Abstract
Objective
Based on both endoscopic findings and serum auto-antibody levels, we determined the prevalence of autoimmune gastritis (AIG), which has not been previously reported, in individuals who underwent health checkup examinations in Japan.
Methods
At total of 6,739 subjects (4,288 males, 2,451 females; mean age 52.1 years) underwent an upper gastrointestinal endoscopic examination as part of an annual medical checkup. Those suspected to have AIG based on endoscopic evidence of proximal-predominant gastric mucosal atrophy were further examined for the presence of anti-parietal cells and anti-intrinsic factor antibodies, with a final diagnosis of AIG made in cases found to be positive for either or both of those factors.
Results
Of the 6,739 examined subjects, 46 were suspected to have AIG based on the endoscopic findings, of whom 33 were finally diagnosed with AIG, for an overall prevalence 0.49% (females 0.65%, males 0.40%). Seven with AIG also had thyroid disease, including Hashimoto's and Basedow disease, while none with AIG showed anemia in blood test findings. The prevalence of AIG was not different regardless of the H. pylori infection status (negative, positive, post-eradicated).
Conclusion
In individuals who underwent an upper gastrointestinal endoscopic examination as part of an annual checkup in Japan, the prevalence of AIG was 0.49%. We concluded that it is not uncommon for asymptomatic and healthy individuals to have AIG, and propose that additional studies are needed to clarify its prevalence as well as to establish the criteria used for diagnosis.
Keywords: autoimmune gastritis, anti-parietal cells antibody, anti-intrinsic factor antibody, gastrin, Helicobacter pylori
Introduction
Autoimmune gastritis (AIG), type A gastritis, is a chronic inflammatory disease of the stomach caused by cellular and humoral immune responses to gastric parietal cells (1-3). Inflammation and continuous atrophy is restricted to the corpus and fundus in patients with AIG, since parietal cells are a type of epithelial cell located in glands of the corpus and fundus, but not in the antrum (2,3). Parietal cells produce hydrochloric acid and an intrinsic factor, with final total loss of parietal cells resulting from disease progression, which provokes iron deficiency anemia and vitamin B12 deficiency (pernicious anemia) (4,5).
No definitive criteria for the diagnosis of AIG have been established and its prevalence in the general population has not been fully determined, though the disease is reported to be more common in women and older individuals (6-8). Based on positive findings of anti-parietal cells antibodies in the serum, the prevalence of AIG in German subjects aged 50-74 years was reported to be 19.5% (9), while that in individuals in the Canary islands was 7.8% (10). Therefore, it seems likely that there are many asymptomatic individuals with AIG among the general population. We conducted the present study to examine the prevalence of AIG based on the endoscopic finding of proximal-predominant gastric mucosal atrophy, as well as positive findings of antibodies to parietal cells or intrinsic factor in the serum in individuals who came to our medical center in Japan for an annual medical checkup, as the prevalence in these subjects was considered to be similar to that of the general population.
Materials and Methods
The present subjects were individuals who visited the Health Center of Shimane Environment and Health Public Corporation for a detailed medical checkup examination during the 2-year period from May 2016 to April 2018. The majority were socially active and productive, and considered to be socioeconomically middle class. Any cases with a history of total gastrectomy were excluded from the study subjects. During the study period, the subjects underwent a total of 9,784 upper GI endoscopic examinations, of which 3,045 were follow-up examinations. Therefore, the examination findings of 6,739 subjects (4,288 males, 2,451 females; mean age 52.1 years, range 22-86 years) were included in this study. A precise medical history concerning the status of H. pylori infection (negative, positive, post-eradication) was obtained in an interview with the patient conducted by a public health nurse. Serum anti-H. pylori IgG antibody detection was performed using a SphereLight H. pylori antibody JⓇ kit (FUJIFILM Wako Pure Chemical Corporation, Osaka) (11-13). The antibody titer was automatically determined using a chemiluminescent enzyme immunoassay (CLEIA), with a value of 4.0 U/mL defined as positive, according to the manufacturer's instructions. The status of H. pylori infection was decided based on the medical history and results of the serum anti-H. pylori IgG antibody test. Those without successful eradication were included in the group with H. pylori infection, even if they had undergone eradication therapy. When eradication therapy was confirmed to be successful, we recommended that the patient undergo an H. pylori stool antigen test at our institution. The presence or absence of H. pylori infection and successful eradication were also confirmed based on the endoscopic findings obtained in an upper GI endoscopic examination (14-18).
