NASH showed that the risk of disease developing into cirrhosis decreased with the provision of nonabsorbable antibiotics [11]. Other studies in humans with hepatic steatosis indicate that the condition of small intestinal bacterial overgrowth (SIBO) can be suppressed by administration of antibiotics [12].
In general, the gastrointestinal microbiota consists of 3 domains of life: Bacteria, Archaea, and Eukarya [13]. The human gastrointestinal microbiota has a fairly high variety of bacteria species—around 200 dominant species and 1,000 nondominant species—and they are varying between individuals [7]. Anaerobic bacteria dominate the gastrointestinal microbiota population. More than 90% of the gastrointestinal microbiota are part of the phyla Firmicutes (gram positive) and Bacteroidetes (gram negative) [13], followed by Actinobacteria (gram positive) and Proteobacteria (gram negative). Firmicutes produces butyrate as its main metabolite, while Bacteroidetes produce acetate and propionate [14]. The gastrointestinal microbiota generally plays a role in suppressing the growth of pathogenic microorganisms, constantly educating the gastrointestinal immune system, regulating intestinal hormone production, and producing neurotransmitters for gastrointestinal innervation. Also, the gastrointestinal microbiota plays a role in the production of vitamin K and B, and short-chain fatty acids (SCFAs) [13]. Normally, a small portion of the gut microbiota can reach the liver but will be eliminated by Kupffer cells [10]. In the case of disturbances of the gut barrier and the composition of the gut microbiota, the amount of gut microbiota and its products reaching the liver will be greater and potentially cause tissue damage in the liver.
The composition of the gut microbiota is specific for each person [10]. Dysbiosis can be interpreted as a relative change in the composition of an individual’s commensal microbiota compared with others in the community [15]. Dysbiosis can also be defined as a disturbance in the composition of microbiota, which can be loss of beneficial microbiota, increased pathogenic microbiota, or decreased microbiota variety [9, 15]. A previous study found that the gut microbiota of NAFLD patients has reduced diversity compared with that of healthy individuals [16]. Under dysbiotic conditions, the gut microbiota is unable to maintain its role in local homeostasis, and thus disruption in intestinal barrier occurs [17].
Humans and animal model studies have suggested an association between dysbiosis of gut microbiota and NAFLD [20]. In the general population of NAFLD patients, there is an increase in Proteobacteria, Enterobacteriaceae, Lachnospiraceae, Escherichia, and Bacteroidetes—though some studies found a reduction or no change in Bacteroidetes [18, 19], and also a decrease in Prevotella and Firmicutes [20, 21]. Da Silva et al. found a decrease in some groups of gut microbiota, one of which were comprised of Firmicutes and Bacteroidetes phyla, and an increase in another group of microbiota in patients with simple steatosis and NASH compared with healthy controls. The study suggested that a specific gut microbiota community may have a potential role in the pathogenesis of NAFLD [22]. A study by Sobhonslidsuk found an increase in the Bacteroidetes/Firmicutes ratio in NASH patients independent of diabetes risk factors or metformin use [23]. A study by Boursier et al. tated that the degree of NAFLD is related to microbiota dysbiosis and changes in the metabolic function of the gastrointestinal microbiota. Bacteroidetes was independently associated with NASH, and Ruminococcus was associated with significant fibrosis [24].
An investigation of the association of metabolic risk factors with the gut microbiota in patients with type 2 diabetes revealed a decrease in the number of butyrate-producing bacteria (Clostridium sp., Eubacterium rectale, Faecalibacterium prausnitzii, Roseburia intestinalis, Roseburia inulinivorans) and an increase in sulfate-lowering bacteria (Desulfovibrio, Lactobacillus gasseri, Lactobacillus reuteri, Lactobacillus plantarum) [23]. Alteration in the Firmicutes/Bacteroidetes ratio has also been reported in individuals with obesity and impaired glucose metabolism [13]. Other studies in populations without obesity have found that there was an increase in Bacteroidetes and a decrease in Firmicutes in patients with NAFLD compared with non-NAFLD patients [25]. These studies suggested that the gut microbiota has a role in NAFLD as well as metabolic syndrome development.
PATHOGENESIS OF NAFLD
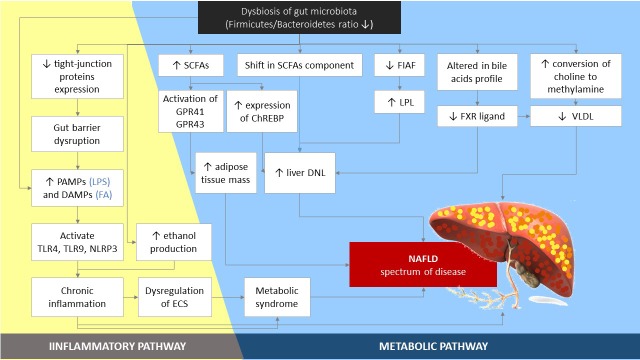
Studies show that the gut microbiota contributes to NAFLD through various mechanisms, including (1) regulation of energy homeostasis via carbohydrate fermentation SCFAs which then induces DNL in the liver; (2) modulation of the endocannabinoid system; (3) modulation of choline metabolism needed for the synthesis of very-low-density lipoprotein (VLDL) and liver lipid export; (4) modulation of bile acid homeostasis; (5) endogenous ethanol formation; and (6) increase of lipopolysaccharide (LPS), which activates the production of pro-inflammatory cytokines in liver macrophages, causing inflammation of hepatocytes (Fig. 1) [10, 26].
