Abstract
GlycA, a composite biomarker of systemic inflammation, is associated with cardiovascular disease (CVD) and mortality but its relationship with peripheral artery disease (PAD) is unknown. We assessed whether GlycA is associated with ankle-brachial index (ABI), carotid plaque (CP) and incident clinical PAD among 6,466 Multi-Ethnic Study of Atherosclerosis participants without CVD at baseline. Plasma GlycA, ABI, and CP were measured at baseline. ABI and CP were re-measured at 10-years. Incident clinical PAD was ascertained from hospital records. We used logistic, Cox, and linear mixed models, adjusted for demographic and lifestyle factors. Mean±SD was 62±10 years for age and 381±61 μmol/L for GlycA; 53% were women. GlycA was associated with both prevalent low ABI ≤0.8 (prevalence odds ratio [95% CI] per SD increment in GlycA, 1.65 [1.39–1.97]) and CP (1.19 [1.11–1.27]) at baseline. There were no significant associations of GlycA with incident low ABI, incident CP, or 10-year change in ABI or CP score. We identified 110 incident cases of PAD after 79,590 person-years. The hazard ratio (95% CI) of incident PAD per SD increment in GlycA was 1.38 (1.14–1.66). In conclusion, GlycA is associated with prevalent low ABI, prevalent CP, and with incident PAD after a median of 14-years.
Keywords: Peripheral artery disease, inflammation, GlycA, carotid plaque, ankle branchial index
Introduction
Despite advances in public health interventions and preventive pharmacotherapies for cardiovascular disease (CVD), such as the use of statins and aspirin, CVD still remains a leading cause of morbidity and mortality in the United States and around the world.1 A failure to identify novel predictors of CVD, which may be modifiable and risk stratify individuals better, may be a contributing factor.2 Inflammation plays an important role in the development of atherosclerotic disease,3 and several inflammatory markers have been shown to be associated with CVD.4 Additionally, an anti-inflammatory therapy targeting interleukin-1β was shown to reduce the risk of recurrent CVD events, independent of lipid-lowering therapy, in the Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS).5
Peripheral artery disease (PAD) is an independent predictor of CVD events and mortality, even when asymptomatic.6–8 PAD commonly affects the lower extremities and can be assessed using the ankle-brachial index (ABI).6 Atherosclerosis can also result in plaque formation in the carotid arteries, which may lead to stenosis or plaque rupture and ultimately stroke.9 Prior studies have shown that both carotid plaque and ABI independently predict CVD events.6, 7, 10, 11 The identification of novel biomarkers that predict PAD and/or carotid plaque formation may identify individuals who are at an earlier stage in the disease process, at a time when preventive therapies could be instituted. Several epidemiological studies have provided evidence of an association between inflammatory markers, such as high-sensitivity C-reactive protein (hsCRP) and interleukin-6 (IL-6), with PAD12, 13 but findings have been inconsistent for carotid atherosclerosis.14–17
GlycA, a nuclear magnetic resonance (NMR) composite biomarker of systemic inflammation, reflects serum concentration and glycosylation state of main acute-phase reactants such as α1-acid glycoprotein, haptoglobin, α1-antitrypsin, α1-antichymotrypsin and transferrin.18 This novel biomarker has several advantages compared with hsCRP, including its composite nature, lower analytic imprecision, and lower intra-individual variability.18
In prior work from the Multi-Ethnic Study of Atherosclerosis (MESA), plasma GlycA levels were associated with poorer cardiovascular health at baseline19 and with increased risk for incident CVD events and all-cause mortality, even after adjustment for other inflammatory markers such as hsCRP, d-dimer, and IL-6.4 GlycA has also been shown to be associated with several measures of subclinical atherosclerosis, including prevalent coronary artery calcium (CAC)20 as well as prevalent calcification in the thoracic aorta and aortic valve.21 However, the associations of GlycA with atherosclerotic disease specifically located in the peripheral arteries and carotid arteries, have not yet been established. Despite many shared risk factors, risk factors for atherosclerosis across different vascular beds are not necessarily overlapping22, 23 and warrant further study. We hypothesized that plasma GlycA levels would be associated with risk of PAD and carotid plaque in a diverse community-based cohort free of CVD at baseline.
Methods
The MESA cohort included 6,814 men and women aged 45–84 years and free of clinical CVD at enrollment. Participants were enrolled from 6 field sites in the United States including Los Angeles County, CA (UCLA); Chicago, IL (Northwestern University); Baltimore/Baltimore County, MD (Johns Hopkins University); St. Paul, MN (University of Minnesota); Forsyth County, NC (Wake Forest University); and Northern Manhattan/Bronx, NY (Columbia University). The MESA study design has been previously published.24 Briefly, MESA includes 2,622 (38%) non-Hispanic white, 1,892 (28%) African, 1,493 (22%) Hispanic, and 801 (12%) Chinese Americans, who participated in up to 6 in-person exams occurring approximately every 2 years from 2000 to 2018.
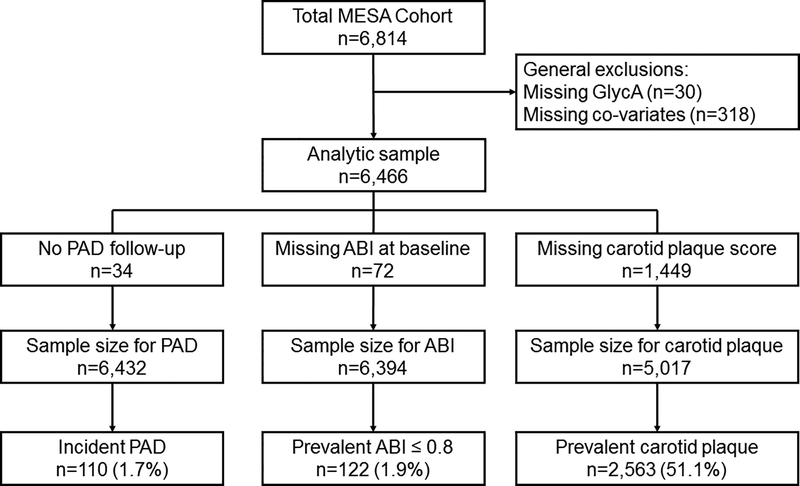
The current study includes 6,466 participants with measured GlycA levels and includes data from Exams 1, 3, and 5. For each respective analysis (carotid plaque, ABI, and incident clinical PAD), the sample size varied slightly based on available data. See Figure 1 for inclusions/exclusions. The study was approved by the Institutional Review Boards at each research center and informed consent was obtained from all participants.