All upper endoscopic examinations were performed by licensed experienced endoscopists using an EG-L580NW endoscope (Fujifilm, Tokyo, Japan). Three expert endoscopists simultaneously reviewed the endoscopic images from all subjects during the study period, and the suspected AIG cases were determined by consensus. When the degree of gastric mucosal atrophy, estimated based on visible submucosal vessels in endoscopic images, was predominant in the corpus and fundus in comparison to the antral part, those cases were noted as suspected AIG (Fig. 1, 2). In addition, the presence or absence of the endoscopic findings of the residual fundic gland mucosa in the fundic area was determined (Fig. 2). Serum samples were obtained from suspected AIG cases and stored at -80℃, then the level of gastrin, and concentrations of anti-parietal cells and the anti-intrinsic factor antibody were determined by SRL, Tokyo. For the analysis of gastrin, a radioimmunoassay with a polyethyleneglycol method was used, while anti-parietal cells and anti-intrinsic factor antibodies were analyzed with a fluorescent antibody test and the CLEIA method, respectively. A positive finding for anti-parietal cells was determined when the titer was ≥10 U, while serum gastrin was defined as normal when the level was ≤200 pg/mL. In this study, we diagnosed a subject as having AIG when anti-parietal cells and/or anti-intrinsic factor antibodies were found to be positive. The serum levels of pepsinogen I and II were also determined using SphereLight Pepsinogen I and IIⓇ kits, respectively (FUJIFILM Wako Pure Chemical Corporation, Osaka) at our institute, and the pepsinogen I/II ratio was calculated. The pepsinogen test findings were defined as positive when the pepsinogen I/II ratio was ≤3.0 and subjects with AIG were divided into 3 groups according to that ratio (≤1.0, 1.1-3.0, >3.0).
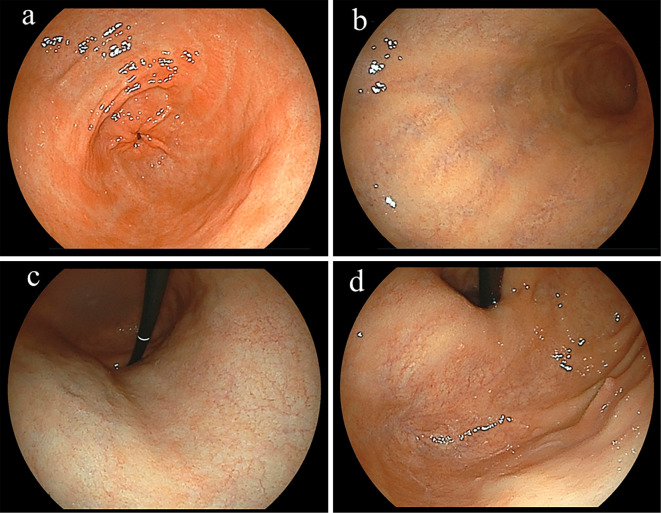
Figure 1.
Representative subject. A 55-year-old female without Helicobacter pylori infection. The endoscopic findings revealed proximal-predominant gastric mucosal atrophy (a: antrum, b: greater curvature of middle body, c: lesser curvature of upper body, d: fornix). No endoscopic findings of residual fundic gland mucosa in the atrophic fundic area were observed. Anti-parietal cells antibody was positive (titer 80-fold), while the anti-intrinsic factor antibody was negative. Therefore, this subject was diagnosed as having autoimmune gastritis. The pepsinogen I/II ratio was 0.4 and the level of gastrin in serum was 2,900 pg/mL.