Fig. 1.
The role of gut microbiota in the pathogenesis of NAFLD. Dysbiosis of gut microbiota leads to low-grade chronic inflammation. The inflammatory condition itself can also be caused by an increment in ethanol production resulting from gut microbiota activity. The inflammatory condition predisposes individuals to development of metabolic syndrome features and NAFLD. Based on metabolic pathway, an increase in SCFAs and alteration of their components give rise to an increase in adipose tissue and liver DNL. Liver DNL is also caused by suppression of FIAF, enhancing LPL activity. Dysbiosis alters the bile acids profile and lowers FXR signaling causing an increase in liver DNL. Choline conversion to methylamine increases, decreasing VLDL synthesis, and this factor predisposes individuals to the development of fatty liver. PAMPs: pathogen-associated molecular patterns; DAMPs: damage-associated molecular patterns; LPS: lipopolysaccharides; FA: fatty acid; TLR: Toll-like receptor; NLRP-3: nucleotide-binding domain and leucine-rich repeat pyrin 3 domain; ECS: endocannabinoid system; SCFAs: short-chain fatty acids; ChREBP: carbohydrate response element binding protein; DNL: de novo lipogenesis; FIAF: fasting-induced adipose factor; LPL: lipoprotein lipase; FXR: farnesoid X receptor; VLDL: very-low-density lipoprotein; NAFLD: nonalcoholic fatty liver disease.
Dysregulation in production of SCFAs
Obesogenic food sources—namely high-fat diet, high fructose intake, high-calorie diet, and high meal frequency [6]—greatly affect the composition of the gastrointestinal microbiota and integrity of the intestinal wall [14, 26], especially when they are a long-term habit [6]. The composition of the gut microbiota related to a high-fat diet is mainly gram-negative bacteria, which in turn increases LPS levels [14]. The NASH patients generally experience SIBO that interferes with the integrity of the gastrointestinal wall [26]. Small intestinal bacterial overgrowth is defined as increase in the number and/or changes in the composition of the microbiota of the proximal gastrointestinal tract [27]. A dysfunctional gut barrier makes it easier for microbiota products including LPS and intraluminal bacterial to translocate into the enterohepatic circulation [7]. Obesogenic food sources per se trigger an intestinal inflammatory response which causes intestinal tight junctions to be impaired, increasing permeability. Apart from food intermediaries, some microbiota can also reduce the genes for expression of tight junction proteins such as zonula occludens (ZO-1) that interfere with the integrity of the intestinal wall [14].
NAFLD patients have also been shown to have higher SCFA levels and SCFA-producing bacteria [18] and dysbiosis of gut microbiota can cause abnormalities of SCFA components [28]. Short-chain fatty acids are useful as an energy source and anti-inflammatory, angiogenic, and vasodilator agent; promotility agent; and wound healing agent [18]. SCFAs, such as butyrate, propionate, and acetate, are metabolites of oligo-fermentation and fermentation of specific polysaccharides (complex or indigestible carbohydrates) by the gut microbiota that are absorbed in the distal ileum and colon [29]. Butyrate is produced mainly by Firmicutes, while acetate and propionate are predominant products of Bacteroidetes. Faecalibacterium prausnitzii is the most representative of the Firmicutes phylum [14], and treatment of F. prausnitzii in mice was shown to improves hepatic fat content [30].
Butyrate is the main energy source of enterocytes. It affects both insulin sensitivity and energy balance [16]. Butyrate is considered anti-obesogenic [10], and its level has been found to be lower than those of acetate and propionate in patients with NAFLD [16]. Butyrate supplementation in obese mice helps improve the integrity of the gastrointestinal wall and improves insulin secretion from beta cells, decreasing the amount of adipose tissue in the body, and lack of butyrate causes decreased gut integrity [28]. Meanwhile, acetate and propionate function as substrates of gluconeogenesis and lipogenesis in the liver [13]. Increased gluconeogenesis and lipogenesis due to increased acetate and propionate production may lead to NAFLD and obesity and subsequently insulin resistance and type 2 diabetes mellitus. In turn, obesity, insulin resistance, and type 2 diabetes mellitus also have a reciprocal impact on the pathogenesis of NAFLD [28]. Additionally, some obesity-related gut microbiota have a greater capacity to extract energy from food [7], as they can digest other polysaccharides other than complex polysaccharides into SCFA, means that more energy is extracted from the diet [6].
