Abstract
Background:
Prompt initiation of antiretroviral therapy (ART) for HIV-infected infants is strongly recommended but diagnostic confirmation is important as committing children to life-long ART carries serious health and social implications.
Methods:
Two HIV-exposed infants in Johannesburg, South Africa were identified presenting with unusual trajectories of diagnostic nucleic acid amplification tests (NAAT) and viral load (VL) results.
Results:
Case 1 had repeat indeterminate NAAT results during the first three weeks of life; repeat testing thereafter was negative with undetectable VL including after daily nevirapine prophylaxis ended. ART was not initiated at this time. Case 2 had a single positive NAAT result at one month of age that prompted initiation of ART. Subsequent results were negative and ART was discontinued. Repeat negative NAAT with VL below the limit of quantification or undetectable continued to be obtained. Shortly after and around weaning, positive NAAT results with high VL (7.1 and 6.03 log10 copies/ml for Case 1 and 2, respectively) were observed in both children. Both mothers were treated with tenofovir, emtricitabine and efavirenz during breastfeeding. Testing with ultrasensitive assays on early samples conclusively revealed HIV-1 proviral DNA in Case 1. Testing with ultrasensitive assays after the early period but prior to weaning did not detect HIV in either infant.
Conclusion:
We hypothesize that breast milk from the mothers of these two rare cases had HIV-specific or non-specific factors that led to the undetectable results in already infected infants until breastfeeding ended. Our results raise the importance of repeat testing of HIV-exposed breastfed infants after complete cessation of all breastfeeding.
Introduction
Prompt initiation of antiretroviral therapy (ART) is strongly recommended for HIV-infected infants[1].Confirmation of diagnosis is important as committing children to life-long ART has serious social and biological implications[2]. Here we describe two HIV-exposed infants initially positive or indeterminate on standard diagnostic nucleic acid amplification tests (NAAT) in the first month of life but whose follow-up confirmatory tests during breastfeeding were repeatedly negative. The infants presented with definitive evidence of infection around a year of age peri-weaning.
Methods and Results
Two infants were identified during screening for a treatment study at Rahima Moosa Mother and Child Hospital in Johannesburg, South Africa. Written informed consent was obtained from mothers. The study was approved by Institutional Review Boards of Columbia University and University of the Witwatersrand. All clinical management was at the discretion of the treating physicians.
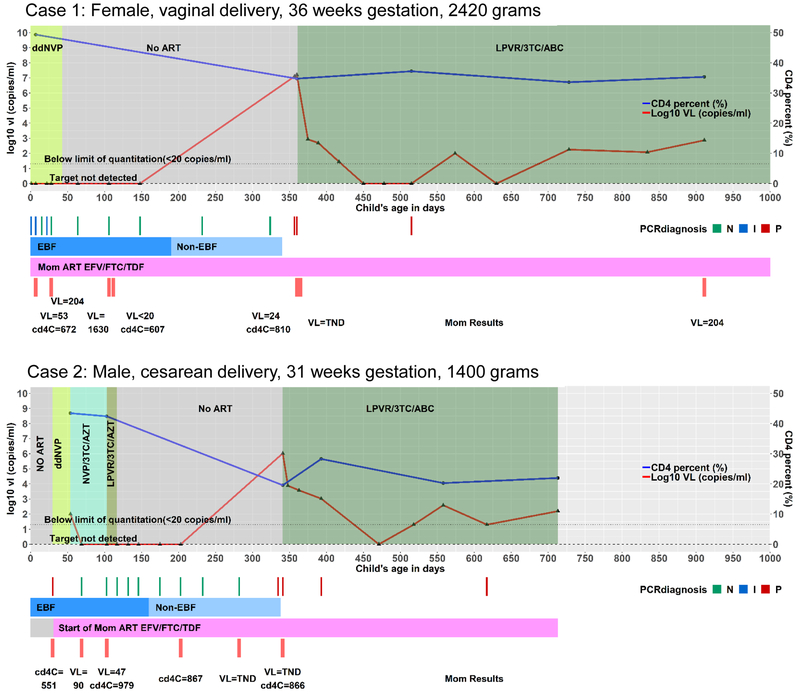
Case 1 was identified as part of an early infant diagnosis program at the site that tested 5743 HIV-exposed newborns between 5 June 2014 and 31 August 2016[3]. Case 1 had an indeterminate NAAT (COBAS AmpliPrep/TaqMan HIV-1, v2.0, Roche Molecular Systems, Branchburg, NJ) on a sample collected nine hours after birth. Subsequent NAAT results included two indeterminate results at days 7 and 22, a negative result at day 15 and six negative results between days 28 and 324. All viral load (VL) tests (COBAS® AmpliPrep/COBAS® TaqMan® HIV-1 Test, Roche Molecular Systems, Branchburg, NJ) over this time were target not detected (TND). Daily nevirapine syrup to the infant was started at 3 hours and continued for 43 days (Figure 1).