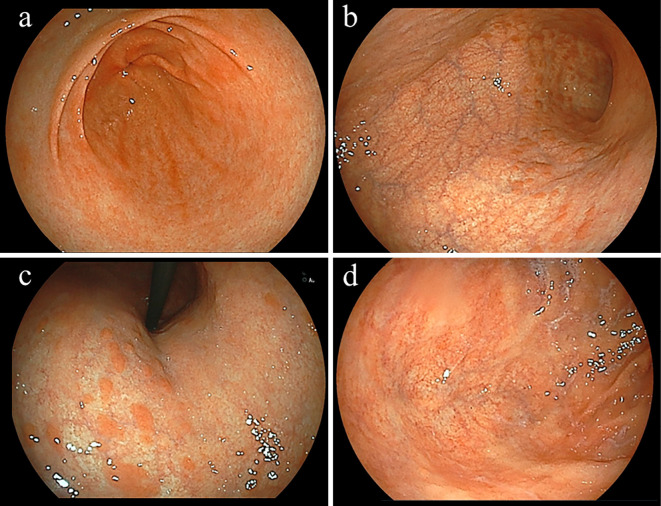
Figure 2.
Representative subject. A 54-year-old female without Helicobacter pylori infection. The endoscopic findings revealed proximal-predominant gastric mucosal atrophy (a: antrum, b: greater curvature of middle body, c: lesser curvature of middle body, d: fornix). Multiple reddish elevated lesions, which were diagnosed as residual fundic mucosa, were observed in the atrophic fundic area of the body. Anti-parietal cells antibody was positive (titer 80-fold), while the anti-intrinsic factor antibody was negative. Therefore, this subject was diagnosed as having autoimmune gastritis. The pepsinogen I/II ratio was 1.13 and level of gastrin in serum was 81 pg/mL.
Statistical analyses were performed using chi-squared, Fisher's exact test, Kruskal-Wallis, and Mann-Whitney U tests. Analyses of correlations between groups were done with Spearman's rank correlation test. All calculations were performed using the Stat View 5.0 software program (Abacus Concepts, Berkeley, USA) for Macintosh, with a p value <0.05 considered to indicate statistical significance.
This study was performed in accordance with the Declaration of Helsinki, and the protocol was approved by the ethics committee of the Shimane Environment and Health Public Corporation. Written informed consent indicating that clinical data would be used for a clinical study without release of individual information was obtained from all subjects before performing the medical checkup examinations.
Results
Of the 6,739 subjects, 46 were initially suspected to have AIG based on the endoscopic findings of the corpus and fornix showing predominant gastric mucosal atrophy. The presence of anti-parietal cells and anti-intrinsic factor antibodies in serum was further investigated in these 46, and the numbers positive for those were 32 and 6, respectively. Five subjects were positive for both anti-parietal cells and the anti-intrinsic factor antibody. Therefore, 33 (0.49%) of the 6,739 subjects were diagnosed with AIG according to the criteria noted above and their characteristics are shown in Table 1. The prevalence of females with AIG was greater than for males (0.65% vs. 0.40%), although the difference was not significant. Thirteen among 46 suspected AIG cases were not diagnosed as AIG due to a negative finding for both anti-parietal cells and anti-intrinsic factor antibodies. The status of H. pylori infection in the majority of non-AIG cases were positive and post-eradicated, and the majority of those showed a normal pepsinogen I/II ratio (>3.0) and normal serum gastrin level (≤200 pg/mL) (Table 1).
Table 1.
Characteristics of 46 Suspected Autoimmune Gastritis (AIG) Cases.
