Abstract
Recent large clinical trials failed to show clear benefits of percutaneous transluminal renal angioplasty (PTRA) as compared with medical therapy on patients with renal artery stenosis. It was also reported that proteinuria is an adverse prognostic factor after PTRA, and PTRA is less effective in patients with overt proteinuria. From the renoprotective point of view, to reduce proteinuria after PTRA is an important therapeutic goal in patients with renal artery stenosis with overt proteinuria. We hereby describe two patients successfully treated by combination therapy with PTRA and administration of angiotensin-converting enzyme (ACE) inhibitor for bilateral renal artery disease with overt proteinuria.
Keywords: renal artery disease, proteinuria, angioplasty, angiotensin-converting-enzyme inhibitor
Introduction
It is well known that the renal artery stenosis causes refractory hypertension and pulmonary edema (1,2). Percutaneous transluminal renal angioplasty (PTRA) for the renal artery stenosis is effective to control blood pressure as well as to reduce hypertensive emergency episode. However, previous clinical studies have reported that PTRA has barely discernible effect. Overt proteinuria occurs in various kidney diseases, and further reduces renal function. The first-line treatment for the overt proteinuria is an angiotensin converting enzyme (ACE) inhibitor or angiotensin II receptor blocker (ARB). The overt proteinuria is considered less likely to occur in patients with the renal artery stenosis, because of reduced renal perfusion pressure as well as lower glomerular filtration pressure (3-5). If the overt proteinuria is associated with bilateral renal artery disease in a patient, ACE inhibitor or ARB cannot be used. Furthermore, no report the effectiveness of PTRA to reduce the proteinuria in such patient (6-8).
We hereby describe two patients successfully treated by combination therapy with PTRA and administration of ACE inhibitor for bilateral renal artery stenosis with overt proteinuria.
Case Reports
Case1
A 67-year-old male presented with high fever and back pain. He was treated under the diagnosis of hypertension and type 2 diabetes as he underwent coronary artery bypass graft 10 years ago. Initial laboratory evaluation showed a severe inflammatory response [C-reactive protein (CRP) 15.2 mg/dL] and renal dysfunction [estimated glomerular filtration rate (eGFR) 40.3 mL/min/1.73 m2]. Since further examination eliminated infectious disease and malignant disease, he was suspected of vasculitis, then, high dose prednisolone was started. The blood pressure was 206/111 mmHg on admission. We firstly administered cilnidipine of 20 mg/day and nifedipine of 80 mg/day, then, added on carvedilol of 20 mg/day, but his blood pressure control was suboptimal. CT angiography showed bilateral renal artery stenosis, but there were no differences in kidney size and contrast effect between right and left kidneys. One month after admission, inflammatory findings gradually improved, and the dosage of prednisolone was tapered, but his eGFR decreased from 40.3 to 24.1 mL/min/1.73 m2. Two months after admission, CT angiography showed right renal artery stenosis and left artery total occlusion (Fig. 1A), and renal ultrasonography showed severe stenosis of the proximal portion of the right renal artery [peak systolic velocity (PSV) of 3.25 m/s; renal aortic ratio (RAR) of 6.0] and total occlusion of the left renal artery (Fig. 1B). His eGFR decreased to 33.1 mL/min/1.73 m2 and overt proteinuria (5.92 g/gCr) was also found.
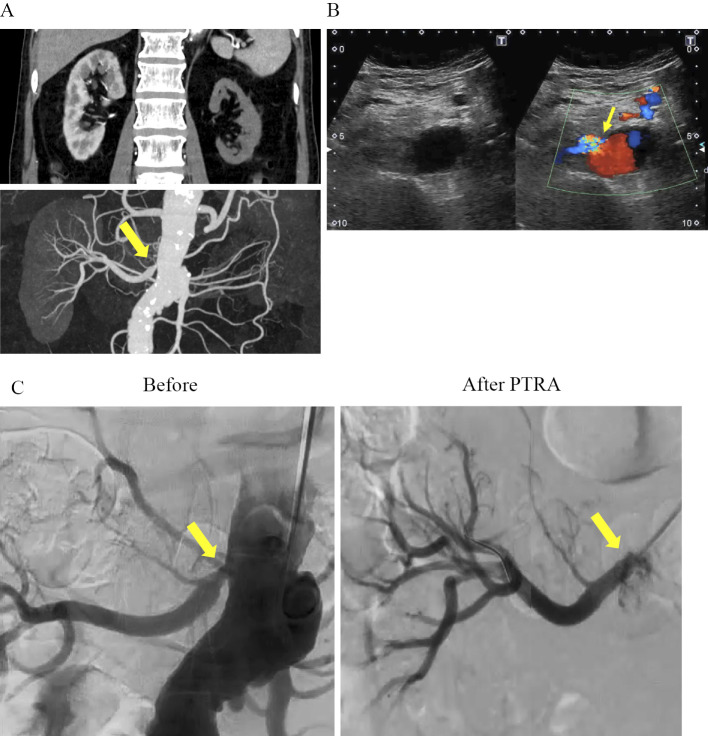
Figure 1.