Figure 1.
Participant flow chart of inclusions and exclusions.
GlycA and covariate ascertainment
For this analysis, we used laboratory data from blood samples that were collected at the baseline exam (2000–2002) after a 12-h overnight fast; participants were also recommended to abstain from smoking for 12 h before the blood draw.
GlycA (μmol/L) was measured from NMR LipoProfile® spectra acquired from EDTA-plasma samples at LipoScience (Raleigh, NC; now LabCorp) using proprietary deconvolution software described previously in detail.18 The intra-assay and inter-assay coefficients of variation (CV) for GlycA measurement were 1.9% and 2.6%, respectively.
Serum levels of hsCRP (mg/L) were measured at baseline using the BNII nephelometer (Dade Behring, Deerfield, IL) at the Core Lab at the University of Vermont, Burlington, Vermont. Intra-assay CV of hsCRP ranged from 2.3% to 4.4% and inter-assay CV ranged from 2.1% to 5.7%.19 Baseline serum levels of IL-6 (pg/mL) were measured using ultrasensitive ELISA (Quantikine HS Human IL-6 Immunoassay, R&D Systems) with inter-assay CV of 6.3% while fibrinogen levels (mg/dL) were measured using the BNII nephelometer (Dade Behring, Deerfield, IL) with inter-assay CV of 2.6%.25
Interview and questionnaire data from the baseline exam were used for information on age, sex, race/ethnicity and smoking status. Total MET-minutes/week of moderate and vigorous physical activity was derived from the Typical Week Physical Activity survey, while medication use was ascertained by a medication inventory approach. Body mass index (BMI) was calculated as weight in kg per height in m squared (kg/m2). Blood pressure was measured 3 times using a Dinamap automated blood pressure device and determined by averaging the last 2 measurements. Total cholesterol and high-density lipoprotein cholesterol (HDL-C) were measured at a central core lab (the Collaborative Studies Clinical Laboratory at Fairview-University Medical Center, Minneapolis, MN). HDL-C was measured using the cholesterol oxidase method after precipitation of non-HDL-C with magnesium/dextran. Diabetes was defined as use of diabetes medication or insulin, self-report or a fasting glucose ≥126 mg/dL. Estimated glomerular filtration rate (eGFR) was calculated with the CKD-Epi equation.26
Ankle-brachial index
ABI was measured at baseline, at Exam 3 (approximately 5 years later) and at Exam 5 (approximately 10 years later). Methods for the measurement of ABI using a Doppler probe have been described.6, 27 For both legs separately, ABI was calculated as the maximum systolic blood pressure in the posterior tibial artery and dorsalis pedis for each respective leg, divided by the average of the left and right brachial pressures. In the event that left and right brachial pressures differed by 10 mmHg or more, the higher of the brachial pressures was used. Non-compressible ABIs (i.e. pulse still detected despite cuff inflation to 300 mm Hg) were excluded from this analysis. Low ABI was diagnosed if either leg had an ABI ≤0.8.
Clinical PAD events
After the baseline exam, participants were contacted every 9–12 months by telephone to inquire about interim hospital admissions and outpatient cardiovascular diagnoses and procedures. If the study participant was deceased, family members were contacted. Medical records were requested for those who reported a hospitalization or a new outpatient cardiovascular diagnosis. From these medical records, PAD was classified as definite or probable and included symptomatic disease such as lower extremity claudication, atherosclerosis of the lower extremity, arterial embolism and/or thrombosis of the lower extremity and abdominal aortic aneurysm.28 Probable PAD required only a documented physician diagnosis of a PAD condition with symptoms. Definite PAD required one or more other criteria, such as ultrasound evidence of obstruction; an exercise test positive for claudication; revascularization for PAD; amputation for ischemia; ABI ≤0.8 (low ABI); imaging of an aortic aneurysm; or a vascular procedure for abdominal aortic aneurysm. Follow-up information regarding clinical PAD events was obtained from Exam 1 (2000–2002) until a study endpoint, death or December 31, 2015.
Carotid plaque
Carotid plaque was assessed at baseline and approximately 10-years later at Exam 5. A full description of the methods for ascertaining carotid plaque score has been reported.29 Briefly, B-mode ultrasound images of the right and left common, bifurcation, and internal carotid artery segments were imaged with a Logiq 700 ultrasound system using the M12L transducer (General Electric Medical Systems; frequency, 13 MHz). Ultrasound images were reviewed and interpreted by the MESA Carotid Ultrasound Reading Center at the University of Wisconsin Atherosclerosis Imaging Research Program. Images were imported into Syngo Ultrasound Workplace reading stations and interpreted with Arterial Health Package software (Siemens Medical, Malvern, PA) for plaque scoring. Carotid ultrasound images from Exam 1 and Exam 5 were matched side by side on a video monitor and measured concurrently.29
Carotid plaque presence was defined as a discrete, focal abnormal intima-medial wall thickness >1.5 mm or a focal thickening of >50% of the surrounding intima-media thickness. Carotid plaque score (CPS), which ranged from 0–12, was defined as the number of carotid plaques in the internal, bifurcation, and common segments of both carotid arteries. A total plaque score was calculated to describe carotid plaque burden as follows: 1 point per plaque for the near and far walls of each segment (common carotid artery, bulb, and internal carotid artery) for the right and left carotid arteries. For example, if a participant had a plaque on the near and far wall of the bulb and the far wall of the left internal carotid artery, the total plaque score would be calculated as 3.29
Statistical analyses
We presented the baseline characteristics by GlycA quartiles. Continuous variables were presented as means (standard deviations, SD) and categorical variables as frequencies (%). Skewed variables were presented as median (25th - 75th percentile).
We used adjusted logistic regression models to estimate the prevalence odds ratios (OR) and their 95% confidence intervals (CI) for low ABI and carotid plaque presence (CPS >0) at baseline using the lowest quartile of GlycA as the reference group, as well as per 1 SD increment in GlycA (continuous). Similar models were used to assess associations with incident low ABI (after excluding participants with baseline prevalent low ABI) and incident carotid plaque (after excluding participants with baseline prevalent plaque) at Exam 5 (2010–2012). Linear mixed effect models were used to assess the associations between GlycA and ABI (natural log-transformed [ABI]) and carotid plaque score (natural log-transformed [CPS+1]) at baseline and for the 10-year change in these outcomes. These models leverage all available data (i.e. up to 3 measurements for ABI and up to 2 measurements for CPS) using continuous measures, while accounting for baseline differences.