In addition to SCFA abnormalities, increased SCFA levels themselves can stimulate G-protein coupled receptors (GPRs), and carbohydrate response element binding protein (ChREBP) expression. The SCFAs act as a ligand for GPR43 and GPR41, which are expressed in adipocyte, endocrine, and immune cells. Activation of GPR43 by SCFAs leads to inhibition of lipolysis and adipocyte differentiation and hence an increase in hence tissue mass [31]. Monosaccharides from microbial fermentation activate the hepatic ChREBP and thereby increase the proteins involved in liver lipogenesis [27].
Dysregulation of appetite signaling
Our central nervous system constantly responds to neural and chemical signals from the gut while maintaining food-reward homeostasis [32]. The gut microbiota can alter appetite via through metabolites of food conversion such as SCFAs, γ-aminobutyric acid (GABA), serotonin (5-HT), and other neurotransmitters (NTs). These metabolites affect central regulation directly via vagal stimulation or indirectly through immune-neuroendocrine mechanisms [6, 33].
The metabolites of gut microbiota activate enteroendocrine L cells to produce gut hormones such as glucagon-like peptide-1 (GLP-1), peptide YY (PYY), and cholecystokinin (CCK). These gut hormones then transmit signals through the vagus nerve and blood circulation to the nucleus tractus solitarius (NTS) in the brain. Information from the NTS is further transmitted to the appetite and energy balance center in the arcuate nucleus (ARC) of the hypothalamus, where neuropeptide Y (NPY), agouti-related protein (AgRP), anorexigenic peptides, cocaine-amphetamine-regulated transcript (CART), and pro-opiomelanocortin (POMC) neurons lie [33, 34]. The binding of SCFAs to GPRs can also trigger the release of PYY, which modulates intestinal motility and nutrient absorption [27].
Besides the gut microbiota’s metabolites, some strains of bacteria can regulate appetite via the hypothalamus neuroendocrine pathway [34]. Although it was previously mentioned that propionate is a substrate for lipogenesis in the liver, a study by Chambers et al. found that propionate can stimulate the release of PYY and GLP-1, which means that it can reduce energy intake. Long-term administration of colonic propionate can prevent the gaining of more weights in overweight adults and significantly reduce liver fat content in NAFLD patients [35]. Another study found that propionate positively attenuates appetite independent from plasma PYY and GLP-1 [39]. Acetate, as the main SCFAs produced by gut microbiota, has also been found to be an appetite suppressan [36]; however, another study showed that acetate promotes hyperphagia, obesity, and related sequelae [37]. The lowering of appetite may be balanced by a proportional increase in energy harvesting as the Firmicute/Bacteroidetes ratio decreases in patients with NAFLD [38]. Studies of the gut-brain axis involvement in the pathogenesis of NAFLD have reported inconsistent results, and this still needs further research.
Suppression of fasting-induced adipose factor (FIAF)
In addition to promoting the absorption of monosaccharides in the gastrointestinal tract and accelerating de novo lipogenesis (DNL), the gut microbiota can also suppress the fasting-induced adipocyte factor (FIAF) produced by intestinal cells, which causes an increase of lipoprotein lipase (LPL) activity, activation of sterol regulatory element-binding protein 1 (SREBP-1), and also ChREBP [38]. Increased LPL activity causes an increase in FFA uptake in adipose tissue and the liver and hence induces accumulation of triglycerides in the liver [7, 27, 39].
Modulation of the endocannabinoid system (ECS)
The endocannabinoid system (ECS) is a lipid mediator comprising of cannabinoid (CB) receptors which are present in the brain and peripheral tissue including the liver, and this system is activated in a variety of liver diseases by various underlying etiologies. It has a role in proliferation, differentiation, and secretion of adipocyte hormone. The CB receptors are the same receptors which mediates marijuana [40]. Active marijuana use was shown to give protective effects against NAFLD independent of metabolic risk factors [41].
Cannabinoid receptor 1 (CB)-1 is predominant in the brain and plays a role in psychotropic effects of cannabinoids. Meanwhile, CB-2 presents mainly in peripheral tissue and plays a role in the modulation of innate immunity and bone mass. In the liver, CB-1 presents in hepatocytes and endothelial cells, while CB-2 is detected in Kupffer cells [42].
The ECS is proposed to be important because of its association with obesity and LPS. Endocannabinoid levels are found to be increased in obese individuals [26], while dysregulation in the ECS has been reported to be associated with dysbiosis of gut microbiota [43]. Both in vivo and in vitro studies has reported that the ECS controls gut barrier function via a CB1-dependent mechanism [44]. One animal study showed that gut microbiota influence the ECS, which in turn regulates gut permeability. LPS then increases along with the increase in gut permeability. The LPS itself, which is involved in creating inflammatory conditions, has also been found to control adipose tissue metabolism through cannabinoid-driven adipogenesis [26, 45]. In addition, the change in LPS leads to the production of endogenous ligands for CB, such as anandamine (AEA), from adipose tissue macrophages, which contributes to insulin resistance [43]. The CB stimulation causes inhibition in adipokine secretion, especially adiponectin, and affects the onset of metabolic syndrome–which predisposes individuals to NAFLD [26]. Stimulation of CB also promotes energy preservation and induces obesity when excess energy sources are present [46]. The relation between ECS and dysbiosis of gut microbiota is also represented by the reduction of AEA in rats caused by administration of prebiotics [45]. The first evidence of the relationship between ECS and specific gut microbiota was shown in a study by Rousseaux et al., in which the administration of a Lactobacillus acidophilus strain modulated CB receptors expression in the intestinal cells of rats [47]. Specific bacteria, namely Akkermansia muciniphila, were suspected to improve the functionality of intestinal wall tight junctions by inducing production of several bioactive lipids known as “gatekeepers”, such as N-palmitoylethanolamine (PEA), 2-arachidonoylglycerol (2-AG), 2-oleoylglycerol (2-OG), 2-palmitoylglycerol (2-PG), and glycerol ester of prostaglandin D2 (PGD2-G) [48].