Renal artery CT angiography and percutaneous transluminal renal angioplasty. (A) Renal artery CT angiography shows severe stenosis of the right renal artery (arrow), and occlusion of left renal artery. (B) Renal ultrasonography shows severe stenosis of the proximal portion of right renal artery (PSV 3.25 m/s; RAR 6.0) (arrow). (C) Angiography before and after Percutaneous Transluminal Renal Angioplasty. Genesis® 6.0*18 was successfully inserted into the right renal artery (arrows). (D) Blood pressure and renal function before and after percutaneous transluminal renal angioplasty. CT: computed tomography
After remission of the inflammatory disease was achieved, we performed PTRA for the right renal artery. Intravascular ultrasound (IVUS) showed an intimal-media thickness that suggested inflammatory change in the proximal site of right renal artery. Dilatation with a 5.0 mm balloon was attempted, but sufficient dilatation could not be achieved because of hard elastic recoil. Insertion of a stent (GenesisⓇ 6.0*18) was therefore necessary, and optimal dilatation was finally achieved (Fig. 1C). Just after the PTRA, an ACE inhibitor (captopril; 37.5 mg three times a day) was started, consequently, his blood pressure gradually improved (140/90 mmHg). As shown in Table 1, although the level of serum creatinine did not change, his proteinuria decreased after PTRA and ACE inhibitor administration. (pre PTRA 5.92 g/gCr, 1 month: 0.58 g/gCr, 6 months 0.22 g/gCr, and 12 months0.26 g/gCr). One year after the PTRA, the patency of the treated lesion was confirmed by renal ultrasonography.
Table 1.
Change in Laboratory Data and Medical Treatment in Case 1.
| PTRA before | PTRA after 1Mo | PTRA after 6Mo | PTRA after 12Mo | |
|---|---|---|---|---|
| Systolic BP (mmHg) | 190 | 150 | 170 | 143 |
| Diastolic BP (mmHg) | 117 | 88 | 96 | 78 |
| BUN (mg/dL) | 25.5 | 15 | 27.1 | 24 |
| sCr (mg/dL) | 1.67 | 1.79 | 1.68 | 1.71 |
| eGFR (mL/min/1.73m2) | 33.1 | 30.7 | 32.9 | 30.0 |
| UTP/CRE (g/gCr) | 5.92 | 0.58 | 0.22 | 0.26 |
| BS (mg/dL) | 161 | 153 | 154 | 141 |
| HbA1c | 7.0 | 6.8 | 6.4 | 6.4 |
| PRA (ng/mL/h) | 7.4 | 16 | 18 | 15 |
| PAC (ng/dL) | 19.2 | 7.6 | 7.3 | 8.8 |
| Medication (mg/day) | Cilnidipine 20 Nifedipine 80 Carvedilol 20 |
Nifedipine 40 Carvedilol 20 Captopril 37.5 |
Nifedipine 40 Carvedilol 20 Imidapril 5 |
Nifedipine 40 Carvedilol 20 Imidapril 5 |
Case 2
A 65-year-old male patient presented to the emergency department with rapidly developing orthopnea and reduced consciousness level. He had hypertension and type 2 diabetes, but he rejected taking any medicine. Nine years ago, he was admitted to the other hospital with primary diagnosis with heart failure and inferior old myocardial infarction. At that time, coronary angiography showed that his distal right coronary artery was occluded, and renal ultrasonography showed stenosis in the right renal artery. But he had never been followed after discharge. On admission to our hospital, his blood pressure was 263/131 mmHg, and peripheral oxygen saturation measured