Abstract
A 51-year-old woman had been taking proton pump inhibitor since August 2008. In May, 2016, endoscopic findings showed no atrophy and no intestinal metaplasia in the stomach, and multiple fundic gland polyps were identified in the stomach. A biopsy of a pedunculated polyp measuring 10 millimeters in diameter at the greater curvature of the middle gastric body demonstrated well differentiated tubular adenocarcinoma. In July 2016, we treated this lesion and two other semipedunculated polyps located near the first polyp and also measuring 10 mm in diameter by endoscopic mucosal resection. The final diagnosis of all lesions was a fundic gland polyp with low grade dysplasia and the cutting end was negative.
Keywords: fundic gland polyp, dysplasia, endoscopic mucosal resection, Helicobacter pylori negative
Introduction
Fundic gland polyp (FGP) is usually asymptomatic and it is normally discovered incidentally during esophagogastroduodenoscopy (EGD). FGP has been reported to increase in number or newly occur in association with the use of long term proton pump inhibitor (PPI) (1); however, the prevalence of dysplasia is extremely rare (2,3). We herein report a rare case of a patient with three synchronous gastric fundic gland polyps with low grade dysplasia that were successfully treated by endoscopic mucosal resection.
Case Report
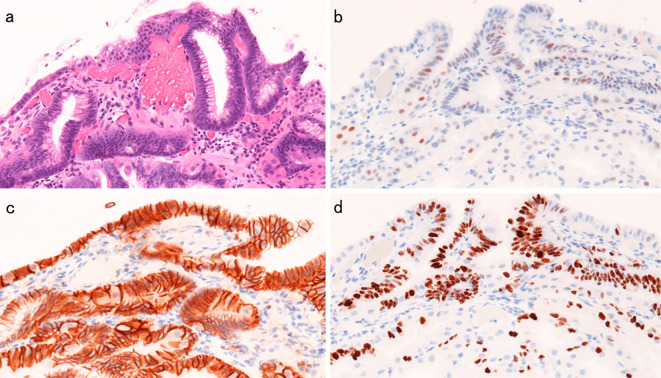
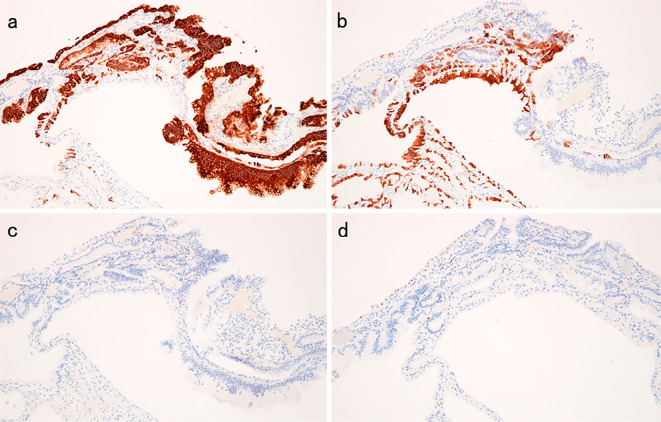
A 51-year-old woman had been suffering from multicentric Castleman’s disease and thus had been continually taking PPI (Lansoprazole 15 mg once daily) because of epigastric discomfort since August 2008. In September 2008, EGD revealed no polyp in the stomach. PPI was then changed to Esomeprazole 20 mg once daily in December 2012, and to Vonoprazan 20 mg once daily in March 2016 because sufficient symptom control was difficult. In May 2016, she was admitted to our department for further examination and treatment of a gastric neoplasia located on the greater curvature of the gastric body, based on the findings of EGD after an upper gastrointestinal series. The endoscopic findings showed no atrophy (the criteria of Kimura and Takemoto C-0) and no intestinal metaplasia in the stomach, and multiple FGPs were identified in the stomach. In addition. a pedunculated polyp measuring 10 mm in diameter was found at the greater curvature of the middle gastric body (Fig. 1). According to magnified narrow band imaging (NBI) findings, a demarcation line was present, but a microsurface pattern was absent. Regarding the microvascular pattern, both expansion and some irregularity of run were observed (Fig. 2). The biopsy result was well differentiated tubular adenocarcinoma (Fig. 3). A complete blood count and biochemistry demonstrated no abnormal findings. Pepsinogen-I, II, and I/II were 301.8 ng/mL, 57.4 ng/mL, and 5.3 (negative). Serum gastrin was 2,000 pg/mL. About Helicobacter pylori (H. pylori), serum antibody was less than 3 U/mL, a stool antigen test and histological examination (Hematoxylin and Eosin staining and immunohistochemistry) were negative. She had no past history of familial adenomatous polyposis (FAP) and no colon polyp was found on colonoscopy. In July 2016, we treated this lesion and another two semipedunculated polyps located near to the initial polyp and also measuring 10 mm in diameter because the patient had requested endoscopic mucosal resection (Fig. 4, 5). A histological examination of all lesions showed the same finding. Hyperplastic change and a cystically dilated fundic gland were observed. An increase quantity of chromatin, nuclear swelling, and front formation in a part of the foveola epithelium were recognized (Fig. 6). In the area of dysplasia, immunohistochemical staining revealed p53, pepsinogen I, and H+/K+-ATPase to be negative, while Ki-67 was positive. As for beta-catenin, no accumulation was observed in the nucleus and only the cell membrane was dyed (Fig. 7). The mucus phenotype was gastric type (foveola epithelium type) (Fig. 8). The final diagnosis of all lesions was a fundic gland polyp with low grade dysplasia and the cutting end was negative. No dysplasia has not detected after endoscopic mucosal resection according to follow-up EGD (Fig. 9).
