Abstract
Metabolic diseases occur when normal metabolic processes are disrupted in the human body, which can be congenital or acquired. The incidence of metabolic diseases worldwide has reached epidemic proportions. So far, various methods including systemic drug therapy and surgery are exploited to prevent and treat metabolic diseases. However, current pharmacotherapeutic options for treatment of these metabolic disorders remain limited and ineffective, especially reducing patient compliance to treatment. Therefore, it is desirable to exploit effective drug delivery approaches to effectively treat metabolic diseases and reduce side effects. This brief review summarizes novel delivery strategies including local, targeted, and oral drug delivery strategies, as well as intelligent stimulus-responsive drug delivery strategy, for the treatment of metabolic disorders including diabetes, obesity, and atherosclerosis.
Keywords: biomaterials, metabolic diseases, drug delivery, nanomedicine
Graphical Abstract
1. Introduction
Metabolic diseases, such as diabetes, obesity, and atherosclerosis, pose a significant threat to global human health, and their incidence is rapidly increasing. In a healthy metabolism, the proper function of pathways or mechanisms involved in nutrient sensing and management is essential to integrating the metabolic systems into pathogen-sensing and immune responses [1]. In general, metabolic disorders are caused by the dysfunction of either metabolism, immunity, or both [1–2]. Diabetes mellitus is characterized by hyperglycemia involved in high blood glucose levels, which is attributed to defects in insulin secretion and insulin action. The World Health Organization (WHO) estimates that over 400 million adults are expected to suffer from diabetes by 2030 [3]. Obesity is a related metabolic disorder that has also been a serious threat to human health worldwide and is comorbid with diabetes. It is caused by an excess of adipose tissue and an imbalance between energy intake and expenditure [4], which increases the risk of developing type 2 diabetes, hypertension and cardiovascular diseases [5]. As the leading cause of coronary artery disease, atherosclerosis processes responsible for the largest ratio of deaths in the United States[6]. Lipid metabolic disorders are the pathological basis of atherosclerosis, which is a deleterious condition caused by the deposition of foam cells and extracellular materials mainly in arteries[7].
Various methods including systemic drug treatment and surgery are used to prevent and treat the aforementioned metabolic diseases. For diabetes, the subcutaneous injection of insulin is the most common approach for controlling hyperglycemia in the past decades; for the mitigation of obesity, physical exercise, hormonal therapy and clinical surgery in gastrointestinal and fat elimination are alternative options; for atherosclerosis, the morbidity and mortality of this kind of cardiovascular events still remain unacceptably high even though interventional and surgical treatment options were used. However, current pharmacotherapeutic options for the management of these diseases remain limited and ineffective. For example, diabetes management typically requires daily drug administration, meaning many injections must be made at the same or similar site each time, causing the patient discomfort and reducing their compliance to treatment. Anti-inflammatory therapeutics is the major strategy to treat atherosclerosis. However, systemic inhibition of inflammation causes some side effects [8]. These methods for treating metabolic disorders are often insufficient in preserving metabolic equilibrium and preventing life-threatening complications [9]. Thus, new effective drugs or/and strategies to improve the management ability of metabolic disorders are critical for the treatment of related diseases.
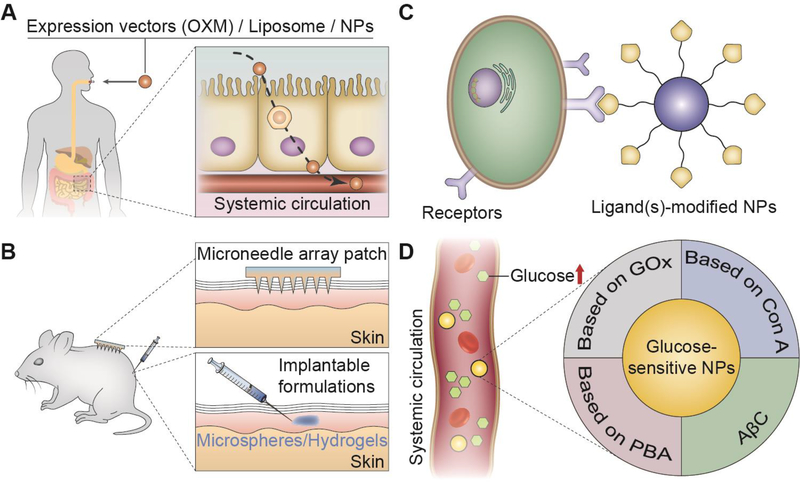
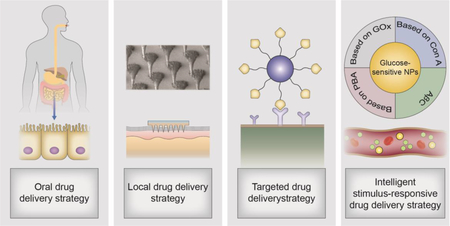
Over recent decades, it has gradually become more important for drug delivery strategies to face the challenges posed by different diseases. For example, insulin pumps release the required insulin from the replaceable reservoir to control blood glucose levels in people with diabetes [10]. Alternatively, using continuous glucose monitors (CGMs) along with insulin pumps to automatically release appropriate doses of insulin is another insulin delivery strategy for the treatment of blood glucose disorder [11]. Delivery systems were also developed to carry drugs to the brain to regulate appetite and thermogenesis [12]. Genes and pathways have also been studied as targets in treating obesity [13]. These approaches have achieved high therapeutic efficiency but are still not sufficient to cure these metabolic diseases. Therefore, emerging novel delivery strategies have been intensively studied nowadays. In this review, we majorly focus on the new paradigms for the treatment of diabetes, obesity, and atherosclerosis. The following novel delivery strategies based on nano-biotechnologies are briefly summarized and discussed: oral drug delivery strategy, local drug delivery strategy, targeted drug delivery strategy, and intelligent stimulus-responsive drug delivery strategy (Figure 1).
Figure 1.
Drug delivery strategies for the treatment of metabolic diseases: (A) oral drug delivery strategies, (B) local drug delivery strategies, (C) targeted drug delivery strategies, and (D) intelligent stimulus-responsive drug delivery strategies.
2. Oral Drug Delivery Strategies for the Treatment of Metabolic Diseases
Oral delivery of therapeutics represents the most convenient route for drug administration [14], offering increased patient compliance (compared to injections) and higher treatment efficacy, especially for diseases (e.g., diabetes) requiring frequent administration [15]. However, biologic drugs such as proteins (insulin) and peptides are not orally bioavailable, due to the physiological barriers presented by the gastrointestinal (GI) tract including extreme pH, enzymatic degradation, and poor permeability of the intestinal epithelium [16].
