Abstract
BACKGROUND:
Polysubstance use is common among opioid-using women, yet its association with pharmacotherapy for neonatal abstinence syndrome (NAS) remains unclear. We hypothesized that benzodiazepine exposure would increase risk of an infant developing pharmacologically treated NAS.
METHODS:
We conducted a retrospective cohort study of maternal-infant dyads enrolled in Tennessee Medicaid, using individual-level data linkage of vital records and administrative (ie, outpatient, inpatient, and prescription) data from 2009 to 2011. These data underwent chart review from 2013 to 2016 to obtain clinically relevant exposure data (eg, toxicology testing). The association of antenatal exposures with pharmacologically treated NAS was evaluated by using multivariable logistic regression, controlling for maternal and infant factors and clustered by hospital.
RESULTS:
Among 112 029 maternal-infant dyads, we confirmed 822 cases of NAS, of which 598 (72.7%) were cases of pharmacologically treated NAS. Infants who developed pharmacologically treated NAS were more likely to have been exposed to antenatal benzodiazepines compared with infants with confirmed NAS not treated pharmacologically (40.9% vs 30.8%; P = .008). In adjusted analyses, benzodiazepine exposure was associated with greater risk of developing pharmacologically treated NAS (odds ratio: 1.51; 95% confidence interval: 1.04–2.21). Alternatively, exposure to tobacco, marijuana, cocaine, gabapentin, and selective serotonin reuptake inhibitors were not associated with increased risk of developing pharmacologically treated NAS.
CONCLUSIONS:
Among a population of infants with intrauterine polysubstance exposure, benzodiazepine exposure was an independent predictor of an infant developing pharmacologically treated NAS. Obtaining history of antenatal benzodiazepine exposure among opioid-exposed infants may allow for risk stratification and development of personalized care plans.
Opioid use in the United States increased sharply over the previous 2 decades, including among pregnant women.1,2 Pregnant women who use opioids have higher rates of polysubstance use compared with pregnant women who do not use opioids.3,4 For example, when compared with pregnant women who do not use an opioid, pregnant women who use or are prescribed opioids are more likely to use legal substances (eg, alcohol and tobacco), illicit substances (eg, cocaine and marijuana), and be prescribed psychoactive medications (eg, gabapentin, antidepressants, and benzodiazepines).5,6 Use of multiple substances places a pregnant woman and her developing fetus at an increased risk for adverse outcomes, including maternal overdose, preterm labor, and stillbirth.7–9
Neonatal abstinence syndrome (NAS) is a postnatal withdrawal syndrome exhibited by a subset of opioid-exposed infants.10 NAS is characterized by hyperactivity of the central, autonomic, and gastrointestinal systems with a wide spectrum of clinical symptoms ranging from increased tone and tremors to more severe manifestations, including poor feeding, sleep disturbances, and seizures. Infants with severe manifestations of the syndrome often require pharmacotherapy, prolonging their hospital course.11 Over the last decade, the incidence of NAS increased nearly sevenfold and, in 2014, accounted for an estimated $500 million in hospital costs nationwide.12–14 However, factors that contribute to disease severity remain poorly understood.15 In previous studies, researchers evaluated the association of polysubstance use among infants with NAS and demonstrated that exposure to additional substances may alter the severity and timing of onset of withdrawal symptoms.16–18 However, much of this literature has been conducted in infants exposed to maternal methadone, which may limit generalizability to other populations. In other research, the association of multiple substance use was evaluated by grouping together the effect of coexposure to benzodiazepines, cocaine, or opioids in addition to methadone, limiting the ability to understand the association of individual substances with NAS severity.19 To fill these gaps, we studied a cohort of pregnant women with infants who were diagnosed with NAS to determine which substance exposures increased risk of pharmacologically treated NAS. We hypothesized that benzodiazepine exposure would increase risk of an infant developing pharmacologically treated NAS.
Methods
Study Design and Setting
This retrospective cohort study was conducted by using data from TennCare, Tennessee’s Medicaid program. The Medicaid program is an ideal source of data for infants with NAS because >80% of infants diagnosed with the syndrome are in the program in the United States.14 Medicaid administrative data were combined with vital records to create maternal-infant dyads for women and infants enrolled in TennCare. These data included outpatient, inpatient, and prescription data and underwent extensive chart review between 2013 and 2016. By using a standardized data collection process, chart review allowed for detailed understanding of antenatal exposures and outcomes. The study was approved with a waiver of informed consent by our medical center institutional review board, the Tennessee Department of Health, and the Bureau of TennCare.