with pulse oximeter was 58%. Chest X-ray showed congestive pulmonary edema and massive pleural effusion. Cardiac ultrasound showed severe pulmonary hypertension with severe mitral regurgitation due to the tethering of posterior mitral leaflet. It was likely that the etiology of pulmonary edema was caused by several conditions, including fluid retention due to decreasing in urine volume for several days, rapid increasing in afterload, and functional severe mitral regurgitation due to inferior old myocardial infarction. Immediately, his respiration was supported with invasive mechanical ventilation and intravenous furosemide was started. However, anuria continued, then continuous hemodiafiltration was initiated. Renal ultrasonography showed severe stenosis of the proximal portion of the left renal artery (PSV 5.70 m/s; RAR 5.8) and total occlusion of the right renal artery. His status was anuria due to functionally solitary kidney with severe renal artery stenosis, accordingly, PTRA should be performed immediately for withdrawal of continuous hemodiafiltration. We conducted emergent PTRA for the left renal artery stenosis, and deployed a stent (GenesisⓇ 5.0*18). A large amount of diuresis was obtained after the PTRA, and his condition improved dramatically. After the first PTRA, his blood pressure was controlled around 140/80 mmHg, and continuous infusion of nicardipine was weaned and changed to oral medications (cilnidipine of 20 mg, nifedipine of 40 mg, bisoprolol of 2.5 mg, and trichlormethiazide of 1 mg, once a day). Introduction of ACE inhibitor was considered, but not administrated, for fear of hyperkalemia and decreasing in glomerular filtration at that time.
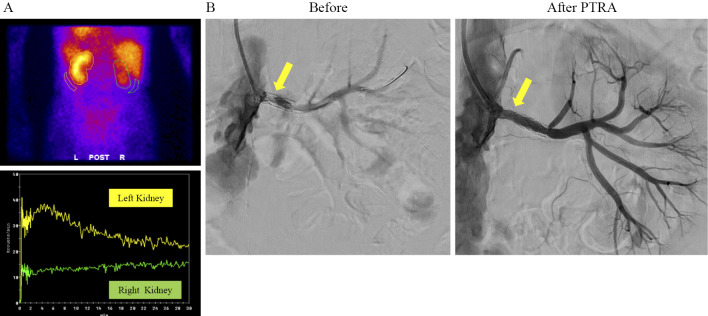
Six months later, he re-admitted to the hospital with orthopnea, and renal ultrasonography showed severe in-stent restenosis in the left renal artery (PSV 4.99 m/s; RAR 6.9). His eGFR was 20.9 mL/min/1.73 m2 and massive proteinuria (10.82 g/gCr) appeared. 99mTecnetium-mercaptoacetyltryglycine (99mTc-MAG3) renogram demonstrated remarkably decreasing uptake in left kidney, and no uptake in right kidney (Fig. 2A). We performed PTRA with 5.0 mm PTA balloon for in-stent-restenosis of the left renal artery (Fig. 2B), and got an optimal dilatation and started ACE inhibitor (enalapril; 2.5 mg once a day). One month after the treatment, his blood pressure was well controlled and his proteinuria level remarkably reduced from 10.82 to 1.77 g/gCr. One-year after PTRA, his proteinuria kept 2.0 to 3.0 g/gCr, and the introduction of renal replacement therapy was avoided (Table 2).
Figure 2.
Renogram and percutaneous transluminal renal angioplasty. (A) 99mTecnetium-mercaptoacetyltryglycine (99mTc-MAG3) renogram demonstrated remarkably decreasing uptake in left kidney, and no uptake in right kidney. (B) Before and after angioplasty with 5.0 mm PTA balloon for in-stent-restenosis of left renal artery (arrows). (C) Blood pressure and renal function before and after percutaneous transluminal renal angioplasty.
Table 2.