Figure 1:
Summary of infant and maternal tests and antiretroviral drug exposures in Case 1 and Case 2. Abbreviations: ddNVP – daily nevirapine prophylaxis; VL – Viral load; ART – Antiretroviral therapy; LPVR – lopinavir/ritonavir; 3TC – lamivudine; ABC – abacavir; PCR diagnosis N – negative, I – indeterminate, P – positive; EFV – efavirenz; FTC –emtricitabine; TDF – tenofovir; EBF – Exclusive breastfeeding; Non-EBF – breastfeeding continues but is not exclusive.
Case 2 was referred to the site with a history of a positive NAAT on a sample collected at 30 days of age coinciding with the first identification of their mother’s positive status. Daily nevirapine prophylaxis was initiated at age 30 days and ART (zidovudine/lamivudine/nevirapine) at 54 days when the child’s results were disclosed. Pre-treatment infant VL was <100 copies/mL (usually <20 copies/ml on this assay but due to inadequate volume was run in 1:5 dilution). At day 69 (on ART), NAAT was negative and VL was TND. Ritonavir-lopinavir was substituted for nevirapine at day 103. At 117 days of age (63 days on ART), the decision was made to discontinue ART and repeat tests to establish diagnosis. Between day 103 and 282, eight repeat NAAT tests were done and all were negative. All VL tests conducted at these timepoints were TND (Figure 1).
The mother of Case 1 had discontinued ART prior to this pregnancy and tenofovir/emtricitabine/efavirenz was restarted 80 days prior to delivery. The mother of Case 2 tested positive on an HIV rapid test 30 days post-delivery after a negative HIV test 8 days prior to delivery. She was initiated on tenofovir/emtricitibine/efavirenz at 30 days post-delivery. She reported two prior intrauterine deaths, and had pregnancy-related hypertension and untreated hypothyroidism. Case 2 was hospitalized for 21 days after birth for management of respiratory distress and jaundice related to prematurity. Infant weight and length were persistently less than −2 and −3 standard deviations below the mean Z-score for age, respectively. The child was found to have elevated thyroid stimulating hormone and thyroid hormone replacement therapy was started. Maternal investigations did not suggest an autoimmune cause for hypothyroidism.
Both infants had definitive evidence of HIV infection only around a year of age. Case 1 presented at day 357 with a three-day history of diarrhea, post-prandial vomiting, loss of appetite, cough and fever. This was 18 days after breastfeeding was reported to have ended. NAAT was positive and VL >12 million copies/mL. Repeat testing 3 days later confirmed the positive NAAT, VL >16 million copies/mL and CD4+ T-cell count (percentage) of 2777 cells/μL (34.8%). Maternal plasma VL was 24 copies/mL and maternal CD4+ T-cell count was 810 cells/μL. Breastmilk was collected at the confirmatory visit and tested using Abbott RealTime® HIV-1 platform (Abbott Molecular, Des Plaines, IL) on milk supernatant and the result was TND. No history of wet nursing or sexual abuse was obtained.