Cohort Assembly and Outcome
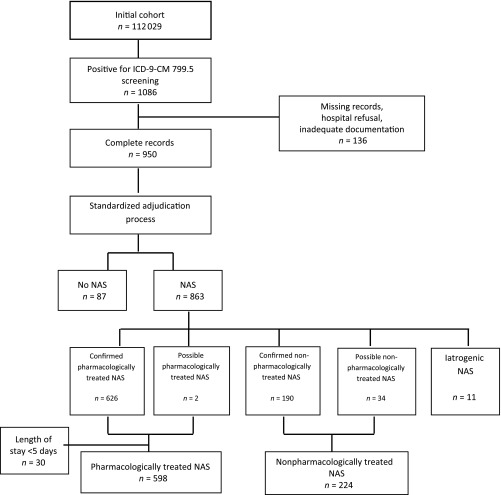
Maternal and infant dyads were included in the study if (1) the mother was 15 to 44 years old at the time of delivery; (2) the mother was enrolled in TennCare at least 30 days before delivery; (3) the infant was enrolled in TennCare within 30 days of delivery; and (4) the infant was born between January 1, 2009, and December 31, 2011. By using these criteria, 112 029 maternal-infant dyads were identified. Infants with NAS were identified by using a 2-step process. First, all administrative records were screened for the presence of the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) code 779.5, drug withdrawal syndrome in the newborn, in any diagnostic field. Medical records were requested for infants when 779.5 appeared in any diagnostic field. All medical records obtained (87% of requested) underwent a standardized chart review and adjudication process to confirm the diagnosis of NAS and obtain additional clinical information.20 The adjudication process was performed by 2 independent physicians using a standardized algorithm. On review of the medical charts, some hospitals presumptively treated opioid-exposed infants and discharged them home early. To account for this practice, we defined pharmacologically treated NAS as an infant treated with a medication for NAS that had a length of hospital stay ≥5 days.21 Infants treated nonpharmacologically were defined as infants with confirmed NAS who were not treated with a medication for NAS (Fig 1). Infants with iatrogenic withdrawal were excluded.
FIGURE 1.
Flowchart for cohort derivation. Flowchart depicting how the study population was defined.
Exposures
Maternal substance use was captured by multiple sources, including chart review, prescription claims, birth certificate data, claims data, and maternal and infant toxicology results. Opioid exposures were categorized as immediate release (IR) (eg, Percocet and Vicodin) or sustained release (SR; eg, MS Contin). Maintenance medications (eg, buprenorphine and methadone) were combined with SR. Opioid and benzodiazepine exposures were captured by outpatient prescription claims, positive maternal or infant toxicology test results at the time of delivery, and chart review. Additional prescription medication exposures included selective serotonin reuptake inhibitors (SSRIs) and gabapentin that were captured by outpatient prescriptions filled within the last 30 days of pregnancy or chart review. Illicit drug exposures, including tetrahydrocannabinol (THC), cocaine, methamphetamines, and phencyclidine (PCP), were captured by positive maternal or infant toxicology test results at the time of delivery or chart review. Tobacco use during pregnancy was captured by birth certificate data and claims data by using ICD-9-CM diagnostic codes for tobacco use (305.1, V15.82, 989.84, and 649.0×; Supplemental Tables 4–6).
Maternal and Infant Characteristics
Maternal and infant demographic information was obtained from birth certificates, including maternal age, years of education, race, and infant sex. Birth weight and gestational age were obtained from infant birth certificate. Because the opioid-using population is at increased risk for hepatitis C (HCV),22,23 depression, and anxiety,24 we obtained data for all mothers from birth certificates and hospital administrative records by using the following diagnostic codes: HCV: 070.41, 070.44, 070.51, 070.54, and 070.7×; depression: 296.2×, 296.3×, and 311; and anxiety disorder: 300.×. Respiratory distress and poor feeding are 2 common clinical manifestations of NAS; as such, data on these symptoms were obtained for all infants in the study.15 Clinical signs of respiratory distress and feeding difficulties, including transient tachypnea of newborn (770.6), meconium aspiration syndrome (770.1, 770.11, and 770.12), respiratory distress syndrome (769.×), other neonatal respiratory diagnoses (770.×, excluding the codes mentioned above and 770.7), and feeding difficulty (779.3×), were obtained from hospital administrative records by using the aforementioned diagnostic codes.
