Abstract
Objective
Amyotrophic lateral sclerosis (ALS) is an adult-onset neurodegenerative disease characterized by the progressive loss of the upper and lower motor neurons that progresses to paralysis of almost all skeletal muscles of the extremities, bulbar, and respiratory system. Although most ALS cases are sporadic, about 10% are dominantly inherited. We herein report an atypical phenotype of familial ALS (fALS). To elucidate the phenotype-genotype correlation of this atypical phenotype of fALS, clinical and genetic investigations were performed.
Methods and Patients
Five sibling patients (three men, two women) from a Japanese family and one healthy sibling (a woman) were clinically interviewed and examined. Genetic analyses, including genome-wide linkage analyses and whole-exome sequencing, were performed using genomic DNA extracted from the peripheral blood samples of these siblings.
Results
The clinical features of fALS are characterized by slow progression (mean duration of the disease±standard deviation [SD]: 19.6±3.9 years) and lower extremities-predominant late-onset muscular weakness (mean onset of muscular weakness±SD: 52.8±2.6 years). Genetic analyses revealed novel heterozygous missense mutations of c.2668C>T, p.R890C in the PLEC gene and c.421G>C, p.V141L in the ST3GAL6 gene in all affected siblings.
Conclusion
A new atypical fALS family with a benign clinical course is herein reported. We identified two candidate gene mutations of PLEC and ST3GAL6 linked to this phenotype.
Keywords: familial amyotrophic lateral sclerosis, fALS, PLEC, ST3GAL6
Introduction
Amyotrophic lateral sclerosis (ALS) is an adult-onset neurodegenerative disease characterized by progressive loss of the upper and lower motor neurons that progresses to paralysis of almost all skeletal muscles of the extremities and bulbar regions. Symptoms of respiratory failure are eventually observed in almost all ALS cases within two to three years from the onset (1). Although most ALS cases are sporadic, about 10% are dominantly inherited. Several genes, such as SOD1, TARDBP, FUS, OPTN, C9ORF72, and ANG, have been identified as causative genes of familial ALS (fALS) (2). However, the genetic etiologies of one-third of familial cases remain to be elucidated (2).
The clinical phenotypes, including the age at onset, and temporal forms of symptom progression in fALS are substantially heterogeneous. For example, the SOD1 Asp90Ala and His46Arg mutations are reportedly associated with a very long survival, whereas the SOD1 Ala4Val mutation can cause aggressive disease progression (2-4).
We herein report a new family with fALS in which five Japanese siblings presented with slowly progressing upper and lower motor neuron deficits with muscular atrophy. The mode of inheritance in this family is consistent with the autosomal dominant pattern. None of the patients suffered from respiratory failure associated with lower motor neuron deficit. Whole-exome sequencing (WES) and linkage analyses were conducted, and heterozygous missense alterations of the PLEC gene (OMIM 601282, c.2668C>T, p.R890C) and ST3GAL6 gene (OMIM 607156, c.421G>C, p.V141L) were identified as candidate variants.
Materials and Methods
The evaluation of the clinical features
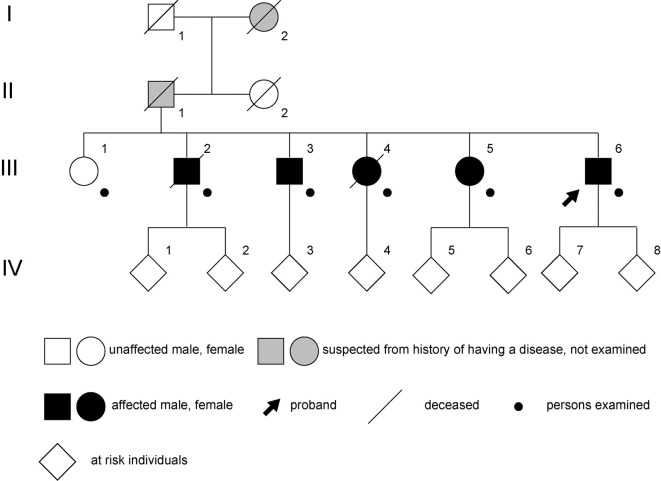
Two neurologists (J.T. and T.O.) interviewed and examined six siblings, including five afflicted patients and one healthy woman. The clinical history of their father, aunt, and grandparents was obtained from interviews with the patients (Fig. 1). The protocol of this study was reviewed and approved by the Institutional Review Boards of Kagoshima University and the University of Tokyo. Written informed consent was obtained from all participants in this study, or in patients with cognitive impairment, from their guardians.