| AIG cases* | Non-AIG cases* | p value | |
|---|---|---|---|
| Gender, male/female | 16/17 | 5/8 | 0.775 |
| Age, years | 58.9±1.8 (39-81) | 56.9±2.6 (43-72) | 0.550 |
| H. pylori status | 0.046 | ||
| Negative | 17 | 2 | |
| Positive | 1 | 2 | |
| Post-eradicated | 15 | 9 | |
| Thyroid disease** | 7 | 1 | 0.409 |
| Serum pepsinogen I***, ng/mL | 21.7±4.5 (1.1-96.0) | 21.1±2.0 (8.9-33.8) | 0.234 |
| Serum pepsinogen II***, ng/mL | 11.3±1.8 (4.8-56.2) | 6.1±0.5 (3.9-9.2) | 0.005 |
| Pepsinogen I/II ratio*** | 2.0±0.3 (0.1-6.2) | 3.7±0.3 (1.0-5.5) | 0.006 |
| ≤ 1.0, 1.1-3.0,>3.0 | 14/7/9 | 0/3/10 | 0.005 |
| Serum gastrin*, pg/mL | 1,274±250 (10-4,700) | 126±15 (76-280) | 0.001 |
| ≤200/201-1,000/>1,000 pg/mL | 7/14/9 | 12/1/0 | <0.001 |
Values are expressed as the mean±SE (range) or number of subjects. *AIG cases were diagnosed by the positive of anti-parietal cell and/or anti-intrinsic factor antibodies, and non-AIG cases were determined by the negative of both antibodies. **Accompanied by Hashimoto’s disease or Basedow disease. ***Pepsinogen I, II, I/II ratio, and gastrin were examined in 43 subjects, after excluding 3 AIG cases with a history of proton pump inhibitor administration and with a remnant stomach (post-operative status).
The status of H. pylori infection was determined from medical records and results of the test for the anti-H. pylori IgG antibody. The cases with antral gastric mucosal atrophy and a negative finding for the anti-H. pylori IgG antibody test were excluded to calculate the prevalence of AIG in different H. pylori statuses, as these cases are considered to either have a post-eradication status or be positive for H. pylori infection (11,19). As a result, the status of H. pylori infection was able to be determined in 4,197 of subjects, in whom a negative, positive, and a post-eradicated status numbered 1,780, 620, and 1,797, respectively. For the 33 subjects with AIG, a negative, positive, and with a post-eradicated status was observed in 17, 1, and 15, respectively. When the subjects identified to have H. pylori infection were analyzed, the prevalence of AIG based on the infection status was 0.96%, 0.16%, and 0.83%, respectively (p=0.149). A 69-year-old female had a history of distal gastrectomy due to gastric cancer in her 50s, though the details regarding gastric cancer in that case were not available, while none of the other 32 subjects showed any evidence of gastric cancer or a neuroendocrine cell tumor in the endoscopic findings. Seven of the subjects with AIG had Hashimoto's or Basedow disease, and the rate of thyroid disease in subjects with was significantly greater than in those without AIG (21.2% vs. 1.7%; p<0.001). No subject with AIG showed anemia (cut-off hemoglobin level in males and females, <13.0 and <12.0 g/dL, respectively) in blood samples obtained at the time of the medical checkup. In addition, no large red blood cells (mean corpuscular volume >100), which are associated with vitamin B12 deficient status, were observed in our cases with AIG.
Two of the 33 subjects with AIG have received proton pump inhibitor treatment and 1 had a history of gastric surgery. Therefore, the serum levels of gastrin, and pepsinogen I and II, and the pepsinogen I/II ratio were analyzed in the other 30, with the results shown in Table 1. In these subjects, a low level of serum pepsinogen I and low I/II ratio, and a high level of serum gastrin were observed, though these values showed a wide range and some subjects had normal levels (pepsinogen I/II ratio >3.0, serum gastrin ≤200 pg/mL).
Comparative studies among the different H. pylori status, serum gastrin level and pepsinogen I/II ratio in cases with AIG are shown in Table 2. When the subjects with AIG were divided into 2 groups (H. pylori-negative group and H. pylori-positive and post-eradicated group), there were no significant differences between the groups in gender, age, pepsinogen I/II ratio and serum gastrin level. When the subjects with AIG were divided based on the serum gastrin levels (≤200/201-1,000/>1,000 pg/mL) and pepsinogen I/II ratio (≤1.0, 1.1-3.0, >3.0), high serum gastrin group (>1,000 pg/mL) and low pepsinogen I/II ratio group (≤1.0) tended to be older than the other groups. When the residual fundic gland mucosa in the atrophic fundic area was endoscopically examined, its presence was observed in all cases of the low serum gastrin group (≤200 pg/mL) and high pepsinogen I/II ratio group (>3.0). The pepsinogen I/II ratio and serum gastrin level were found to be inversely correlated with the findings of a Spearman's rank correlation test (ρ=-0.764, p<0.001) (Fig. 3).