Thus, therapies targetting dysbiosis as well as CB receptors stimulation seems promising. Inhibition of CB-1 has been shown to have beneficial effects, such as increased energy expenditure, that may improve metabolic syndrome [49], give rise to resistance against diet-induced obesity, inhibit de novo lipogenesis [50], reduce hepatic TNF-α, reduce insulin resistance, delay the progression of steatosis into fibrosis and cirrhosis (via antagonism by Rimonabant) [51], and even reverse hepatic steatosis [52, 53]. Meanwhile, studies on stimulation and inhibition of CB-2 were inconsistent [42].
Modulation of choline metabolism
Choline deficiency is known to cause chronic liver disease [7]. Exogenous choline can be obtained from dairy products, meat, fish, wheat, soybeans, and beans, while endogenous choline in the form of phosphatidylcholine is obtained from bile fatty acids, exfoliated epithelial cells, and gastrointestinal microbiota [27]. Choline is one of the phospholipids that make up cell membranes and precursors of the neurotransmitter acetylcholine. Choline also has an important role in the synthesis of VLDL and lipid transport in the liver, thus preventing fat accumulation [6, 39].
Enzymes produced by the gut microbiota—which are changed by a high-fat diet [7]—act as a catalyst for the conversion of dietary choline into toxic metabolites, namely dimethylamine and trimethylamine, which are subsequently converted in the liver into trimethylamine oxide (TMAO). TMAO is a substance that contributes to the inflammation of hepatocytes and progression of NAFLD into NASH. Both the microbiota and and a low choline diet are factors associated with the incidence of hepatic steatosis and NAFLD [6, 39]. The increased conversion of choline to methylamine also means that the levels of phosphatidylcholine, which play a role in VLDL synthesis will be reduced thus maintain the fat accumulation in liver [7].
Modulation of bile acid metabolism
Bile acids are important molecules that play a role in activating various pathways regulating lipid metabolism, carbohydrate metabolism, and inflammatory responses. Bile acids facilitate the absorption of intestinal lipid and lipid-soluble vitamins [54]. Besides, bile acids also maintain the integrity of the intestinal wall, thus preventing gastrointestinal bacteria translocation. Obesogenic food sources can also influence the composition of bile acids, which further affect the gut microbiota composition [6]. Bile acids have strong antimicrobial activity, as they contain immunoglobulin A (IgA) antibodies produced by Peyer’s patches of the gallbladder submucosal which can modulate the load of gut microbiota [10]. In turn, the gut microbiota can also affect bile acid metabolism and IgA content by binding to certain receptors [6, 10]. Bile acid deficiency is associated with inflammation and microbiota overgrowth [54].
Primary bile acids are synthesized in the liver from cholesterol and absorbed into the enterohepatic circulation in the distal ileum. The gut microbiota metabolizes primary bile acids into conjugated or secondary bile acids which later bind to various signaling molecules. Primary bile acids act as a ligands for the nuclear farnesoid X receptor (FXR) [6, 39] and have a high affinity for FXR [2]. FXR is widely expressed in the liver and intestine, and it maintains control of liver de novo lipogenesis (DNL), export of VLDL, as well as plasma triglyceride turnover [27]. Meanwhile, secondary bile acids act as signaling molecules for the Takeda G-protein-coupled bile acid receptor (TGR5) [6, 27, 39].
Dysbiosis of gut microbiota can lead to hepatic fat accumulation via the FXR signaling pathway. The bond between conjugated bile acid and FXR inhibits gluconeogenesis and glycogenolysis in the liver, and it increases insulin sensitivity in adipose tissue and skeletal muscle [6]. Studies in fatty liver mice show that changes in bile acid composition due to antibiotics can cause inhibition of FXR signaling, so triglyceride accumulation in the liver is reduced [55, 56]. Meanwhile, activation of TGR increases the GLP-1 secretion from L-cells, enteroendocrine cells, which protects against obesity [13, 33]. Through this signaling pathway, bile acids can not only regulate the synthesis of bile acids themselves but can also affect fat and glucose homeostasis in the liver [27].