Multivariable Cox proportional hazards regression models were used to estimate the adjusted hazard ratios (HR) and 95% CI for the association between GlycA quartiles and incident clinical PAD events, as well as per 1 SD increment in GlycA (continuous). We assessed the proportional hazards assumption using Schoenfeld residuals; and the proportional hazards assumption was met. Additionally, we performed restricted cubic splines with knots placed at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles to allow a more flexible distribution in characterizing the association between GlycA (continuous) and incident clinical PAD.
In a secondary aim of our study, we determined if individuals with high GlycA and high hsCRP have a higher risk compared to each marker alone, for clinical PAD. In this analysis, we used Cox regression models to assess the joint association of hsCRP (≥2 mg/L) and GlycA (≥ population median; 374.9 μmol/L) with PAD. We categorized participants based on their hsCRP and GlycA levels into Low GlycA/ High hsCRP, High GlycA/ Low hsCRP, High GlycA/ High hsCRP and compared them with Low GlycA/ Low hsCRP. We also examined the association of hsCRP alone with risk of incident PAD, for comparison purposes with GlycA. In another sensitivity analysis for incident clinical PAD, we excluded participants with a low ABI at baseline.
In all of our analyses, models were progressively adjusted as follows. In model 1, we adjusted for the demographic variables of age, sex, and race/ethnicity. In model 2, our main analytic model, we additionally adjusted for the socioeconomic and behavioral risk factors of education (<high school; high school or vocational school; college, graduate or professional school), BMI (as a continuous variable), cigarette smoking (current; former; never), pack years of smoking (natural log transformed), and physical activity level (MET-minutes/week of moderate or vigorous activity; natural log transformed).
Additionally we performed 2 supplemental models. Model 3 further adjusted for CVD risk factors that may be intermediate variables in the relationship between inflammation and atherosclerosis; these included systolic blood pressure (continuous), use of anti-hypertensive medications (yes, no), diabetes (yes, no), eGFR (continuous), total cholesterol and HDL-cholesterol (continuous), and use of lipid-lowering therapy (yes, no). In model 4 we further adjusted for other inflammatory markers which may be correlated with both GlycA and PAD -- hsCRP, IL-6, and fibrinogen (all natural log transformed).
We tested for linear trends across GlycA quartiles by using an ordinal variable for each category and modeling this as a continuous variable for each of our outcomes. Analyses were performed using STATA Version 15, and we considered p-values <0.05 to be significant.
Results
Baseline characteristics
Among the 6,466 participants eligible for this analysis, the mean (SD) age was 62 (10) years, 53% of participants were women, 39% were white, 27% black, 22% Hispanic, and 12% Chinese. As shown in Table 1, higher plasma GlycA levels were associated with a higher mean systolic blood pressure, BMI, and total cholesterol and lower mean HDL-C and eGFR. Higher plasma GlycA levels were also associated with higher median smoking pack-years, and lower median physical activity. Compared to the lower plasma GlycA quartiles, the higher GlycA quartiles had higher proportions of women, current smokers, participants who were Hispanic, participants with less than high school, high school or vocational school education, and participants on anti-hypertensive and lipid-lowering medications, and participants with diabetes.
Table 1.
Baseline characteristics of participants stratified by GlycA quartiles, Multi-Ethnic Study of Atherosclerosis (2000–2002)*,†.
| Characteristics | Total | GlycA (μmol/L) | p-for-trend across quartiles | |||
|---|---|---|---|---|---|---|
| Q1 (204.7 – 337.0) |
Q2 (338.0–374.9) |
Q3 (375.0 – 418.6) |
Q4 (419.8–787.8) |
|||
| n | 6466 | 1637 | 1599 | 1628 | 1602 | - |
| Age, years | 62.2 (10.3) | 61.4 (10.5) | 62.4 (10.2) | 62.6 (10.2) | 62.2 (10.1) | 0.01 |
| Female | 3426 (53%) | 629 (38.4%) | 754 (47.2%) | 910 (55.9%) | 1133 (70.7%) | <0.001 |
| Race | <0.001 | |||||
| White | 2515 (38.9%) | 605 (37%) | 656 (41%) | 611 (37.5%) | 643 (40.1%) | - |
| Black | 1746 (27%) | 416 (25.4%) | 384 (24%) | 469 (28.8%) | 477 (29.8%) | - |
| Hispanic | 1416 (21.9%) | 292 (17.8%) | 339 (21.2%) | 378 (23.2%) | 407 (25.4%) | - |
| Chinese | 789 (12.2%) | 324 (19.8%) | 220 (13.8%) | 170 (10.4%) | 75 (4.7%) | - |
| Education | <0.001 | |||||
| Less than high school | 1175 (18.2%) | 244 (14.9%) | 280 (17.5%) | 319 (19.6%) | 332 (20.7%) | - |
| High school or vocational school | 2663 (41.2%) | 581 (35.5%) | 631 (39.5%) | 702 (43.1%) | 749 (46.8%) | - |
| College, graduate or professional school | 2628 (40.6%) | 812 (49.6%) | 688 (43%) | 607 (37.3%) | 521 (32.5%) | - |
| BMI, kg/m2 | 28.3 (5.4) | 26.4 (4.7) | 27.6 (4.9) | 28.8 (5.3) | 30.4 (6.0) | <0.001 |
| Current smoker | 816 (12.6%) | 137 (8.4%) | 175 (10.9%) | 223 (13.7%) | 281 (17.5%) | <0.001 |
| Former smoker | 2349 (36.3%) | 630 (38.5%) | 591 (37.0%) | 585 (35.9%) | 543 (33.9%) | <0.001 |
| Pack-years of smoking‡ | 16.5 (6.0 – 33.0) | 13.5 (5.0 – 26.3) | 16.0 (5.6 – 31.5) | 17.0 (6.5 – 34.0) | 19.8 (7.5 – 38.0) | <0.001 |
| Physical activity, MET-minutes/week‡,§ | 4020 (1980 – 7515) | 4268 (2228 – 8190) | 4155 (1995 – 7650) | 4073 (1980 – 7320) | 3638 (1700 – 6893) | <0.001 |
