Abstract
Reactive oxygen species (ROS), such as hydroxyl and superoxide anion radicals, are highly reactive molecules derived from the metabolism of oxygen. ROS play positive roles in cell physiology, but they may also damage cell membranes and DNA, inducing oxidation that causes membrane lipid peroxidation and decreases membrane fluidity. Soymilk yogurt, which is soymilk fermented using lactic acid bacteria (LAB), is an excellent food item with numerous functional substances with antioxidant effects. In this study, the antioxidative activities of soymilk yogurt were investigated. Sixteen of the 26 tested LAB strains solidified soymilk. In antioxidant capacity tests for bacterial cells, Leuconostoc mesenteroides MYU 60 and Pediococcus pentosaceus MYU 759 showed the highest values in the oxygen radical antioxidant capacity (ORAC) and hydroxyl radical antioxidant capacity (HORAC) tests, respectively. The supernatant of soymilk yogurt made with Lactobacillus gasseri MYU 1 showed the highest ORAC and HORAC values. L. mesenteroides MYU 60, Lactobacillus plantarum MYU 74, Lactobacillus reuteri MYU 220, and P. pentosaceus MYU 759 showed significantly high N-acetylcysteine equivalent values compared with the control in a total ROS reducing assay (p<0.05). These strains were selected, and a comet assay was performed, which exhibited decreased values in all selected strains compared with the control, indicating DNA protection. An acidic exopolysaccharide produced by P. pentosaceus MYU 759 showed high antioxidant capacity. The antioxidant substances produced by LAB fermentation may be exopolysaccharides, antioxidant peptides, and isoflavone aglycones. Soymilk yogurt can be used as a functional food useful for various diseases related to oxidation.
Keywords: oxidative stress, soymilk yogurt, fermented soymilk, lactic acid bacteria, antioxidant substance, exopolysaccharide
INTRODUCTION
Reactive oxygen species (ROS) such as superoxide anion radicals, hydrogen peroxide (H2O2), hydroxyl radicals, and singlet oxygen are highly reactive byproducts generated during the process of oxygen consumption in aerobic organisms. ROS are used as part of the immune mechanism to prevent bacterial and viral infection [1]. However, excessive ROS oxidizes important biological components such as DNA, lipids, and proteins. Such oxidative damage is closely involved in the acceleration of senescence and the development of various diseases including lifestyle diseases such as cancer [2], diabetes [3], hypertension [4], and arteriosclerosis [5].
Lactic acid bacteria (LAB) are common microbes used as probiotics, which show numerous beneficial effects such as managing lactose intolerance [6], lowering cholesterol [7], improving immune function [8], preventing colon cancer [9], inhibiting the adherence of some pathogens [10,11,12], and biosorption of heavy metals [13, 14]. LAB also have antioxidants on their cell surface and produce antioxidant substances such as peptides, L-3-(4-hydroxyphenyl) lactic acid (HPLA), L-indole-3-lactic acid (ILA), and exopolysaccharides (EPSs) [15,16,17]. While soymilk is an excellent food that contains not only abundant nutrients but also antioxidant substances such as isoflavones, soybean saponins, vitamin E, antioxidant peptides, and polyamines, fermented soymilk called soymilk yogurt may be expected to have higher functionality. Further, isoflavones in unfermented soymilk exist in the form of glycosides, while isoflavones contained in soymilk yogurt are mostly aglycones with high absorbability in the small intestine [18,19,20]. It is also known that polyamines (putrescine, spermidine, and spermine) are abundant in soymilk and increased or decreased by lactic acid fermentation [21]. Polyamine possesses not only antioxidant activity [22, 23] but also anti-inflammatory activity [24, 25] and the ability to enhance cell proliferation [26], provide protection against damaging radiation [27], and promote longevity [28, 29].
Furthermore, because soymilk yogurt possesses the functionality of LAB, it may be an excellent food. Therefore, in this study, we examined the cytoprotective effects of soymilk yogurt against oxidative stress with the aim of discovering new uses for fermented soy foods.
MATERIALS AND METHODS
Bacterial strains and culture conditions
Twenty-six LAB strains were used in this study (Table 1). The bacterial strains were propagated twice at 37°C for 24 hr in De Man, Rogosa and Sharpe (MRS) broth with 2% (v/v) inoculum before the experiments.
Table 1. Lactic acid bacteria used in this study and solidification of soymilk.
| Strain number | Species | Isolated source | pH after fermentation | Coagulability* |
|---|---|---|---|---|
| MYU 1 | Lactobacillus gasseri | Japanese takuan pickle | 4.76 | + |
| MYU 10 | Lactobacillus sakei | Japanese takuan pickle | 5.78 | + |
| MYU 17 | Lactobacillus gasseri | Kimchi | 4.72 | + |
| MYU 20 | Lactobacillus curvatus | Kimchi | 6.12 | – |
| MYU 26 | Lactobacillus curvatus | Kimchi | 6.15 | – |
| MYU 29 | Lactobacillus paracasei | Pickled nozawana vegetable | 4.86 | + |
| MYU 51 | Leuconostoc sp. | Kimchi | 5.24 | + |
| MYU 57 | Lactobacillus sakei | Rice | 4.83 | + |
| MYU 60 | Leuconostoc mesenteroides | Kimchi | 5.31 | + |
| MYU 65 | Lactobacillus sakei | Kimchi | 4.51 | + |
| MYU 67 | Lactobacillus sakei | Kimchi | 4.87 | + |
| MYU 69 | Lactobacillus sakei | Kimchi | 5.03 | + |
| MYU 71 | Lactobacillus sakei | Japanese amazake (non-heated) | 4.68 | + |
| MYU 74 | Lactobacillus plantarum | Japanese pickle | 4.34 | + |
| MYU 87 | Pediococcus pentosaceus | Pickled celery | 6.07 | – |
| MYU 88 | Pediococcus pentosaceus | Pickled celery | 6.19 | – |
| MYU 89 | Pediococcus pentosaceus | Pickled celery | 5.97 | – |
| MYU 95 | Pediococcus pentosaceus | Nuka-doko (fermented rice bran bed) | 6.09 | – |
| MYU 111 | Lactobacillus plantarum | Soy sauce pickled radish | 4.59 | + |
| MYU 117 | Lactobacillus pentosus/L. plantarum | Soy sauce pickled radish | 4.50 | + |
| MYU 220 | Lactobacillus reuteri | Porcine intestine (called horumon in Japan) | 5.38 | + |
| MYU 381 | Lactobacillus reuteri | Porcine intestine (called horumon in Japan) | 5.93 | – |
| MYU 382 | Lactobacillus reuteri | Porcine intestine (called horumon in Japan) | 6.25 | – |
| MYU 390 | Lactobacillus reuteri | Porcine intestine (called horumon in Japan) | 5.94 | – |
| MYU 758 | Pediococcus pentosaceus | Rice | 6.10 | – |
| MYU 759 | Pediococcus pentosaceus | Rice | 4.70 | + |
*Clotting strains are indicated with +, and non-clotting strains are indicated with –.