Change in Laboratory Data and Medical Treatment in Case 2.
| PTRA before | PTRA after 1Mo | PTRA after 6Mo | PTRA after 12Mo | |
|---|---|---|---|---|
| Systolic BP (mmHg) | 147 | 141 | 128 | 128 |
| Diastolic BP (mmHg) | 78 | 55 | 67 | 72 |
| BUN (mg/dL) | 29.8 | 60.4 | 40.5 | 35.7 |
| sCr (mg/dL) | 2.27 | 3.71 | 3.05 | 3.11 |
| eGFR (mL/min/1.73m2) | 23.9 | 14.0 | 17.3 | 16.8 |
| UTP/CRE (g/gCr) | 10.82 | 1.77 | 2.70 | 2.59 |
| BS (mg/dL) | 94 | 118 | 113 | 139 |
| HbA1c | 6.5 | 6.6 | 7.0 | 7.2 |
| PRA (ng/mL/h) | 20< | 2.7 | 12 | N/A* |
| PAC (ng/dL) | 6.8 | 7.5 | 16.5 | N/A* |
| Medication (mg/day) | Nifedipine 40 Cilnidipine 10 Bisoprolol 2.5 Trichlormethiazide1 |
Cilnidipine 10 Bisoprolol 2.5 Trichlormethiazide1 Enalapril 2.5 |
Cilnidipine 20 Bisoprolol 2.5 Trichlormethiazide1 Enalapril 2.5 |
Cilnidipine 20 Bisoprolol 0.625 Enalapril 2.5 |
*N/A: not available
Discussion
We presented two male patients treated by combination therapy with PTRA and administration of ACE inhibitor for bilateral renal artery disease, severe stenosis in one side of renal artery and obliteration in another side of renal artery with non-functional kidney, with overt proteinuria. In both cases, the proteinuria dramatically improved after the combination therapy.
The renal artery stenosis is frequently associated with refractory hypertension, renal dysfunction, and flash pulmonary edema. Several retrospective trials demonstrated clinical benefit of PTRA in the management of blood pressure and stabilization of renal function in patients with atherosclerotic renal artery stenosis (8,10-12). In contrast, recent randomized clinical trials, which compared clinical outcomes between PTRA and medical therapy alone in patients with atherosclerotic renal artery stenosis (ARAS), failed to demonstrate significant clinical benefits of PTRA as compared medical therapy (6,7,13). Even when the result of the randomized controlled trials did not support the PTRA for the stable ARAS lesion, it is well known that PTRA is recommended to the cases with functionally solitary kidney or pulmonary edema. Unlike ARAS, renal artery stenosis associated with vasculitis and fibromuscular dysplasia (FMD) is still preferable indication for PTRA because of the favorable response to PTRA (14,15). Recent trials assessed only eGFR was assessed instead of proteinuria as clinical outcome. The proteinuria is another important maker of the kidney disease progression, since it is strongly associated with cardiovascular event, all-cause mortality and end-stage renal disease (16). Several studies reported that proteinuria is an adverse prognostic factor after PTRA, and PTRA is less effective in patients with overt proteinuria (17,18). From the renoprotective point of view, to reduce proteinuria after PTRA is an important therapeutic goal in patients with renal artery stenosis with overt proteinuria.
In our two cases, one kidney with total occluded renal artery may functionally secrete renin. Improving in hypoperfusion by PTRA for the other kidney may have two benefits, one is to decrease in renin secretion with reducing systemic blood pressure and the other is to recover glomerular blood pressure. However, dilation of stenotic renal artery would rather cause glomerular hypertension if the systemic blood pressure was not sufficiently reduced. Previous report seems to be such a case (9). In such clinical setting, combined with ACE inhibitor may reduce proteinuria by improving glomerular hypertension via both reducing systemic blood pressure and dilating efferent arteriole even after dilation of renal artery.
In both cases, PTRA and administration of ACE inhibitor significantly improved proteinuria and prevented from continuous renal replacement therapy. However, changes in blood pressure and eGFR changes before and after PTRA differed between the two cases. Namely, in case 1, blood pressure was definitely improved and eGFR was preserved after PTRA and administration of ACE inhibitor. On the other hands, case 2 showed little change in blood pressure and eGFR decreased in case 2. In case 1, blood pressure in pre-PTRA was extremely high and residual glomerulus function was preserved. It is possible that decrease in systemic blood pressure influenced to reduction of proteinuria. It was previously reported that the baseline high systolic blood pressure is a predictor for improvement of blood pressure after PTRA (8). On the other hand, in case 2, renal damage was already advanced and systemic blood pressure was not so high in pre-PTRA period. In spite of poor response in blood pressure, his proteinuria was significantly reduced by improving glomerular hypertension. In advanced stage of chronic kidney disease, ACE inhibitor can cause short time worsening in eGFR after PTRA, but long-term stabilization of renal function can be expected due to control of proteinuria and intraglomerular pressure.