Endogenous ethanol formation
Alcohol can induce triglyceride accumulation in the liver. It acts as a “second hit” which increases the susceptibility of the liver to progressing from steatosis to steatohepatitis [7]. Plasma ethanol levels have been found to be higher in NASH patients [34]. The gut microbiota produces some hepatotoxic substances, such as ethanol, phenol, and ammonia. All three of these hepatotoxic substances can stimulate Kupffer cells to produce nitric oxide (NO) and cytokines such as tumor necrosis factor-α (TNF-α). A high-fat diet is also thought to play a role in increasing the number of Escherichia genus members of the Proteobacteria phylum [34], alcohol-producing bacteria, in NAFLD patients. Ethanol undergoes the oxidation process to produce acetate and acetaldehyde as its main metabolites. Acetate is the substrate for fatty acids synthesis, while acetaldehyde can induce the formation of reactive oxygen species (ROS) [6, 27].
Inflammatory pathways
The molecular pathway of NAFLD development and progression is considered as a complex mechanism [5]. Low-grade chronic inflammations are considered to be the primary factor of NAFLD and to be pathogenetically associated with obesity that occurs due to continuous stimulation of innate immune responses by LPS via the gut-liver axis [4, 57]. Proteobacteria members are said to comprise to predominant population that expresses endotoxin (LPS) [6].
The gastrointestinal microbiota communicates with host cells via activation of pattern recognition receptors (PRRs) [23]. PRRs are membrane proteins that are widely expressed in innate immune system cells to detect pathogen-associated molecular patterns (PAMPs) and damage-associated molecular pattern (DAMPs). There are several types of PRRs, including a membrane-bound type that includes PRRs such as the Toll-like receptors (TLRs), as well as a cytosolic type that includes PRRs such as NOD-like receptors (NLRs) [58]. In the liver, TLR is mostly expressed by Kupffer cells, for example, TLR-4 and TLR- 9 [2]. However, in addition to Kupffer cells, TLR is also found in stellate cells and hepatocytes [59].
The activation of TLR is key in the development of NAFLD [17]. The term DAMPs refers to endogenous molecules released by injured cells—in this case, fatty acids—which induce oxidative stress in hepatocytes, while PAMPs refer to LPS and other gut microbiota products such as bacterial’s LPS, peptidoglycan, and DNA [17, 60]. LPS is a triggering factor for insulin resistance in adipose tissue [54]. LPS is the component of gram-negative bacteria which is currently the most studied PAMP in the pathogenesis of NAFLD [6]. The DAMPs and PAMPs trigger activation of liver cells both directly and indirectly through their binding with TLR or NLR expressed by Kupffer cells, stellate cells, and hepatocytes. Activation of Kupffer cells by the binding of LPS with TLR4 induces an inflammatory cascade and release of massive amount of IL-1β and TNF-α [17, 60].
Meanwhile, NLR proteins (NLRPs) are cytoplasmic PRRs and are part of inflammasomes—cytoplasmic multiprotein complexes—that play a role in caspase 1 activation and the breakdown of IL-1β and IL-18 pre-cytokines into their active forms [27, 60]. NLRP-3 is an inflammasomes specifically involved in NAFLD development towards fibrosis [17, 61]. A study using high-fat diet mice showed that inhibition of NLRP-3 inflammasome signaling leads to improvement of liver steatosis [62]. It is suggested that activation of PRRs due to dysbiosis of gut microbiota and gut barrier disruption events, in essence, will trigger the production of cytokines such as IL-1β, IL-6, and TNF-α [5] and the process and recruitment of acute inflammatory cells that leads to a more severe NAFLD spectrum [27]. Overexpression of TNF-α is considered an inflammatory hallmark in the progression of NAFLD and obesity. TNF-α acts as an antagonist of an anti-inflammatory cytokines, adiponectin, which plays a role in sensitizing cells to insulin. It also activates the intracellular inflammatory pathway of nuclear factor-kappa B (NF-κB) which regulates inflammation, metabolism, cell viability, and production of various cytokines. Both TNF-α and NF-κB are activated, and together they promote insulin resistance and are involved in liver inflammation [59]. Aside from cytokine release, TLR activation also induces upregulation of the NF-κB intracellular inflammatory pathway, c-Jun N-terminal kinase (JNK), and macrophages that cause insulin resistance and obesity [14]. The existence of insulin resistance and obesity means that the more free lipid metabolites are produced by the body and more pro-inflammatory cytokines are circulating. Pro-inflammatory cytokines along with lipotoxicity factors contribute to promoting inflammation, apoptosis, and fibrosis in hepatocytes [5].
Besides binding with TLRs, free fatty acids which reach the liver also disrupt the fluidity of the endoplasmic reticulum (ER) membrane, an organelle that plays a role in protein synthesis and protein folding. Dysfunction of ER results in dysfunctional proteins production. When greater amounts of dysfunctional protein are produced than can be overcome by the cell degradation mechanism, cell apoptosis ensues [59]. Excessive FFA triggers mitochondrial dysfunction and damage to lysosomes, producing oxidative stress which activates NLRPs and results in an inflammatory response. ROS will induce lipid peroxidation and activate stellate cells, inducing fibrosis, and inhibit liver VLDL secretion, inducing steatosis [2]. These events lead to progression of simple steatosis into its more severe phenotypes.