| Systolic blood pressure, mmHg | 126.5 (21.5) | 122.8 (20.8) | 125.7 (21.3) | 128.2 (21.6) | 129.3 (21.9) | <0.001 |
| eGFR, ml/min per 1.73 m2 | 77.7 (16.2) | 79.0 (15.0) | 78.0 (14.8) | 77.1 (16.5) | 76.7 (18.1) | <0.001 |
| Total chol, mg/dL | 194.3 (35.5) | 186.2 (32.1) | 194 (33.7) | 196.5 (34.9) | 200.6 (39.3) | <0.001 |
| HDL-C, mg/dL | 51.0 (14.8) | 52.6 (15.5) | 51.2 (15.1) | 50.3 (14.6) | 50.0 (13.8) | <0.001 |
| Diabetes status | 808 (12.5%) | 136 (8.3%) | 164 (10.3%) | 214 (13.1%) | 294 (18.4%) | <0.001 |
| Antihypertensive | 2390 (37%) | 442 (27%) | 542 (33.9%) | 649 (39.9%) | 757 (47.3%) | <0.001 |
| Lipid-lowering | 1061 (16.4%) | 228 (13.9%) | 227 (14.2%) | 291 (17.9%) | 315 (19.7%) | <0.001 |
| hsCRP, mg/L‡ | 1.90 (0.83 – 4.18) | 0.85 (0.45 – 1.65) | 1.42 (0.74 – 2.79) | 2.41 (1.14 – 4.59) | 4.56 (2.35 – 9.29) | <0.001 |
| IL-6, pg/mL‡ | 1.20 (0.77 – 1.88) | 0.88 (0.59 – 1.4) | 1.06 (0.72 – 1.65) | 1.28 (0.86 – 1.93) | 1.65 (1.15 – 2.54) | <0.001 |
| Fibrinogen, mg/dL‡ | 338 (295 – 388) | 300 (268 – 337) | 327 (291 – 370) | 351 (308 – 395) | 385 (337 – 440) | <0.001 |
Abbreviations: BMI =body mass index; Chol =cholesterol; eGFR =estimated glomerular filtration rate; HDL-C = high density lipoprotein cholesterol; hsCRP =high-sensitivity C-reactive protein; IL-6 =interleukin-6; MET =metabolic equivalent of task.
Data are presented as mean (standard deviation) for continuous variables and as count (percentages) for categorical variables, unless otherwise specified.
Data presented as median (25th - 75th percentile).
Results are for participants with values greater than zero.
The mean (SD) GlycA in this study was 381 (61) μmol/L. Participants in the higher GlycA quartiles had higher median hsCRP, IL-6, and fibrinogen compared with participants in the lower GlycA quartiles (Table 1). GlycA was moderately correlated with the other inflammatory markers. The Spearman’s correlation of GlycA with hsCRP, IL-6, and fibrinogen was of 0.54, 0.35, and 0.46 respectively (all p<0.001).
ABI
Of the 6,394 participants with ABI measures at baseline, 122 (1.9%) had a low ABI ≤0.8. Participants with higher GlycA levels had greater odds of having prevalent low ABI (Table 2). GlycA was also inversely associated with ABI when ABI was assessed continuously as a log-transformed measure (Table 3). In our main model (model 2), the adjusted prevalence OR (95% CI) of low ABI for the highest GlycA quartile compared with the first quartile was 4.87 (2.56, 9.25). Further adjustment for CVD risk factors in model 3 yielded an OR (95% CI) of 3.49 (1.80, 6.75). Results remained significant after additional adjustment for other inflammatory markers, hsCRP, IL-6, and fibrinogen, with an OR (95% CI) of 2.88 (1.40, 5.93). In all adjusted models, the prevalence OR of low ABI were statistically significant when we used continuous GlycA, per 1 SD increment (Table 2). The average adjusted difference (95% CI) in log-transformed ABI (ln_ABI) comparing the highest GlycA quartile to the lowest was −0.029 (−0.037, −0.021) in model 2. Findings remained statistically significant after additional adjustment for CVD risk factors and inflammatory markers in model 4 [−0.013 (−0.022, −0.004)] (Table 3). However, there was no association between GlycA and odds of incident low ABI at Exam 5 (Supplemental Table 1s) or change in ABI over 10 years (Supplemental Table 2s) (all models p >0.05).
Table 2.
Prevalence odds ratios (95% confidence interval) of ABI ≤0.8 and carotid plaque by baseline GlycA: MESA (2000–2002)*.
| Q1 | Q2 | Q3 | Q4 | p-for-trend | Per 1SD increment | |
|---|---|---|---|---|---|---|
| ABI (N) | 1,631 | 1,581 | 1,610 | 1,572 | - | 6,394 |
| Case, n (%) | 14 (0.9%) | 22 (1.4%) | 30 (1.9%) | 56 (3.6%) | - | 122 (1.9%) |
| Model 1† | 1 (reference) | 1.73 (0.87 – 3.44) | 2.40 (1.25 – 4.63) | 5.61 (3.00 – 10.49) | <0.001 | 1.74 (1.47 – 2.06) |
| Model 2‡ | 1 (reference) | 1.70 (0.85 – 3.41) | 2.16 (1.11 – 4.23) | 4.87 (2.56 – 9.25) | <0.001 | 1.65 (1.39 – 1.97) |
| Model 3§ | 1 (reference) | 1.49 (0.74 – 3.00) | 1.74 (0.88 – 3.43) | 3.49 (1.80 – 6.75) | <0.001 | 1.47 (1.22 – 1.76) |
| Model 4ǁ | 1 (reference) | 1.41 (0.69 – 2.86) | 1.61 (0.80 – 3.25) | 2.88 (1.40 – 5.93) | 0.005 | 1.37 (1.10 – 1.71) |
| Carotid plaque (N) | 1,263 | 1,222 | 1,292 | 1,240 | - | 5,017 |
| Case, n (%) | 555 (43.9%) | 621 (50.8%) | 677 (52.4%) | 710 (57.3%) | - | 2,563 (51.1%) |
| Model 1† | 1 (reference) | 1.27 (1.07 – 1.51) | 1.38 (1.17 – 1.63) | 1.78 (1.50 – 2.13) | <0.001 | 1.23 (1.15 – 1.31) |
| Model 2‡ | 1 (reference) | 1.24 (1.05 – 1.48) | 1.30 (1.09 – 1.54) | 1.64 (1.36 – 1.97) | <0.001 | 1.19 (1.11 – 1.27) |
| Model 3§ | 1 (reference) | 1.16 (0.98 – 1.38) | 1.14 (0.95 – 1.36) | 1.35 (1.11 – 1.63) | 0.005 | 1.09 (1.02 – 1.17) |
| Model 4ǁ | 1 (reference) | 1.10 (0.92 – 1.32) | 1.04 (0.86 – 1.25) | 1.15 (0.93 – 1.43) | 0.28 | 1.02 (0.94 – 1.11) |
ABI= ankle brachial index; SD=standard deviation; In=naturally log-transformed; MESA= Multi-Ethnic Study of Atherosclerosis
Statistically significant results are in bold font
Model 1: adjusted for age, sex and race/ethnicity
Model 2: model 1 plus education, BMI, smoking status, ln_pack-years, and ln_physical activity
Model 3: model 2 plus systolic blood pressure, use of antihypertensive medication, total cholesterol, HDL-cholesterol, use of lipid-lowering medications, diabetes and eGFR
Model 4: model 3 plus ln_hsCRP, ln_IL-6 and ln_fibrinogen
Table 3.
