Abstract
An unconscious 55-year-old man with a history of neurofibromatosis type 1 (NF1) was transported to the emergency department and was diagnosed with acute renal failure owing to a large bladder tumor. A submucosal tumor was also identified in the duodenum. The patient was diagnosed with a primary gastrointestinal stromal tumor (GIST) of the bladder and duodenum. After six cycles of regorafenib therapy, 18F-fluorodeoxyglucose accumulation in the duodenal GIST on positron emission tomography-computed tomography (PET-CT) showed a partial metabolic response. Currently, no standard drug therapy for unresectable or relapsed NF1-associated GIST has been established. Regorafenib may thus be considered as and appropriate initial therapy.
Keywords: neurofibromatosis type 1, gastrointestinal stromal tumor, regorafenib, tyrosine kinase inhibitor
Introduction
Neurofibromatosis type 1 (NF1) is a hereditary autosomal dominant disease with an incidence rate of from 1/2,000 to 1/5,000 in most population base studies (1). It is characterized by various dermatological, neurological, and skeletal manifestations, such as cutaneous neurofibromas and café au lait spots (1). The pathogenesis of this disease is based on biallelic mutation of the NF1 gene located on chromosome 17q11.2, which leads to a loss of neurofibromin, a tumor suppressor protein (1,2). Therefore, patients with NF1 have a higher risk of developing various benign or malignant neoplasms, with gastrointestinal stromal tumors (GISTs) developing in approximately 5-25% of patients with NF1 (3).
Activating mutations in either the KIT or PDGFRA gene are present in most GISTs and they are considered to be the primary drivers of oncogenesis in the disease (4,5); therefore, patients with unresectable or relapsed GISTs are initially treated with imatinib (6). However, in NF1-associated GISTs (NF1-GISTs), oncogenesis is attributed to the activation of the RAS/RAF/MAP kinase signaling pathway through loss-of-function NF1 gene mutations, rather than KIT or PDGFRA gene mutations (7). Therefore, no standard drug therapy for unresectable or relapsed NF1-GISTs has been established.
We herein present a case of NF1-GISTs arising in the bladder and duodenum, for which treatment with regorafenib was effective. To our knowledge, no previous report of successful regorafenib therapy for NF1-GISTs has so far been published.
Case Report
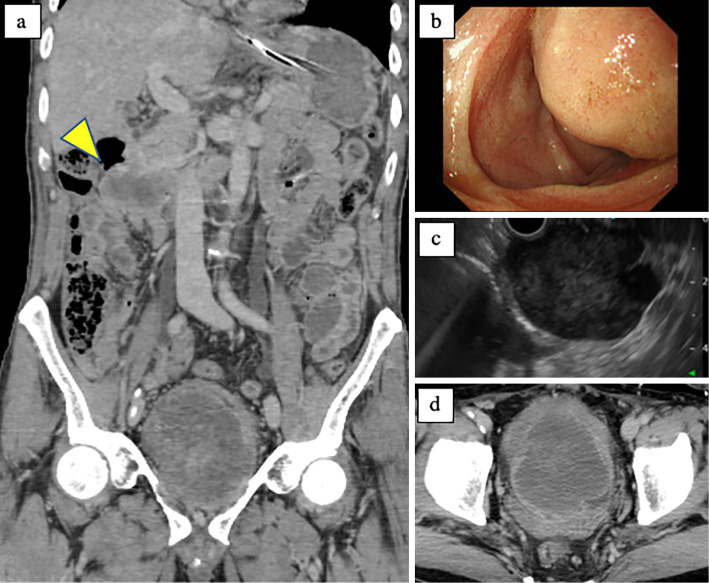
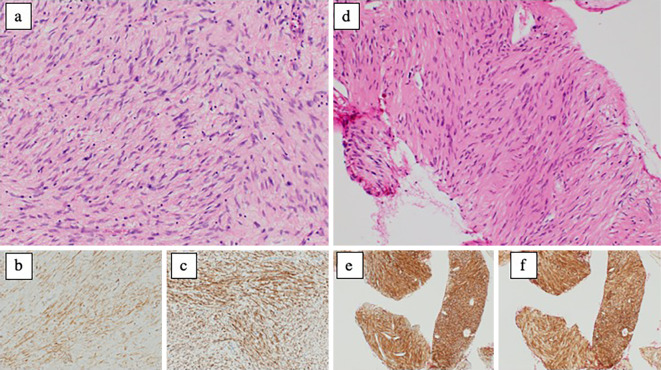
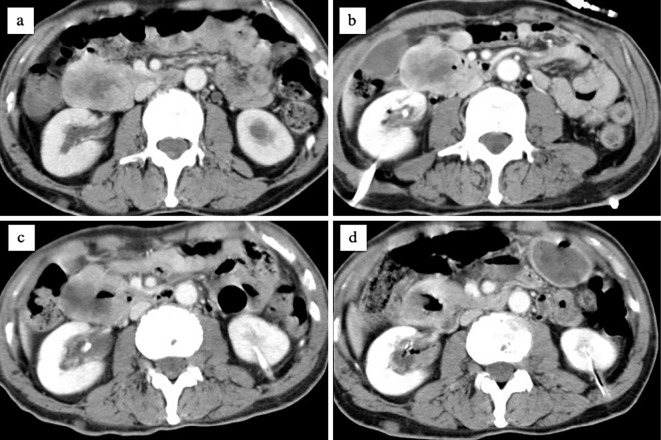
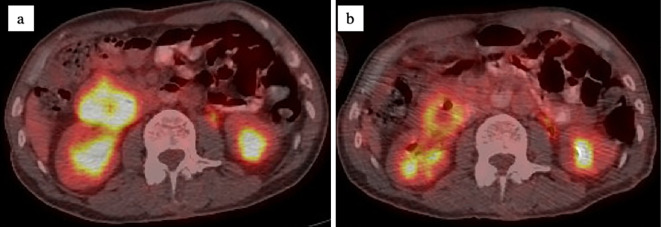
A 55-year-old man was transported to the emergency department of an outside hospital by air ambulance owing to unconsciousness and convulsions. The patient had a history of NF1 with intellectual disability, amblyopia, and multiple cutaneous neurofibromas and café au lait spots (Fig. 1), and he had a sister with NF1 and GISTs, for which she underwent surgery. The laboratory data on admission showed severe renal dysfunction with hyperkalemia and acidemia (Cr 9.9 mg/dL, K 7.6 mEq/L, pH 6.820). Abdominal computed tomography (CT) revealed bilateral hydronephrosis due to a large mass within the bladder measuring 8.9×7.3 cm in diameter (Fig. 2a and d). Surgery was promptly performed; the bladder wall was highly thickened and the bladder cavity contained an extremely large, solid tumor. The tumor was only partially excised piece by piece due to the fact that it was too large to resect en bloc, and the tumor appeared to arise from the bladder neck with direct invasion into the prostate. Finally, a