In sum, the gut microbiota products activate inflammasome pathways in the liver, triggering liver injury and deterioration of NAFLD condition [6]. Disruption of the gut barrier together with dysbiosis of gut microbiota is correlated with the severity of steatosis and fibrosis condition [63]. A high-fat diet also tends to increase gram-negative microbiota, causing more LPS to be present [7], while SIBO is known to induce TLR4 expression and release of the pro-inflammatory cytokine IL-8 in the liver [39].
CONCLUSION
NAFLD is an implication of many risk factors, most notably metabolic syndrome, which are not related to dysbiosis of the gut microbiota. This review illustrates there are many mechanisms related to dysbiosis that contribute to the formation and development of NAFLD. Lifestyle modification is now the basis of the primary therapy for NAFLD, but mitigation of dysbiosis of the gastrointestinal microbiota can be considered as a target for further research on more comprehensive NAFLD therapies.
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. 2018. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67: 328–357. [DOI] [PubMed] [Google Scholar]
- 2.Lucas C, Lucas G, Lucas N, Krzowska-Firych J, Tomasiewicz K. 2018. A systematic review of the present and future of non-alcoholic fatty liver disease. Clin Exp Hepatol 4: 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong MJ, Adams LA, Canbay A, Syn WK. 2014. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology 59: 1174–1197. [DOI] [PubMed] [Google Scholar]
- 4.Ashtari S, Pourhoseingholi MA, Zali MR. 2015. Non-alcohol fatty liver disease in Asia: prevention and planning. World J Hepatol 7: 1788–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buzzetti E, Pinzani M, Tsochatzis EA. 2016. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 65: 1038–1048. [DOI] [PubMed] [Google Scholar]
- 6.Mazzotti A, Caletti MT, Sasdelli AS, Brodosi L, Marchesini G. 2016. Pathophysiology of nonalcoholic fatty liver disease: lifestyle-gut-gene interaction. Dig Dis 34Suppl 1: 3–10. [DOI] [PubMed] [Google Scholar]
- 7.Abdul-Hai A, Abdallah A, Malnick SD. 2015. Influence of gut bacteria on development and progression of non-alcoholic fatty liver disease. World J Hepatol 7: 1679–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Membrez M, Blancher F, Jaquet M, Bibiloni R, Cani PD, Burcelin RG, Corthesy I, Macé K, Chou CJ. 2008. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. FASEB J 22: 2416–2426. [DOI] [PubMed] [Google Scholar]
- 9.García-Castillo V, Sanhueza E, McNerney E, Onate SA, García A. 2016. Microbiota dysbiosis: a new piece in the understanding of the carcinogenesis puzzle. J Med Microbiol 65: 1347–1362. [DOI] [PubMed] [Google Scholar]
- 10.Poeta M, Pierri L, Vajro P. 2017. Gut-liver axis derangement in non-alcoholic fatty liver disease. Children, Basel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell WR, Hoyles L, Flint HJ, Dumas ME. 2013. Colonic bacterial metabolites and human health. Curr Opin Microbiol 16: 246–254. [DOI] [PubMed] [Google Scholar]
- 12.Drenick EJ, Fisler J, Johnson D. 1982. Hepatic steatosis after intestinal bypass—prevention and reversal by metronidazole, irrespective of protein-calorie malnutrition. Gastroenterology 82: 535–548. [PubMed] [Google Scholar]
- 13.Allin KH, Nielsen T, Pedersen O. 2015. Mechanisms in endocrinology: gut microbiota in patients with type 2 diabetes mellitus. Eur J Endocrinol 172: R167–R177. [DOI] [PubMed] [Google Scholar]
- 14.Chakraborti CK. 2015. New-found link between microbiota and obesity. World J Gastrointest Pathophysiol 6: 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen C, Round JL. 2014. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol 16: 1024–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rau M, Rehman A, Dittrich M, Groen AK, Hermanns HM, Seyfried F, Beyersdorf N, Dandekar T, Rosenstiel P, Geier A. 2018. Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease. United European Gastroenterol J 6: 1496–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saltzman ET, Palacios T, Thomsen M, Vitetta L. 2018. Intestinal microbiome shifts, dysbiosis, inflammation, and non-alcoholic fatty liver disease. Front Microbiol 9: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mouzaki M, Comelli EM, Arendt BM, Bonengel J, Fung SK, Fischer SE, McGilvray ID, Allard JP. 2013. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology 58: 120–127. [DOI] [PubMed] [Google Scholar]
- 19.Wong VW, Tse CH, Lam TT, Wong GL, Chim AM, Chu WC, Yeung DK, Law PT, Kwan HS, Yu J, Sung JJ, Chan HL. 2013. Molecular characterization of the fecal microbiota in patients with nonalcoholic steatohepatitis—a longitudinal study. PLoS One 8: e62885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodhouse CA, Patel VC, Singanayagam A, Shawcross DL. 2018. Review article: the gut microbiome as a therapeutic target in the pathogenesis and treatment of chronic liver disease. Aliment Pharmacol Ther 47: 192–202. [DOI] [PubMed] [Google Scholar]