Preparation of soymilk yogurt and confirmation of coagulability
The cultured LAB strains were added to sterilized plain soymilk (Organic Soymilk, Tokyo Meiraku, Tokyo, Japan), which was cultured at 37°C for 24 hr, followed by inoculation into fresh plain soymilk and culture at 37°C for 24 hr to prepare the soymilk yogurt. The mixture was centrifuged (5,800 × g, 5 min, 4°C), and the supernatant was used as the sample stock solution in the antioxidant capacity tests. The sample was diluted 100-fold with 75 mM potassium phosphate buffer (pH 7.0) for the oxygen radical antioxidant capacity (ORAC) method and was diluted 4-fold for the hydroxyl radical antioxidant capacity (HORAC) method so as to be within the range of the calibration curve. In the cytoprotective and comet assays using HCT 116 cells, the sample was diluted 20-fold with phosphate-buffered saline (PBS, pH 7.4), taking into consideration dilution in the body.
In the ORAC/HORAC test in bacterial cells, bacterial cells were cultured with MRS broth, recovered, washed, and suspended in 75 mM potassium phosphate buffer at 5.0 × 108 cells/ml to prepare a sample.
The pH of the soymilk yogurt was measured to confirm fermentability, and the coagulability of the soymilk was visually confirmed. The clotting and non-clotting strains were indicated with + and − signs, respectively.
Measurement of antioxidant capacity using ORAC and HORAC methods
ORAC and HORAC methods were used to measure the antioxidant capacity against peroxyl and hydroxyl hydroradicals, respectively.
In the ORAC method, 200 µl of a fluorescein solution was added to a 96-well black plate (BRAND, Wertheim, Germany) containing 20 µl of each sample, and then the fluorescence intensity was immediately measured after shaking using a fluorescence plate reader (Thermo Fisher Scientific, Waltham, MA, USA; excitation and emission wavelengths: 485 and 538 nm, respectively) at 37°C. After measurement, 75 µl 2,2’-azobis (2-methylpropionamidine) dihydrochloride (AAPH; Wako Pure Chemical Industries, Ltd., Osaka, Japan) solution was added to each well, and the fluorescence intensity was measured 45 times at intervals of 2 min. For blanks, 75 mM potassium phosphate buffer was used instead of the sample; Trolox solution was used as the standard solution. The supernatant of the soymilk was adjusted to pH 4.0 with hydrochloric acid (HCl) and used as a control in order to compare before and after fermentation without the effects of pH. The control sample was diluted 100-fold with 75 mM potassium phosphate buffer (pH 7.0) in the ORAC method and was diluted 4-fold in the HORAC method.
In the HORAC method, Fenton’s reagent consisting of 4.65 mM cobalt (II) fluoride tetrahydrate (Sigma-Aldrich, St. Louis, MO, USA) and 4.06 mM picolinic acid (Kanto Chemical Co., Inc., Tokyo, Japan) in assay buffer and 0.55 M H2O2 (Kanto Chemical Co., Inc.) in assay buffer were used instead of AAPH; gallic acid (GA) was used instead of Trolox. The test was performed in a manner similar to the ORAC method.
Examination of cytoprotective effects against oxidative stress
An assay was performed to evaluate cytoprotective effects using a total ROS detection kit (Enzo Life Sciences, Farmingdale, NY, USA). HCT 116 cells (American Type Culture Collection [ATCC], Manassas, VA, USA) were cultured using McCoy’s 5A culture media (ATCC) with 10% fetal bovine serum (FBS; Gibco, Burlington, ON, Canada) and penicillin-streptomycin (Gibco) at 37°C with exposure to 5% CO2. After the cells attained 80% confluency, 1 × 104 HCT 116 cells were added to a 96-well black plate (Greiner Bio-One International GmbH, Kremsmünster, Austria) and cultured overnight at 37°C with exposure to 5% CO2. After washing HCT 116 cells with PBS, 25 µl of the control, N-acetylcysteine (NAC), or the sample was added and incubated for 30 min under the same conditions. Then, 25 µl of 200 μM pyocyanine was added, and the mixture was incubated for 30 min. Next, 50 µl of the detection solution was added, and the mixture was incubated for 60 min in the dark. The fluorescence intensity was then measured (excitation and emission wavelengths: 485 and 538 nm). The data were expressed as NAC equivalents converted to concentrations of NAC, which is an ROS scavenger.
Examination of DNA protection using a comet assay
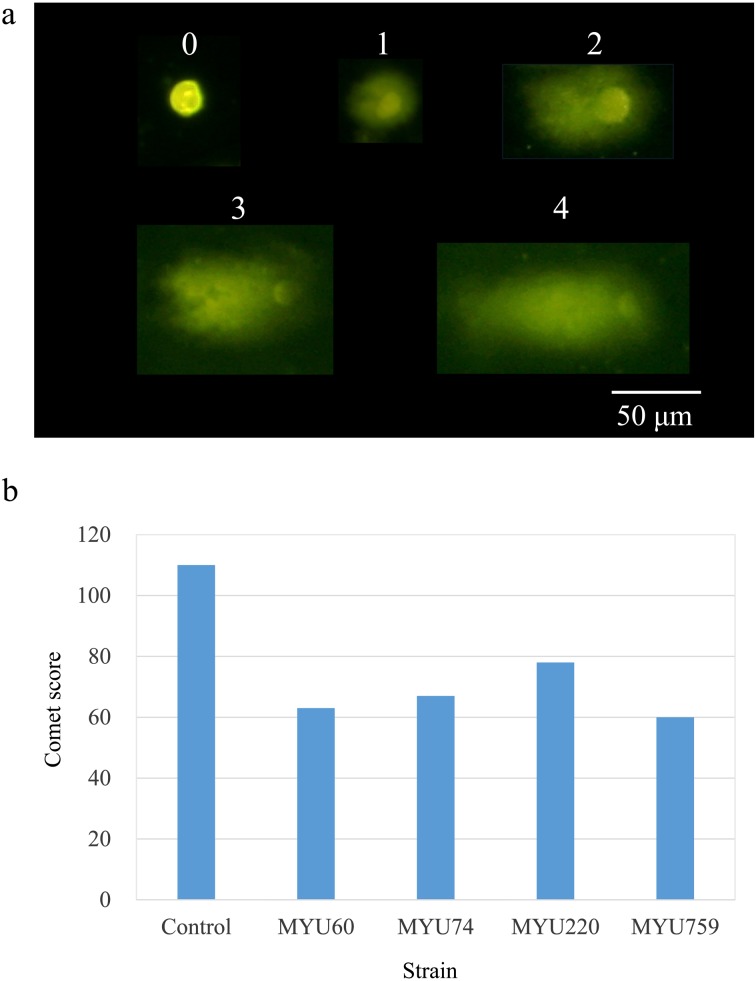
The DNA protective effect was measured using a comet assay kit (CometAssay®, Trevigen, Gaithersburg, MD, USA). Briefly, 1 × 104 HCT 116 cells were cultured overnight in a 12-well plate in McCoy’s 5A culture medium (ATCC) with 10% FBS and penicillin-streptomycin at 37°C with exposure to 5% CO2. After washing the cells, 0.5 ml each of the samples and McCoy’s 5A culture medium were added and incubated at 37°C with exposure to 5% CO2 for 60 min. Then, the supernatant was removed, 0.1 mM H2O2 was added, and the mixture was reacted again for 10 min. After recovering the cells, they were suspended in LMA agarose and spread on a comet slide, which was immersed in lysis solution for 60 min, immersed in alkaline unwinding solution for 20 min, and then electrophoresed in cold alkaline running buffer (pH>13, 25 V, 20 min). After electrophoresis, the comet slide was immersed in 70% ethanol and then distilled water for 5 min each. This treatment was repeated once. After drying, the slide was stained with SYBR Gold (Life Technologies, Grand Island, NY, USA) and observed using a fluorescence microscope. A total of 50 cells were counted per slide and scored according to their damage condition (comet score, see Fig. 4a), and the total score was indicated as a cumulative value of the score for 50 cells. The supernatant of the soymilk was adjusted to pH 4.5, diluted 20-fold with PBS (pH 7.4), and used as a control.