Data Analysis
We used t tests for continuous variables and χ2 tests for categorical variables for univariate analysis. A logistic regression model was fit with exposures chosen a priori on the basis of the literature and clinical relevance. The model outcome was the development of pharmacologically treated NAS and opioid type, infant sex,25 infant birth weight, infant gestational age,26 maternal HCV, antenatal tobacco,16 benzodiazepine, THC, cocaine, SSRI,16 and gabapentin27 exposure as predictors. Maternal HCV status was included in the model as a proxy for intravenous drug use. The model was clustered by hospital site. Two sensitivity analyses were performed, one in which the infants without an opioid exposure confirmed in the medical record were excluded and another in which possible cases and controls were excluded as defined by the adjudication process (Supplemental Tables 4–6).
Results
We collected data for 598 infants with pharmacologically treated NAS, 224 nonpharmacologically treated infants with NAS, and their respective mothers. Mothers of infants with nonpharmacologically treated NAS and pharmacologically treated NAS were similar with regard to race, years of education, and rates of depression or anxiety (Table 1). Mothers of infants with pharmacologically treated NAS were significantly more likely than mothers of infants with nonpharmacologically treated NAS to have HCV (14% vs 8%, P = .03) and were older (average age of 26.2 vs 25.5 years, P = .05). Infants had similar rates of prematurity, low birth weight status, sex, and rates of feeding difficulties; however, infants with pharmacologically treated NAS were more likely to have respiratory complications than infants with nonpharmacologically treated NAS (27% vs 19%; P = .02).
TABLE 1.
Maternal and Infant Characteristics Among Infants Diagnosed With Pharmacologically Treated NAS and Nonpharmacologically Treated NAS
| Maternal Characteristics | Nonpharmacologically Treated NAS, n = 224 | Pharmacologically Treated NAS, n = 598 | P |
|---|---|---|---|
| Age in y, mean | 25.5 | 26.2 | .05 |
| Education in y, mean | 12 | 12 | .98 |
| Race, n (%) | |||
| African American | <10a (1.8) | 15 (2.5) | .54 |
| White | 220 (98) | 583 (97) | .47 |
| Depression or anxiety, n (%) | 41 (18) | 120 (20) | .57 |
| HCV, n (%) | 19 (8) | 84 (14) | .03 |
| Infant characteristics, n (%) | |||
| Preterm (<37 wk gestational age) | 41 (18) | 92 (15) | .31 |
| Low birth wt (<2500 g) | 50 (22) | 131 (21) | .89 |
| Female | 110 (49) | 274 (45) | .40 |
| Respiratory complications | 44 (19) | 165 (27) | .02 |
| Feeding difficulties | 16 (7) | 54 (9) | .39 |
| Seizures | <10a (0.45) | 24 (4) | .008 |
Values of <10 suppressed.
The type of opioid exposure varied among the cohort. Exclusive exposure to IR opioids was more common among infants with nonpharmacologically treated NAS compared with infants with pharmacologically treated NAS (47.7% vs 36.6%, P = .004). Exposure to both IR and SR opioids was more common among infants with pharmacologically treated NAS as compared with infants with nonpharmacologically treated NAS (40.1% vs 25.4%, P < .001). Across both groups, 3% of infants did not have an opioid exposure confirmed by medical record review. Sensitivity analyses excluding infants without an opioid exposure confirmed in medical record were similar to our primary analysis (Supplemental Tables 4–6).
Infants with nonpharmacologically treated NAS and pharmacologically treated NAS were frequently exposed to multiple substances. Both groups had similar rates of exposure to tobacco, THC, cocaine, methamphetamines, PCP, SSRIs, tricyclic antidepressants, and gabapentin. However, infants with pharmacologically treated NAS were significantly more likely to have had antenatal exposure to benzodiazepines as compared with infants with nonpharmacologically treated NAS (40.9% vs 30.8%, P = .008; Table 2). In adjusted analyses that accounted for maternal HCV, prematurity, infant sex, opioid type, and exposures to other substances (including tobacco, marijuana, cocaine, gabapentin, and SSRIs), pharmacologically treated NAS was associated with opioid and benzodiazepine exposures. Benzodiazepine exposure was associated with 51% increased odds of an infant developing pharmacologically treated NAS (odds ratio: 1.51; 95% confidence interval: 1.04–2.21). Exposure to IR opioids was associated with greater risk of pharmacologically treated NAS (odds ratio: 1.65; 95% confidence interval: 1.01–2.69), as was exposure to SR opioids (odds ratio: 2.06; 95% confidence interval 1.41–3.02; Table 3).