- 21.Lau LHS, Wong SH. 2018. Microbiota, obesity and NAFLD. Adv Exp Med Biol 1061: 111–125. [DOI] [PubMed] [Google Scholar]
- 22.Da Silva HE, Teterina A, Comelli EM, Taibi A, Arendt BM, Fischer SE, Lou W, Allard JP. 2018. Nonalcoholic fatty liver disease is associated with dysbiosis independent of body mass index and insulin resistance. Sci Rep 8: 1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sobhonslidsuk A, Chanprasertyothin S, Pongrujikorn T, Kaewduang P, Promson K, Petraksa S, Ongphiphadhanakul B. 2018. The association of gut microbiota with nonalcoholic steatohepatitis in Thais. BioMed Res Int 2018: 9340316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LA, Hunault G, Oberti F, Calès P, Diehl AM. 2016. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 63: 764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang B, Jiang X, Cao M, Ge J, Bao Q, Tang L, Chen Y, Li L. 2016. Altered fecal microbiota correlates with liver biochemistry in nonobese patients with non-alcoholic fatty liver disease. Sci Rep 6: 32002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirpich IA, Marsano LS, McClain CJ. 2015. Gut-liver axis, nutrition, and non-alcoholic fatty liver disease. Clin Biochem 48: 923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arslan N. 2014. Obesity, fatty liver disease and intestinal microbiota. World J Gastroenterol 20: 16452–16463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sohail MU, Althani A, Anwar H, Rizzi R, Marei HE. 2017. Role of the gastrointestinal tract microbiome in the pathophysiology of diabetes mellitus. J Diabetes Res 2017: 9631435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutenburg AM, Sonnenblick E, Koven I, Aprahamian HA, Reiner L, Fine J. 1957. The role of intestinal bacteria in the development of dietary cirrhosis in rats. J Exp Med 106: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munukka E, Rintala A, Toivonen R, Nylund M, Yang B, Takanen A, Hänninen A, Vuopio J, Huovinen P, Jalkanen S, Pekkala S. 2017. Faecalibacterium prausnitzii treatment improves hepatic health and reduces adipose tissue inflammation in high-fat fed mice. ISME J 11: 1667–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ge H, Li X, Weiszmann J, Wang P, Baribault H, Chen JL, Tian H, Li Y. 2008. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology 149: 4519–4526. [DOI] [PubMed] [Google Scholar]
- 32.Fetissov SO. 2017. Role of the gut microbiota in host appetite control: bacterial growth to animal feeding behaviour. Nat Rev Endocrinol 13: 11–25. [DOI] [PubMed] [Google Scholar]
- 33.Torres-Fuentes C, Schellekens H, Dinan TG, Cryan JF. 2017. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol Hepatol 2: 747–756. [DOI] [PubMed] [Google Scholar]
- 34.Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. 2013. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology 57: 601–609. [DOI] [PubMed] [Google Scholar]
- 35.Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SE, MacDougall K, Preston T, Tedford C, Finlayson GS, Blundell JE, Bell JD, Thomas EL, Mt-Isa S, Ashby D, Gibson GR, Kolida S, Dhillo WS, Bloom SR, Morley W, Clegg S, Frost G. 2015. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 64: 1744–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, Anastasovska J, Ghourab S, Hankir M, Zhang S, Carling D, Swann JR, Gibson G, Viardot A, Morrison D, Louise Thomas E, Bell JD. 2014. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun 5: 3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL, Shulman GI. 2016. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 534: 213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun L, Ma L, Ma Y, Zhang F, Zhao C, Nie Y. 2018. Insights into the role of gut microbiota in obesity: pathogenesis, mechanisms, and therapeutic perspectives. Protein Cell 9: 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma J, Zhou Q, Li H. 2017. Gut microbiota and nonalcoholic fatty liver disease: insights on mechanisms and therapy. Nutrients 9: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tam J, Liu J, Mukhopadhyay B, Cinar R, Godlewski G, Kunos G. 2011. Endocannabinoids in liver disease. Hepatology 53: 346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim D, Kim W, Kwak MS, Chung GE, Yim JY, Ahmed A. 2017. Inverse association of marijuana use with nonalcoholic fatty liver disease among adults in the United States. PLoS One 12: e0186702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dibba P, Li A, Cholankeril G, Iqbal U, Gadiparthi C, Khan MA, Kim D, Ahmed A. 2018. Mechanistic potential and therapeutic implications of cannabinoids in nonalcoholic fatty liver disease. Medicines, Basel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mehrpouya-Bahrami P, Chitrala KN, Ganewatta MS, Tang C, Murphy EA, Enos RT, Velazquez KT, McCellan J, Nagarkatti M, Nagarkatti P. 2017. Blockade of CB1 cannabinoid receptor alters gut microbiota and attenuates inflammation and diet-induced obesity. Sci Rep 7: 15645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cani PD. 2012. Crosstalk between the gut microbiota and the endocannabinoid system: impact on the gut barrier function and the adipose tissue. Clin Microbiol Infect 18Suppl 4: 50–53. [DOI] [PubMed] [Google Scholar]