Figure 1.
A family tree of the patients.
WES and data analyses
Genomic DNA of the index patient (III-5) and five siblings was extracted from the peripheral blood using QIAGEN's Puregene Core Kit C (QIAGEN, Valencia, USA) according to the manufacturer's protocol. Exonic sequences were captured from three samples (III-1, III-5, III-6) in Ion AmpliSeq Exome RDY plates using Ion AmpliSeq HiFi Mix (Ion Torrent, Carlsbad, USA). The resulting amplicons were treated with FuPa Reagent (Ion Torrent) to partially digest the primers and phosphorylate the amplicons, which were then ligated to Proton adapters and purified according to the manufacturer's instructions (Ion Torrent). After purification libraries were quantified using quantitative polymerase chain reaction (PCR), they were clonally amplified by emulsion PCR through an Ion Chef System and loaded onto an Ion PI chip v2 BC. This chip was then processed for WES on the Ion Proton platform (Life Technologies, Carlsbad, USA).
The primary data analysis, including alignment to the GRCh37/hg19 human reference genome (UCSC Genome Browser) and variant calling, was performed using the Ion Torrent Suite software program, ver. 4.0.2 (Thermo Fisher Scientific, Waltham, USA), with a plug-in “variant caller” program and default parameters. The resulting VCF file was imported into the CLC genomics workbench, version 7.5 (CLC Bio, Aarhus, Denmark), for filtering and annotation. The variants were checked against dbSNP146, the 1,000 Genomes database, the Exome Aggregation Consortium (ExAC), and the Human Genetic Variation Database (HGVD) (5).
Linkage analyses
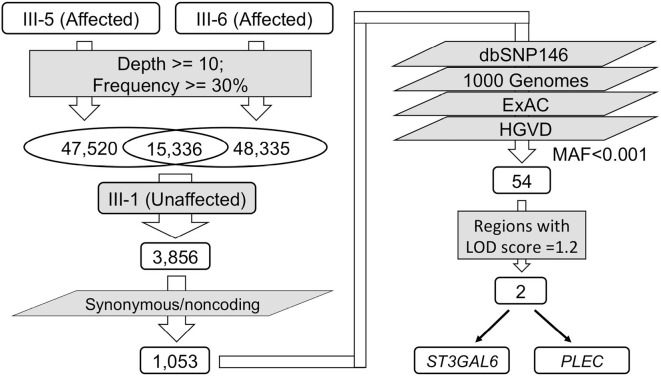
The five affected family members and the unaffected individual were genotyped using the Genome-wide Human SNP array 6.0 (Affymetrix, Santa Clara, USA) according to the manufacturer's instructions. The genotypes of each family member were determined using the Genotyping Console 4.2 (Affymetrix). A parametric multipoint linkage analysis (autosomal dominant model with complete penetrance) was conducted using the pipeline software programs SNP-HiTLink (6) and Allegro version 2 (7), employing single-nucleotide polymorphisms (SNPs) satisfying a p value of >0.001 in the Hardy-Weinberg test, a call rate of >0.98, a genotyping confidence score of <0.02, a minor allele frequency in the controls >0, and intermarker distances of 80 to 120 kb. The candidate variants were validated by PCR followed by Sanger sequencing according to the standard protocols to confirm the genotypes. The analysis workflow is shown in Fig. 2.
Figure 2.
An analysis workflow of the whole-exome sequencing and linkage analyses.
Copy number analyses
Copy number analyses were performed using the SNP typing data with Genotyping Console 4.2 (Affymetrix).
Search for causal variants
The remaining variants were checked against our in-house whole exome or genome databases of 1,153 control subjects and further analyzed in silico for the functional prediction of variants with SIFT (http://sift.jcvi.org/www/SIFT_seq_submit2.html), PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/), and MutationTaster (http://www.mutationtaster.org/) (8).