Table 2.
Comparisons among the Different H. pylori Status, Serum Gastrin Level and Pepsinogen I/II Ratio in Cases with Autoimmune Gastritis (AIG).
| H. pylori infection | negative | positive or eradicated | p value | |
| Number of cases | 17 | 16 | ||
| Gender, male/female | 8/9 | 8/8 | 0.866 | |
| Age, years | 58.5±2.4 (39-77) | 59.4±2.9 (41-81) | 0.787 | |
| Pepsinogen I/II ratio* | 1.6±0.5 (0.3-5.1) | 2.3±1.7 (0.1-6.2) | 0.152 | |
| Serum gastrin*, pg/mL | 1,578±391 (72-4,700) | 970±306 (10-4,200) | 0.407 | |
| Serum gastrin*, pg/mL | ≤200 | 201-1,000 | >1,000 | p value |
| Number of cases | 7 | 14 | 9 | |
| Gender, male/female | 2/5 | 9/5 | 4/5 | 0.281 |
| Age, years | 51.1±3.0 (39-60) | 58.6±2.7 (41-75) | 60.9±3.3 (52-81) | 0.146 |
| Pepsinogen I/II ratio | 3.9±0.5 (1.1-5.1) | 1.9±0.5 (0.3-6.2) | 0.5±0.1 (0.1-0.9) | <0.001 |
| Residual fundic gland mucosa** | ||||
| presence/absence | 7/0 | 12/2 | 4/5 | 0.018 |
| Pepsinogen I/II ratio* | ≤1.0 | 1.1-3.0 | >3.0 | p value |
| Number of cases | 14 | 7 | 9 | |
| Gender, male/female | 8/6 | 3/4 | 4/5 | 0.764 |
| Age, years | 61.6±2.5 (49-81) | 52.1±2.9 (41-61) | 55.6±3.5 (39-70) | 0.142 |
| Serum gastrin, pg/mL | 2,255±388 (250-4,700) | 722±111 (81-930) | 177±56 (10-570) | <0.001 |
| Residual fundic gland mucosa** | ||||
| presence/absence | 8/6 | 6/1 | 9/0 | 0.018 |
*Analysis was performed of 30 subjects after excluding 2 with proton pump inhibitor administration and 1 with a remnant stomach (post-operative status). Values are expressed as the mean±SE (range) or number of subjects. **: Endoscopic finding of residual fundic gland mucosa in the fundic area.
Figure 3.
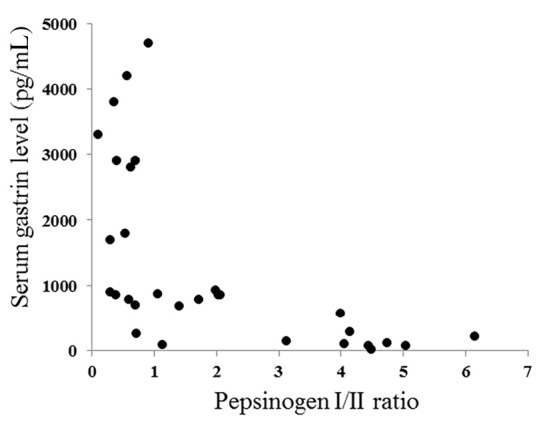
Correlation between the pepsinogen I/II ratio and the serum gastrin level in subjects with autoimmune gastritis. The pepsinogen I/II ratio and serum gastrin level was inversely correlated (Spearman’s rank correlation test; ρ=-0.764, p<0.001).
Discussion
AIG is caused by cellular and humoral immune responses to gastric parietal cells, and results in the development of chronic continuous inflammation and gastric mucosal atrophy in the body and fornix of the stomach (1-3). With disease progression, anacidity, iron deficiency anemia, and pernicious anemia appear in affected individuals (4,5). No definitive criteria for diagnosis of AIG have been established and the prevalence differs based on the criteria used (6). In addition, the prevalence has been reported to vary among different populations and patient groups (2,3). When considering its pathogenesis, the presence of gastric mucosal inflammation and atrophy caused by the immune response to parietal cells is thought to be the most important factor for making a diagnosis.