TABLE 2.
Bivariate Analysis of Licit and Illicit Exposures Among Infants With Pharmacologically Treated NAS and Nonpharmacologically Treated NAS
| Exposure | Nonpharmacologically Treated NAS, n (%) | Pharmacologically Treated NAS, n (%) | P |
|---|---|---|---|
| Illicit substances | |||
| THC | 68 (30) | 155 (25) | .20 |
| Cocaine | 19 (8) | 66 (11) | .28 |
| Methamphetamines | <10a (2) | 20 (3) | .41 |
| PCP | <10a (0.9) | <10a (1.3) | .60 |
| Additional substances | |||
| Benzodiazepines | 69 (30.8) | 245 (40.9) | .008 |
| SSRIs | 33 (14.7) | 102 (17.1) | .4 |
| Tobacco | 176 (78.5) | 491 (82.1) | .25 |
| Gabapentin | <10a (1.3) | 16 (2.7) | .26 |
| Tricyclic antidepressants | <10a (2.2) | <10a (1.5) | .47 |
Values of <10 suppressed.
TABLE 3.
Multivariable Logistic Regression Analysis of Infant Characteristics and Exposures Associated With Pharmacologically Treated NAS
| Exposure | aOR | 95% Confidence Interval | P |
|---|---|---|---|
| Infant characteristics | |||
| Female | 0.88 | 0.65–1.19 | .42 |
| Low birth wt (<2500 g) | 1.08 | 0.70–1.68 | .71 |
| Preterm (<37 wk gestation) | 0.70 | 0.46–1.06 | .09 |
| Opioid exposure type | |||
| IR opioid | 1.65 | 1.01–2.69 | .04 |
| SR opioid | 2.06 | 1.41–3.02 | >.001 |
| Additional licit and illicit exposures | |||
| Tobacco | 1.13 | 0.70–1.83 | .59 |
| SSRIs | 1.17 | 0.70–1.94 | .54 |
| Benzodiazepine | 1.51 | 1.04–2.21 | .02 |
| THC | 0.77 | 0.54–1.09 | .14 |
| Cocaine | 1.38 | 0.81–2.36 | .22 |
| Gabapentin | 1.64 | 0.51–5.2 | .40 |
| Maternal HCV infection | 1.53 | 0.77–3.02 | .22 |
aOR, adjusted odds ratio.
Discussion
In this retrospective cohort study of >800 infants with NAS, infants exposed to benzodiazepines had >50% increased odds of developing pharmacologically treated NAS after controlling for additional exposures and characteristics. This association was significant both in univariate analysis and in the prespecified multivariable logistic regression model, providing evidence that among infants with NAS, antenatal benzodiazepine exposure confers increased odds of an infant developing pharmacologically treated NAS.
This large population-based cohort, which underwent extensive chart review to detail antenatal exposures, builds on earlier work in which researchers identified an association between NAS severity and antenatal benzodiazepine exposure. Seligman et al28 found an adjusted mean length of treatment of NAS to be 14.4 days longer for infants of mothers receiving methadone with concomitant benzodiazepine use compared with those without concomitant use. Importantly, in our study, we demonstrate the relationship between antenatal benzodiazepine exposure and NAS severity in a cohort of women with variable antenatal opioid exposure, not limited to methadone, increasing the generalizability of our results. In another study in methadone-maintained women, Cleary et al19 demonstrated that concomitant drug use (defined as additional opioid, benzodiazepine, or cocaine use in maternal urine toxicology testing in the 4 weeks before delivery or neonatal urine toxicology testing in the first days of life) was associated with a longer median duration of hospitalization (6 vs 5 days, P = .03). Rather than reliance on intermittent toxicology testing, the chart reviews that we performed enabled clear delineation of individual exposures. Our findings differed from those of Dryden et al,29 whose retrospective analysis found that benzodiazepine coexposure in mothers on methadone did not confer increased odds of pharmacologically treated NAS after controlling for confounders with multivariable logistic regression modeling. The difference from our results may be explained by the variety of maternal opioid exposure in our study population.