Although the pathophysiological mechanism of these two cases were different, both cases shared bilateral renal artery disease with functional single kidney and overt proteinuria. We did not have opportunities to perform renal biopsy in these patients because of critical conditions, and thus the mechanism of proteinuria was unclear. However, the over proteinuria in these patients should be attributed to hormonal and hemodynamic disturbances, because it declined after PTRA. In the kidney, the intraglomerular pressure is constantly regulated to approximately 50 mmHg by the balance of the dilatation of efferent arteriole and the constriction of afferent arteriole which are mainly controlled by renin-angiotensin system. In patients with renal artery stenosis, due to highly activated renin angiotensin system, angiotensin II increases the permeability of glomerular membrane and increase intraglomerular pressure by the constriction of efferent arteriole, resulting in proteinuria (19). Previously study reported that the high intraglomerular filtration pressure can cause secondary focal segmental glomerulosclerosis (FSGS). Unilateral renal artery stenosis or obstruction results in FSGS-like changes and the nephrotic syndrome in the contralateral kidney due to hyperfiltration (5). In our patients, after the function of one kidney has been abolished due to the occlusion of the renal artery, distribution of renal flow might change to increase glomerulus hyperfiltration pressure. In such a condition, ACE inhibitor or ARB should be effective to reduce the proteinuria. In a case report, the combination therapy comprising PTRA and ACE inhibitor or ARB in patients with unilateral renal artery stenosis is effective to control their blood pressure and decrease in proteinuria (20). However, there were no report about the efficacy of combination therapy of PTRA and ACE inhibitors in patients with bilateral renal artery disease and overt proteinuria.
Actually, a clinical study showed that use of renin-angiotensin inhibitors have safety and tolerability even in the bilateral cases (21). But, generally, renin-angiotensin inhibitors are contraindicated to patients with bilateral renal artery stenosis, because of their high possibility to induce severe renal dysfunction and hyperkalemia. Therefore, PTRA for at least one side of renal artery stenosis is necessary prior to the administration ACE inhibitor or ARB. After PTRA, it is anticipated that the glomerular filtration pressure increases, but additional ACE inhibitor or ARB dilates efferent arterioles to decrease intraglomerular pressure. This may contribute to reduce proteinuria. After PTRA for one side of renal artery stenosis, it is important to evaluate the renal function and tolerability for ACE inhibitor. The suitable conditions, in which the patient is in diuretic phase and in normal range of serum potassium concentration, are necessary for administration of ACE inhibitor after PTRA. Actually, in case 2, ACE inhibitor was not administrated after first PTRA, in considerations of hyperkalemia and decreasing in glomerular filtration. However, before second PTRA, the patients demonstrated progressive proteinuria. In such a situation, the administration of ACE inhibitor thought to be indispensable. In functional single kidney with proteinuria, ACE inhibitor or ARB should be considered for protecting renal function after PTRA. However, ACE inhibitor and ARB can cause apparent decrease in eGFR. Therefore, careful monitoring of renal function and patency of renal artery are required, especially after PTRA and ACE inhibitor treatment.