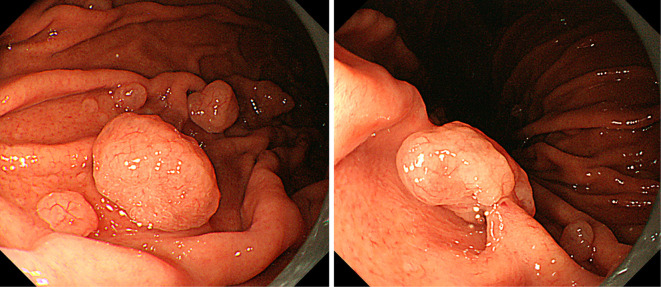
Figure 1.
A pedunculated polyp measuring 10 mm in diameter was identified at the greater curvature of the middle gastric body.
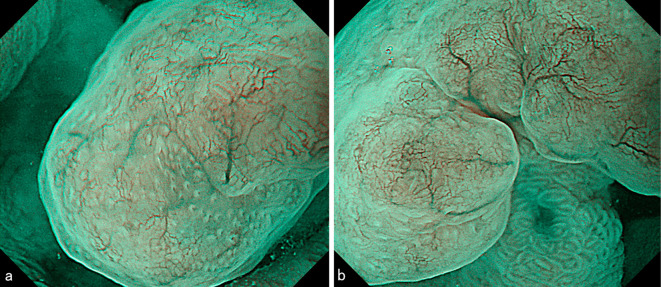
Figure 2.
Narrow band imaging showed a demarcation line to be present, while a microsurface pattern was absent. Regarding the microvascular pattern, both expansion and some irregularity of run were observed.
Figure 3.
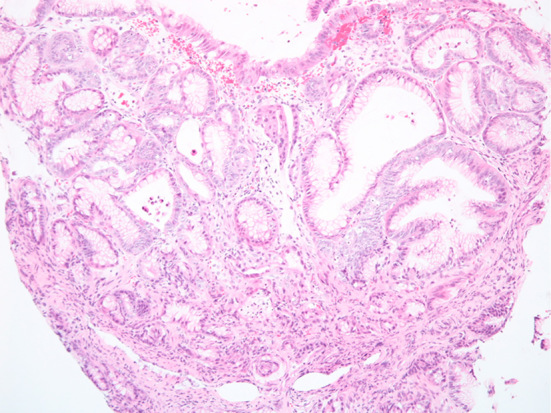
An increase in the quantity of the chromatin and nuclear swelling in a part of the foveola epithelium was recognized (Hematoxylin and Eosin staining, original magnification ×100).
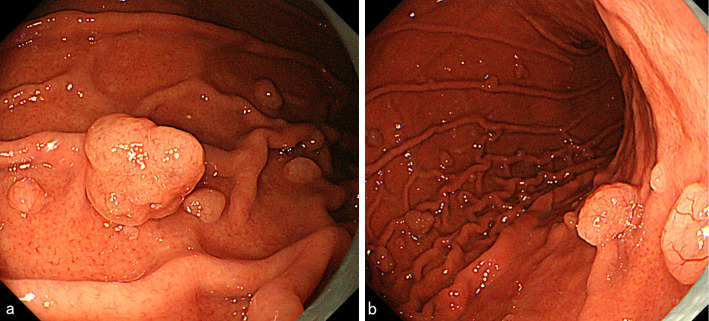
Figure 4.