Baseline cross-sectional association between GlycA, ABI and carotid plaque score: MESA (2000–2002).
| Q1 | Q2 | Q3 | Q4 | p-for-trend | Per 1SD increment | |
|---|---|---|---|---|---|---|
| ln ABI | ||||||
| Model 1† | 0 (reference) | −0.008 (−0.016, −0.001) | −0.010 (−0.017, −0.002) | −0.030 (−0.038, −0.022) | <0.001 | −0.011 (−0.014, −0.008) |
| Model 2‡ | 0 (reference) | −0.008 (−0.016, −0.001) | −0.009 (−0.017, −0.002) | −0.029 (−0.037, −0.021) | <0.001 | −0.011 (−0.013, −0.008) |
| Model 3§ | 0 (reference) | −0.005 (−0.012, 0.003) | −0.003 (−0.010, 0.005) | −0.020 (−0.028, −0.012) | <0.001 | −0.007 (−0.009, −0.004) |
| Model 4ǁ | 0 (reference) | −0.003 (−0.010, 0.005) | 0.001 (−0.007, 0.009) | −0.013 (−0.022, −0.004) | 0.03 | −0.004 (−0.007, −0.0004) |
| In [CPS+1] | ||||||
| Model 1† | 0 (reference) | 0.057 (0.009, 0.105) | 0.086 (0.039, 0.134) | 0.187 (0.137, 0.236) | <0.001 | 0.070 (0.053, 0.088) |
| Model 2† | 0 (reference) | 0.047 (0.000, 0.094) | 0.062 (0.014, 0.109) | 0.151 (0.101, 0.202) | <0.001 | 0.057 (0.039, 0.075) |
| Model 3§ | 0 (reference) | 0.023 (−0.024, 0.069) | 0.017 (−0.030, 0.065) | 0.083 (0.032, 0.134) | 0.004 | 0.029 (0.011, 0.048) |
| Model 4ǁ | 0 (reference) | 0.008 (−0.040, 0.055) | −0.008 (−0.058, 0.041) | 0.037 (−0.020, 0.094) | 0.34 | 0.010 (−0.011, 0.031) |
ABI= ankle brachial index; CPS=carotid plaque score; SD=standard deviation; In=naturally log-transformed; MESA= Multi-Ethnic Study of Atherosclerosis
Statistically significant results are in bold font
Model 1: adjusted for age, sex and race/ethnicity
Model 2: model 1 plus education, BMI, smoking status, ln_pack-years and ln_physical activity
Model 3: model 2 plus systolic blood pressure, use of antihypertensive medication, total cholesterol, HDL-cholesterol, use of lipid-lowering medications, diabetes and eGFR
Model 4: model 3 plus ln_hsCRP, ln_IL-6 and ln_fibrinogen
Carotid plaque
At baseline, 2,563 (51.1%) participants of 5,017 had carotid plaque score >0. Participants with higher GlycA levels had a greater odds for prevalent carotid plaque (Table 2), as well as with greater CPS when CPS was assessed continuously as a log transformed measure (Table 3). The adjusted prevalence ORs of having carotid plaque across increasing GlycA quartiles were 1 (reference), 1.24 (1.05, 1.48), 1.30 (1.09, 1.54), and 1.64 (1.36, 1.97) in our primary model (model 2). After further adjustment for intermediate CVD risk factors (model 3), results remained statistically significant only for the highest GlycA quartile, OR (95% CI): 1.35 (1.11, 1.63) and was attenuated after adjusting for other inflammatory markers in model 4, OR (95% CI): 1.15 (0.93, 1.43) (Table 2). Similarly, the average adjusted difference in log-transformed carotid plaque score [ln (CPS+1)] was 0.151 (0.101, 0.202) when comparing the highest quartiles of GlycA to the lowest in our main model (Table 3). Results remained statistically significant after further adjustment for CVD risk factors and was also attenuated after accounting for inflammatory markers (Table 3). Similar to our ABI findings, there was no significant associations between GlycA and the odds of incident CPS >0 (Supplemental Table 1s) or 10-year change in CPS (Supplemental Table 2s) (all models p >0.05).