Fig. 4.
DNA-protective effects of supernatants of soymilk yogurt by comet assay. Criteria for scoring comet assay results (a) and sum of the scores for each sample (n=50) (b).
Purification of the EPSs and performance of antioxidant tests
Pediococcus pentosaceus MYU 759 was propagated at 37°C for 24 hr using one liter of MRS broth. An equal volume of 99.5% cold ethanol (Kanto Chemical Co., Inc.) was added to the supernatant after centrifugation (1,500 × g, 15 min, 4°C), and the mixture was allowed to stand at 4°C overnight, followed by centrifugation (12,200 × g, 30 min, 4°C) to obtain a precipitate. After treatment with 10% trichloroacetic acid, the sugar fraction was obtained by ethanol precipitation. Next, it was applied to DEAE-TOYOPEAL 650 M (Tosoh Bioscience, King of Prussia, PA, USA) followed by TOYOPEARL HW-65S (Tosoh Bioscience). The fractions with a high sugar content were dialyzed against distilled water at 4°C for 2 days and then lyophilized. The purified EPSs were dissolved with assay buffer (0.125, 0.25, and 0.5 mg/ml), and the antioxidant capacity was measured by the ORAC and HORAC methods. The data were indicated as the Trolox equivalent antioxidant capacity (TEAC; μM TE) and gallic acid equivalent antioxidant capacity (GAEAC; μM GAE).
Statistical analyses
The assays were performed in triplicate and repeated two or more times. The data are expressed as the means ± standard deviation (SD). Multiple comparison tests were performed using the Dunnett T3 (ORAC assay of supernatants and ORAC assay of EPSs), Dunnett T (two-tailed test: oxidative stress and HORAC assays of supernatants), Games-Howell (bacterial ORAC), and Tukey honest significant difference (HSD; HORAC assays of cells and EPSs) tests. The IBM SPSS Statistics software ver. 22 was used for the statistical analysis (IBM Corp., Armonk, NY, USA).
RESULTS
Test of the fermentability of soymilk by LAB
Table 1 shows the results of the soymilk coagulation test; 16 of the 26 strains (62%) coagulated the soymilk. The average pH of the strains that coagulated the soymilk was 4.88, whereas that of the strains that did not coagulate the soymilk was 6.08.
Antioxidation of LAB cells and supernatants of soymilk yogurts
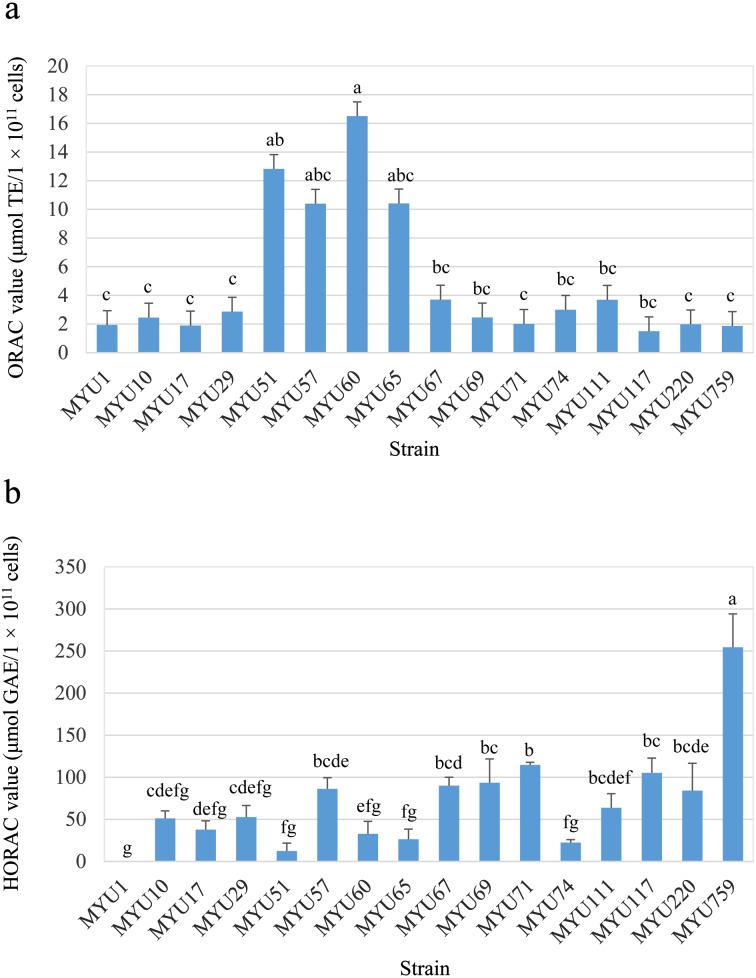
Regarding LAB cells, Leuconostoc sp. MYU 51, Lactobacillus sakei MYU 57, Leuconostoc mesenteroides MYU 60, and L. sakei MYU 67 showed high values in the ORAC test. In particular, L. mesenteroides MYU 60 showed the highest value at 16.50 ± 2.25 μmol TE/1 × 1011 cells (Fig. 1a). The HORAC value was the highest for P. pentosaceus MYU 759, at 254.39 ± 39.75 μmol GAE/1 × 1011 cells (Fig. 1b).
Fig. 1.