Benzodiazepines are known to potentiate the action of the γ-aminobutyric acid receptor, the most common inhibitory neurotransmitter in the central nervous system.30 Isolated benzodiazepine withdrawal has been described in the adult psychiatric literature; however, it is not well described in the pediatric setting.30,31 Isolated benzodiazepine withdrawal is characterized by anxiety, tremors, confusion, insomnia, and convulsions. Thus, the clinical manifestations of the infants with pharmacologically treated NAS in our study may have been due to simultaneous withdrawal of both benzodiazepine and opioid that could not be clinically differentiated.
Our findings have heightened significance in light of emerging data in which the significant rise in the concurrent use of opioids and benzodiazepines is demonstrated.5,32 Sun et al32 found an 80% relative increase in concurrent benzodiazepine and opioid use from 2001 to 2013, which was associated with increased risk of an emergency department visit, inpatient admission, or opioid overdose among a privately insured adult population. The combination of opioids and benzodiazepines is associated with an increased risk of death from overdose.33 As a result, the Centers for Disease Control and Prevention and Food and Drug Administration have made policy changes focused on increasing the public understanding of the dangers of concurrent benzodiazepine and opioid use. These changes include the addition of boxed warnings and creation of patient-focused medication guides for prescription opioids and benzodiazepines.34,35
Our findings contribute to the growing body of research focused on identifying the key drivers of NAS severity.18,36 Notably, in our study, we focused on the role of coexposures modifying the severity of NAS in contrast to the Patrick et al16 study in which researchers identified SSRI and tobacco coexposure increasing the likelihood, not severity, of developing NAS. Future research could use multiple factors, including benzodiazepine exposure, as part of a clinical prediction rule to make care for opioid-exposed infants more efficient after birth. Currently the American Academy of Pediatrics recommends that all opioid-exposed infants be observed in the hospital for 4 to 7 days.10 Without the ability to identify infants at high risk for disease expression and severity, both clinicians and policymakers are limited in their ability to stratify risk or create risk-specific policy statements. As the number of infants with NAS continues to rise, applying knowledge of risk prediction to clinical care will become increasingly important to improving the quality of care delivered to opioid-exposed infants.
Limitations
Our study has several limitations to consider, several of which are specific to the use of administrative data. Factors distinct from infant signs or symptoms that may have influenced the decision to treat are unknown; however, we clustered by hospital to account for hospital-level variation in treatment decisions. Prescription claims data for benzodiazepine provided limited exposure data because of TennCare policies during the study period that restricted payment for this drug. This limited our ability to quantify benzodiazepine exposure and specific timing within the pregnancy. The reliance on self-report of substance exposure during pregnancy may have resulted in some cases of exposure misclassification. Toxicology testing used to define exposures, including testing of urine, umbilical cord, blood, and meconium, was conducted at hospitals across the state with the laboratory using different testing procedures with varying sensitivity and specificity for identifying drugs of interest. The data source is restricted to 1 state, Tennessee, and is reflective of the study period (2009–2011), which may not reflect current drug use patterns, potentially limiting generalizability. However, the use of multiple data sources allowed for a detailed understanding of antenatal exposures and the ability to focus on individual exposures and their association with disease severity among a population-based cohort.
Conclusions
In a large, population-based, retrospective cohort study supplemented by chart review, we found that antenatal benzodiazepine exposure is associated with >50% increased odds of an infant developing pharmacologically treated NAS. Prescribing providers for pregnant women should consider the adverse maternal and neonatal outcomes associated with concomitant benzodiazepine and opioid exposure, exercise caution when prescribing benzodiazepines, and warn pregnant women about the risks of taking them without prescription. Pediatricians caring for opioid-exposed infants should be aware of the increased risk for the development of pharmacologically treated NAS in the setting of antenatal benzodiazepine coexposure.
Acknowledgments
We thank the Tennessee Bureau of TennCare of the Department of Finance and Administration that provided the data. We also thank the Tennessee Department of Health, Office of Health Statistics for providing vital records data. We acknowledge Ann Stark, MD, for her assistance in preparation of the article.