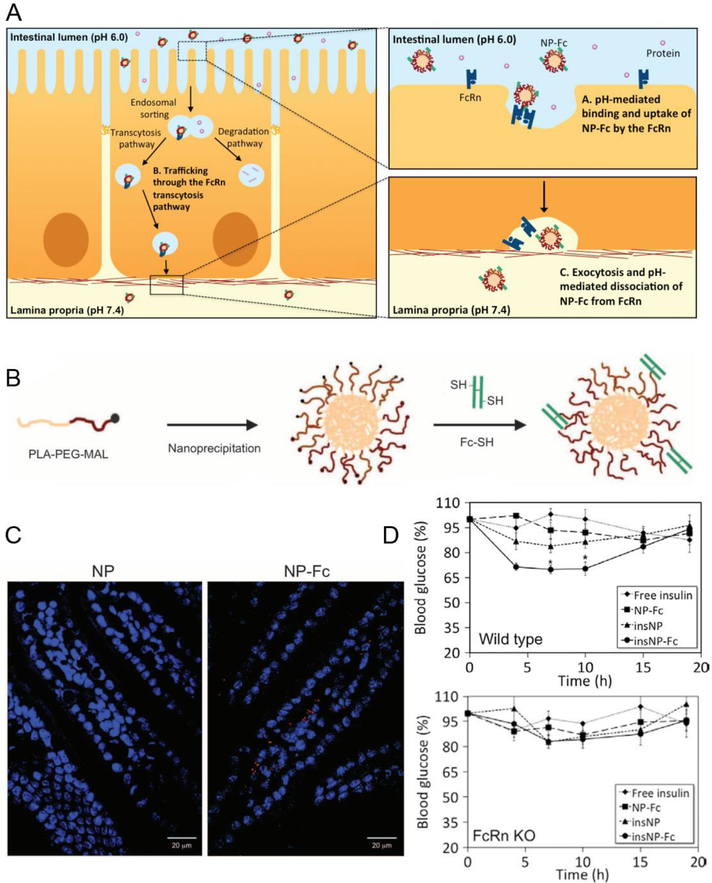
Different types of insulin have pharmacokinetics that varies from 10 min to 4 h. Intraday variations in plasma glucose concentrations of people with diabetes require up to several administrations of distinct types of insulin via injections, which is associated with patient inconvenience, ultimately leading to poor patient compliance and treatment inefficacy. Therefore, the development of oral delivery modalities for insulin holds huge promise for improving the patient compliance and efficacy of existing biologics while accelerating the bench-to-bedside translation of emerging ones. However, insulin is also sensitive to pH and enzymatic degradation in the GI tract, and its inability to cross the GI epithelial barrier presents another considerable challenge for the effective oral administration. Nanomedicine platforms offer a practical possibility of addressing both sets of challenges simultaneously by encapsulating biologics in NPs capable of protecting the biologics against the harsh environment of the GI tract while enabling their active transcytosis across the GI barrier to achieve oral bioavailability [17]. In our previous work [18], we developed a first-in-kind insulin-encapsulated polymeric poly[lactic-co-glycolic acid-b-poly(ethylene glycol)] (PLGA-PEG) nanoparticle (NP) targeted to the neonatal Fc receptor (FcRn). Within the intestinal mucosa, the FcRn is responsible for active transport of IgG antibodies across the intestinal epithelium via transcytosis. Capitalizing on the function of FcRn, our NP system is designed to bind to the FcRn and get transported across the intestinal mucosa without impacting the mucosal integrity and barrier function. NPs can also shield insulin from pH and enzymatic degradation, and targeting the insulin-loaded NPs to FcRn by functionalizing them with the Fc portion of IgG facilitates active transport of NPs across the intestinal epithelium into systemic circulation after oral administration (Figure 2). Our 1st generation NP platform showed a prolonged glycemic reduction response in mouse models after oral gavage of clinically relevant doses of insulin encapsulated NP. The proposed NP platform is largely based on clinically validated polymers PLGA and PEG. This oral delivered NP achieved a high absorption efficiency and induced a hypoglycemic response at a clinically relevant drug dose.
Figure 2.
Fc-targeted NPs deliver insulin across the intestinal epithelium. (A) Schematic of Fc-targeted NP binding to the epithelium cells. (B) Preparation of Fc-targeted NPs which consisted of a PLA core and Fc fragments on the surface. (C) Fluorescently labeled targeted NPs were absorbed in mouse intestinal tissues. (D) The wild type mice and FcRn knockout mice blood glucose response to a different formulation including free insulin solution, nontargeted NPs, and Fc-NPs loaded with insulin. Reproduced with permission.[18] Copyright 2013, American Association for the Advancement of Science.
In parallel, oral administration is also a powerful drug delivery route for obesity and atherosclerosis. To treat obesity, a novel oral delivery system of Bifidobacterium for human oxyntomodulin (OXM) was successfully exploited by Long et. al [14a]. OXM, a gut hormone, released from intestinal L cells, which is benefitted to reduce food intake via intravenous administration. In this system, an Escherichia coli-Bifidobacterium shuttle vector was constructed as carries for OXM gene expression that was secreted in the intestinal tract for obesity and diabetes therapy [14a]. Consequently, this oral delivery strategy for OXM expression could reduce food intake, plasma lipids, and body weight in obese mice. Epigallocatechin gallate (EGCG) is a positive vasculoprotective compound which could reverse endothelial dysfunction and dilate brachial artery [19]. However, its poor bioavailability limits the clinical application. Hong et. al. yielded oral EGCG-loaded NPs based on chitosan (CS) and polyaspartic acid (PAA) (EGCG-CS-PAA NPs) for the treatment of atherosclerosis, which showed a significant decrease of lipid deposition [19]. For oral delivery, chitosan is a widely available, mucoadhesive material which is able to increase cellular permeability.
3. Local Drug Delivery Strategies for the Treatment of Metabolic Diseases
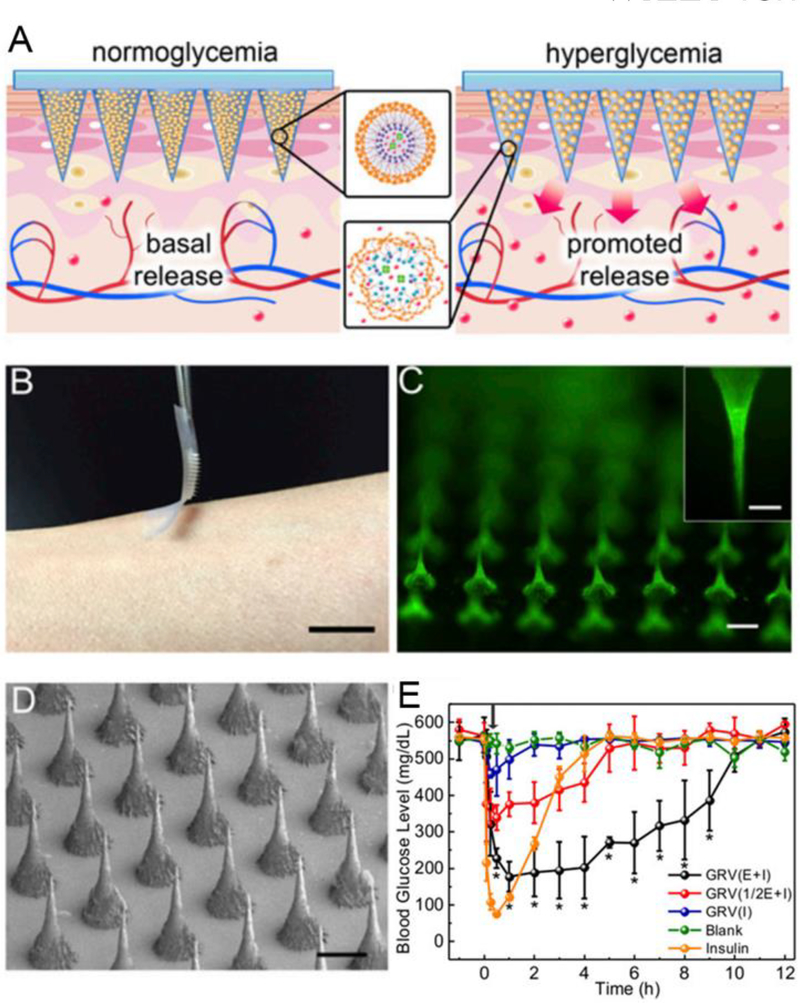
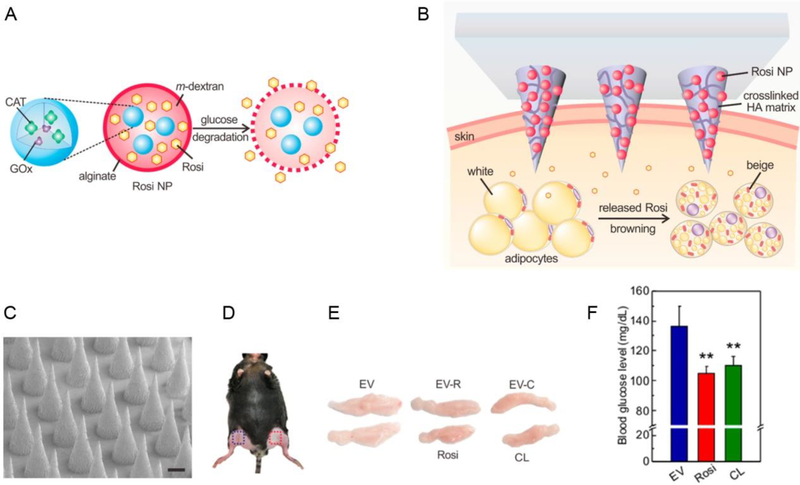