cystostomy was performed. At week 1 postoperatively, transurethral electrocoagulation was performed for a massive hematuria, and an additional resection of the residual tumor of the bladder neck was undertaken as far as was possible. The pathological findings revealed fascicular proliferation of spindle cells (Fig. 3a), with no mitotic figures [0/10 high-power fields (HPFs)]. Immunohistochemically, the tumor cells stained positive for KIT (Fig. 3b), CD34 (Fig. 3c), PDGFRA, DOG1, and vimentin and negative for S-100 protein. The Ki-67 index of the tumor cells was <1%. In addition, a mass measuring approximately 5.0×4.2 cm in diameter was present in the second portion of the duodenum (Fig. 2a and b). Endoscopically, a submucosal tumor (SMT) was observed on the anal side of the papilla of Vater (Fig. 2b), and the SMT had low and partially heterogenous echoic levels according to the findings of ultrasonic endoscopy, and no continuity to the pancreas was observed (Fig. 2c). We then performed endoscopic ultrasound-guided fine needle aspiration and the pathological findings were similar to those of the bladder tumor (Fig. 3d-f). The patient was diagnosed with primary GISTs of the bladder and duodenum. The pathological findings of this case were previously reported in detail by Segawa et al. (8). Following initial surgery and a pathological analysis, the patient was transferred to our hospital to receive drug therapy for both GISTs because the bladder GIST was unresectable. Although no KIT mutations in exons 8, 9, 11, 13, 14, 17, and 18 and no PDGFRA mutations in exons 12, 14, and 18 were detected with the polymerase chain reaction method in either the bladder or duodenal GIST, the patient was initially treated with imatinib because other tyrosine kinase inhibitors (TKIs) have yet to be approved for use as initial GIST therapy in Japan. Imatinib 400 mg was administered once daily for two weeks without any adverse events; however, following a CT scan, no response was observed. Subsequently, we initiated treatment with regorafenib 160 mg once daily on days 1-21 of a 28-day cycle. During cycle 2, the patient developed grade 3 hypertension together with urinary tract infection. Although the hypertension was only temporary, a bilateral percutaneous nephrostomy was performed because of recurrent urine leakage through a vesical fistula. No other adverse events relevant to regorafenib were observed. Following the second cycle of regorafenib therapy, intratumoral air was observed on contrast-enhanced CT (Fig. 4b) which had not been observed prior to treatment (Fig. 4a), suggestive of tumor necrosis. The size of the intratumoral air pocket increased gradually with the continuation of regorafenib therapy accompanied with a decrease in the size of the tumor (Fig. 4c and d). Additionally, after six cycles of regorafenib therapy, 18F-fluorodeoxyglucose accumulation in the duodenal GIST on positron emission tomography-computed tomography (PET-CT) decreased from a maximum standardized uptake value (SUVmax) of 9.1 before treatment (Fig. 5a) to a value of 6.5 (decreased to 71.4%, Fig. 5b), a change fulfilling the criteria of a partial metabolic response as defined by the European Organization for Research and Treatment of Cancer (9). The chemotherapeutic effects on the bladder GIST could not be evaluated because the residual tumor could not be visualized on PET-CT or contrast-enhanced CT. Currently, the patient is undergoing a fourteenth treatment cycle of regorafenib therapy and has shown no disease progression for fifteen months.