Footnotes
Dr Sanlorenzo conceptualized and designed the study, conducted the initial analyses, and drafted the initial manuscript; Dr Patrick conceptualized and designed the study, conducted case adjudication analysis, reviewed and revised the manuscript, and supervised the study; Dr Cooper conceptualized and designed the study and critically reviewed the manuscript; Dr Maalouf conducted case adjudication analysis; Ms Dudley and Ms Stratton supervised data collection and managed the data; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the National Institute On Drug Abuse of the National Institutes of Health (NIH) under awards K23DA038720 (Dr Patrick) and R01DA045729 (Dr Patrick), the National Institute of Child Health and Human Development under award 5T32HD060554-08 (Dr Cooper), and the John and Leslie Hooper Neonatal-Perinatal Medicine Endowment Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Funded by the NIH.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Gallagher BK, Shin Y, Roohan P. Opioid prescriptions among women of reproductive age enrolled in Medicaid - New York, 2008-2013. MMWR Morb Mortal Wkly Rep. 2016;65(16):415–417 [DOI] [PubMed] [Google Scholar]
- 2.Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF. Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstet Gynecol. 2014;123(5):997–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kozhimannil KB, Graves AJ, Levy R, Patrick SW. Nonmedical use of prescription opioids among pregnant U.S. women. Womens Health Issues. 2017;27(3):308–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forray A. Substance use during pregnancy. F1000Res. 2016;5:F1000 Faculty Rev-887 [Google Scholar]
- 5.Hwang CS, Kang EM, Kornegay CJ, Staffa JA, Jones CM, McAninch JK. Trends in the concomitant prescribing of opioids and benzodiazepines, 2002-2014. Am J Prev Med. 2016;51(2):151–160 [DOI] [PubMed] [Google Scholar]
- 6.Jarlenski M, Barry CL, Gollust S, Graves AJ, Kennedy-Hendricks A, Kozhimannil K. Polysubstance use among US women of reproductive age who use opioids for nonmedical reasons. Am J Public Health. 2017;107(8):1308–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bada HS, Das A, Bauer CR, et al. Low birth weight and preterm births: etiologic fraction attributable to prenatal drug exposure. J Perinatol. 2005;25(10):631–637 [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Latif ME, Oei J, Craig F, Lui K; NSW and ACT NAS Epidemiology Group. Profile of infants born to drug-using mothers: a state-wide audit. J Paediatr Child Health. 2013;49(1):E80–E86 [DOI] [PubMed] [Google Scholar]
- 9.Whiteman VE, Salemi JL, Mogos MF, Cain MA, Aliyu MH, Salihu HM. Maternal opioid drug use during pregnancy and its impact on perinatal morbidity, mortality, and the costs of medical care in the United States. J Pregnancy. 2014;2014:906723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hudak ML, Tan RC; Committee on Drugs Committee on Fetus and Newborn American Academy of Pediatrics. Neonatal drug withdrawal [published correction appears in Pediatrics. 2014;133(5):937]. Pediatrics. 2012;129(2). Available at: www.pediatrics.org/cgi/content/full/129/2/e540 [Google Scholar]
- 11.Substance Abuse and Mental Health Services Administration. A collaborative approach to the treatment of pregnant women with opioid use disorders. HHS publication No. 16-4978. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2016
- 12.Patrick SW, Davis MM, Lehmann CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012 [published correction appears in J Perinatol. 2015;35(8):667]. J Perinatol. 2015;35(8):650–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tolia VN, Patrick SW, Bennett MM, et al. Increasing incidence of the neonatal abstinence syndrome in U.S. neonatal ICUs. N Engl J Med. 2015;372(22):2118–2126 [DOI] [PubMed] [Google Scholar]
- 14.Winkelman TNA, Villapiano N, Kozhimannil KB, Davis MM, Patrick SW. Incidence and costs of neonatal abstinence syndrome among infants with Medicaid: 2004-2014. Pediatrics. 2018;141(4):e20173520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McQueen K, Murphy-Oikonen J. Neonatal abstinence syndrome. N Engl J Med. 2016;375(25):2468–2479 [DOI] [PubMed] [Google Scholar]