Oxygen radical antioxidant capacity (ORAC) (a) and hydroxyl radical antioxidant capacity (HORAC) (b) values of lactic acid bacteria (LAB) cells. Data represent average values ± standard deviation (SD). ORAC and HORAC values are indicated as the Trolox equivalent (TE) and gallic acid equivalent (GAE) per 1 × 1011 cells of LAB. Different letters indicate significant differences (p<0.05).
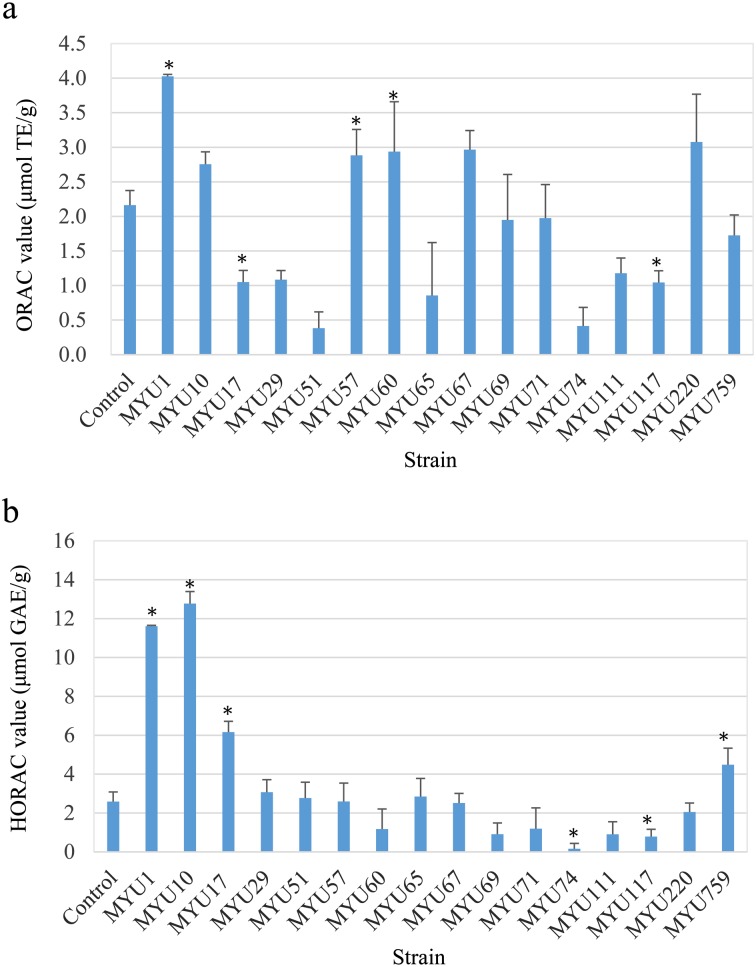
Regarding the supernatants of soymilk yogurts, Lactobacillus gasseri MYU 1, L. sakei MYU 57, and L. mesenteroides MYU 60 showed significantly higher values in the ORAC test than the control (p<0.05, Fig. 2a). L. gasseri MYU 1, in particular, showed the highest value, exhibiting approximately double the value (4.03 ± 0.03 μmol TE/g) of the control. In the HORAC test, the values for L. gasseri MYU 1, L. sakei MYU 10, L. gasseri MYU 17, and P. pentosaceus MYU 759 were significantly higher than that of the control and were 11.61 ± 0.05, 12.77 ± 0.62, 6.16 ± 0.56, and 4.49 ± 0.85 μmol GAE/g, respectively (p<0.05; Fig. 2b).
Fig. 2.
Oxygen radical antioxidant capacity (ORAC) (a) and hydroxyl radical antioxidant capacity (HORAC) (b) values of supernatants of soymilk yogurt. Data represent average values ± standard deviation (SD). ORAC and HORAC values are indicated as the Trolox equivalent (TE) and gallic acid equivalent (GAE) per 1 g of supernatant of soymilk yogurt. *p<0.05 vs. control group.
Cytoprotective effects against oxidative stress
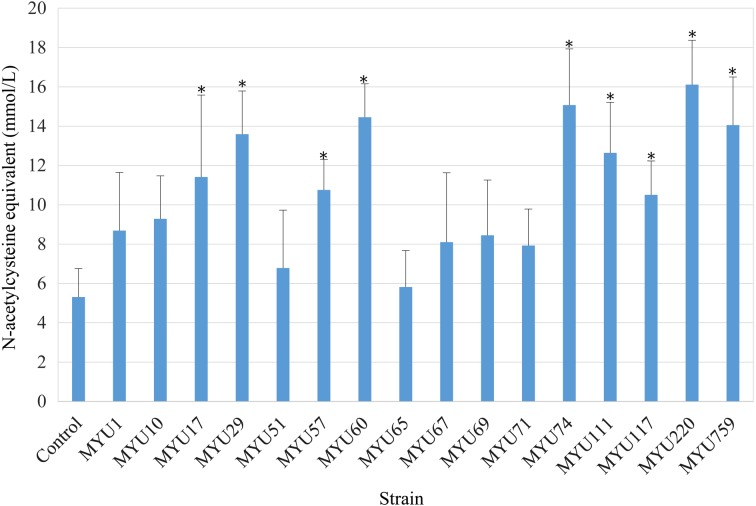
Samples prepared with nine strains showed significantly higher NAC equivalents than that of the control (p<0.05). Lactobacillus reuteri MYU 220 showed the highest value, 16.1 ± 2.3 mM NAC equivalents (Fig. 3). The top four strains (the MYU 220, MYU 74, MYU 60, and MYU 759 strains) were subsequently selected.
Fig. 3.
Reducing effects of total reactive oxygen species (ROS) using supernatants of soymilk yogurt in HCT 116 cells. Data represent average values ± standard deviation (SD) at the N-acetylcysteine (NAC) equivalent (mM). *p<0.05 vs. control.
Examination of DNA protection effects using a comet assay
The DNA protective effect was evaluated using a comet assay (Fig. 4). All the selected bacteria showed lower comet values than the control. The comet scores were 110, 63, 67, 78, and 60 points for the control, L. mesenteroides MYU 60, Lactobacillus plantarum MYU 74, L. reuteri MYU 220, and P. pentosaceus MYU 759, respectively. P. pentosaceus MYU 759 was selected and used for further experiments because its comet score, especially its level 4 DNA damage score, was the lowest in the selected LAB strains (supplementary Table 1).