Results
Clinical features
The proband (III-6) is the sixth sibling of the third generation, which includes one healthy woman and five patients. The proband's father and grandmother were suspected of having the disease based on their history but were not examined by us (Fig. 1).
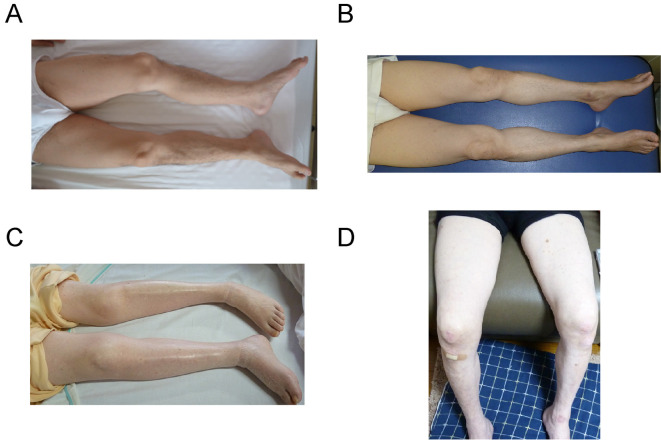
The clinical findings of the patients in the third generation are summarized in Table. Dysarthria, proximal and distal weakness, and muscular atrophy in the lower extremities were observed in all patients (Fig. 3). Hyporeflexia was evident in III-3, III-4, III-5, and III-6, and Babinski's sign was positive in III-4, III-5, and III-6. Tongue atrophy and fasciculation were observed in III-5 (Table). Cerebellar ataxia was not observed in any of the patients.
Table.
Summary of History, Neurological Findings and Neurophysiological Findings of the Patients in Generation III.
| Patients | Age (years) | Duration (years) | |||
|---|---|---|---|---|---|
| At examination | At onset | Onset to cane walk | Onset to wheel-chair | ||
| III-2 | 75 | 57 | 0 | 14 | |
| III-3 | 72 | 52 | 6 | 19 | |
| III-4 | 68 | 53 | NE | NE | |
| III-5 | 64 | 50 | 2 | - | |
| III-6 | 66 | 52 | 4 | 8 | |
| Clinical neurological findings | ||||
|---|---|---|---|---|
| Dysarthria | Tongue atrophy and fasciculation | Dementia | Muscular weakness | |
| + | - | + | B L/E | |
| + | - | - | B L/E | |
| + | - | + | B L/E | |
| + | + | - | B L/E | |
| + | - | - | B L/E | |
| Muscular atrophy | Hyporeflexia | Babinski sign | Fasciculation (except for tongue) |
|
|---|---|---|---|---|
| B L/E | NE | NE | - | |
| B L/E | B U/E L/E | - | - | |
| B L/E | B U/E L/E | L+ | - | |
| B L/E | B U/E L/E | B+ | - | |
| B L/E | B U/E L/E | R+ | - | |
| Neurophysiological examination | |||||
|---|---|---|---|---|---|
| Needle EMG | MEP | ||||
| NE | NE | ||||
| C | NE | ||||
| NE | NE | ||||
| NE | NE | ||||
| C | P | ||||
EMG: electromyography, MEP: motor evoked potential, B: bilateral, R: right, L: left, L/E: lower extremities, U/E: upper extremities, NE: not examined or not identified from clinical history, C: Chronic denervation pattern (High amplitude motor unit potential was observed.), P: prolonged MEP latency and CMCT
Figure 3.
Photographs of the legs of the patients. A: III-6 (proband), B: III-3, C: III-4, D: III-5
III-1, unaffected sibling: 81-year-old woman, right-handed, Japanese
She has no neurological deficit except for conductive hearing loss of the right ear.
III-6, affected, proband: 66-year-old man, right-handed, Japanese
At 52 years of age, he began to feel difficulty walking for the first time. Although he was able to walk with a cane at 56 years of age, he was no longer able to walk even with a cane by 60 years of age. He was admitted to our hospital for an intensive examination at 61 years of age. A neurological examination revealed dysarthria, generalized hyporeflexia, and moderate muscular weakness as well as atrophy in his bilateral lower extremities. Babinski's sign was positive on the right side. A motor and sensory nerve conduction study (NCS) indicated no peripheral nerve lesions. Needle electromyography (NEMG) showed a chronic denervation pattern [high-amplitude motor unit potential (MUP)] but no active denervation patterns. A motor evoked potential (MEP) study showed prolonged latency (27.5 ms) and central motor conduction time (CMCT) (14.15 ms) when recorded from the left abductor pollicis brevis (9). A muscle biopsy was performed from his right quadriceps femoris, with normal findings obtained. Although his weakness of lower extremities has progressively worsened, he can still move about his home using his upper extremities.