Recently, local drug delivery strategy for the treatment of diabetes, obesity and related metabolic disorders has been studied due to its capacity for keeping drug availability and permitting a reduction in dosing frequency [20]. It demonstrates the great potential to deliver therapeutic agents to the desired site over controlled periods of time, which is very useful in controlling blood glucose levels and body energy expenditure [21]. Yuksel et al. administered poly(lactic-co-glycolic-acid)-polyethylene glycol (PLGA/PEG) microspheres directly to the deep muscular fascia to load insulin and related therapeutic agents for the sustained release of the drug (~ 4 weeks) and to induce adipogenic differentiation [22]. As reported by Peng et al., the hypoglycemic effect in diabetic rats subcutaneously injected by poly(hydroxybutyrate-co-hydroxyhexanoate) (PHBHHx) NPs loaded with insulin lasted for more than three days, and this local delivery system allowed for sustained insulin release [23]. Similarly, insulin-PHBHHx NPs entrapped in biodegradable thermosensitive hydrogels can further delay insulin release in a controlled or long-term manner [24]. Transdermal drug delivery and intranasal delivery are other potential strategies for metabolic disease treatment due to the potential for contact between the drugs and the skin or mucosa over large areas of the body. The N-terminal amine of leptin, a derivative of an adipocyte-secreted hormone, was administered intranasally via the nose-to-brain route to cross the blood-brain barrier (BBB) [12a]. This approach enhances the absorption of leptin across the mucosal epithelium and improves the efficiency of obesity treatments. In order to improve diffusion across the skin, a patch-based drug delivery platform has been developed [25]. The use of microneedles is a major technological strategy used in promoting drug permeation across the stratum corneum. For instance, a series of microneedle (MN) patches in studies by the Gu group were used to incorporate pharmacotherapeutic agents for metabolic diseases [21]. An intelligent delivery system based on a microneedle-array patch was designed to release insulin in response to the hypoxic microenvironment in a hyperglycemic state (Figure 3) [21b]. This system, as the first prototype of the glucose-responsive “smart insulin patch”, included glucose-responsive vesicles that were formed by hypoxia-sensitive hyaluronic acid/2-nitroimidazole conjugates. Glucose oxidase enzyme and insulin were both loaded into the vesicles. In a hypoxic condition, these conjugates can be converted to hydrophilic 2-aminoimidazoles. Thus, enzymatic oxidation of glucose could lead to vesicle breakdown and the subsequent release of insulin in a glucose-responsive manner which mimics the function of pancreatic cells. In addition, based on such a smart insulin patch, H2O2-responsive vesicles were exploited for insulin delivery [26]. In addition, the Gu group also developed a transcutaneous browning agent patch based on the microneedle technology to induce adipose tissue transformation (Figure 4) [21a]. CL 316243 or Rosiglitazone (Rosi), the browning agent, was first loaded into the pH-sensitive dextran NPs and was then embedded into a degradable microneedle (MN) patch to continually release drugs into subcutaneous adipose tissue. This functions to prevent adipocyte hypertrophy, increase body energy expenditure and improve type-2 diabetes in vivo.
Figure 3.
Microneedle (MN)-array patch based transdermal delivery of pharmacotherapeutic agents for diabetes treatment. (A) The glucose-responsive vesicles were included in the MN-array patch and will release insulin when triggered by a hyperglycemic state. (B) Transdermal delivery of insulin by an MN-array patch. Scale bar: 1 cm. (C) Fluorescence images of the FITC-labeled insulin-loaded MN-array patch. Scale bar: 200 μm. (D) Scanning electron microscope (SEM) image of the MN array. Scale bar: 200 μm. (E) In vivo transdermal delivery of insulin by MN-array patches for blood glucose control and type I diabetes treatment. Reproduced with permission.[21b] Copyright 2015, National Academy of Sciences.
Figure 4.
Microneedle (MN)-array patch based transdermal delivery of browning agents for obesity treatment. (A-B) The MN-array patch consisted of pH-sensitive dextran NPs and a cross-linked hyaluronic acid (HA) matrix. (C) SEM image of MN array. Scale bar: 200 μm. (D) Local transdermal delivery sites for the MN-array patch. (E) The inhibition of growth of inguinal adipose tissues in obese mice after treatment with a browning agent-MN array. (F) Blood glucose levels of mice treated with the MN array at 16 h post fasting. Reproduced with permission.[21a] Copyright 2017, American Chemical Society.
4. Targeted Drug Delivery Strategies for the Treatment of Metabolic Diseases
Effective delivery of therapeutic drugs is always a formidable challenge. Target delivery of pharmacotherapeutic compounds to the disorder sites is more effective. It is urgently required, but also challenging [27]. Their surfaces were modified with ligands [28], targeting molecules [29] or peptides [30], which could deliver higher levels of drugs to the nidus sites and create an improved therapeutic effect. Therefore, this is also a promising approach to treat metabolic diseases. Barnett et al. developed a targeted intraportal delivery strategy based on the magnetic resonance tracking and guiding for islets using magnetocapsules for restoring normal glycemia [31]. Ligands specific for special cell types can facilitate internalization of carrying drugs, thereby reducing the administrated dose and minimizing side effects. Huang et al. added a CSKSSDYQC (CSK) targeting peptide to modified insulin-loaded chitosan NPs, which produced a better hypoglycemic effect compared to the control group [30].