Purification and antioxidant capacity analysis of EPSs
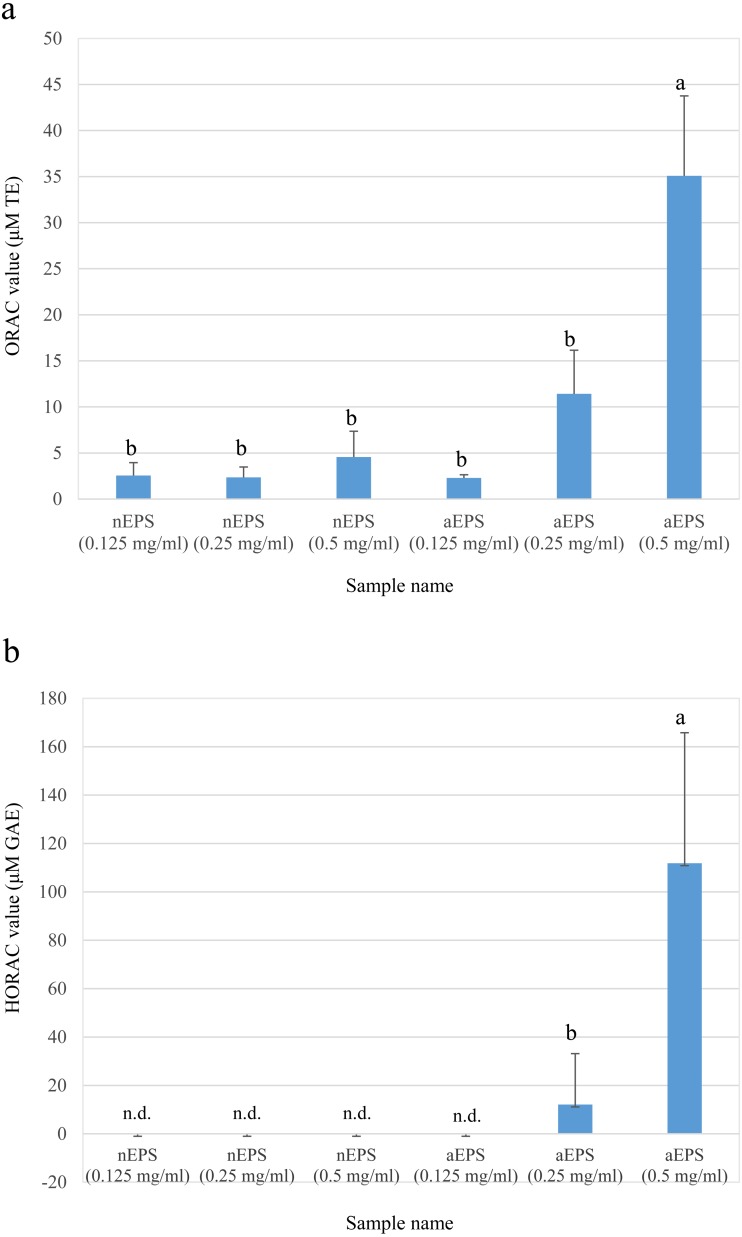
From anion exchange chromatography, it was revealed that P. pentosaceus MYU 759 produces two type of EPSs, a neutral EPS (nEPS) and an acidic EPS (aEPS). In gel filtration chromatography, the molecular weights of nEPS and aEPS were deduced to be approximately 100,000 and 20,000, respectively. The amount of EPSs purified from one liter of culture broth was 623 mg for nEPS and 355 mg for aEPS. Antioxidant capacity analysis revealed high activity of aEPS in both the ORAC and HORAC assays, whereas little or no activity was observed for nEPS (Fig. 5).
Fig. 5.
Oxygen radical antioxidant capacity (ORAC) (a) and hydroxyl radical antioxidant capacity (HORAC) (b) values of purified EPSs produced by P. pentosaceus MYU 759. Data represent the average values ± standard deviation (SD). ORAC and HORAC values are indicated as the TEAC (μM TE) and GAEAC (μM GAE), respectively. n.d.: not detected. Different letters indicate significant differences (p<0.05).
DISCUSSION
The coagulability of soymilk, an excellent food that contains numerous antioxidant substances such as isoflavones, saponins, and polyamines [30], via LAB fermentation was tested, and 16 of the 26 strains (62%) were shown to have coagulated the soymilk (Table 1). The average pH of the strains that coagulated the soymilk was 4.88, whereas that of the strains that did not coagulate the soymilk was 6.08. Oizumi et al. [31] reported the average size of particles in soymilk was remarkably increased at pH 5.6 and that the fluidity index was severely reduced at pH 5.8 or less. Angeles and Marth [32] reported that the presence of 0.23–0.25% titratable acid, corresponding to a pH of 5.7, caused coagulation of soymilk fermented by LAB. These results correspond to our results (pH range of the strains that coagulated the soymilk, 4.34–5.78; Table 1). Therefore, the coagulation of many of the soymilk samples in this study was considered to have been the result of acid clotting induced by the lactic acid produced by LAB. However, some studies reported another coagulation mechanism. Murata et al. [33] reported that various commercial proteinases originating from microorganisms, plants, and animals can coagulate a soymilk. Hatanaka et al. [34] also reported that an intracellular 45 kDa protease of Saccharomyces bayanus SCY003 coagulated soymilk at a pH greater than 6.0. In this study, however, there was no coagulation at pH 6 or more. Therefore, it was considered that the main factor related to coagulation of soymilk was the pH decrease in this study, although the possibility that protease was involved could not be completely excluded.
In the ORAC and HORAC antioxidant tests using bacterial cells (Fig. 1), four strains (MYU 51, MYU 57, MYU 60, and MYU 65) in the ORAC assays and one strain (MYU 759) in the HORAC assays showed high activities. Some papers have shown that LAB cells have antioxidative activities. Annuk et al. [35] reported that the antioxidative activity of intestinal lactobacilli (ca. 109 CFU/ml) is strain specific among facultatively and obligately heterofermentative lactobacilli but that obligately homofermentative lactobacilli had high antioxidative activity. Lactobacillus paracasei ssp. paracasei YBJ01 showed free radical and superoxide anion scavenging activities in vitro, significantly increased serum superoxide dismutase (SOD), glutathione peroxidase, and total-antioxidant capability, and inhibited generation of malondialdehyde (MDA) in a dose-dependent manner in vivo [36]. Lactobacillus rhamnosus GG was shown to alleviate intestinal diseases caused by alcohol-induced oxidative stress, suggesting that the bacterium relieves intestinal oxidation [37]. Finally, Lin and Yen [38] reported that intracellular cell-free extracts of LAB and bifidobacteria strains showed metal ion chelating ability and ROS scavenging ability.
Living LAB strains are capable of producing antioxidants. Ljungh and Wadström [39] reported that P. pentosaceus 16:1 and L. plantarum 2592 (107 cells) produced antioxidants after 18 hr growth corresponding to 100 µg of vitamin C in a colorimetric assay. Suzuki et al. [15] identified two antioxidant substances, HPLA and ILA, from MRS culture of many strains of L. plantarum and Lactobacillus paraplantarum. It was also reported that antioxidative activities of soymilk were increased by fermentation. Wang et al. [40] reported that fermented soymilk products produced with LAB and bifidobacteria showed higher antioxidant properties than unfermented soymilk. Tsai et al. [41] reported that administration of soymilk fermented with LAB to a hamster fed a high-fat meal relieved oxidative stress and atherosclerosis. Liu et al. [42] reported that milk kefir and soymilk kefir had significantly higher antioxidant activity than plain milk and soymilk. In cheese, it is established that the degree of ripening and rate of soluble peptide production are related to the antioxidant activity [43,44,45]. In this study, many of the prepared soymilk yogurt supernatants showed higher ORAC and HORAC values than that of the control (Fig. 2), and nine soymilk yogurt supernatants showed significantly high activity in the ROS elimination test using HCT 116 cells (p<0.05; Fig. 3). These findings suggest that antioxidant substances such as peptides, HPLA, and ILA may be produced by fermentation.