III-2, affected: died at 77 years of age, man, right-handed, Japanese
He began to feel difficulty walking and started using a cane to walk at 57 years of age. He became confined to a wheelchair and had difficulty speaking 71 years of age, and dementia developed around this time. He was examined by us at 75 years of age. Since he had moderate dementia, his medical history was obtained through an interview with his wife. A neurological examination revealed mild dementia, agitation, dysarthria, moderate muscular weakness, and atrophy in his bilateral lower extremities. We did not examine his deep tendon reflexes or Babinski's sign because he became irritated during the examination. He died at 77 years of age from pneumonia.
III-3, affected: 77-year-old man, right-handed, Japanese
At 52 years of age, he began to feel difficulty walking. He needed a cane to walk from 58 years of age and became confined to a wheelchair at 71 years of age. He felt difficulty speaking at 66 years of age, a symptom that has been progressing slowly. He was hospitalized for an intensive examination at 72 years of age. A neurological examination revealed dysarthria, generalized hyporeflexia, mild muscular weakness, and atrophy in his bilateral lower extremities. An NCS indicated no peripheral nerve lesions. NEMG showed chronic denervation pattern (high-amplitude MUP) but no active denervation patterns.
III-4, affected: died at 73 years of age, woman, right-handed, Japanese
She began to feel difficulty walking and stumbled frequently at 53 years of age. She suffered fractures of the right lower leg and left foot at 54 and 59 years of age, respectively. She subsequently became unable to move without a walker with wheels. She was examined by us at 68 years of age. A neurological examination revealed mild dementia, dysarthria, generalized hyporeflexia, moderate muscular weakness, and atrophy in her bilateral lower extremities and positivity for left Babinski's sign. She died at 73 years of age from pneumonia.
III-5, affected: 69-year-old woman, right-handed, Japanese
She fell down the stairs and suffered a right fourth toe fracture at 50 years of age. Although she had recovered from the injury by one year later, she began to feel difficulty walking at this time. She used a cane to walk from 52 years of age and became unable to walk without a walker with wheels at 58 years of age. She was examined by us at 64 years of age. A neurological examination revealed dysarthria, dysphasia, mild atrophy, and fasciculation of the tongue, generalized hyporeflexia, mild muscular weakness, and atrophy in her bilateral lower extremities along with bilateral positive Babinski's sign. These neurological signs are steadily worsening.
WES and data analyses
WES yielded more than 40 million mapped reads with >95% on target in 2 patients (III-5 and III-6) and their healthy sister (III-1). More than 90% of the target regions were covered ≥10-fold for all three cases, and the average depths were 121.5, 119.7, and 124.0, respectively.
For these 2 patients (III-5 and III-6), a total of 47,520 and 48,335 variants were called, with a variant-calling threshold depth ≥10 and an alteration frequency ≥30%. The 2 patients shared 15,336 heterozygous variants, with 3,856 variants being entirely absent in their healthy sister (III-1). After excluding the variants with a minor allele frequency ≥0.001 in dbSNP146, 1,000 Genomes, ExAC, or HGVD, a total of 54 nonsynonymous or splicing site heterozygous variants remained (Fig. 2) (Supplementary material). None of these variants were identified among known disease-causing genes of Charcot-Marie-Tooth (CMT) disease, hereditary spastic paraplegia (HSP), or fALS.