Case 1’s virus at the confirmatory visit (day 360) was clade C and was phylogenetically-linked to the mother’s proviral DNA sequence from a stored maternal sample collected at delivery (Supplementary Materials). HIV-1 genotyping found no drug resistance mutations in the child’s sample.Drug resistance testing was done as previously described[4].
Tests on stored infant plasma collected at three months of age detected 0.29 mg/L efavirenz with a corresponding maternal efavirenz plasma value (13.5 hours post-dose) of 2.7 mg/L (expected mid-dose interval 1.0–4.0 mg/L). Repeat child testing at eight months showed an efavirenz level of 0.19 mg/L (plasma). Efavirenz concentration was measured using high-performance liquid chromatography[5].
Case 2 had a positive NAAT at a scheduled visit at day 335, 31 weeks after having stopped ART. The mother reported stopping all breastfeeding 3 days later. The infant had generalized lymphadenopathy but was otherwise well. Repeat NAAT six days later was positive with VL 1,060,000 copies/mL and CD4+ T-cell count (percentage) 2576 cells/μL (19.6%). Maternal VL at this time was TND (CD4+ T-cell count 866 cells/μL).
Both children were initiated on lamivudine/abacavir/ritonavir-lopinavir and VL declined. In Case 1, new onset seizures were reported on day 52 and 105 post ART initiation. They were temporally-associated with an episode of head trauma and documented pyrexia of 38.0–38.9°C. Follow-up investigations included a brain computed tomography scan, electroencephalography and laboratory investigations to exclude hypoglycaemia, electrolyte abnormalities and infective aetiologies. Tests showed no abnormal results and the seizures were classified as febrile seizures by a paediatric neurologist. No medications were initiated and seizures did not recur.
Further testing was done on select PBMC and CD4+ T-cells prepared using Ficoll-Paque™ (GE Healthcare Biosciences, Chicago, IL) density gradient centrifugation and the Whole Blood CD4+ T-cell magnetic bead isolation (Miltenyi Biotec, Cologne, Germany), respectively. Nested and semi-nested real-time PCR assays had been developed in-house for the reverse transcriptase (RT) gene [6], highly-conserved integrase (IN) [7] and the 2-LTR episomal HIV-1 DNA at the 2-LTR junction (Supplementary Materials).
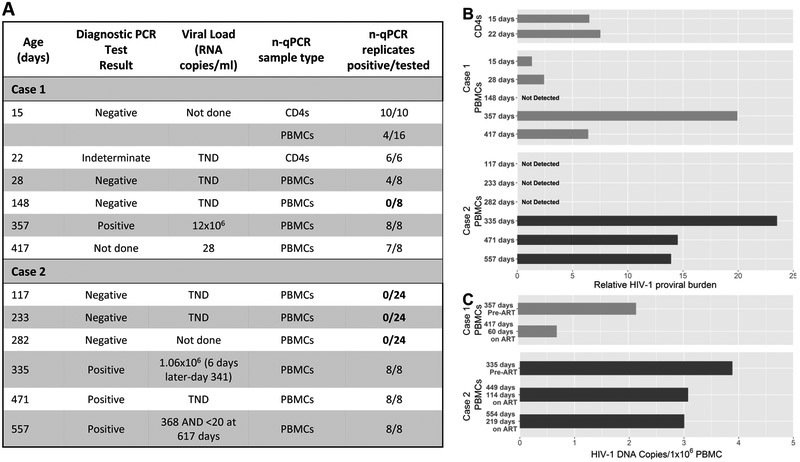
In Case 1, CD4+ T-cells stored from samples collected at day 15 and 22 were tested using the nested PCR targeting integrase. All replicates yielded positive results (10/10 at day 15 and 6/6 at day 22). PBMCs from day 15 and 28 yielded positive results in 4/16 and 4/8 replicates, respectively (Figures 2A/2B). CD4+ T-cells and PBMCs tested by the nested PCR targeting 2-LTR episomal HIV-1 DNA found one of four CD4+ T-cell replicates with positive results from the day 15 sample. All four PBMC replicates from day 15 were negative and 8 replicates on the day 22 CD4+ T cell sample were negative for 2-LTR episomal DNA. Select amplification plots are shown in supplementary materials (Figure S1).