Some studies have shown the antioxidant effects of EPSs produced by LAB. Zhang et al. [16] reported that a neutral EPS of L. plantarum C88 exhibited scavenging abilities on hydroxyl and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals. Furthermore, the EPS showed a protective effect against H2O2-induced Caco-2 cell oxidative injury. The results revealed that the EPS inhibited the formation of MDA and raised the activities of SOD and total antioxidant capacities (T-AOCs) in a dose-dependent manner. Wang et al. [46] also reported that an EPS of L. plantarum KX041 exhibited high antioxidant activity with free radical scavenging capacity for 2,2-azinobis (3-ethylbenzthiazoline)-6-sulfonic acid (ABTS), DPPH, hydroxyl, and super-oxide free radicals. Xu et al. [47] reported that neutral EPS and acidic EPS of Bifidobacterium animalis RH showed antioxidant activities in vitro and in vivo. Oral administration of the EPSs of B. animalis RH in a galactose-induced aged mouse model significantly increased the activities of antioxidant enzymes such as SOD and catalase (CAT), the total antioxidant capacity in serums, and glutathione S-transferase (GST) in the liver. They also could inhibit significantly the formation of MDA in serums and the liver and reduce the activity of monoamine oxidase (MAO) and lipofuscin accumulation in the mouse brain. Although the mechanism of the antioxidative effects of EPS is not clear, some papers have suggested the chelating ability on ferrous ion [48]; free radical scavenging by abstraction of the anomeric hydrogen of polysaccharides [49]; conjugation with other components such as polyphenol, flavonoid, proteins, and peptides [48, 50]; and chemical modifications such as phosphorylation and sulfation [48]. We clarified that P. pentosaceus MYU 759 produced two types of EPSs and that aEPS showed a concentration-dependent increase in ORAC and HORAC even at 0.25 mg/l (Fig. 5). EPSs of LAB are often phosphorylated and sulfated [51, 52], and phosphorylation and sulfation of polysaccharides increase antioxidative activities [53, 54]. Our results agree with these findings. These results suggest that aEPS could be one of the antioxidant substances in soymilk yogurt produced by P. pentosaceus MYU 759, although it is necessary to measure the concentration of EPSs in soymilk yogurt instead of MRS broth.
It is also considered that aglyconeization of isoflavone glycoside may contribute to antioxidation. Cheng et al. [55] reported that an increase in aglycone isoflavones in ethanol extracts of soymilk yogurt stimulated nitric oxide (NO) production and endothelial NO synthase (eNOS) activity in human umbilical vein endothelial cells. It also had a stimulating effect on superoxide anion scavenging and prostaglandin E2 production and enhanced mRNA expression of the E-prostanoid 4 receptor in rat thoracic aorta smooth muscle cells. Marazza et al. [19] reported that aglycone isoflavones in fermented soymilk showed high antioxidant capacity and exerted a DNA protection effect. Moreover, Murota et al. [56] reported that the transport of isoflavone aglycones, genistein and daidzein, through Caco-2 monolayers was more than ten times that of their glucosides, genistin and daidzin. In this study, since the selected soymilk yogurt supernatant showed a higher DNA protection than the control soymilk (Fig. 4), improvement of absorption of isoflavones by aglyconeization might have contributed to this effect. Therefore, we tested aglyconeization of isoflavones in P. pentosaceus MYU 759 by HPLC analysis. Isoflavone aglycones (daizein, glycitein, and genistein) in this strain increased compared with the unfermented control sample (rates of 3.4% and 22.4%, respectively). We also clarified that P. pentosaceus MYU 759 produces β-glucosidase (3.6 mU/ml). Many reports have shown that isoflavone glucosides are converted to aglycones by β-glucosidase of LAB, bifidobacteria, and yeast [57,58,59,60]. Although aglycone rates and β-glucosidase activities are not so high in P. pentosaceus MYU 759, it may be one of the reasons that fermented soymilk showed high antioxidation, as isoflavone aglycones can be more easily absorbed from the intestine than isoflavones in their glycoside form [18, 61].
In this study, we demonstrated that antioxidant substances are produced by fermenting soymilk with the selected LAB, such as P. pentosaceus MYU 759. The antioxidant substances produced by LAB fermentation may be EPSs, antioxidant peptides, and isoflavone aglycones. Therefore, soymilk yogurt made with the LAB strains selected in this study could be used as a functional food for various diseases related to oxidation.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Supplementary
Acknowledgments
This study was supported by a Grant for Young Scientists from the Fuji Foundation for Protein Research in 2015 and a grant from the Agricultural Research Organization, Tokai University.