Identifying putative causative mutations
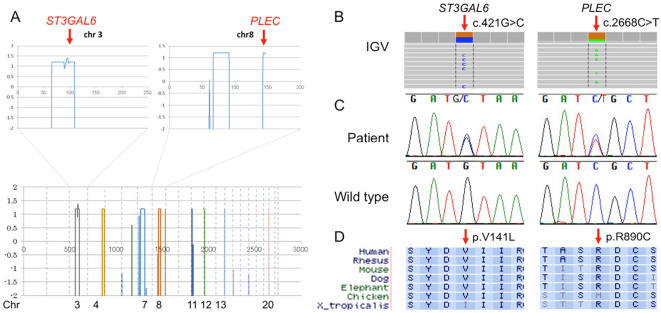
A parametric linkage analyses using SNP data revealed candidate regions with maximum Logarithm of Odds (LOD) scores of 1.2 on chromosomes 3, 4, 7, 8, 11, 12, 13, and 20 spanning 170 Mb. Among the 54 remaining exome variants, only 2 heterozygous missense variants were located within the candidate regions determined by the linkage analysis: c.2668C>T (p.R890C) of PLEC on chromosome 8 and c.421G>C (p.V141L) of ST3GAL6 on chromosome 3 (Fig. 4A). Both variants were validated using Sanger sequencing in all five affected siblings (Fig. 4B and C). However, aside from CHMP2B, there are no known disease-causing fALS or familial frontotemporal dementia/ALS gene loci located in these regions, including SOD1, SETX, FUS, VAPB, ANG, TARDBP, FIG4, OPTN, VCP, PFN1, ERBB4, HNRNPA1, MATR3, TUBA4A, C9orf72, CHCHD10, SQSTM1, TBK1, and CCNF. We also checked known CMT or HSP genes located in these linked regions, and no suspected variants were found. In addition, we did not observe any novel copy number variations involving exons in the candidate regions.
Figure 4.
A linkage analysis and identification of variants in ST3GAL6 and PLEC. (A) The linkage analysis reveals multiple regions with a maximum LOD score of 1.2, in which two variants in ST3GAL6 (chromosome 3) and PLEC (chromosome 8) were found within the exome data. (B) Variants in ST3GAL6 (c.421G>C) and PLEC (c.2668C>T), visualized using IGV. (C) Sequencing chromatograms of the present patients and wild type. (D) Cross-species conservation of V141 (plectin) and R890 (sialyltransferases) (UCSC Genome Browser).
The two candidate variants were observed in neither the in-house exome nor the genome database of 1,153 control subjects. Both variants involve highly conserved amino acid residues (Fig. 4D). We also noted no other variants in ST3GAL6 in the 10 index patients with genetically undiagnosed fALS by Sanger sequencing. In addition, based on exome sequencing of 33 independent index patients with genetically undiagnosed fALS, we did not find any other variants in ST3GAL6 or variants considered to be likely pathogenic in PLEC. The PLEC variant was predicted to be functionally deleterious by all three in silico tools [PolyPhen-2 (Score: 1.0), SIFT (Score: 0), and MutationTaster (Score: 180; disease causing)]. The variant in ST3GAL6 was predicted to be deleterious in both SIFT (Score: 0.004) and MutationTaster (Score: 32; disease causing) but benign in PolyPhen-2 (Score: 0.002).
Discussion
The patients of the family examined suffered from predominant lower motor neuron symptoms. With regard to upper motor neuron symptoms, III-4, III-5, and III-6 were positive for Babinski's sign. The prolonged CMCT observed in III-5 may also have been caused by upper motor neuron disturbance. According to the El Escorial criteria, III-4, III-5, and III-6 who were positive for Babinski's sign were diagnosed with possible ALS (10). The prolonged latency of MEP and CMCT observed in III-6 may support the diagnosis of ALS (9). III-2 and III-3 were diagnosed with suspected ALS because they had lower motor neuron symptoms but no upper neuron symptoms (10). None of the patients had sensory disturbances.
We performed NCS and NEMG in III-3 and III-6 and a muscle biopsy in III-6 to rule out myopathy or peripheral neuropathy. Spinocerebellar ataxias (SCA) was another potential differential diagnosis. In particular, SCA36 is known to affect motor neurons in addition to causing cerebellar ataxia (11). However, none of the afflicted family members showed ataxic speech, such as explosive or scanning speech, although all showed dysarthria caused by lower motor neuron involvement. None showed any other cerebellar signs. Although the symptoms and neurological findings characterized by weakness of the bilateral lower extremities and generalized hyporeflexia resembled those of CMT disease, an NCS of our patients showed no prolonged latency, decrement of conduction velocity, or amplitude of compound muscle action potential.