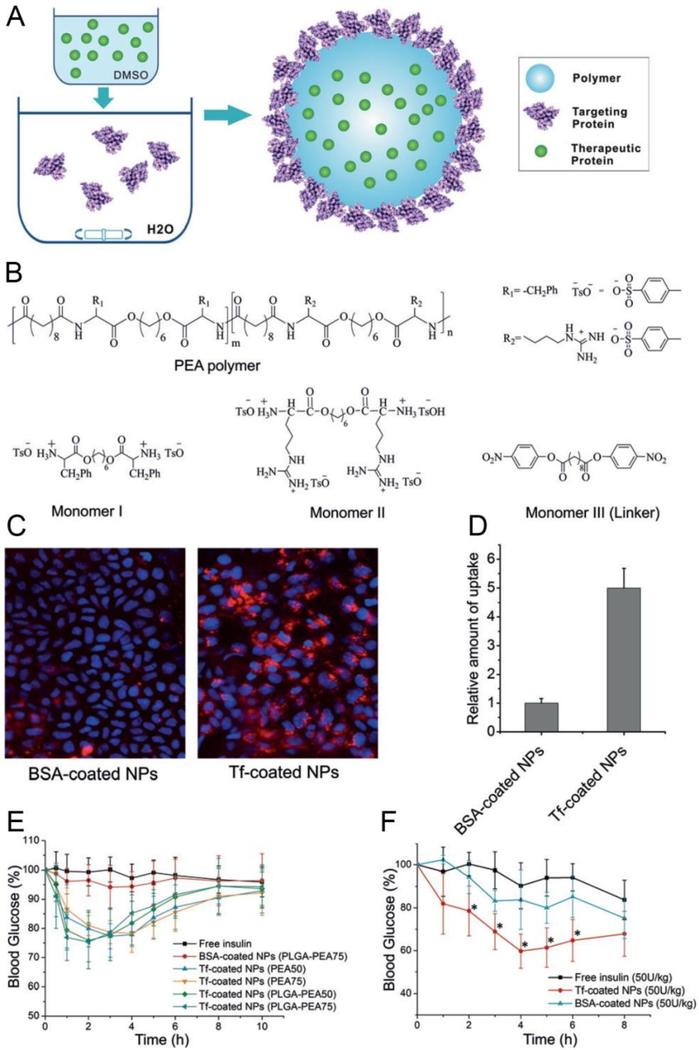
As described before, we showed that the immunoglobulin G antibody-modified NPs could target to the neonatal Fc receptor (FcRn) and across epithelial barriers when orally administered [18]. Generally, the FcRn interacts with Fc in a pH-dependent manner, with a high affinity in acidic (pH <6.5). In this delivery system, Fc fragments were grafted to the drug-loaded NPs (NP-Fc), giving them a strong affinity to the high-level FcRn apical surface of absorptive epithelial cells. Consequently, the absorption efficiency was improved by more than 10 times for FcRn-targeted NPs when compared to nontargeted NPs. In subsequent work, our group studied another targeted delivery system[32]. This system consisted of installing protein ligands such as transferrin (Tf) on the particle surface which could alter the NP behaviors within the cell (Figure 5). This NP platform consisted of poly (ester amide)s (PEAs) as the NP core in addition to arginine (Arg) and phenylalanine (Phe). Insulin was incorporated into the NP core and Tf proteins were captured on the NP surface (core-shell structure). This structure benefitted the absorption by epithelial cells due to the high binding affinity to Tf receptors and the sustained release of insulin. After oral administration, the hypoglycemic response of Tf-coated NP was also more sensitive than normal insulin-loaded NPs.
Figure 5.
Preparation of Tf-coated polymeric NPs and targeting delivery of insulin to the epithelial cells. (A) Self-assembly formation of Tf-NPs loaded with therapeutic proteins. (B) Chemical structures of monomers and the components of NPs. (C) Tf-coated NPs promote the uptake of targeted NPs by Caco-2 cells. (D) Quantitative analysis of the relative uptake of Tf-coated NPs versus their nontargeted counterpart. (E) Blood glucose response of healthy animals to different formulations including free insulin, nontargeted NPs, and Tf-NPs via oral administration. (F) A hypoglycemic response of diabetic animals to different formulations including free insulin, nontargeted NPs and Tf-NPs via oral administration (* p< 0.05 vs. free insulin). Reproduced with permission. [32] Copyright 2016, Wiley-VCH.
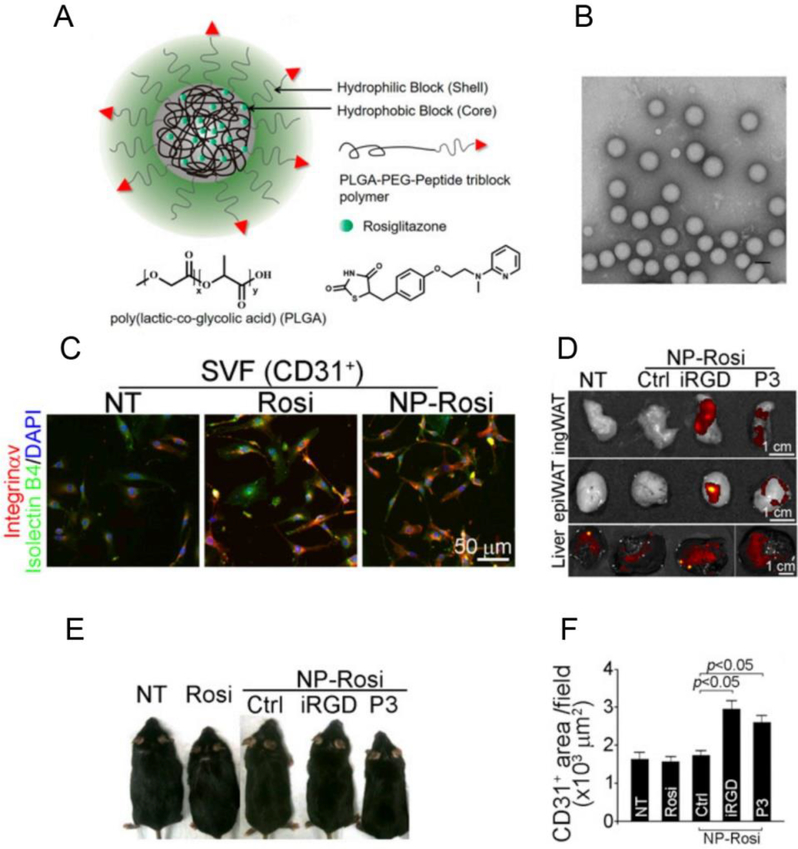
Recently, a targeted strategy was utilized to treat obesity in our group [33]. Angiogenesis, playing a major role in the expansion and regression of adipose tissue, makes the vasculature a potential therapeutic target for obesity and related metabolic diseases [5a, 33–34]. Stimulation of angiogenesis results in transforming adipose tissue from energy storage status into energy expenditure status, which is expected for obesity treatment [33]. Based on this concept, poly(lactide-co-glycolide)-b-poly(ethylene glycol) (PLGA-b-PEG) was used to prepare NPs to entrap therapeutic compounds such as Rosi and prostaglandin E2 analog (PGE2). Then, the NPs were coated with a targeting peptide such as iRGD which binds to antigens specifically expressed on the endothelium of angiogenic vasculature (Figure 6). Herein, iRGD or P3, two adipose vasculature-targeted peptides, were covalently conjugated to the polymeric NPs. As a result, more targeted NPs homed into local adipose tissue and released Rosi, which amplified the white adipose tissue (WAT) browning process which facilitates resistance to obesity.
Figure 6.
Targeting delivery of peptide-conjugated polymeric NPs loaded with Rosi for obesity treatment. (A) Formation of PLGA-b-PEG-peptide/Rosiglitazone NPs (Peptide-NP-Rosi). (B) Transmission electron microscope (TEM) image of iRGD-NP-Rosi. (C) Fluorescent localization of iRGD-NP-Rosi in the adipose tissues where CD31+ SVF was stained with integrin αv antibody in red, Isolectin B4 in green and nuclei in blue. (D) In vivo fluorescence imaging of adipose tissues and livers. (E) Antiobesity effect of iRGD-NP-Rosi in obese mice. (F) The area of CD31-positive blood vessels was quantified. Reproduced with permission.[33] Copyright 2016, National Academy of Sciences.