References
- 1.Schwarz KB. 1996. Oxidative stress during viral infection: a review. Free Radic Biol Med 21: 641–649. [DOI] [PubMed] [Google Scholar]
- 2.Seishima R, Wada T, Tsuchihashi K, Okazaki S, Yoshikawa M, Oshima H, Oshima M, Sato T, Hasegawa H, Kitagawa Y, Goldenring JR, Saya H, Nagano O. 2015. Ink4a/Arf-dependent loss of parietal cells induced by oxidative stress promotes CD44-dependent gastric tumorigenesis. Cancer Prev Res (Phila) 8: 492–501. [DOI] [PubMed] [Google Scholar]
- 3.Mangialardi G, Spinetti G, Reni C, Madeddu P. 2014. Reactive oxygen species adversely impacts bone marrow microenvironment in diabetes. Antioxid Redox Signal 21: 1620–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuo L, Rose BA, Roberts WJ, He F, Banes-Berceli AK. 2014. Molecular characterization of reactive oxygen species in systemic and pulmonary hypertension. Am J Hypertens 27: 643–650. [DOI] [PubMed] [Google Scholar]
- 5.Jacinto TA, Meireles GS, Dias AT, Aires R, Porto ML, Gava AL, Vasquez EC, Pereira TMC, Campagnaro BP, Meyrelles SS. 2018. Increased ROS production and DNA damage in monocytes are biomarkers of aging and atherosclerosis. Biol Res 51: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savaiano DA, Kotz C. 1989. Recent advances in the management of lactose intolerance. ASDC J Dent Child 56: 228–233. [PubMed] [Google Scholar]
- 7.Danielson AD, Peo ER, Jr, Shahani KM, Lewis AJ, Whalen PJ, Amer MA. 1989. Anticholesteremic property of Lactobacillus acidophilus yogurt fed to mature boars. J Anim Sci 67: 966–974. [DOI] [PubMed] [Google Scholar]
- 8.Perdigón G, Maldonado Galdeano C, Valdez JC, Medici M. 2002. Interaction of lactic acid bacteria with the gut immune system. Eur J Clin Nutr 56Suppl 4: S21–S26. [DOI] [PubMed] [Google Scholar]
- 9.Lim BK, Mahendran R, Lee YK, Bay BH. 2002. Chemopreventive effect of Lactobacillus rhamnosus on growth of a subcutaneously implanted bladder cancer cell line in the mouse. Jpn J Cancer Res 93: 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mack DR, Michail S, Wei S, McDougall L, Hollingsworth MA. 1999. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am J Physiol 276: G941–G950. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Xu J, Shuai J, Chen J, Zhang Z, Fang W. 2007. The S-layer proteins of Lactobacillus crispatus strain ZJ001 is responsible for competitive exclusion against Escherichia coli O157:H7 and Salmonella typhimurium. Int J Food Microbiol 115: 307–312. [DOI] [PubMed] [Google Scholar]
- 12.Varma P, Dinesh KR, Menon KK, Biswas R. 2010. Lactobacillus fermentum isolated from human colonic mucosal biopsy inhibits the growth and adhesion of enteric and foodborne pathogens. J Food Sci 75: M546–M551. [DOI] [PubMed] [Google Scholar]
- 13.Kinoshita H, Ohtake F, Ariga Y, Kimura K. 2016. Comparison and characterization of biosorption by Weissella viridescens MYU 205 of periodic group 12 metal ions. Anim Sci J 87: 271–276. [DOI] [PubMed] [Google Scholar]
- 14.Kinoshita H, Sohma Y, Ohtake F, Ishida M, Kawai Y, Kitazawa H, Saito T, Kimura K. 2013. Biosorption of heavy metals by lactic acid bacteria and identification of mercury binding protein. Res Microbiol 164: 701–709. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki Y, Kosaka M, Shindo K, Kawasumi T, Kimoto-Nira H, Suzuki C. 2013. Identification of antioxidants produced by Lactobacillus plantarum. Biosci Biotechnol Biochem 77: 1299–1302. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Liu C, Li D, Zhao Y, Zhang X, Zeng X, Yang Z, Li S. 2013. Antioxidant activity of an exopolysaccharide isolated from Lactobacillus plantarum C88. Int J Biol Macromol 54: 270–275. [DOI] [PubMed] [Google Scholar]
- 17.Pessione E, Cirrincione S. 2016. Bioactive molecules released in food by lactic acid bacteria: encrypted peptides and biogenic amines. Front Microbiol 7: 876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kano M, Takayanagi T, Harada K, Sawada S, Ishikawa F. 2006. Bioavailability of isoflavones after ingestion of soy beverages in healthy adults. J Nutr 136: 2291–2296. [DOI] [PubMed] [Google Scholar]
- 19.Marazza JA, Nazareno MA, de Giori GS, Garro MS. 2012. Enhancement of the antioxidant capacity of soymilk by fermentation with Lactobacillus rhamnosus. J Funct Foods 4: 594–601. [Google Scholar]
- 20.Chen Y, Shih T, Chiu CP, Pan T, Tsai T. 2013. Effects of lactic acid bacteria-fermented soy milk on melanogenesis in B16F0 melanocytes. J Funct Foods 5: 395–405. [Google Scholar]
- 21.Okamoto A, Sugi E, Koizumi Y, Yanagida F, Udaka S. 1997. Polyamine content of ordinary foodstuffs and various fermented foods. Biosci Biotechnol Biochem 61: 1582–1584. [DOI] [PubMed] [Google Scholar]
- 22.Ha HC, Sirisoma NS, Kuppusamy P, Zweier JL, Woster PM, Casero RA., Jr1998. The natural polyamine spermine functions directly as a free radical scavenger. Proc Natl Acad Sci USA 95: 11140–11145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujisawa S, Kadoma Y. 2005. Kinetic evaluation of polyamines as radical scavengers. Anticancer Res 252A: 965–969. [PubMed] [Google Scholar]
- 24.Zhang M, Caragine T, Wang H, Cohen PS, Botchkina G, Soda K, Bianchi M, Ulrich P, Cerami A, Sherry B, Tracey KJ. 1997. Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: a counterregulatory mechanism that restrains the immune response. J Exp Med 185: 1759–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soda K, Kano Y, Nakamura T, Kasono K, Kawakami M, Konishi F. 2005. Spermine, a natural polyamine, suppresses LFA-1 expression on human lymphocyte. J Immunol 175: 237–245. [DOI] [PubMed] [Google Scholar]
- 26.Roseeuw DI, Marcelo CL, Rhodes LM, Voorhees JJ. 1983. Epidermal keratinocytes actively maintain their intracellular polyamine levels. Cell Tissue Kinet 16: 493–504. [PubMed] [Google Scholar]
- 27.Douki T, Bretonniere Y, Cadet J. 2000. Protection against radiation-induced degradation of DNA bases by polyamines. Radiat Res 153: 29–35. [DOI] [PubMed] [Google Scholar]
- 28.Soda K, Dobashi Y, Kano Y, Tsujinaka S, Konishi F. 2009. Polyamine-rich food decreases age-associated pathology and mortality in aged mice. Exp Gerontol 44: 727–732. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto M, Kurihara S, Kibe R, Ashida H, Benno Y. 2011. Longevity in mice is promoted by probiotic-induced suppression of colonic senescence dependent on upregulation of gut bacterial polyamine production. PLoS One 6: e23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chun J, Kim GM, Lee KW, Choi ID, Kwon GH, Park JY, Jeong SJ, Kim JS, Kim JH. 2007. Conversion of isoflavone glucosides to aglycones in soymilk by fermentation with lactic acid bacteria. J Food Sci 72: M39–M44. [DOI] [PubMed] [Google Scholar]