Figure 1.
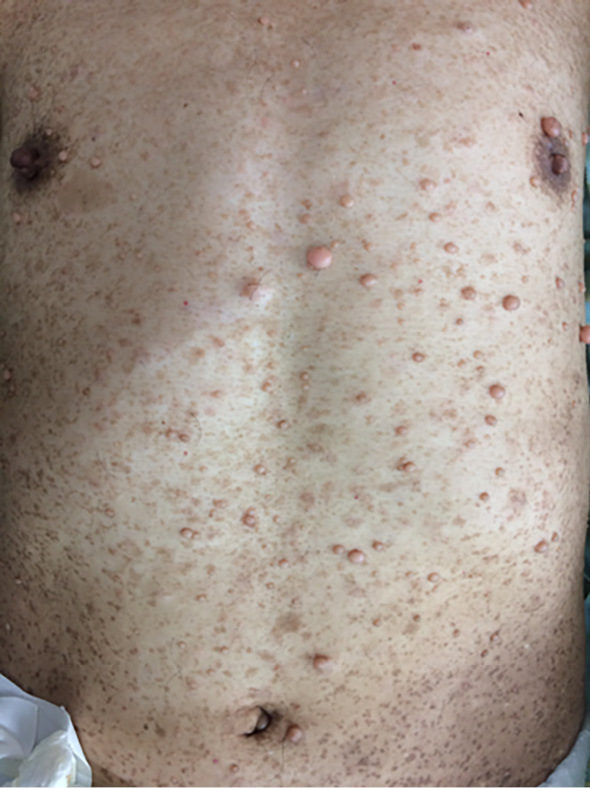
Cutaneous findings of the patient: Multiple neurofibromas and café au lait spots were observed.
Figure 2.
Abdominal CT, esophagogastroduodenoscopy, and ultrasonic endoscopy findings on admission: A 8.9×7.3-cm-sized mass in the bladder and 5.0×4.2-cm-sized mass in the duodenum (arrow head) were observed (a, d). A SMT was apparent on the anal side of the papilla of Vater in the second portion of the duodenum (b). The SMT had low but partially heterogeneous echoic levels with no continuity to the pancreas. The SMT had a smooth mucosa with no ulcerations. CT: computed tomography, SMT: submucosal tumor
Figure 3.
Pathological findings of the tumors: (a-c) bladder tumor, (d-f) duodenal tumor, (a, d) Hematoxylin and Eosin staining ×400, (b, e) KIT, (c, f) CD34. Fascicular proliferation of spindle-shaped tumor cells was visible. No mitotic figures were observed (0/10 HPFs). Immunohistochemically, tumor cells stained positive for KIT, CD34, PDGFRA, DOG1, and vimentin, and stained negative for S-100 protein. The Ki-67 index of the tumor cells was 1%.
Figure 4.
Contrast-enhanced CT performed before (a) and after 2 cycles (b), 4 cycles (c), and 6 cycles (d) of regorafenib therapy: intratumoral air appeared on contrast-enhanced CT after 2 cycles of regorafenib therapy (b) which had not been observed prior to treatment (a), suggestive of tumor necrosis. The size of the intratumoral airspace increased gradually with the continuation of regorafenib therapy with a decreasing size of the tumor (c, d). CT: computed tomography
Figure 5.