In conclusion, recent large clinical trials failed to show beneficial effects of PTRA on patients with renal artery stenosis on restoring renal function or improving clinical outcomes. However, there are a certain batch of patients with bilateral renal artery disease who have benefit from PTRA with ACE inhibitor or ARB as shown in our report. In the treatment with patients with renal artery disease, we should carefully pay attention to not only serum creatinine concentration but also the level of proteinuria.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Bloch MJ, Trost DW, Pickering TG, Sos TA, August P. Prevention of recurrent pulmonary edema in patients with bilateral renovascular disease through renal artery stent placement. Am J Hypertens 12: 1-7, 1999. [DOI] [PubMed] [Google Scholar]
- 2. Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med 344: 431-442, 2001. [DOI] [PubMed] [Google Scholar]
- 3. Halimi JM, Ribstein J, Du Cailar G, Mimran A. Nephrotic-range proteinuria in patients with renovascular disease. Am J Med 108: 120-126, 2000. [DOI] [PubMed] [Google Scholar]
- 4. Alkhunaizi AM, Chapman A. Renal artery stenosis and unilateral focal and segmental glomerulosclerosis. Am J Kidney Dis 29: 936-941, 1997. [DOI] [PubMed] [Google Scholar]
- 5. Iwami D, Harada H, Usubuchi H, et al. Regional secondary focal segmental glomerulosclerosis in a transplanted kidney: resolution with treatment of a segmental renal artery stenosis. BMC Nephrology 13: 38, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 361: 1953-1962, 2009. [DOI] [PubMed] [Google Scholar]
- 7. Cooper CJ, Murphy TP, Cutlip DE, et al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 370: 13-22, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fujihara M, Yokoi Y, Abe T, et al. Clinical outcome of renal artery stenting for hypertension and chronic kidney disease up to 12 months in the J-RAS Study - prospective, single-arm, multicenter clinical study. Circ J 79: 351-359, 2015. [DOI] [PubMed] [Google Scholar]
- 9. Almirall J, Mendez I, Comet R, Andreu X. Nephrotic syndrome after renal percutaneous transluminal angioplasty. Nephrol Dial Transplant 15: 1696-1699, 2000. [DOI] [PubMed] [Google Scholar]
- 10. Burket MW, Cooper CJ, Kennedy DJ, et al. Renal artery angioplasty and stent placement: predictors of a favorable outcome. Am Heart J 139: 64-71, 2000. [DOI] [PubMed] [Google Scholar]
- 11. Bush RL, Najibi S, MacDonald MJ, et al. Endovascular revascularization of renal artery stenosis: technical and clinical results. J Vasc Surg 33: 1041-1049, 2001. [DOI] [PubMed] [Google Scholar]
- 12. Rocha-Singh K, Jaff MR, Rosenfield K. Evaluation of the safety and effectiveness of renal artery stenting after unsuccessful balloon angioplasty: the ASPIRE-2 study. J Am Coll Cardiol 46: 776-783, 2005. [DOI] [PubMed] [Google Scholar]
- 13. Bax L, Woittiez AJ, Kouwenberg HJ, et al. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med 150: 840-848, w150-w151, 2009. [DOI] [PubMed] [Google Scholar]
- 14. Gavalas MV, Gasparis AP, Tassiopoulos AK, Loh S, Labropoulos N. Long-term follow-up for percutaneous transluminal angioplasty in renal artery fibromuscular dysplasia. Int Angiol 34: 529-537, 2015. [PubMed] [Google Scholar]
- 15. Tyagi S, Singh B, Kaul UA, Sethi KK, Arora R, Khalilullah M. Balloon angioplasty for renovascular hypertension in Takayasu's arteritis. Am Heart J 125: 1386-1393, 1993. [DOI] [PubMed] [Google Scholar]
- 16. Andrassy KM. Comments on ‘KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease’. Kidney Int 84: 622-623, 2013. [DOI] [PubMed] [Google Scholar]
- 17. Murphy TP, Cooper CJ, Pencina KM, et al. Relationship of albuminuria and renal artery stent outcomes: results from the CORAL randomized clinical trial (Cardiovascular Outcomes With Renal Artery Lesions). Hypertension 68: 1145-1152, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iwashima Y, Fukuda T, Horio T, et al. Association between renal function and outcomes after percutaneous transluminal renal angioplasty in hypertensive patients with renal artery stenosis. J Hypertens 36: 126-135, 2018. [DOI] [PubMed] [Google Scholar]
- 19. Hwang S, Ham JS, Hwang KB, et al. Renal artery stenosis presenting with nephrotic-range proteinuria: a case report. Kidney Res Clin Pract 35: 119-122, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wakui H, Hosokawa Y, Oshikawa J, et al. Endovascular treatment of renal artery stenosis improves contralateral renal hypertrophy with nephrotic syndrome. CEN Case Rep 3: 53-55, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Evans KL, Tuttle KR, Folt DA, et al. Use of renin-angiotensin inhibitors in people with renal artery stenosis. Clin J Am Soc Nephrol 9: 1199-1206, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]