- 31.Oizumi K, Idogawa S, Iwamoto Y, Ito K, Fujii T. 2016. Influence of pH on the colloidal stability of soymilk. Nippon Shokuhin Kagaku Kogaku Kaishi 63: 142–149(In Japanese). [Google Scholar]
- 32.Angeles AG, Marth EH. 1971. Growth and acid production. J Milk Food Technol 34: 30–36. [Google Scholar]
- 33.Murata K, Kusakabe I, Kobayashi H, Akaike M, YM. P, Murakami K. 1987. Studies on the coagulation of soymilk-protein by commercial proteinases. Agric BioI Chem 51: 385–389. [Google Scholar]
- 34.Hatanaka S, Maegawa M, Kanauchi M, Kasahara S, Shimoyamada M, Ishida M. 2014. Characteristics and purification of soybean milk curdling enzyme-producing yeast Saccharomyces bayanus SCY003. Food Sci Technol Res 20: 927–938. [Google Scholar]
- 35.Annuk H, Shchepetova J, Kullisaar T, Songisepp E, Zilmer M, Mikelsaar M. 2003. Characterization of intestinal lactobacilli as putative probiotic candidates. J Appl Microbiol 94: 403–412. [DOI] [PubMed] [Google Scholar]
- 36.Suo H, Liu S, Li J, Ding Y, Wang H, Zhang Y, Zhao X, Song JL. 2018. Lactobacillus paracasei ssp. paracasei YBJ01 reduced d-galactose-induced oxidation in male Kuming mice. J Dairy Sci 101: 10664–10674. [DOI] [PubMed] [Google Scholar]
- 37.Forsyth CB, Farhadi A, Jakate SM, Tang Y, Shaikh M, Keshavarzian A. 2009. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol 43: 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin MY, Yen CL. 1999. Antioxidative ability of lactic acid bacteria. J Agric Food Chem 47: 1460–1466. [DOI] [PubMed] [Google Scholar]
- 39.Ljungh A, Wadström T. 2006. Lactic acid bacteria as probiotics. Curr Issues Intest Microbiol 7: 73–89. [PubMed] [Google Scholar]
- 40.Wang YC, Yu RC, Chou CC. 2006. Antioxidative activities of soymilk fermented with lactic acid bacteria and bifidobacteria. Food Microbiol 23: 128–135. [DOI] [PubMed] [Google Scholar]
- 41.Tsai TY, Chu LH, Lee CL, Pan TM. 2009. Atherosclerosis-preventing activity of lactic acid bacteria-fermented milk-soymilk supplemented with Momordica charantia. J Agric Food Chem 57: 2065–2071. [DOI] [PubMed] [Google Scholar]
- 42.Liu JR, Chen MJ, Lin CW. 2005. Antimutagenic and antioxidant properties of milk-kefir and soymilk-kefir. J Agric Food Chem 53: 2467–2474. [DOI] [PubMed] [Google Scholar]
- 43.Pritchard S, Phillips M, Kailasapathy K. 2010. Identification of bioactive peptides in commercial Cheddar cheese. Food Res Int 43: 1545–1548. [Google Scholar]
- 44.Igoshi K, Kondo Y, Kobayashi H, Kabata K, Kawakami H. 2008. Antioxidative activity of cheese. Milchwissenscha 63: 424–427. [Google Scholar]
- 45.Huma N, Rafiq S, Sameen A, Pasha I, Khan MI. 2018. Antioxidant potential of buffalo and cow milk Cheddar cheeses to tackle human colon adenocarcinoma (Caco-2) cells. Asian-Australas J Anim Sci 31: 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Shao C, Liu L, Guo X, Xu Y, Lü X. 2017. Optimization, partial characterization and antioxidant activity of an exopolysaccharide from Lactobacillus plantarum KX041. Int J Biol Macromol 103: 1173–1184. [DOI] [PubMed] [Google Scholar]
- 47.Xu R, Shang N, Li P. 2011. In vitro and in vivo antioxidant activity of exopolysaccharide fractions from Bifidobacterium animalis RH. Anaerobe 17: 226–231. [DOI] [PubMed] [Google Scholar]
- 48.Wang J, Hu S, Nie S, Yu Q, Xie M. 2016. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxid Med Cell Longev 2016: 5692852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsiapali E, Whaley S, Kalbfleisch J, Ensley HE, Browder IW, Williams DL. 2001. Glucans exhibit weak antioxidant activity, but stimulate macrophage free radical activity. Free Radic Biol Med 30: 393–402. [DOI] [PubMed] [Google Scholar]
- 50.Lin T, Chen Y. 2017. Comparison of antioxidant activity of exopolysaccharides between Lactobacillus acidophilus La and Bifidobacterium adolescentis Ba in vitro. In ICMHI ‘17 Proceedings of the 1st International Conference on Medical and Health Informatics 2017, ACM, New York, pp.107–111.
- 51.Makino S, Ikegami S, Kano H, Sashihara T, Sugano H, Horiuchi H, Saito T, Oda M. 2006. Immunomodulatory effects of polysaccharides produced by Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. J Dairy Sci 89: 2873–2881. [DOI] [PubMed] [Google Scholar]
- 52.Wang K, Li W, Rui X, Chen X, Jiang M, Dong M. 2014. Structural characterization and bioactivity of released exopolysaccharides from Lactobacillus plantarum 70810. Int J Biol Macromol 67: 71–78. [DOI] [PubMed] [Google Scholar]
- 53.He Y, Ye M, Jing L, Du Z, Surahio M, Xu H, Li J. 2015. Preparation, characterization and bioactivities of derivatives of an exopolysaccharide from Lachnum. Carbohydr Polym 117: 788–796. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Z, Liu Z, Tao X, Wei H. 2016. Characterization and sulfated modification of an exopolysaccharide from Lactobacillus plantarum ZDY2013 and its biological activities. Carbohydr Polym 153: 25–33. [DOI] [PubMed] [Google Scholar]
- 55.Cheng CP, Tsai SW, Chiu CP, Pan TM, Tsai TY. 2013. The effect of probiotic-fermented soy milk on enhancing the NO-mediated vascular relaxation factors. J Sci Food Agric 93: 1219–1225. [DOI] [PubMed] [Google Scholar]
- 56.Murota K, Shimizu S, Miyamoto S, Izumi T, Obata A, Kikuchi M, Terao J. 2002. Unique uptake and transport of isoflavone aglycones by human intestinal caco-2 cells: comparison of isoflavonoids and flavonoids. J Nutr 132: 1956–1961. [DOI] [PubMed] [Google Scholar]
- 57.Chun J, Kim JS, Kim JH. 2008. Enrichment of isoflavone aglycones in soymilk by fermentation with single and mixed cultures of Streptococcus infantarius 12 and Weissella sp. 4. Food Chem 109: 278–284. [DOI] [PubMed] [Google Scholar]
- 58.Pyo Y, Lee T, Lee Y. 2005. Enrichment of bioactive isoflavones in soymilk fermented with β-glucosidase-producing lactic acid bacteria. Food Res Int 38: 551–559. [Google Scholar]
- 59.Rekha CR, Vijayalakshmi G. 2010. Bioconversion of isoflavone glycosides to aglycones, mineral bioavailability and vitamin B complex in fermented soymilk by probiotic bacteria and yeast. J Appl Microbiol 109: 1198–1208. [DOI] [PubMed] [Google Scholar]
- 60.Tsangalis D, Ashton J, Mcgill A, Shah N. 2002. Enzymic transformation of isoflavone phytoestrogens in soymilk by β‐glucosidase‐producing bifidobacteria. J Food Sci 67: 3104–3113. [Google Scholar]
- 61.Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, Kataoka S, Kubota Y, Kikuchi M. 2000. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr 130: 1695–1699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.