- 16.Patrick SW, Dudley J, Martin PR, et al. Prescription opioid epidemic and infant outcomes. Pediatrics. 2015;135(5):842–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaltenbach K, Jones HE. Neonatal abstinence syndrome: presentation and treatment considerations. J Addict Med. 2016;10(4):217–223 [DOI] [PubMed] [Google Scholar]
- 18.Huybrechts KF, Bateman BT, Desai RJ, et al. Risk of neonatal drug withdrawal after intrauterine co-exposure to opioids and psychotropic medications: cohort study. BMJ. 2017;358:j3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cleary BJ, Eogan M, O’Connell MP, et al. Methadone and perinatal outcomes: a prospective cohort study. Addiction. 2012;107(8):1482–1492 [DOI] [PubMed] [Google Scholar]
- 20.Maalouf FI, Cooper WO, Stratton SM, et al. Positive predictive value of administrative data for neonatal abstinence syndrome. Pediatrics. 2019;143(1):e20174183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Summey J, Chen L, Mayo R, et al. Early treatment innovation for opioid-dependent newborns: a retrospective comparison of outcomes, utilization, quality, and safety, 2006-2014. Jt Comm J Qual Patient Saf. 2018;44(6):312–320 [DOI] [PubMed] [Google Scholar]
- 22.Suryaprasad AG, White JZ, Xu F, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006-2012. Clin Infect Dis. 2014;59(10):1411–1419 [DOI] [PubMed] [Google Scholar]
- 23.Puzhko S, Roy É, Jutras-Aswad D, et al. High hepatitis C incidence in relation to prescription opioid injection and poly-drug use: assessing barriers to hepatitis C prevention. Int J Drug Policy. 2017;47:61–68 [DOI] [PubMed] [Google Scholar]
- 24.Sullivan MD, Edlund MJ, Steffick D, Unützer J. Regular use of prescribed opioids: association with common psychiatric disorders. Pain. 2005;119(1–3):95–103 [DOI] [PubMed] [Google Scholar]
- 25.Charles MK, Cooper WO, Jansson LM, Dudley J, Slaughter JC, Patrick SW. Male sex associated with increased risk of neonatal abstinence syndrome. Hosp Pediatr. 2017;7(6):328–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaltenbach K, Holbrook AM, Coyle MG, et al. Predicting treatment for neonatal abstinence syndrome in infants born to women maintained on opioid agonist medication. Addiction. 2012;107(suppl 1):45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carrasco M, Rao SC, Bearer CF, Sundararajan S. Neonatal gabapentin withdrawal syndrome. Pediatr Neurol. 2015;53(5):445–447 [DOI] [PubMed] [Google Scholar]
- 28.Seligman NS, Salva N, Hayes EJ, Dysart KC, Pequignot EC, Baxter JK. Predicting length of treatment for neonatal abstinence syndrome in methadone-exposed neonates. Am J Obstet Gynecol. 2008;199(4):396.e1–396.e7 [DOI] [PubMed] [Google Scholar]
- 29.Dryden C, Young D, Hepburn M, Mactier H. Maternal methadone use in pregnancy: factors associated with the development of neonatal abstinence syndrome and implications for healthcare resources. BJOG. 2009;116(5):665–671 [DOI] [PubMed] [Google Scholar]
- 30.Pétursson H. The benzodiazepine withdrawal syndrome. Addiction. 1994;89(11):1455–1459 [DOI] [PubMed] [Google Scholar]
- 31.Ista E, van Dijk M, Gamel C, Tibboel D, de Hoog M. Withdrawal symptoms in children after long-term administration of sedatives and/or analgesics: a literature review. “Assessment remains troublesome”. Intensive Care Med. 2007;33(8):1396–1406 [DOI] [PubMed] [Google Scholar]
- 32.Sun EC, Dixit A, Humphreys K, Darnall BD, Baker LC, Mackey S. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ. 2017;356:j760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49 [DOI] [PubMed] [Google Scholar]
- 35.US Food and Drug Administration. FDA requires strong warnings for opioid analgesics, prescription opioid cough products, and benzodiazepine labeling related to serious risks and death from combined use. 2016. Available at: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm518697.htm. Accessed August 31, 2016
- 36.Desai RJ, Huybrechts KF, Hernandez-Diaz S, et al. Exposure to prescription opioid analgesics in utero and risk of neonatal abstinence syndrome: population based cohort study. BMJ. 2015;350:h2102. [DOI] [PMC free article] [PubMed] [Google Scholar]