For the present study, we diagnosed AIG based on not only the presence of proximal-predominant gastric mucosal atrophy, but also positive findings for anti-parietal cells and the anti-intrinsic factor antibody. Our results indicate that the rate of prevalence of AIG in individuals in Japan is 0.49%, based on our findings of individuals who underwent an upper gastrointestinal endoscopic examination as part of an annual checkup. Nearly all of the present subjects diagnosed with AIG did not have AIG-related diseases, such as iron deficiency or pernicious anemia, nor symptoms requiring a visit to a medical center, though a complication with thyroid disease was frequently observed. Considering the high prevalence of positive results for the anti-parietal cells antibody in serum in previous reports (9,10), we consider that there may be many undiagnosed individuals with AIG who do not have apparent proximal-predominant gastric mucosal atrophy.
Gastric mucosal atrophy in the area of the corpus and fundus gradually progresses in patients with AIG, and loss of parietal cells with disease progression provokes hypoacidity and anacidity (2,4-6). Therefore, determining the serum pepsinogen and gastrin levels is reported to be useful for evaluating the degree of gastric mucosal atrophy in cases with AIG (20-22). The serum pepsinogen I level and the ratio of pepsinogen I and II have been reported to be extremely low, while hypergastrinemia caused by gastric hypoacidity has also been demonstrated in cases of AIG (14-16), which were also seen in the present study. However, one-third of our subjects with AIG also had a normal serum gastrin level (<200 pg/mL) and pepsinogen I/II ratio (>3.0). These AIG cases with normal serum gastrin levels and pepsinogen I/II ratios tended to be younger than the subjects with hypergastrinemia and low pepsinogen I/II ratios (≤1.0). In addition, the endoscopic finding of residual fundic gastric gland was more frequently observed in AIG cases with a normal serum gastrin level and pepsinogen I/II ratio. Thus, gastric acid secretion in cases with AIG may not be disturbed during the early stage of the disease (2). We found that 33 of the 46 subjects initially suspected to have AIG were finally diagnosed based on the presence of the auto-antibody. Therefore, endoscopic findings showing proximal-predominant gastric mucosal atrophy are considered useful for th escreening of AIG, even when the serum pepsinogen I/II ratio and gastrin level are normal. Thirteen of the AIG suspected 46 cases were not diagnosed as AIG in this study, and the majority of these cases had either a positive or post-eradicated status regarding H. pylori infection. Therefore, endoscopic observations of proximal-predominant gastric mucosal atrophy should be carefully performed in cases with a positive and post-eradicated status of H. pylori infection.
Molecular mimicry of H. pylori antigens and gastric H/K ATPase has been demonstrated, and the H/K ATPase autoantibody was reported to be present in 20-30% of H. pylori infected patients, thus H. pylori infection is considered to have some role in the occurrence of AIG (23,24). In the present study, the prevalence of AIG was not different among subjects with differing H. pylori statuses (negative, positive, post-eradicated). In addition, AIG has not been demonstrated to have a high prevalence in populations with a high rate of H. pylori infection. Therefore, H. pylori infection might not be directly correlated with the occurrence of AIG. On the other hand, only one case with H. pylori infection was diagnosed as AIG in this study. Thus, an endoscopic diagnosis of AIG in cases with H. pylori infection is considered to be difficult due to gastric mucosal inflammation and the extension of mucosal atrophy caused by H. pylori infection. Several previous studies have demonstrated a variety of autoimmune diseases occurring in association accompanied with AIG (3,6,25,26), which was confirmed by our findings. Additional studies are therefore needed to clarify the relationship between the occurrence of AIG and H. pylori infection, as well as the mechanisms of disease occurrence.