The clinical characteristics of the patients in this family are late-onset muscular weakness, atrophy, and very slow progression. Despite 14-20 years passing since the onset of muscular weakness, none of the patients showed respiratory failure due to motor neuron dysfunction. The mean (± standard deviation) timing of disease onset of muscular weakness and the duration of affliction in this family are 52.8±2.6 and 19.6±3.9 years, respectively.
Based on WES and linkage analyses using specimens of five patients and one healthy sibling of the third generation, we identified two mutations of the PLEC and ST3GAL6 genes as the putative causal mutations for this family. These mutations have never been reported to be associated with fALS. All five patients had mutations in these two genes, whereas the one healthy sibling lacked both.
PLEC encodes plectin, which is a versatile cytoskeletal linker protein of substantial size (>500 kDa) abundantly expressed in a wide variety of mammalian tissues and cell types (12). Homozygous or compound heterozygous mutations, of which the majority are loss-of-function mutations, have been identified in patients with epidermolysis bullosa simplex with muscular dystrophy (13-17). Plectin deficiency can reportedly cause structural and functional alterations of mitochondria, resulting in myofiber degeneration. Compound heterozygous missense mutations of PLEC were recently reported in a family with early-onset limb-girdle muscular dystrophy (18). However, no mutations of PLEC related to motor neuron disease (MND) have been reported thus far. PLEC plays an important role in cell adhesion, so mutations in this gene could conceivably affect signal processing between cells, leading to the malfunction of motor neurons.
The protein encoded by the ST3GAL6 gene is a member of the sialyltransferase family. Sialyltransferases are enzymes that transfer the sialic acid moiety from cytidine 5'-monophospho-N-acetylneuraminic acid to the terminal positions on sugar chains of glycoproteins and glycolipids (19). A ST3GAL6-deficient mouse study suggested that the protein encoded by this gene plays an important role in the synthesis of functional selectin ligands (20). Although the expression of ST3GAL6 is predominantly observed in the placenta, liver, heart, and skeletal muscle, it is also found in the brain (19). Thus, mutations in ST3GAL6 may influence the motor neuron function to some degree.
The present cases of fALS showed autosomal dominant inheritance, although the mutations were heterozygous. Mutations in PLEC and/or ST3GAL6 may have caused gain-of-function effects.
Several limitations associated with the present study warrant mention. First, although patients III-2 and III-4 had dementia, we were unable to evaluate their cognitive function in detail. Second, whether the pathogenic causal gene of this family is either or both PLEC or ST3GAL6 is unclear. Further functional studies are needed to draw a specific conclusion concerning how variants in PLEC and ST3GAL6 contribute to the clinical phenotype. We were also unable to exclude the possibility of other genes located in the phenotype-linked chromosome regions completely because only the exonic regions were analyzed in the present study. However, based on the results currently available, PLEC and ST3GAL6 are the only genes strongly speculated to be associated with this atypical case of fALS.
Conclusion
We herein report a rare fALS phenotype with a relatively benign clinical course with predominant lower motor involvement in the lower extremities. This rare fALS phenotype may be misdiagnosed as hereditary SCA with motor neuron involvement (11), as dysarthria and weakness of the lower extremities are the main symptoms. Thus, attending physicians should be aware of the heterogeneity of the phenotypes of fALS and differentiate other diseases carefully. Further investigation will be necessary to determine the role of PLEC and ST3GAL6 variants for this phenotype and demonstrate their pathogenesis.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This study was supported in part by grants from the research on Applying Health and Technology (201331010B) of Ministry of Health, Welfare and Labour, Japan. This research was also supported by the research program for conquering intractable disease from the Japan Agency for Medical Research and development (AMED) (201442014A, 201442071A). H.I. reports receiving grants from the Japan Society for the Promotion of Science during the conduct of the study.
Supplementary Material
The 54 rare variants with allele frequencies <0.001 detected in the affected siblings using whole-exome sequencing.