- 45.Muccioli GG, Naslain D, Bäckhed F, Reigstad CS, Lambert DM, Delzenne NM, Cani PD. 2010. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol 6: 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matias I, Belluomo I, Cota D. 2016. The fat side of the endocannabinoid system: role of endocannabinoids in adipocyte. Cannabis Cannabinoid Res 1: 176–185. [Google Scholar]
- 47.Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, Dubuquoy L, Dubuquoy C, Merour E, Geboes K, Chamaillard M, Ouwehand A, Leyer G, Carcano D, Colombel JF, Ardid D, Desreumaux P. 2007. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med 13: 35–37. [DOI] [PubMed] [Google Scholar]
- 48.Cani PD, Plovier H, Van Hul M, Geurts L, Delzenne NM, Druart C, Everard A. 2016. Endocannabinoids—at the crossroads between the gut microbiota and host metabolism. Nat Rev Endocrinol 12: 133–143. [DOI] [PubMed] [Google Scholar]
- 49.Mallat A, Teixeira-Clerc F, Deveaux V, Manin S, Lotersztajn S. 2011. The endocannabinoid system as a key mediator during liver diseases: new insights and therapeutic openings. Br J Pharmacol 163: 1432–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Bátkai S, Harvey-White J, Mackie K, Offertáler L, Wang L, Kunos G. 2005. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest 115: 1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gary-Bobo M, Elachouri G, Gallas JF, Janiak P, Marini P, Ravinet-Trillou C, Chabbert M, Cruccioli N, Pfersdorff C, Roque C, Arnone M, Croci T, Soubrié P, Oury-Donat F, Maffrand JP, Scatton B, Lacheretz F, Le Fur G, Herbert JM, Bensaid M. 2007. Rimonabant reduces obesity-associated hepatic steatosis and features of metabolic syndrome in obese Zucker fa/fa rats. Hepatology 46: 122–129. [DOI] [PubMed] [Google Scholar]
- 52.Cluny NL, Vemuri VK, Chambers AP, Limebeer CL, Bedard H, Wood JT, Lutz B, Zimmer A, Parker LA, Makriyannis A, Sharkey KA. 2010. A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br J Pharmacol 161: 629–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tam J, Vemuri VK, Liu J, Bátkai S, Mukhopadhyay B, Godlewski G, Osei-Hyiaman D, Ohnuma S, Ambudkar SV, Pickel J, Makriyannis A, Kunos G. 2010. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest 120: 2953–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hersoug LG, Møller P, Loft S. 2016. Gut microbiota-derived lipopolysaccharide uptake and trafficking to adipose tissue: implications for inflammation and obesity. Obes Rev 17: 297–312. [DOI] [PubMed] [Google Scholar]
- 55.Jiang C, Xie C, Li F, Zhang L, Nichols RG, Krausz KW, Cai J, Qi Y, Fang ZZ, Takahashi S, Tanaka N, Desai D, Amin SG, Albert I, Patterson AD, Gonzalez FJ. 2015. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest 125: 386–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park MY, Kim SJ, Ko EK, Ahn SH, Seo H, Sung MK. 2016. Gut microbiota-associated bile acid deconjugation accelerates hepatic steatosis in ob/ob mice. J Appl Microbiol 121: 800–810. [DOI] [PubMed] [Google Scholar]
- 57.Bieghs V, Trautwein C. 2014. Innate immune signaling and gut-liver interactions in non-alcoholic fatty liver disease. Hepatobiliary Surg Nutr 3: 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Macleod C, Bryant CE. 2017. Visualising pattern recognition receptor signalling. Biochem Soc Trans 45: 1077–1085. [DOI] [PubMed] [Google Scholar]
- 59.Duarte N, Coelho IC, Patarrão RS, Almeida JI, Penha-Gonçalves C, Macedo MP. 2015. How inflammation impinges on NAFLD: a role for Kupffer cells. BioMed Res Int 2015: 984578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mehal WZ. 2014. The inflammasome in liver injury and non-alcoholic fatty liver disease. Dig Dis 32: 507–515. [DOI] [PubMed] [Google Scholar]
- 61.Wree A, McGeough MD, Peña CA, Schlattjan M, Li H, Inzaugarat ME, Messer K, Canbay A, Hoffman HM, Feldstein AE. 2014. NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J Mol Med (Berl) 92: 1069–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang G, Lee HE, Lee JY. 2016. A pharmacological inhibitor of NLRP3 inflammasome prevents non-alcoholic fatty liver disease in a mouse model induced by high fat diet. Sci Rep 6: 24399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gäbele E, Dostert K, Hofmann C, Wiest R, Schölmerich J, Hellerbrand C, Obermeier F. 2011. DSS induced colitis increases portal LPS levels and enhances hepatic inflammation and fibrogenesis in experimental NASH. J Hepatol 55: 1391–1399. [DOI] [PubMed] [Google Scholar]