Neuroendocrine cell hyperplasia and tumors caused by hypergastrinemia are well-known complications seen in patients with AIG (2,6,7,27,28). None of the present subjects had an endoscopically detected neuroendocrine cell tumor. However, we did not perform histological examinations of the gastric body, because nearly all of our subjects were healthy and visited our medical center for an annual checkup. Therefore, we did not analyze the prevalence of neuroendocrine cell hyperplasia in those diagnosed with AIG. However, some likely were affected by histological neuroendocrine cell hyperplasia, since two-thirds with AIG showed hypergastrinemia. We think that a histological study of the stomach is needed in future follow-up endoscopic examinations of subjects who show hypergastrinemia to investigate the presence of neuroendocrine cell hyperplasia. In addition, the incidence of gastric cancer in cases with AIG has been reported to be higher than that in the general population (2,3,7,29). Although not detected in any of our subjects during the study period, 1 had a history of gastric cancer. Additionally the number of elderly individuals was small among our AIG cases (≥70 years old, n=6), which might have been related to the absence of findings of gastric cancer. Nevertheless, we think that a follow-up endoscopic examination is essential to detect gastric cancer at an early stage in individuals diagnosed with AIG.
The present study is associated with several limitations. It was performed at a single medical center, and a majority of the present subjects were socially active and productive, thus it included relatively few young and elderly individuals. On the other hand, the present subjects are considered to be representative of the general population in comparison with patients who visit a medical clinic for specific symptoms. In addition, the prevalence of AIG was different when the criteria used for the diagnosis of AIG differed. A comparative study is thus needed to clarify the difference in the prevalence of AIG among the different criteria and in a variety of populations.
In conclusion, the present results showed the prevalence of AIG to be 0.49% in a large number of individuals in Japan who underwent an upper gastrointestinal endoscopic examination as part of an annual checkup. It is likely that many asymptomatic healthy individuals are thus affected by AIG.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We wish to thank Kiyoe Kawase, Noriko Hara, Yuki Funaki, Noriko Yamauchi, and Naoyuki Notsu of the Shimane Environment and Health Public Corporation, as well as Rika Tohma and Keiko Masuzaki of the Second Department of Internal Medicine, Shimane University Faculty of Medicine, for their helpful technical support.
References
- 1. Strickland RG, Mackay IR. A reappraisal of the nature and significance of chronic atrophic gastritis. Am J Dig Dis 18: 426-440, 1973. [DOI] [PubMed] [Google Scholar]
- 2. Toh BH. Diagnosis and classification of autoimmune gastritis. Autoimmun Rev 13: 459-462, 2014. [DOI] [PubMed] [Google Scholar]
- 3. Kulnigg-Dabsch S. Autoimmune gastritis. Wien Med Wochenschr 166: 424-430, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Irvine WJ, Cullen DR, Mawhinney H. Natural history of autoimmune achlorhydric atrophic gastritis. A 1-15-year follow-up study. Lancet 2: 482-485, 1974. [DOI] [PubMed] [Google Scholar]
- 5. Toh BH, van Driel IR, Gleeson PA. Pernicious anemia. N Engl J Med 337: 1441-1448, 1997. [DOI] [PubMed] [Google Scholar]
- 6. Imamura H. Diagnosis of autoimmune (type A) gastritis. Gatroenterol Endosc 60: 1444-1449, 2018(in Japanese, Abstract in English). [Google Scholar]
- 7. Coati I, Fassan M, Farinati F, Graham DY, Genta RM, Rugge M. Autoimmune gastritis: pathologist's viewpoint. World J Gastroenterol 21: 12179-12189, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carmel R. Prevalence of undiagnosed pernicious anemia in the elderly. Arch Intern Med 156: 1097-1100, 1996. [PubMed] [Google Scholar]
- 9. Zhang Y, Weck MN, Schöttker B, Rothenbacher D, Brenner H. Gastric parietal cell antibodies, Helicobacter pylori infection, and chronic atrophic gastritis: evidence from a large population-based study in Germany. Cancer Epidemiol Biomarkers Prev 22: 821-826, 2013. [DOI] [PubMed] [Google Scholar]