Clinical peripheral artery disease
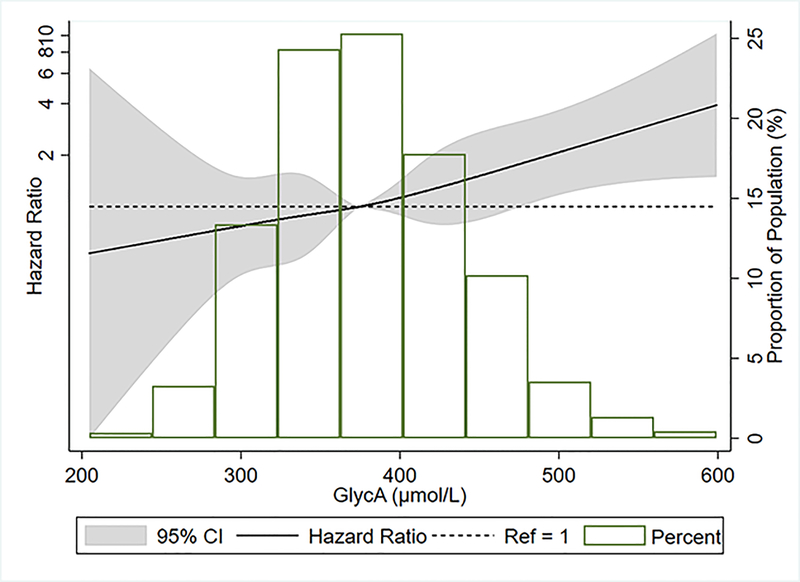
We identified 110 incident cases of clinical PAD during 79,590 person-years of follow-up. Higher GlycA levels were associated with an increased risk of incident clinical PAD (Table 4, Figure 2). The unadjusted incidence rates (95% CI) per 1,000 person-years for clinical PAD were 0.92 (0.59, 1.45), 1.04 (0.68, 1.60), 1.55 (1.09, 2.20), and 2.06 (1.51, 2.82) for GlycA quartiles 1 to 4, respectively. In our primary model (model 2), compared to the first quartile, the adjusted HR (95% CI) of incident clinical PAD for highest quartile was 2.01 (1.12, 3.60) and for one SD higher GlycA (continuous) was 1.38 (1.14, 1.66) (Table 4). These associations were attenuated and lost statistical significance after further adjustment for CVD risk factors and inflammatory markers in models 3 and 4, respectively (Table 4). In a sensitivity analysis which excluded participants with low ABI at baseline and adjusted for variables in our primary model, the HR (95% CI) of incident PAD was 1.40 (1.13, 1.73) for one SD increment in GlycA (Supplemental Table 3s). Similarly, this association was no longer significant with further adjustment for other covariates in models 3 and 4.
Table 4.
Hazard ratios (95% confidence interval) of incident peripheral artery disease by baseline GlycA: MESA (2000–2015)*
| Q1 | Q2 | Q3 | Q4 | p-for-trend | Per 1SD increment | |
|---|---|---|---|---|---|---|
| N | 1,627 | 1,595 | 1,619 | 1,591 | - | 6,432 |
| Case, n (%) | 19 (1.2%) | 21 (1.3%) | 31 (1.9%) | 39 (2.5%) | - | 110 (1.7%) |
| Incidence rate† | 0.92 (0.59 – 1.45) | 1.04 (0.68 – 1.60) | 1.55 (1.09 – 2.20) | 2.06 (1.51 – 2.82) | - | 1.38 (1.15 – 1.67) |
| Model 1‡ | 1 (reference) | 1.12 (0.60 – 2.09) | 1.79 (1.01 – 3.19) | 2.74 (1.55 – 4.84) | <0.001 | 1.51 (1.26 – 1.80) |
| Model 2§ | 1 (reference) | 0.99 (0.53 – 1.85) | 1.43 (0.80 – 2.57) | 2.01 (1.12 – 3.60) | 0.01 | 1.38 (1.14 – 1.66) |
| Model 3ǁ | 1 (reference) | 0.85 (0.46 – 1.60) | 1.15 (0.64 – 2.06) | 1.39 (0.77 – 2.54) | 0.14 | 1.19 (0.97 – 1.44) |
| Model 4# | 1 (reference) | 0.79 (0.42 – 1.49) | 1.00 (0.54 – 1.84) | 1.01 (0.52 – 1.96) | 0.75 | 1.04 (0.82 – 1.31) |
SD=standard deviation; In=naturally log-transformed
Statistically significant results are in bold font
Unadjusted Incidence rate per 1,000 person-years.
Model 1: adjusted for age, sex, and race/ethnicity
Model 2: model 1 plus education, BMI, smoking status, ln_pack-years, and ln_physical activity
Model 3: model 2 plus systolic blood pressure, use of antihypertensive medication, total cholesterol, HDL-cholesterol, use of lipid-lowering medications, diabetes, and eGFR
Model 4: model 3 plus ln_hsCRP, ln_IL-6, and ln_fibrinogen
Figure 2.
Restricted cubic spline* of GlycA with incident peripheral artery disease, the Multi-Ethnic Study of Atherosclerosis, 2000‐2015
* The median (374.9 μmol/L) was used as reference in a Cox proportional hazards model adjusted for age, sex, race/center, education, BMI, smoking status, ln pack-years, and ln physical activity. Knots were placed at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles. High extreme values (GlycA levels > 600 μmol/L) were excluded (n=17) from analysis.
In assessing the joint associations of hsCRP and GlycA with clinical PAD risk (p-for-interaction=0.73), participants with both high GlycA and high hsCRP had the highest risk for incident PAD compared with participants with low GlycA and low hsCRP [HR (95% CI): 2.39 (1.41, 4.05)], which remained significant after further adjusting for CVD risk factors [1.92 (1.12, 3.27)] (Supplemental Table 4s). Results were stronger after excluding participants with low ABI (Supplemental Table 5s). In another analysis we examined the association of GlycA and hsCRP, both natural log transformed, with incident PAD. The hazard conferred by GlycA was slightly higher than seen with hsCRP, but confidence intervals overlapped. In our primary model, the HR (95% CI) of incident clinical PAD per SD increment in ln GlycA was 1.39 (1.13, 1.71) and for ln hsCRP 1.28 (1.03, 1.58). Both inflammatory markers were no longer statistically significantly associated with incident PAD after adjusting for CVD risk factors in model 3 (Supplemental Table 6s).
Discussion
In this community-based, multi-ethnic prospective cohort study, higher levels of GlycA were associated with a greater odds of prevalent low ABI and carotid plaque presence at baseline, as well as an increased hazard for incident clinical PAD events after a median of 14 years of follow-up. We found no associations of GlycA with odds of incident low ABI or incident carotid plaque, nor with 10-year change in ABI and carotid plaque score. We also found that participants with high levels of both hsCRP and GlycA had the highest risk of incident clinical PAD.