PET-CT performed before (a) and after 6 cycles of regorafenib therapy (b): 18F-fluorodeoxyglucose accumulation in the duodenal GIST on PET-CT decreased from SUV-max of 9.1 before treatment to 6.5 after 6 cycles of regorafenib therapy (decreased to 71.4%). PET-CT: positron emission tomography-computed tomography, SUVmax: maximum standardized uptake value
Discussion
GISTs are the most common mesenchymal neoplasms of the gastrointestinal tract, originating from the progenitors of interstitial cells of Cajal, which regulate digestive tract motility (4). Activating gene mutations in two receptor tyrosine kinases are well known oncogenic mechanisms of GISTs; that is, approximately 80-85% of GISTs have sporadic mutations in KIT, while PDGFRA mutations are a less common driver of oncogenesis (5,6). Both gain-of-function gene mutations lead to the constitutive activation of kinases, resulting in cell growth via the PI3K-AKT, RAS/RAF/MAP kinase, and JAK-STAT3 signaling pathways (7). The remaining 10-15% of GISTs have no KIT or PDGFRA oncogenic mutations, and they are known as wild-type GISTs (wt-GISTs) (10). NF1-GISTs are classified as wt-GISTs at a frequency of less than 1% or 1-2% of all GISTs (6,10,11).
Neurofibromin, encoded by the NF1 gene, functions as a negative regulator of the RAS/RAF/MAP kinase pathway through the inhibition of the RAS oncogene (2). The loss of neurofibromin as a result of NF1 gene mutation in patients with NF1 leads to the constitutive activation of the RAS oncogene, and the subsequent activation of the RAS/RAF/MAP kinase pathway, which exposes these patients to a higher risk of various benign and malignant tumor development (7). Maertens et al. detailed the precise molecular oncogenic pathway of NF1-GISTs (7). Namely, the somatic inactivation of the wild-type NF1 allele and subsequent inactivation of neurofibromin occur, and hyperactivation of RAS/RAF/MAP kinase pathway is demonstrated with an expression level of phosphorylated MAPK 3 to 18 times higher in NF1-GISTs than in sporadic GISTs. Meanwhile, the PI3K-AKT and JAK-STAT3 pathways are not as highly activated in NF1-GISTs as in sporadic GISTs, and no mutations in either KIT or PDGFRA have been demonstrated (7). As the oncogenic mechanisms of NF1-GISTs are different from those of sporadic GISTs, the clinical manifestations also differ. For instance, the median age of onset of NF1-GISTs has been reported to be 49 years (12), compared to 60-65 years for sporadic GISTs (13), and most NF1-GISTs develop in the small intestine, especially in the duodenum and jejunum, with multiple lesions (12). Although the molecular pathogenesis in the present case was uncertain, the clinical manifestations corresponded to typical NF1-GISTs. Meanwhile, in this case, a GIST developed within the bladder and thus led to a lower urinary tract obstruction, resulting in acute renal failure. GISTs arising outside of the gastrointestinal tract are called extra-gastrointestinal stromal tumors (EGISTs) and have a frequency of approximately 5-10% of the sporadic GISTs (14), whereas EGISTs in patients with NF1 are rarely reported (15).
The only curative treatment for GISTs is complete surgical resection of the primary lesion. Patients with unresectable or relapsed GISTs are initially treated with imatinib, a novel TKI capable of inhibiting KIT, PDGFRA, and BCR/ABL, which showed an overall response rate exceeding 50% and a median progression-free survival of 1.7 years at a median follow-up time of 10.9 years (16). In cases of refractory tumors or patients intolerant to imatinib, sunitinib and regorafenib could be administered as second- and third-line treatments, respectively (6). However, the European Society for Medical Oncology guidelines recommend not administering imatinib for NF1-GISTs (13) because the imatinib provides no effect for NF1-GISTs that have no KIT or PDGFRA gene mutations. In addition, the efficacy of other TKIs for NF1-GISTs has also not been previously reported; thus, no treatment strategy for unresectable or relapsed NF1-GISTs has yet been established.
The molecular mechanisms underlying the antitumor effect of regorafenib on the NF1-GIST in the present case are obscure. Regorafenib is a multikinase inhibitor, which potently inhibits RAF-1, BRAF, and members of the vascular endothelial growth factor receptor (VEGFR) family, in addition to inhibiting KIT and PDGFRA (17). Although the absence of neurofibromin in the present case was not examined, the constitutive activation of the MAP kinase pathway including RAF-1 and BRAF might exist, and these targets could be inhibited by regorafenib (17). It could also be speculated that regorafenib might have influenced tumor angiogenesis through the inhibition of some VEGFR family members. Sunitinib, another multikinase inhibitor, also could inhibit KIT, PDGFRA, and the VEGFR family (18), but its activity in inhibiting RAF has not been elucidated. A single case report of NF1-GIST successfully treated with sunitinib after disease progression during imatinib therapy was previously published, but mutations of KIT or PDGFRA were not confirmed (19).