Figure 2.
A: Diagnostic HIV-1 PCR test results, viral load tests and samples tested using integrase-based nested PCR at different time points. B: Using the number of replicates that tested positive (as a percentage) divided by the average of the Ct values obtained from the positive replicates, a value representing the relative proviral burden of HIV-1 cell-associated DNA was calculated and plotted on a bar graph for the different time points tested for both cases. C: Quantitation of total cell-associated HIV-1 DNA plotted on a bar graph for the time points tested for both cases.
At day 148, none of the eight replicates carried out on PBMCs using the nested PCR targeting integrase were positive. At day 357, 8/8 PBMC replicates were again positive and 60 days after ART was started, 7/8 PBMC replicates were positive (Figures 2A/2B).
In Case 2, PBMCs stored from samples collected at day 117, 233 and 282 were tested using the nested PCR targeting integrase. Twenty-four replicates at each time point were tested and no positive results were attained (Figure 2A/2B).
PBMC samples were also tested with a semi-nested real-time quantitative hydrolysis probe (TaqMan) PCR assay designed to quantitate total HIV-1 DNA[6]. There were six replicates to allow detection to one copy/9.1×105 cells. Total HIV-1 DNA copies/million PBMCs declined after ART was introduced most markedly in Case 1 (Figure 2C).
Discussion
We present two cases of HIV-infected infants with repeat negative HIV tests while being breast-fed by ART-treated mothers. There have been previous reports suggesting that maternal ART during pregnancy and infant prophylaxis may reduce the sensitivity of HIV diagnostic assays[8-11]. Our cases differ from these reports in that negative results were observed at older ages. Our cases had initial positive results in the first month of life when effects of transplacental drug passage and infant prophylaxis are expected to be greatest.
In addition to transplacental passage, penetrance of breast milk by antiretroviral drugs is known to occur[12-14]. However, it seems unlikely that drug effects alone explain the pattern of results. Efavirenz concentrations in Case 1 were below the target mid-dose therapeutic threshold[15]. Moreover, the infection that finally was detected had no evidence of drug selection. We hypothesize that, although maternal ART likely played a role, constituents of breast milk or immune processes induced by breastfeeding were active to explain the trajectory of these results.
Breast milk contains a plethora of immunomodulatory and HIV inhibitory factors that may explain the inefficiency of post-natal HIV transmission[16]. For example, neutralizing antibodies, immunoglobulins, T-cell responses, and potent HIV inhibitory activity have been correlated with lower rates of breastfeeding HIV transmission[17-20]. Survival of untreated HIV-infected children is better when they are breastfed than when they are not[21]. Prognosis of infants who acquire HIV-infection during breastfeeding is better than those who acquired the infection intrauterine or intrapartum[22]. We hypothesize that breast milk from the mothers of these two rare cases had HIV-specific or non-specific factors that led to the undetectable results we observed in already infected infants until breastfeeding ended.
An alternative hypothesis is late post-natal infection through breastfeeding. Breast milk concentrations of HIV spike during weaning and transmissions can cluster around this time[23]. However, VL in maternal plasma and the post-weaning breast milk sample were consistently low. We did not measure cell-associated HIV concentrations in breast milk, and these are known to be strongly correlated with postnatal transmission[24]. Nevertheless, it is rare for post-natal transmission to occur with suppressive maternal ART although this has been described[25].
The post-natal infection hypothesis also requires assuming that the initial positive signals were falsely positive. Indeterminate diagnostic tests pose clinical challenges as they indicate either true low positive results or non-specific amplification signals[26]. For Case 1 with indeterminate results, other testing conclusively demonstrated true infection early on. Detection of 2-LTR episomal DNA in CD4+ T-cells, although at very low levels, provides evidence of viral replication as these episomal circles are a by-product of viral replication. It is thus surprising that the later samples showed no evidence of HIV infection using ultrasensitive methods. While more sensitive assays may have diagnostic utility, our results indicate that they will be limited by the timing of when they are used.