References
- 1. Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med 344: 1688-1700, 2001. [DOI] [PubMed] [Google Scholar]
- 2. Renton AE, Chio A, Traynor BJ. State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci 17: 17-23, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Swinnen B, Robberecht W. The phenotypic variability of amyotrophic lateral sclerosis. Nat Rev Neurol 10: 661-670, 2014. [DOI] [PubMed] [Google Scholar]
- 4. Ohi T, Nabeshima K, Kato S, Yazawa S, Takechi S. Familial amyotrophic lateral sclerosis with His46Arg mutation in Cu/Zn superoxide dismutase presenting characteristic clinical features and Lewy body-like hyaline inclusions. J Neurol Sci 225: 19-25, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Higasa K, Miyake N, Yoshimura J, et al. Human genetic variation database, a reference database of genetic variations in the Japanese population. J Hum Genet 61: 547-553, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fukuda Y, Nakahara Y, Date H, et al. SNP HiTLink: a high-throughput linkage analysis system employing dense SNP data. BMC Bioinformatics 10: 121, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gudbjartsson DF, Thorvaldsson T, Kong A, Gunnarsson G, Ingolfsdottir A. Allegro version 2. Nat Genet 37: 1015-1016, 2005. [DOI] [PubMed] [Google Scholar]
- 8. Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods 11: 361-362, 2014. [DOI] [PubMed] [Google Scholar]
- 9. Wassermann E, Epstein C, Ziemann U. Oxford handbook of transcranial stimulation. Oxford University Press, 2008. [Google Scholar]
- 10. Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1: 293-299, 2000. [DOI] [PubMed] [Google Scholar]
- 11. Kobayashi H, Abe K, Matsuura T, et al. Expansion of intronic GGCCTG hexanucleotide repeat in NOP56 causes SCA36, a type of spinocerebellar ataxia accompanied by motor neuron involvement. Am J Hum Genet 89: 121-130, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wiche G. Role of plectin in cytoskeleton organization and dynamics. J Cell Sci 111 (Pt 17): 2477-2486, 1998. [DOI] [PubMed] [Google Scholar]
- 13. Pulkkinen L, Smith FJ, Shimizu H, et al. Homozygous deletion mutations in the plectin gene (PLEC1) in patients with epidermolysis bullosa simplex associated with late-onset muscular dystrophy. Hum Mol Genet 5: 1539-1546, 1996. [DOI] [PubMed] [Google Scholar]
- 14. McLean WH, Pulkkinen L, Smith FJ, et al. Loss of plectin causes epidermolysis bullosa with muscular dystrophy: cDNA cloning and genomic organization. Genes Dev 10: 1724-1735, 1996. [DOI] [PubMed] [Google Scholar]
- 15. Smith FJ, Eady RA, Leigh IM, et al. Plectin deficiency results in muscular dystrophy with epidermolysis bullosa. Nat Genet 13: 450-457, 1996. [DOI] [PubMed] [Google Scholar]
- 16. Chavanas S, Pulkkinen L, Gache Y, et al. A homozygous nonsense mutation in the PLEC1 gene in patients with epidermolysis bullosa simplex with muscular dystrophy. J Clin Invest 98: 2196-2200, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gache Y, Chavanas S, Lacour JP, et al. Defective expression of plectin/HD1 in epidermolysis bullosa simplex with muscular dystrophy. J Clin Invest 97: 2289-2298, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhong J, Chen G, Dang Y, Liao H, Zhang J, Lan D. Novel compound heterozygous PLEC mutations lead to earlyonset limbgirdle muscular dystrophy 2Q. Mol Med Rep 15: 2760-2764, 2017. [DOI] [PubMed] [Google Scholar]
- 19. Okajima T, Fukumoto S, Miyazaki H, et al. Molecular cloning of a novel alpha2,3-sialyltransferase (ST3Gal VI) that sialylates type II lactosamine structures on glycoproteins and glycolipids. J Biol Chem 274: 11479-11486, 1999. [DOI] [PubMed] [Google Scholar]
- 20. Yang WH, Nussbaum C, Grewal PK, Marth JD, Sperandio M. Coordinated roles of ST3Gal-VI and ST3Gal-IV sialyltransferases in the synthesis of selectin ligands. Blood 120: 1015-1026, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The 54 rare variants with allele frequencies <0.001 detected in the affected siblings using whole-exome sequencing.