In the present case, the authors reluctantly administered imatinib as first-line therapy since another TKI has yet to be approved for use as initial GIST therapy in Japan. After the cessation of imatinib, we administered regorafenib owing to its ability to inhibit RAF-1 and BRAF, and consequently obtained the expected therapeutic response. Although further investigation into the pathogenesis of NF1-GISTs and the precise molecular mechanisms of these multikinase inhibitors in antitumor activities is anticipated, regorafenib could currently be considered as an initial therapy for unresectable or relapsed NF1-GISTs.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Rasmussen SA, Friedman JM. NF1 gene and neurofibromatosis 1. Am J Epidemiol 151: 33-40, 2000. [DOI] [PubMed] [Google Scholar]
- 2. Viskochil D. Genetics of neurofibromatosis 1 and the NF1 gene. J Child Neurol 17: 562-570, 2002. [DOI] [PubMed] [Google Scholar]
- 3. Yantiss RK, Rosenberg AE, Sarran L, Besmer P, Antonescu CR. Multiple gastrointestinal stromal tumors in type I neurofibromatosis: a pathologic and molecular study. Mod Pathol 18: 475-484, 2005. [DOI] [PubMed] [Google Scholar]
- 4. Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol 22: 3813-3825, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279: 577-580, 1998. [DOI] [PubMed] [Google Scholar]
- 6. Nishida T, Blay JY, Hirota S, Kitagawa Y, Kang YK. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer 19: 3-14, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maertens O, Prenen H, Debiec-Rychter M, et al. Molecular pathogenesis of multiple gastrointestinal stromal tumors in NF1 patients. Hum Mol Genet 15: 1015-1023, 2006. [DOI] [PubMed] [Google Scholar]
- 8. Segawa K, Sugita S, Sugawara T, et al. Multiple gastrointestinal stromal tumors involving extragastrointestinal sites in neurofibromatosis type 1. Pathol Int 68: 142-144, 2018. [DOI] [PubMed] [Google Scholar]
- 9. Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer 35: 1773-1782, 1999. [DOI] [PubMed] [Google Scholar]
- 10. Huss S, Elges S, Trautmann M, Sperveslage J, Hartmann W, Wardelmann E. Classification of KIT/PDGFRA wild-type gastrointestinal stromal tumors: implications for therapy. Expert Rev Anticancer Ther 15: 623-628, 2015. [DOI] [PubMed] [Google Scholar]
- 11. Corless CL. Gastrointestinal stromal tumors: what do we know now? Mod Pathol 27: S1-S16, 2014. [DOI] [PubMed] [Google Scholar]
- 12. Miettinen M, Fetsch JF, Sobin LH, Lasota J. Gastrointestinal stromal tumors in patients with neurofibromatosis 1: a clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol 30: 90-96, 2006. [DOI] [PubMed] [Google Scholar]
- 13. The ESMO/European Sarcoma Network Working Group Ann Oncol 25(Supple): iii21, 2014.25210085 [Google Scholar]
- 14. Etit D, Kar H, Ekinci N, Yenipazar AE, Çakalağaoğlu F. Extra-gastrointestinal stromal tumor of prostate. Balkan Med J 34: 168-171, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Malhotra A, Wright J, Gajra A. Extra gastrointestinal stromal tumor treated with imatinib in a patient with neurofibromatosis type 1. J Gastrointest Oncol 3: 373-376, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Casali PG, Zalcberg J, Le Cesne A, et al. Ten-year progression-free and overall survival in patients with unresectable or metastatic GI stromal tumors: long-term analysis of the European Organisation for Research and Treatment of Cancer, Italian Sarcoma Group, and Australasian Gastrointestinal Trials Group Intergroup Phase III randomized trial on imatinib at two dose levels. J Clin Oncol 35: 1713-1720, 2017. [DOI] [PubMed] [Google Scholar]
- 17. Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 129: 245-255, 2011. [DOI] [PubMed] [Google Scholar]
- 18. Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 9: 327-337, 2003. [PubMed] [Google Scholar]
- 19. Kalender M, Sevinc A, Tutar E, Sirikci A, Camci C. Effect of sunitinib on metastatic gastrointestinal stromal tumor in patients with neurofibromatosis type 1: a case report. World J Gastroenterol 13: 2629-2632, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]