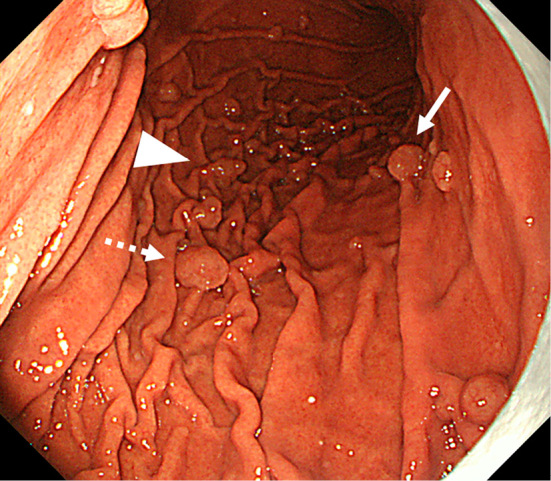
The dotted arrow indicates the lesion of Fig. 1. Two semipedunculated polyps were located near the initial polyp and also measured 10 mm in diameter (arrow and arrowhead).
Figure 5.
a: Arrowhead of Fig. 4. b: Arrow of Fig. 4.
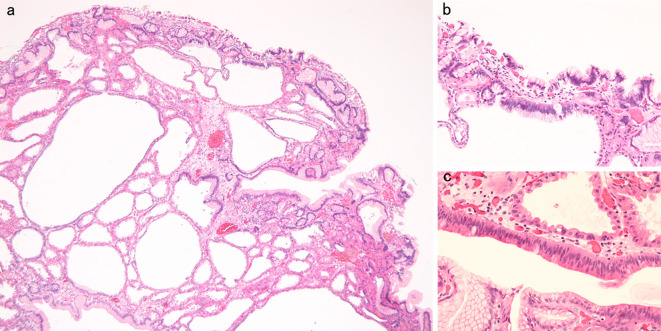
Figure 6.
Histopathological appearance of the lesion of Fig. 1. a: Hyperplastic change and cystic dilation of the fundic gland were observed [Hematoxylin and Eosin (H&E) staining, original magnification ×40]. b, c: An increase in the quantity of the chromatin, nuclear swelling, and front formation in a part of the foveola epithelium were recognized (H&E staining, original magnification b: ×100, c: ×200).
Figure 7.
Histopathological appearance of the area of dysplasia of the lesion iin Fig. 1. a: Hematoxylin and Eosin staining. b: p53 was negative. c: No nuclear beta-catenin staining was observed. d: Ki-67 was positive.
Figure 8.
a: MUC5AC. b: MUC6. c: MUC2. d: CDX2.
Figure 9.
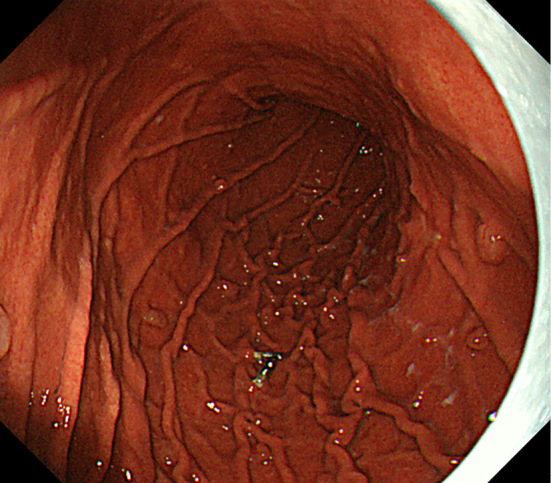
Multiple fundic gland polyps remained, but they all decreased in size at 28 months after endoscopic mucosal resection.
Discussion
FGP is the most common polyp found in the stomach. It has been reported in 0.8-1.9% of patients who undergo EGD (2). It is small and smooth, measuring only a few millimeters across. Histologically, it is an elevated lesion that features hyperplasia of fundic gland tissue and dilated cystic glands. It is usually found in the normal gastric mucosa free from H. pylori infection, inflammation, or atrophy (4). In this case, the histological findings were typical of FGP. In addition, the endoscopic findings showed no atrophy (the criteria of Kimura and Takemoto C-0) and no intestinal metaplasia in the stomach. Regarding H. pylori, serum antibody, stool antigen test, and histological examinations were all negative. As a result, she was diagnosed to not demonstrate any H. pylori infection.