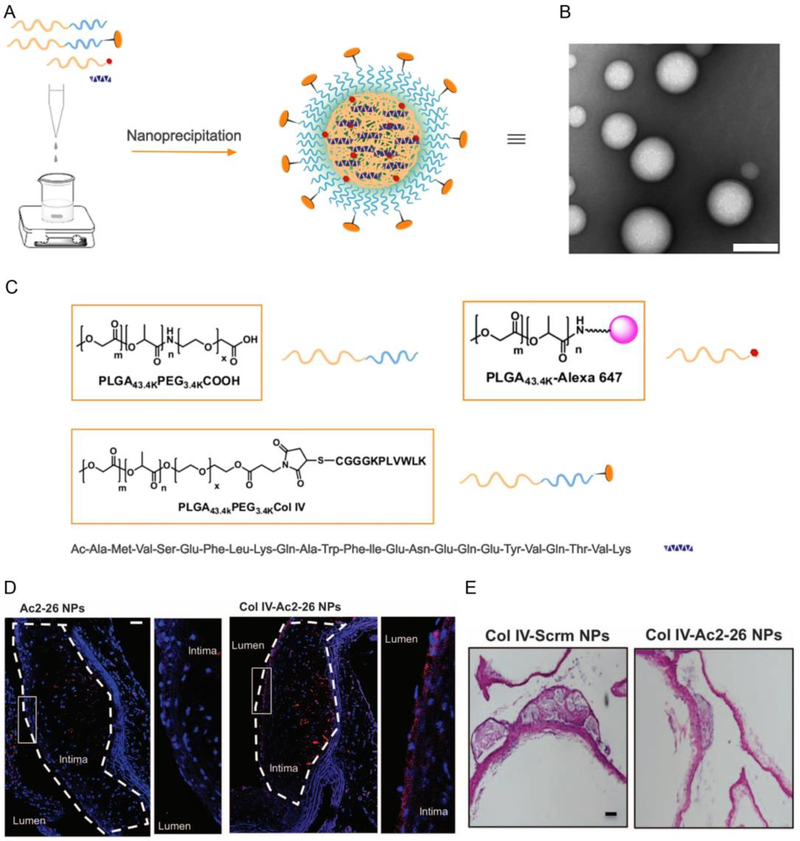
Atherosclerosis is a lipoprotein metabolic disorder caused by the subendothelial retention of apolipoprotein B (apoB)-containing lipoproteins (LPs) in the focal areas of arteries [35]. Hyperleptinemia may play an important role in obesity-associated cardiovascular diseases such as atherosclerosis [36]. This disease can stimulate an inflammatory response in the body and cause the thinning of the protective “cap” of subendothelial collagen thereby leading to oxidative stress and necrosis. One of the potential therapeutics for atherosclerosis is targeted delivery strategies to atherosclerotic lesions. In our group, amino acids 2–26 (Ac2–26), mimicking the receptor N-formyl peptide receptor 2 (FPR2/ALX) of annexin A1, were incorporated into collagen IV (Col IV)-targeted NPs (Col IV-Ac2–26 NPs) to stabilize the vulnerable plaques in atherosclerotic lesions (Figure 7) [37]. As expected, Col IV–Ac2–26 NPs could target lesions and increase the protective collagen layer overlying lesions, suppress oxidative stress and decrease plaque necrosis. This is a new way of therapy for chronic, advanced atherosclerosis.
Figure 7.
Col IV-targeted NP preparation and their therapeutic effects on advanced atherosclerosis in fat-fed Ldlr−/− mice. (A) Preparation of Col-IV NPs loaded with Ac2–26. (B) A TEM image of Col IV-Ac-2–26 NPs. (C) Structures of the materials used. (D) Location of Col IV-Ac2–26 NPs in atherosclerotic lesions. The nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI) and NPs are shown in red. (E) Cross-sectioned specimen evaluation of aortic root and brachiocephalic (BCA) lesions after different NP administrations (Col IV Scrm NPs and Col IV-Ac2–26 NPs). Reproduced with permission. [37] Copyright 2015, American Association for the Advancement of Science.
5. Intelligent Stimulus-Responsive Drug Delivery Strategies for the Treatment of Metabolic Diseases
The traditional treatment of diabetes often lacks successful automatic monitoring of blood glucose. An ideal drug delivery system for diabetes would intelligently release the drug when blood glucose levels are higher than normal, and stop the release from the device after normal glycemia is restored. Ever increasing numbers of functional delivery strategies are emerging to meet this requirement. Glucose oxidase (GOx) is widely used in the smart insulin delivery systems because of its induction of enzymatic oxidation through alteration of the microenvironment. As previously mentioned, the Gu group produced a glucose-sensitive insulin delivery device with the aid of GOx [21b]. In a hyperglycemic state, glucose suffered from enzymatic oxidation with GOx. This could lead to a local hypoxic microenvironment, which triggers the dissociation of vesicles and subsequent release of insulin. Duan et al. developed a metal-organic framework (MOF)-based glucose-responsive insulin nanosystem to release insulin when high levels of glucose were transferred into gluconic acid (lower pH) under GOx action [38]. MOF was sensitive to low pH, inducing decomposition of the composite to deliver insulin. These are the most typical examples of a glucose-responsive platform for such a GOx-based insulin delivery strategy. Thus, glucose-sensitive drug delivery systems like artificial pancreas can mimic the biofeedback systems with the control of blood concentration at a regular level [39]. However, the enzyme reaction-induced change in the microenvironment is always limited, restricting the further application of these smart insulin systems.
Concanavalin A (Con A), a well-known plant lectin protein, is another element used to trigger the glucose-sensitive response and shows a specific binding ability with glucose, mannose, and polysaccharide. Con A can quickly bind to glucose in place of the saccharide on the delivery system, leading to the breakdown of its original structure and subsequent insulin release [40]. Tanna et al. covalently synthesized a cross-linked polymer based on Con A and dextran to prepare a closed-loop drug delivery device for insulin [41]. The viscosity of the materials was relatively high and would change after lectin-polysaccharide interaction in response to the glucose concentration. Based on the specific saccharide-binding affinity of Con A, Yin et al. used cationic materials of N-(2-(dimethylamino) ethyl)-methacrylamide (DMAEMA) to prepare glucose and pH dual-responsive hydrogels for insulin delivery, which is very responsive to changes in glucose concentrations [42].
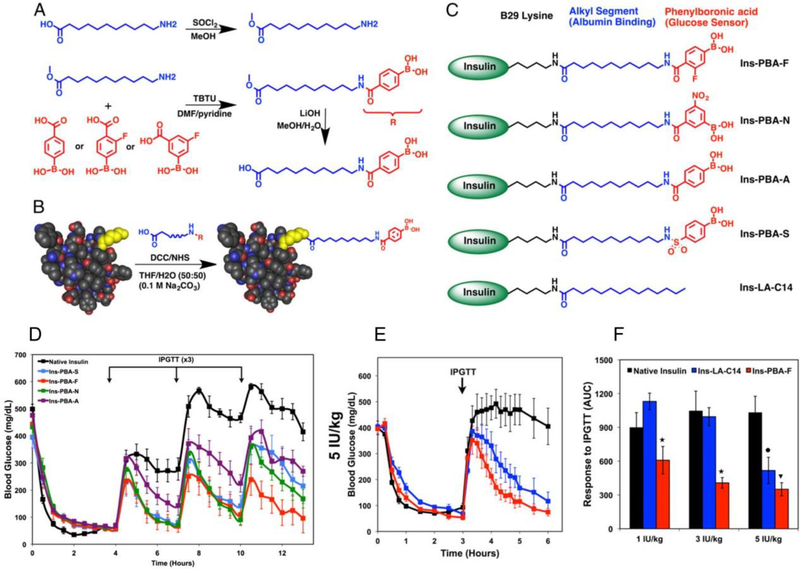
Recently, phenylboronic acid (PBA) and its derivatives have been used frequently to construct glucose-sensitive elements due to their versatile chemical structures. In aqueous solutions, PBA and its derivatives can bind reversibly to cis-1,2- and cis-1,3-diols, including sugars, to form boronate ester bonds. In a previous study, adipoylamidophenylboronic acid (AAPBA) was crosslinked with glycopoly peptide to form glucose-sensitive nanogels [40]. Insulin was quickly released from the nanogel as the glucose concentration increased. This study shows that the AAPBA related intelligent glucose-triggered delivery strategy is an excellent candidate for diabetes treatment. Similarly, a core-shell nanogel showed high glucose sensitivity when incorporated with PBA [43]. PBA was also directly conjugated to insulin molecules to trigger the required insulin release (Figure 8) [44]. The chemical modification of insulin with PBA can provide long-lasting glucose-sensing and glucose-responsive activities. This delivery approach ultimately reduces the number of administrations required and improves the reliability of glycemic control in insulin therapy.