Prior work has already established that assessment for PAD and carotid plaque provides prognostic information regarding CVD risk.30–32 Due to the asymptomatic nature of PAD and limitations to current PAD diagnostic modalities,33, 34 preventive medical and lifestyle management may not be initiated early in these patients. Thus there is a need for other markers of PAD that may predict the disease early in its onset. The composite nature of GlycA makes it a potentially useful target for the additional evaluation of systemic inflammation and peripheral vascular risk. Furthermore, in addition to the role that inflammatory markers may play in the identification of a higher-risk individual who may benefit from more intensive preventive therapies, inflammatory markers may be a potential target for treatment as experimental studies have further confirmed a causal role of inflammation in the progression of atherosclerosis.5
Prior epidemiological studies have examined the relationship between GlycA and subclinical or clinical CVD, and have shown positive relationships.4, 35–39 In several cross-sectional studies, there were strong positive associations between GlycA and coronary atherosclerosis among individuals with rheumatoid arthritis,35 psoriasis,40 and HIV infection.20 In a study of participants undergoing cardiac catheterization, GlycA was strongly associated with the presence and extent of coronary artery disease, and positively associated with cardiovascular and non-cardiovascular mortality.36 Similarly, a prospective cohort study found an association between baseline GlycA and incident CVD risk occurring over a median follow-up time of 17.2 years.37 Several other studies have shown similar positive associations between GlycA and incident CVD, mortality, and cancer4 and have shown that the relationship with CVD was stronger for GlycA than that reported for hsCRP38 and is independent of hsCRP.39
Despite the aforementioned established association of GlycA with incident CVD events4 and with subclinical coronary and extra-coronary atherosclerosis,20, 21, 40 the relationship of GlycA with subclinical and clinical PAD, specifically, had not been previously established. Although there are some common shared cardiovascular risk factors that promote atherosclerosis across various vascular beds, risk factors are not necessarily overlapping.22, 23 Thus, this present study provides new insight of the relationships of GlycA with PAD and carotid plaque, specifically.
However, whether therapeutic lowering of GlycA (by lifestyle or pharmacotherapy) can slow or prevent peripheral atherosclerosis remains uncertain and needs to be studied further before GlycA is adopted as a routinely used clinical test for the purposes of risk assessment, management, or follow-up of patients. Of note, therapies targeting tumor necrosis factor have been shown to reduce GlycA levels and improve vascular inflammation in patients with psoriasis;40 thus, these therapies could be of potential clinical use if confirmed in other studies of patients at risk for vascular disease. The GlycA test is offered nationally in the U.S. by LabCorp®. The current (2019) cost of a GlycA test is $35.06 when ordered “a la carte”, although the cost will likely decrease in the future if it is included as part of an NMR-measured cardiometabolic profile.
Our study has many strengths including the use of a gender- and racially-diverse study population, with well-characterized lifestyle and CVD risk factors, and measurement of other inflammatory markers. We were able to examine the association of GlycA with prevalent and incident subclinical peripheral and carotid atherosclerosis and with incident clinical PAD events. Our study adds to the growing body of literature of the potential use of this composite inflammatory marker for risk prediction, and in this case, risk prediction specifically for PAD.
However, some limitations warrant consideration. First, given the composite nature of GlycA, we are unable to identify the contributions of its component biomarkers to incident CVD for this analysis. Differences in contributions of the acute-phase reactants, precipitating inflammatory stimuli, and co-existing morbidities may alter the expression, and the effects of the component inflammatory biomarkers. Nevertheless, GlycA has been shown to be a useful marker of chronic inflammation as well.41 Second, we failed to find a positive association with incident low ABI and new carotid plaque presence despite positive cross-sectional associations and positive associations with incident clinical PAD. Third, our study was observational and we cannot therefore ascribe a direct causal relationship between plasma GlycA elevation and atherosclerosis. Residual confounding may explain in part the associations found. For example, we were unable to adjust for use of anti-inflammatory medications which might influence GlycA levels. Fourth, we used a single measure of GlycA which precludes the assessment of the effect of changes in GlycA levels over time with the PAD end-points. Fifth, there were a low number of prevalent, as well as incident, PAD events, which limited our statistical power for those analyses. Sixth, we performed multiple tests and results may be due to chance; however findings were consistent with our a priori hypotheses and with other studies that have established a link between GlycA and atherosclerosis.20, 21 Lastly, MESA recruitment took place in the early 2000s, which limits the study’s generalizability to current day where primary prevention therapies are more prevalent and are more widely used.
In conclusion, higher GlycA levels were cross-sectionally associated with prevalent low ABI and carotid plaque presence, as well as associated with increased risk for incident PAD events after a median of 14 years of follow-up. Our findings provide potential support for the use of GlycA as an early biomarker of vascular risk.
Supplementary Material
Acknowledgements
The authors thank the investigators, staff, and participants of the MESA study for their valuable contributions.
Funding
Drs. Michos and Zhao were supported by the Blumenthal Scholars Fund for Preventive Cardiology at Johns Hopkins University. This MESA study was funded by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from NCATS. Dr. Mora was supported by grants K24 HL136852 and R01HL134811 from the National Heart, Lung, and Blood Institute.