- 10. Cabrera de León A, Almeida González D, Almeida AA, et al. Factors associated with parietal cell autoantibodies in the general population. Immunol Lett 147: 63-66, 2012. [DOI] [PubMed] [Google Scholar]
- 11. Adachi K, Mishiro T, Tanaka S, Kinoshita Y. Analysis of negative result in serum anti-H. pylori IgG antibody test in cases with gastric mucosal atrophy. J Clin Biochem Nutr 59: 145-148, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adachi K, Mishiro T, Okada M, Kinoshita Y. Prevalence of multiple white and flat elevated lesions in individuals undergoing medical checkup. Intern Med 57: 1213-1218, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adachi K, Mishiro T, Okimoto E, Kinoshita Y. Influence of degree of gastric mucosal atrophy on serum lipid levels before and after eradication of Helicobacter pylori infection. Intern Med 57: 3067-3073, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kyoto Classification of Gastritis. Haruma K, Ed. Nihon Medical Center, Tokyo, 2017. [Google Scholar]
- 15. Watanabe K, Nagata N, Nakashima R, et al. Predictive findings for Helicobacter pylori-uninfected, -infected and -eradicated gastric mucosa: validation study. World J Gastroenterol 19: 4374-4379, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kato M, Terao S, Adachi K, et al. Changes in endoscopic findings of gastritis after cure of H. pylori infection: multicenter prospective trial. Dig Endosc 25: 264-273, 2013. [DOI] [PubMed] [Google Scholar]
- 17. Kamada T, Haruma K, Inoue K, Shiotani A. Helicobacter pylori infection and endoscopic gastritis -Kyoto classification of gastritis. Nihon Shokakibyo Gakkai Zasshi 112: 982-993, 2015(in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 18. Sugimoto M, Ban H, Ichikawa H, et al. Efficacy of the Kyoto Classification of Gastritis in identifying patients at high risk for gastric cancer. Intern Med 56: 579-586, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Toyoshima O, Nishizawa T, Arita M, et al. Helicobacter pylori infection in subjects negative for high titer serum antibody. World J Gastroenterol 24: 1419-1428, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Antico A, Tampoia M, Villalta D, Tonutti E, Tozzoli R, Bizzaro N. Clinical usefulness of the serological gastric biopsy for the diagnosis of chronic autoimmune gastritis. Clin Dev Immunol 2012: 520970, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Agréus L, Kuipers EJ, Kupcinskas L, et al. Rationale in diagnosis and screening of atrophic gastritis with stomach-specific plasma biomarkers. Scand J Gastroenterol 47: 136-147, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nasrollahzadeh D, Aghcheli K, Sotoudeh MS, et al. Accuracy and cut-off values of pepsinogens I, II and gastrin 17 for diagnosis of gastric fundic atrophy: influence of gastritis. PLoS One 6: e26957, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Amedei A, Bergman MP, Appelmelk BJ, et al. Molecular mimicry between Helicobacter pylori antigens and H+, K+ -adenosine triphosphatase in human gastric autoimmunity. J Exp Med 198: 1147-1156, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Veijola LI, Oksanen AM, Sipponen PI, Rautelin HI. Association of autoimmune type atrophic corpus gastritis with Helicobacter pylori infection. World J Gastroenterol 16: 83-88, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Centanni M, Marignani M, Gargano L, et al. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med 159: 1726-1730, 1999. [DOI] [PubMed] [Google Scholar]
- 26. Tursi A, Grattagliano I, De Polo M, et al. Noninvasive prediction of chronic atrophic gastritis in autoimmune thyroid disease in primary care. Scand J Gastroenterol 49: 1394-1396, 2014. [DOI] [PubMed] [Google Scholar]
- 27. Creutzfeldt W. The achlorhydria-carcinoid sequence: role of gastrin. Digestion 39: 61-79, 1988. [DOI] [PubMed] [Google Scholar]
- 28. Bordi C, D'Adda T, Azzoni C, Pilato FP, Caruana P. Hypergastrinemia and gastric enterochromaffin-like cells. Am J Surg Pathol 19(Suppl 1): S8-S19, 1995. [PubMed] [Google Scholar]
- 29. Vannella L, Lahner E, Osborn J, Annibale B. Systematic review: gastric cancer incidence in pernicious anaemia. Aliment Pharmacol Ther 37: 375-382, 2013. [DOI] [PubMed] [Google Scholar]