Figure 8.
Covalent modification of aliphatic phenylboronic acid (PBA)-related conjugates for glucose-responsive insulin delivery. (A) Schematic formation of aliphatic phenylboronic acids. (B) A generalized illustration of insulin conjugated with PBAs. (C) Structures of PBA-modified long-acting insulin derivatives. (D) Blood glucose screening in STZ-induced diabetic mice after the injection of insulin derivatives including native insulin. (E) Blood glucose levels in diabetic mice after the administration of different insulin formulations. (F) The responsiveness of each insulin formulation. Reproduced with permission.[44] Copyright 2015, National Academy of Sciences.
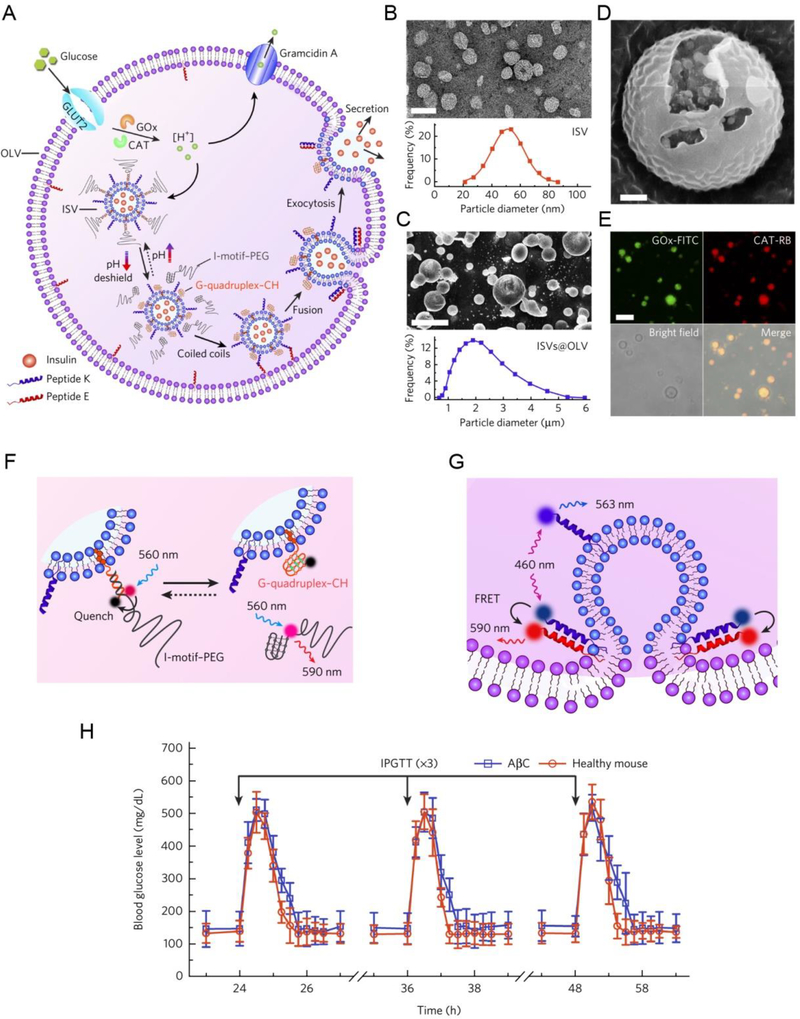
Another interesting approach for monitoring and regulating blood glucose levels relying on the pathological state is the use of artificial pancreatic cells [45]. Chen et al. synthesized artificial pancreatic beta cells (AβCs) using polyethylene glycol 5000–conjugated cytosine-rich DNA (PEG–CDNA) and cholesterol-ended guanine-rich DNA (GDNA–CH). These artificial AβCs contain two major superstructures: the inner small liposomal vesicles (ISVs) and the outer large vesicle (OLV) (Figure 9). Insulin was incorporated into ISVs that mimic the storage granules inside the mature beta cells, while OLV was used to mimic the plasma membrane. When the glucose was absorbed and went through enzymatic oxidation and proton efflux in a sequential cascade manner, the AβCs were able to make a distinction effectively between high and normal blood glucose levels. In this way, ISVs and OLV worked together and were stimulated by hyperglycemic conditions followed by fusion and insulin ‘exocytosis’.
Figure 9.
AβC generation and its in vivo type I diabetes treatment. (A) Design and biochemical processes of AβC. (B) A TEM image of ISVs and size distribution of insulin-loaded ISVs. (C) Cryo-SEM image of the vesicles-in-vesicle superstructures and size distribution of the superstructures. (D) Small liposomal vesicles existed in the large liposome. (E) Fluorescein isothiocyanate-labeled glucose oxidase (GOx-FITC) and rhodamine B-labeled catalase (CAT-RB) were loaded into the liposome and were observed by CLSM. (F) Schematic illustration for reversible PEG association and disassociation tuned by glucose metabolism. (G) Schematic illustration of the quenching of the peptide donor nitrobenzofuran (blue) on peptide K by the peptide acceptor tetramethylrhodamine (red) on peptide e induced by peptide assembly after PEG dissociation. (H) AβC treatment was performed on diabetic mice for in vivo intraperitoneal glucose tolerance test (IPGTT). Healthy mice were set as control. Reproduced with permission.[45] Copyright 2018, Springer Nature.
6. Conclusion
The development of delivery strategies for the management of metabolic diseases has attracted considerable attention over the past few years. As a result, more new bio-devices and nanomaterials have been tested to provide a targeted or intelligent platform to release pharmacotherapeutic agents (Table 1). Even if conventional oral administration remains the preferred method, the gastrointestinal (GI) tract limits the bioavailability of labile drugs due to the presence of protease which is a degradative enzyme. Thus, an alternative drug delivery approach must be developed to improve bioavailability. Compared to the conventional oral administration of drugs, local delivery by microneedle (MN) technology is better tolerated by patients due to its convenience and mechanism for long-term release of drugs. In addition, MN-based delivery strategies have provided satisfactory therapeutic effects in the treatment of metabolic disorders such as diabetes and obesity.
Table 1.