To mitigate concerns about potential effects of maternal ART and infant prophylaxis on sensitivity of HIV diagnostic assays, delay of testing until prophylaxis has ended, or, in some circumstances, monitored ART cessation if an infant has been started on ART, is recommended[27]. In both cases, the most consistent pattern of negative results were observed after discontinuation of antiretrovirals given to the infants. Our results raise the importance of repeat testing of HIV-exposed breastfed infants after complete cessation of all breastfeeding.
Supplementary Material
Acknowledgements:
We would like to thank Drs. Jennifer Norman, Gillian Hunt and Carole Wallis for assistance with these investigations; the National Health Laboratory Services (NHLS) for the routine birth diagnostic testing; and the South African National Department of Health antiretroviral treatment program. The study was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institute of Allergy and Infectious Disease, National Institutes of Health (U01HD080441) and the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa.
References cited:
- 1.Penazzato M, Prendergast AJ, Muhe LM, Tindyebwa D, Abrams EJ. Optimization of antiretroviral therapy in HIV-infected children under 3 years of age: a systematic review. Aids 2014,28 Suppl 2:S137–146. [DOI] [PubMed] [Google Scholar]
- 2.Dunning L, Francke JA, Mallampati D, MacLean RL, Penazzato M, Hou T, et al. The value of confirmatory testing in early infant HIV diagnosis programmes in South Africa: A cost-effectiveness analysis. PLoS Med 2017,14:e1002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Technau KG, Mazanderani AH, Kuhn L, Hans L, Strehlau R, Abrams EJ, et al. Prevalence and outcomes of HIV‐1 diagnostic challenges during universal birth testing–an urban South African observational cohort. Journal of the International AIDS Society 2017,20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Z, Wagar N, DeVos JR, Rottinghaus E, Diallo K, Nguyen DB, et al. Optimization of a low cost and broadly sensitive genotyping assay for HIV-1 drug resistance surveillance and monitoring in resource-limited settings. PLoS One 2011,6:e28184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinillos F, Dandara C, Swart M, Strehlau R, Kuhn L, Patel F, et al. Case report: Severe central nervous system manifestations associated with aberrant efavirenz metabolism in children: the role of CYP2B6 genetic variation. BMC Infect Dis 2016,16:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuhn L, Paximadis M, Da Costa Dias B, Loubser S, Strehlau R, Patel F, et al. Age at antiretroviral therapy initiation and cell-associated HIV-1 DNA levels in HIV-1-infected children. PLoS One 2018,13:e0195514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Violari A, Cotton MF, Kuhn L, Schramm DB, Paximadis M, Loubser S, et al. A child with perinatal HIV infection and long-term sustained virological control following antiretroviral treatment cessation. Nat Commun 2019,10:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King CC, Kourtis AP, Persaud D, Nelson JA, Ziemniak C, Hudgens MG, et al. Delayed HIV detection among infants exposed to postnatal antiretroviral prophylaxis during breastfeeding. AIDS (London, England) 2015,29:1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balasubramanian R, Fowler MG, Dominguez K, Lockman S, Tookey PA, Huong NNG, et al. Time to first positive HIV-1 DNA PCR may differ with antiretroviral regimen in infants infected with non-B subtype HIV-1. Aids 2017,31:2465–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell C, Dross S, Beck IA, Micek MA, Frenkel LM. Low concentrations of HIV-1 DNA at birth delays diagnosis, complicating identification of infants for antiretroviral therapy to potentially prevent the establishment of viral reservoirs. Clin Infect Dis 2014,58:1190–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgard M, Blanche S, Jasseron C, Descamps P, Allemon MC, Ciraru-Vigneron N, et al. Performance of HIV-1 DNA or HIV-1 RNA tests for early diagnosis of perinatal HIV-1 infection during anti-retroviral prophylaxis. J Pediatr 2012,160:60–66.e61. [DOI] [PubMed] [Google Scholar]