FGP had been reported to increase or newly occur in association with long term PPI use. According to a recent prospective study in Japan, 13.6% patients had developed new FGP after 104 weeks of PPI use (1). Parietal cell hyperplasia has been reported to result from acid suppression during PPI use and such hyperplastic parietal cells can be the cause of fundic gland polyposis (5). In this case, EGD revealed no polyps in the stomach at 1 month after beginning the administration of PPI. Then, about 8 years after, multiple FGPs were identified in the stomach. As a result, she was thought to have developed multiple new FGPs due to the long term PPI use. Dysplasia was observed in this case and the FGPs were reported to dramatically decrease in size after stopping PPI (5). As a result, we thought that stopping the administration of PPI was necessary in this case. However, she has been suffering from multicentric Castleman’s disease, and she is therefore continuing to take PPI on demand since it is difficult to control the symptoms of this disease.
About FGP of FAP, dysplasia had been observed in up to 54% (3,6). In contrast, dysplasia in FGP from non-FAP patients is extremely rare. The prevalence of dysplasia has been reported to be 0.3-1.0% (2,3). Lloyd et al. followed 25 FGPs with dysplasia up for an average of 4.4 years. 68% of these patients used PPI. No clinical evidence of FAP, gastric cancer, death, or surgery occurred, so they reported that FGPs with dysplasia were not necessarily harbingers of FAP (7). FAP patients developed FGP with dysplasia earlier and more frequently than non-FAP patients. Regarding beta-catenin immunohistochemical staining, FGP with dysplasia in FAP and non-FAP patients also has a low rate of nuclear positivity (8). In this case, no clinical evidence of FAP occurred and beta-catenin immunohistochemical staining revealed that there was no nuclear positivity. In March 2018, a PubMed search using each of the keywords “fundic gland polyp” and “dysplasia” retrieved no report of synchronous three gastric FGPs with low grade dysplasia from non-FAP patients. Therefore, we herein report the first known case of a patient with three synchronous gastric fundic gland polyps with low grade dysplasia that was successfully treated by endoscopic mucosal resection.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We would like to sincerely thank Dr. Keiko Kojima and Dr. Masahiko Tsujimoto, Department of Pathology, NTT West Osaka hospital, and Dr. Ryoji Kushima, Department of Clinical Laboratory Medicine and Diagnostic Pathology, Shiga University of Medical Science, for their valuable help and support for this study.
References
- 1. Hongo M, Fujimoto K; Gastric Polyps Study Group.. Incidence and risk factor of fundic gland polyp and hyperplastic polyp in long-term proton pump inhibitor therapy: a prospective study in Japan. J Gastroenterol 45: 618-624, 2010. [DOI] [PubMed] [Google Scholar]
- 2. Levy MD, Bhattacharya B. Sporadic fundic gland polyps with low-grade dysplasia: a large case series evaluating pathologic and immunohistochemical findings and clinical behavior. Am J Clin Pathol 144: 592-600, 2015. [DOI] [PubMed] [Google Scholar]
- 3. Wu TT, Kornacki S, Rashid A, et al. . Dysplasia and dysregulation of proliferation in foveolar and surface epithelia of fundic gland polyps from patients with familial adenomatous polyposis. Am J Surg Pathol 22: 293-298, 1998. [DOI] [PubMed] [Google Scholar]
- 4. Kato M, Inoue K, Murakami K, et al. . Kyoto classification of gastritis. Nihon Medical Center, Tokyo, 2017: 68-70. [Google Scholar]
- 5. Hamada K, Takeuchi Y, Akasaka T, et al. . Fundic gland polyposis associated with proton-pump inhibitor use. Eur J Intern Med 4: 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jalving M, Koornstra JJ, Götz JM, et al. . High-grade dysplasia in sporadic fundic gland polyps: a case report and review of the literature. Eur J Gastroenterol Hepatol 15: 1229-1233, 2003. [DOI] [PubMed] [Google Scholar]
- 7. Lloyd IE, Kohlmann WK, Gligorich K, et al. . A clinicopathologic evaluation of incidental fundic gland polyps with dysplasia: implications for clinical management. Am J Gastroenterol 112: 1094-1102, 2017. [DOI] [PubMed] [Google Scholar]
- 8. Straub SF, Drage MG, Gonzalez RS. Comparison of dysplastic fundic gland polyps in patients with and without familial adenomatous polyposis. Histopathology 72: 1172-1179, 2018. [DOI] [PubMed] [Google Scholar]