Summary of drug delivery strategies for the treatment of metabolic diseases.
| Platform | Drug(s) | Application | Strategy | Refs. |
|---|---|---|---|---|
| PLGA-PEG nanoparticles | Insulin | Diabetes | Oral delivery | [18] |
| Escherichia coli-bifidobacterium shuttle vector | Expressing OXM | Obesity | Oral delivery | [14a] |
| Chitosan/polyaspartic acid NPs | EGCG | Atherosclerosis | Oral delivery | [19] |
| PLGA/PEG microspheres | Insulin, IGF-1 | adipogenic differentiation | Local delivery | [22] |
| PHBHHx Nanoparticles, PHBHHx NPs-loaded hydrogels | Insulin | Diabetes | Local delivery | [23,24] |
| Hypoxia-sensitive hyaluronic acid/2-nitroimidazole formed microneedle patches | Insulin | Diabetes | Local delivery | [21b] |
| pH-sensitive dextran NPs-embedded microneedles | CL 316243 or Rosi | Obesity and improve type-2 diabetes | Local delivery | [21a] |
| CSK-modified chitosan NPs | Insulin | Diabetes | Targeted delivery | [30] |
| NP-Fc | Insulin | Diabetes | Targeted delivery | [18] |
| Tf-PEAs/Arg/Phe-NPs | Insulin | Diabetes | Targeted delivery | [32] |
| iRGD (or P3)-PLGA-b-PEG NPs | Rosi, PGE2 | Obesity | Targeted delivery | [33] |
| Col IV-Ac2–26 NPs | Amino acids 2–26 | Atherosclerosis | Targeted delivery | [37] |
| MOF-based Glucose-responsive nanoplatforms | Insulin | Diabetes | Intelligent stimulus-responsive delivery | [38] |
| A closed-loop drug delivery device based on Con A and dextran | Insulin | Diabetes | Intelligent stimulus-responsive delivery | [41] |
| AAPBA crosslinked with glycopolypeptide to form glucose-sensitive nanogels | Insulin | Diabetes | Intelligent stimulus-responsive delivery | [40] |
| Artificial pancreatic beta cells (AβCs) | Insulin | Diabetes | Intelligent stimulus-responsive delivery | [45] |
To further improve the absorption and bioavailability of therapeutic agents, many targeted delivery platforms were fabricated using functional ligands or peptides. When conjugated with a targeting ligand, the delivery platform, such as NPs, shows improvement in internalization by specific cells via receptor-mediated endocytosis. For oral administration of insulin, it is crucial for the drug to overcome the transport barrier of the intestinal epithelium. Such receptor-mediated transcytosis can promote transepithelial absorption for precise control of hyperglycemia. In parallel, iRGD or P3 modified NPs can target adipose angiogenic vessels to modulate the metabolism of energy. Collagen IV (Col IV)-targeted NPs containing Ac2–26 could protect against advanced atherosclerosis. Furthermore, more efforts have been devoted to exploiting intelligent delivery strategies such as glucose-responsive platforms for metabolic diseases. They act like a pancreas to “secrete” insulin from the device to restore normal glycemia.
Given recent technical advancements obtained from the bench, we believe that smart and functional targeted delivery systems will revolutionize the therapeutic field of metabolic diseases including diabetes, obesity, atherosclerosis, and other related metabolic disorders. However, the stability and large-scale production of these formulations still limit their application. To accelerate their clinical translation to improve patients’ quality of life, more systematical researches need to be performed to solve the current shortcomings, as well as safety issues. Fortunately, there are already many successful pre-clinical studies reported with these delivery strategies and the future explorations from bench to bedside are worth the wait. Increasing numbers of exciting discoveries and inventions are still on the way.
Acknowledgements
S.S. and N.K. contributed equally to this work. This work was supported by the National Institute of Health (NIH) grant HL127464 (O.C.F.) and METAvivor Early Career Investigator (W.T.). The authors would like to thank Dr. Junqing Wang (Harvard Medical School) for his assistance of figure design.
Footnotes
Conflict of Interest
In compliance with the Brigham and Women’s Hospital and Harvard Medical School institutional guidelines, O.C.F. discloses his financial interest in Selecta Biosciences, Tarveda Therapeutics, Placon Therapeutics, and Seer. The rest of the authors declare no conflicts of interest.
References
- [1].Hotamisligil GS, Erbay E, Nature reviews. Immunology 2008, 8, 923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gregor MF, Hotamisligil GS, Annual review of immunology 2011, 29, 415. [DOI] [PubMed] [Google Scholar]
- [3].a) Shaw JE, Sicree RA, Zimmet PZ, Diabetes research and clinical practice 2010, 87, 4; [DOI] [PubMed] [Google Scholar]; b) Veiseh O, Tang BC, Whitehead KA, Anderson DG, Langer R, Nature reviews. Drug discovery 2015, 14, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].a) Enriori PJ, Evans AE, Sinnayah P, Cowley MA, Obesity (Silver Spring) 2006, 14 Suppl 5, 254S; [DOI] [PubMed] [Google Scholar]; b) Puhl RM, Heuer CA, Obesity (Silver Spring) 2009, 17, 941. [DOI] [PubMed] [Google Scholar]
- [5].a) Cao Y, Nature reviews. Drug discovery 2010, 9, 107; [DOI] [PubMed] [Google Scholar]; b) Trasande L, Chatterjee S, Obesity (Silver Spring) 2009, 17, 1749. [DOI] [PubMed] [Google Scholar]
- [6].Yu M, Amengual J, Menon A, Kamaly N, Zhou F, Xu X, Saw PE, Lee SJ, Si K, Ortega CA, Choi WI, Lee IH, Bdour Y, Shi J, Mahmoudi M, Jon S, Fisher EA, Farokhzad OC, Advanced healthcare materials 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].a) Kamaly N, Fredman G, Subramanian M, Gadde S, Pesic A, Cheung L, Fayad ZA, Langer R, Tabas I, Farokhzad OC, Proceedings of the National Academy of Sciences of the United States of America 2013, 110, 6506; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kamaly N, Fredman G, Fojas JJ, Subramanian M, Choi WI, Zepeda K, Vilos C, Yu M, Gadde S, Wu J, Milton J, Leitao R. Carvalho, Fernandes L. Rosa, Hasan M, Gao H, Nguyen V, Harris J, Tabas I, Farokhzad OC, ACS Nano 2016, 10, 5280; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Libby P, Tabas I, Fredman G, Edward A. Fisher, Circulation Research 2014, 114, 1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schiener M, Hossann M, Viola JR, Ortega-Gomez A, Weber C, Lauber K, Lindner LH, Soehnlein O, Trends in molecular medicine 2014, 20, 271. [DOI] [PubMed] [Google Scholar]
- [9].Cooke D, Bloom S, Nature reviews. Drug discovery 2006, 5, 919. [DOI] [PubMed] [Google Scholar]
- [10].a) Umpierrez GE, Klonoff DC, Diabetes care 2018, 41, 1579;29936424 [Google Scholar]; b) Cengiz E, Bode B, Van Name M, Tamborlane WV, Expert review of medical devices 2016, 13, 57; [DOI] [PubMed] [Google Scholar]; c) McAdams BH, Rizvi AA, Journal of clinical medicine 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].a) Hovorka R, Nodale M, Haidar A, Wilinska ME, Diabetes technology & therapeutics 2013, 15, 4; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hovorka R, Nature reviews. Endocrinology 2011, 7, 385. [DOI] [PubMed] [Google Scholar]