- 12.Gandhi M, Mwesigwa J, Aweeka F, Plenty A, Charlebois E, Ruel TD, et al. Hair and plasma data show that lopinavir, ritonavir and efavirenz all transfer from mother to infant in utero, but only efavirenz transfers via breastfeeding. Journal of acquired immune deficiency syndromes (1999) 2013,63:578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider S, Peltier A, Gras A, Arendt V, Karasi-Omes C, Mujawamariwa A, et al. Efavirenz in human breast milk, mothers’, and newborns’ plasma. J Acquir Immune Defic Syndr 2008,48:450–454. [DOI] [PubMed] [Google Scholar]
- 14.Mirochnick M, Thomas T, Capparelli E, Zeh C, Holland D, Masaba R, et al. Antiretroviral concentrations in breast-feeding infants of mothers receiving highly active antiretroviral therapy. Antimicrobial agents and chemotherapy 2009,53:1170–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bienczak A, Denti P, Cook A, Wiesner L, Mulenga V, Kityo C, et al. Plasma efavirenz exposure, sex, and age predict virological response in HIV-infected African children. Journal of acquired immune deficiency syndromes (1999) 2016,73:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kourtis AP, Butera S, Ibegbu C, Belec L, Duerr A. Breast milk and HIV-1: vector of transmission or vehicle of protection? Lancet Infect Dis 2003,3:786–793. [DOI] [PubMed] [Google Scholar]
- 17.Lohman-Payne B, Slyker JA, Moore S, Maleche-Obimbo E, Wamalwa DC, Richardson BA, et al. Breast milk cellular HIV-specific interferon gamma responses are associated with protection from peripartum HIV transmission. Aids 2012,26:2007–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollara J, McGuire E, Fouda GG, Rountree W, Eudailey J, Overman RG, et al. Association of HIV-1 Envelope-Specific Breast Milk IgA Responses with Reduced Risk of Postnatal Mother-to-Child Transmission of HIV-1. J Virol 2015,89:9952–9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. HIV-specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads. PLoS Pathog 2012,8:e1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wahl A, Baker C, Spagnuolo RA, Stamper LW, Fouda GG, Permar SR, et al. Breast milk of HIV-positive mothers has potent and species-specific in vivo HIV-inhibitory activity. Journal of virology 2015,89:10868–10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Semrau K, Mwiya M, et al. Effects of early, abrupt weaning on HIV-free survival of children in Zambia. N Engl J Med 2008,359:130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marston M, Becquet R, Zaba B, Moulton LH, Gray G, Coovadia H, et al. Net survival of perinatally and postnatally HIV-infected children: a pooled analysis of individual data from sub-Saharan Africa. Int J Epidemiol 2011,40:385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuhn L, Kim H-Y, Walter J, Thea DM, Sinkala M, Mwiya M, et al. HIV-1 concentrations in human breast milk before and after weaning. Science translational medicine 2013,5:181ra151–181ra151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ndirangu J, Viljoen J, Bland RM, Danaviah S, Thorne C, Van de Perre P, et al. Cell-free (RNA) and cell-associated (DNA) HIV-1 and postnatal transmission through breastfeeding. PLoS One 2012,7:e51493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bispo S, Chikhungu L, Rollins N, Siegfried N, Newell ML. Postnatal HIV transmission in breastfed infants of HIV-infected women on ART: a systematic review and meta-analysis. J Int AIDS Soc 2017,20:21251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maritz J, Preiser W, van Zyl GU. Establishing diagnostic cut-off criteria for the COBAS AmpliPrep/COBAS TaqMan HIV-1 Qualitative test through validation against the Amplicor DNA test v1.5 for infant diagnosis using dried blood spots. Journal of Clinical Virology 2012,53:106–109. [DOI] [PubMed] [Google Scholar]
- 27.Mazanderani AH, Technau K-G, Hsiao N-Y, Maritz J, Carmona S, Sherman GG. Recommendations for the management of indeterminate HIV PCR results within South Africa’s early infant diagnosis programme. Southern African Journal of HIV Medicine 2016,17:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.