Glossary
- ABI
ankle brachial index
- BMI
body mass index
- CI
confidence interval
- Chol
cholesterol
- CPS
carotid plaque score
- eGFR
estimated glomerular filtration rate
- HDL-C
high density lipoprotein cholesterol
- hsCRP
high-sensitivity C-reactive protein
- IL-6
interleukin-6
- In
naturally log-transformed
- MESA
Multi-Ethnic Study of Atherosclerosis
- MET
metabolic equivalent of task
- n
number
- Ref
reference
- SD
standard deviation
Footnotes
Trial Registration. The MESA cohort design is registered at clinicaltrials.gov
Declaration of Conflicts of Interest
Dr. Otvos is employed by LabCorp (formerly LipoScience). Dr. Mora received research grants from Atherotech, consulting fees from Quest and has a patent application on the use of GlycA for predicting risk of colorectal cancer. Dr. Stein receives royalties from the Wisconsin Alumni Research Foundation for patent related to carotid artery wall thickness and arterial age (technology not used in this paper). The other authors do not report any disclosures.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Niiranen TJ, Vasan RS. Epidemiology of cardiovascular disease: recent novel outlooks on risk factors and clinical approaches. Expert Rev Cardiovasc Ther. 2016;14:855–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libby P Interleukin-1 beta as a target for atherosclerosis therapy: biological basis of CANTOS and beyond. J Am Coll Cardiol 2017;70:2278–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duprez DA, Otvos J, Sanchez OA, Mackey RH, Tracy R, Jacobs DR Jr. Comparison of the Predictive Value of GlycA and Other Biomarkers of Inflammation for Total Death, Incident Cardiovascular Events, Noncardiovascular and Noncancer Inflammatory-Related Events, and Total Cancer Events. Clin Chem. 2016;62:1020–1031. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 6.Criqui MH, McClelland RL, McDermott MM, et al. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2010;56:1506–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Neal WT, Efird JT, Nazarian S, Alonso A, Heckbert SR, Soliman EZ. Peripheral arterial disease and risk of atrial fibrillation and stroke: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2014;3:e001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. New Engl J Med. 1992;326:381–386. [DOI] [PubMed] [Google Scholar]
- 9.Gomez CR. Carotid plaque morphology and risk for stroke. Stroke. 1990;21:148–151. [DOI] [PubMed] [Google Scholar]
- 10.Polak JF, Szklo M, Kronmal RA, et al. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2013;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gepner AD, Young R, Delaney JA, et al. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2015;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdellaoui A, Al-Khaffaf H. C-reactive protein (CRP) as a marker in peripheral vascular disease. Eur J Vasc Endovasc Surg. 2007;34:18–22. [DOI] [PubMed] [Google Scholar]
- 13.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation. 2005;112:976–983. [DOI] [PubMed] [Google Scholar]
- 14.Folsom AR, Pankow JS, Tracy RP, et al. Association of C-reactive protein with markers of prevalent atherosclerotic disease. Am J Cardiol. 2001;88:112–117. [DOI] [PubMed] [Google Scholar]
- 15.Chapman CML, Beilby JP, McQuillan BM, Thompson PL, Hung J. Monocyte count, but not C-reactive protein or interleukin-6, is an independent risk marker for subclinical carotid atherosclerosis. Stroke. 2004;35:1619–1624. [DOI] [PubMed] [Google Scholar]
- 16.van der Meer IM, de Maat MPM, Hak AE, et al. C-Reactive Protein Predicts Progression of Atherosclerosis Measured at Various Sites in the Arterial Tree. The Rotterdam Study. Stroke. 2002;33:2750–2755. [DOI] [PubMed] [Google Scholar]
- 17.Ammirati E, Moroni F, Norata GD, Magnoni M, Camici PG. Markers of inflammation associated with plaque progression and instability in patients with carotid atherosclerosis. Mediators Inflamm. 2015;2015:718329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otvos JD, Shalaurova I, Wolak-Dinsmore J, et al. GlycA: A Composite Nuclear Magnetic Resonance Biomarker of Systemic Inflammation. Clin Chem. 2015;61:714–723. [DOI] [PubMed] [Google Scholar]
- 19.Benson EA, Tibuakuu M, Zhao D, et al. Associations of ideal cardiovascular health with GlycA, a novel inflammatory marker: The Multi-Ethnic Study of Atherosclerosis. Clin Cardiol. 2018;41:1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tibuakuu M, Fashanu OE, Zhao D, et al. GlycA, a novel inflammatory marker, is associated with subclinical coronary disease. AIDS. 2019;33:547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ezeigwe A, Fashanu OE, Zhao D, et al. The novel inflammatory marker GlycA and the prevalence and progression of valvular and thoracic aortic calcification: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2019;282:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dirrichs T, Penzkofer T, Reinartz SD, Kraus T, Mahnken AH, Kuhl CK. Extracoronary thoracic and coronary artery calcifications on chest CT for lung cancer screening: association with established cardiovascular risk factors - the “CT-Risk” trial. Acad Radiol. 2015;22:880–889. [DOI] [PubMed] [Google Scholar]
- 23.Wagenknecht LE, Langefeld CD, Freedman BI, Carr JJ, Bowden DW. A comparison of risk factors for calcified atherosclerotic plaque in the coronary, carotid, and abdominal aortic arteries: the diabetes heart study. Am J Epidemiol. 2007;166:340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 25.Whelton SP, Narla V, Blaha MJ, et al. Association between resting heart rate and inflammatory biomarkers (high-sensitivity C-reactive protein, interleukin-6, and fibrinogen) (from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol. 2014;113:644–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wassel CL, Berardi C, Pankow JS, et al. Soluble P-selectin predicts lower extremity peripheral artery disease incidence and change in the ankle brachial index: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2015;239:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vidula H, Liu K, Criqui MH, et al. Metabolic syndrome and incident peripheral artery disease - the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2015;243:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tattersall MC, Gassett A, Korcarz CE, et al. Predictors of carotid thickness and plaque progression during a decade: the Multi-Ethnic Study of Atherosclerosis. Stroke. 2014;45:3257–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ankle Brachial Index Collaboration, Fowkes FG, Murray GD, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gepner AD, Young R, Delaney JA, et al. Comparison of carotid plaque score and coronary artery calcium score for predicting cardiovascular disease events: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hazarika S, Annex BH. Biomarkers and genetics in peripheral artery disease. Clin Chem. 2017;63:236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooke JP, Wilson AM. Biomarkers of peripheral arterial disease. J Am Coll Cardiol. 2010;55:2017–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ormseth MJ, Chung CP, Oeser AM, et al. Utility of a novel inflammatory marker, GlycA, for assessment of rheumatoid arthritis disease activity and coronary atherosclerosis. Arthritis Res Ther. 2015;17:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGarrah RW, Kelly JP, Craig DM, et al. A novel protein glycan-derived inflammation biomarker independently predicts cardiovascular disease and modifies the association of HDL subclasses with mortality. Clin Chem. 2017;63:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akinkuolie AO, Buring JE, Ridker PM, Mora S. A novel protein glycan biomarker and future cardiovascular disease events. J Am Heart Assoc. 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gruppen EG, Riphagen IJ, Connelly MA, Otvos JD, Bakker SJ, Dullaart RP. GlycA, a pro-inflammatory glycoprotein biomarker, and incident cardiovascular disease: relationship with C-reactive protein and renal function. PLoS One. 2015;10:e0139057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akinkuolie AO, Glynn RJ, Padmanabhan L, Ridker PM, Mora S. Circulating n-linked glycoprotein side-chain biomarker, rosuvastatin therapy, and incident cardiovascular disease: an analysis from the JUPITER trial. J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joshi AA, Lerman JB, Aberra TM, et al. GlycA is a novel biomarker of inflammation and subclinical cardiovascular disease in psoriasis. Circ Res. 2016;119: 1242–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ritchie SC, Wurtz P, Nath AP, et al. The Biomarker GlycA Is Associated with Chronic Inflammation and Predicts Long-Term Risk of Severe Infection. Cell Syst. 2015;1:293–301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.