- [12].a) Yuan D, Yi X, Zhao Y, Poon CD, Bullock KM, Hansen KM, Salameh TS, Farr SA, Banks WA, Kabanov AV, Journal of controlled release : official journal of the Controlled Release Society 2017, 263, 172; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Yi X, Yuan D, Farr SA, Banks WA, Poon CD, Kabanov AV, Journal of controlled release : official journal of the Controlled Release Society 2014, 191, 34; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Price TO, Farr SA, Yi X, Vinogradov S, Batrakova E, Banks WA, Kabanov AV, The Journal of pharmacology and experimental therapeutics 2010, 333, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].a) Kharitonenkov A, Shanafelt AB, Curr Opin Investig Drugs 2009, 10, 359; [PubMed] [Google Scholar]; b) Heymsfield SB, Wadden TA, The New England journal of medicine 2017, 376, 1492; [DOI] [PubMed] [Google Scholar]; c) Bonet ML, Oliver P, Palou A, Biochimica et biophysica acta 2013, 1831, 969. [DOI] [PubMed] [Google Scholar]
- [14].a) Long RT, Zeng WS, Chen LY, Guo J, Lin YZ, Huang QS, Luo SQ, Int J Obes (Lond) 2010, 34, 712; [DOI] [PubMed] [Google Scholar]; b) Mitragotri S, Burke PA, Langer R, Nature reviews. Drug discovery 2014, 13, 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Murata T, Tsuzaki K, Yoshioka F, Okada H, Kishi J, Yamada K, Sakane N, Journal of diabetes investigation 2015, 6, 687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].a) Carino GP, Jacob JS, Mathiowitz E, Journal of controlled release : official journal of the Controlled Release Society 2000, 65, 261; [DOI] [PubMed] [Google Scholar]; b) Fonte P, Araujo F, Reis S, Sarmento B, Journal of diabetes science and technology 2013, 7, 520; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Pridgen EM, Alexis F, Farokhzad OC, Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2014, 12, 1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].a) Kamaly N, Yameen B, Wu J, Farokhzad OC, Chemical reviews 2016, 116, 2602; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Damge C, Vranckx H, Balschmidt P, Couvreur P, Journal of pharmaceutical sciences 1997, 86, 1403; [DOI] [PubMed] [Google Scholar]; c) Damge C, Reis CP, Maincent P, Expert opinion on drug delivery 2008, 5, 45; [DOI] [PubMed] [Google Scholar]; d) Damge C, Maincent P, Ubrich N, Journal of controlled release : official journal of the Controlled Release Society 2007, 117, 163. [DOI] [PubMed] [Google Scholar]
- [18].Pridgen EM, Alexis F, Kuo TT, Levy-Nissenbaum E, Karnik R, Blumberg RS, Langer R, Farokhzad OC, Science translational medicine 2013, 5, 213ra167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hong Z, Xu Y, Yin JF, Jin J, Jiang Y, Du Q, Journal of agricultural and food chemistry 2014, 62, 12603. [DOI] [PubMed] [Google Scholar]
- [20].Li H, Liu T, Zhu Y, Fu Q, Wu W, Deng J, Lan L, Shi S, Acta biomaterialia 2017, 58, 136. [DOI] [PubMed] [Google Scholar]
- [21].a) Zhang Y, Liu Q, Yu J, Yu S, Wang J, Qiang L, Gu Z, ACS nano 2017, 11, 9223; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Yu J, Zhang Y, Ye Y, DiSanto R, Sun W, Ranson D, Ligler FS, Buse JB, Gu Z, Proceedings of the National Academy of Sciences of the United States of America 2015, 112, 8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yuksel E, Weinfeld AB, Cleek R, Waugh JM, Jensen J, Boutros S, Shenaq SM, Spira M, Plastic and reconstructive surgery 2000, 105, 1721. [DOI] [PubMed] [Google Scholar]
- [23].Peng Q, Zhang ZR, Gong T, Chen GQ, Sun X, Biomaterials 2012, 33, 1583. [DOI] [PubMed] [Google Scholar]
- [24].Peng Q, Sun X, Gong T, Wu CY, Zhang T, Tan J, Zhang ZR, Acta biomaterialia 2013, 9, 5063. [DOI] [PubMed] [Google Scholar]
- [25].Hultstrom M, Roxhed N, Nordquist L, Journal of diabetes science and technology 2014, 8, 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].a) Hu X, Yu J, Qian C, Lu Y, Kahkoska AR, Xie Z, Jing X, Buse JB, Gu Z, ACS nano 2017, 11, 613; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wang J, Ye Y, Yu J, Kahkoska AR, Zhang X, Wang C, Sun W, Corder RD, Chen Z, Khan SA, Buse JB, Gu Z, ACS nano 2018, 12, 2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shi S, Zhou M, Li X, Hu M, Li C, Li M, Sheng F, Li Z, Wu G, Luo M, Cui H, Fu R, Xiang M, Xu J, Zhang Q, Lu L, Journal of controlled release : official journal of the Controlled Release Society 2016, 235, 1. [DOI] [PubMed] [Google Scholar]
- [28].a) Russell-Jones GJ, Journal of drug targeting 2004, 12, 113; [DOI] [PubMed] [Google Scholar]; b) Zhang N, Ping Q, Huang G, Xu W, Cheng Y, Han X, International journal of pharmaceutics 2006, 327, 153. [DOI] [PubMed] [Google Scholar]
- [29].Petrus AK, Vortherms AR, Fairchild TJ, Doyle RP, ChemMedChem 2007, 2, 1717. [DOI] [PubMed] [Google Scholar]
- [30].Jin Y, Song Y, Zhu X, Zhou D, Chen C, Zhang Z, Huang Y, Biomaterials 2012, 33, 1573. [DOI] [PubMed] [Google Scholar]
- [31].Barnett BP, Arepally A, Karmarkar PV, Qian D, Gilson WD, Walczak P, Howland V, Lawler L, Lauzon C, Stuber M, Kraitchman DL, Bulte JW, Nature medicine 2007, 13, 986. [DOI] [PubMed] [Google Scholar]
- [32].Zhu X, Wu J, Shan W, Tao W, Zhao L, Lim JM, D’Ortenzio M, Karnik R, Huang Y, Shi J, Farokhzad OC, Angew Chem Int Ed Engl 2016, 55, 3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Xue Y, Xu X, Zhang XQ, Farokhzad OC, Langer R, Proceedings of the National Academy of Sciences of the United States of America 2016, 113, 5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].a) Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, Folkman MJ, Proceedings of the National Academy of Sciences of the United States of America 2002, 99, 10730; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Cao Y, The Journal of clinical investigation 2007, 117, 2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tabas I, Garcia-Cardena G, Owens GK, The Journal of cell biology 2015, 209, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Beltowski J, Atherosclerosis 2006, 189, 47. [DOI] [PubMed] [Google Scholar]
- [37].Fredman G, Kamaly N, Spolitu S, Milton J, Ghorpade D, Chiasson R, Kuriakose G, Perretti M, Farokzhad O, Tabas I, Science translational medicine 2015, 7, 275ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Duan Y, Ye F, Huang Y, Qin Y, He C, Zhao S, Chem Commun (Camb) 2018, 54, 5377. [DOI] [PubMed] [Google Scholar]
- [39].Zhao L, Xiao C, Wang L, Gai G, Ding J, Chem Commun (Camb) 2016, 52, 7633. [DOI] [PubMed] [Google Scholar]
- [40].Zhao CXL, Ding J, Zhuang X, Gai G, Wang L, Chen X., Polym. Chem 2015, 6, 3807. [Google Scholar]
- [41].Tanna S, Taylor M. Joan, Sahota TS, Sawicka K, Biomaterials 2006, 27, 1586. [DOI] [PubMed] [Google Scholar]
- [42].Yin R, Tong Z, Yang D, Nie J, International journal of biological macromolecules 2011, 49, 1137. [DOI] [PubMed] [Google Scholar]
- [43].Zhao L, Xiao C, Ding J, He P, Tang Z, Pang X, Zhuang X, Chen X, Acta biomaterialia 2013, 9, 6535. [DOI] [PubMed] [Google Scholar]
- [44].Chou DH, Webber MJ, Tang BC, Lin AB, Thapa LS, Deng D, Truong JV, Cortinas AB, Langer R, Anderson DG, Proceedings of the National Academy of Sciences of the United States of America 2015, 112, 2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chen Z, Wang J, Sun W, Archibong E, Kahkoska AR, Zhang X, Lu Y, Ligler FS, Buse JB, Gu Z, Nature chemical biology 2018, 14, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]