Abstract
Normal vitamin D homeostasis is critical for optimal health; nevertheless, vitamin D deficiency is a worldwide public health problem. Vitamin D insufficiency is most commonly due to inadequate cutaneous synthesis of cholecalciferol and/or insufficient intake of vitamin D, but can also arise as a consequence of pathological states such as obesity. Serum concentrations of 25(OH)D (calcidiol) are low in obesity, and fail to increase appropriately after vitamin D supplementation. Although sequestration of vitamin D in adipose tissues or dilution of ingested or cutaneously synthesized vitamin D in the large fat mass of obese patients has been proposed to explain these findings, here we investigate the alternative mechanism that reduced capacity to convert parent vitamin D to 25(OH)D due to decreased expression of CYP2R1, the principal hepatic vitamin D 25-hydroxylase. To test this hypothesis, we isolated livers from female mice of 6 to 24 weeks of age, weaned onto either a normal chow diet or a high-fat diet, and determined the abundance of Cyp2r1 mRNA using digital droplet-quantitative PCR. We observed a significant (p < 0.001) decrease in Cyp2r1 mRNA in the liver of high-fat diet–fed mice relative to lean-chow–fed female mice. Moreover, there was a significant (p < 0.01) relationship between levels of Cyp2r1 mRNA and serum 25(OH)D concentrations as well as between Cyp2R1 mRNA and the ratio of circulating 25(OH)D3 to cholecalciferol (p < 0.0001). Using linear regression we determined a curve with 25(OH)D3/cholecalciferol versus normalized Cyp2R1 mRNA abundance with an R2 value of 0.85. Finally, we performed ex vivo activity assays of isolated livers and found that obese mice generated significantly less 25(OH)D3 than lean mice (p < 0.05). Our findings indicate that expression of CYP2R1 is reduced in obesity and accounts in part for the decreased circulating 25(OH)D.
Keywords: OBESITY, VITAMIN D, P450, CYP2R1
Introduction
Vitamin D insufficiency is usually due to environmental factors that reduce access to dietary calciferols or decrease cutaneous synthesis of cholecalciferol, and is characterized by reduced serum concentrations of 25-hydroxyvitamin D (calcidiol, 25(OH)D), the major circulating metabolite of vitamin D. Recently, genomewide association studies (GWASs)(1–3) have also implicated a genetic contribution to circulating levels of 25 (OH)D. Among the small number of genes that have been associated with serum levels of 25(OH)D is CYP2R1, which encodes the principal hepatic vitamin D 25-hydroxylase,(4) which converts parent calciferol to 25(OH)D.
Obese individuals have lower circulating concentrations of 25 (OH)D than their race, socioeconomic status (SES), and age-matched peers.(5–7) In addition to this difference in baseline circulating 25(OH) D concentrations, obese subjects show smaller increases in serum levels of 25(OH)D than lean peers when supplemented with either vitamin D2 (ergocalciferol) or vitamin D3 (cholecalciferol).(8–11) Although the basis for this difference is incompletely understood, one possible explanation is that excessive adipose tissue sequesters vitamin D and hence reduces availability of the substrate for 25-hydroxylation in the liver.
We have hypothesized that an additional mechanism for decreased production of 25(OH)D might be reduced expression of CYP2R1, because recent animal studies show that expression of many hepatic P450 enzymes (ie, CYPs) is altered in the context of obesity.(12,13) These changes in CYP expression reflect a chronic inflammatory process induced by obesity that involves both innate and acquired immunity, and which is associated with marked increases in circulating cytokines. Therefore, in the present investigation we determined the effect of diet-induced obesity on conversion of parent vitamin D to 25(OH)D in mice through analysis of hepatic Cyp2r1 mRNA expression as well as CYP2R1 activity in liver homogenates.
Materials and Methods
We generated cohorts of treatment and control C57BL/6 mice in three age groups (6, 13 to 15, and 21 to 24 weeks of age). The mice were bred and maintained in the animal colonies of the University of Pennsylvania (breeding pairs obtained from Jackson Laboratory, Bar Harbor, ME, USA). Mice were all sacrificed between 8:00 a.m. and 10:00 a.m. in age-matched groups. The experimental protocol followed the principles of the Guide for the Care and Use of Laboratory Animals and was approved by the local Institutional Committee for Animal Care and Use. At 3 weeks of age, we placed littermates on either a high-fat diet (Harlan TD06414: 18.4% kcal from protein; 21.3% from carbohydrate [sucrose]; 60.3% from fat [lard and soybean oil]; 5.1 kcal/g, cholecalciferol concentration 2.1 IU/g; Envigo, Huntingdon, UK) or a control diet (Lab Diet 5015: 19.8% kcal from protein; 54.9% from carbohydrate [cornstarch]; 25.3% from fat [soybean oil]; 3.7 kcal/g, cholecalciferol concentration 3.3 IU/ g; LabDiet, St. Louis, MO, USA); further details for diets can be found on the following websites: www.harlan.com and www.labdiet.com. At the time of euthanasia, we obtained serum samples and harvested livers, which were flash frozen.
Analysis of Cyp2r1 transcript abundance
Total RNA was isolated from liver tissue using the RNeasy (Qiagen) reagent, and first-strand cDNA was generated from 1 mg of total RNA using Applied Biosystems high-capacity cDNA reverse transcriptase kit per manufacturer’s instructions. We used the Bio-Rad M96 mouse reference gene plates (100–29130) to determine which reference genes varied least between samples. We performed digital droplet quantitative PCR using Bio-Rad QX100 per manufacturer’s instructions to determine the abundance of the described mRNA transcripts, 18s and Nono (a transcript that encodes an RNA-binding protein housekeeping gene) was used for normalization (the following ABI probe-primer sets were used: for Cyp2r1, Mm01159413_m1 [VIC dye]; for Cyp27a1, Mm00470430_m1 [FAM dye]; for Cyp24a1, Mm00487244_m1; for Cyp3a11, Mm00731567_m1 [VIC dye]; for 18s, Mm03928990_g1 [FAM dye]; and for Nono Mm00834875_g1 [FAM dye]). All probe-primer sets were tested to ensure that the cDNA concentrations were within the linear range of each assay. Cyp2r1 mRNA abundance was normalized to 18s RNA abundance and Nono mRNA abundance.
Vitamin D and vitamin D metabolite assays
Serum concentrations of calciferols, 25(OH)D2, and 25(OH)D3 were determined by isotope-dilution liquid chromatography tandem mass spectrometry (Heartland Assays, Inc).
Immunoblot analyses: immunoblot for murine CYP2R1 protein
Because no commercial available antibody was able to detect CYP2R1 protein, we had a custom antibody produced. In brief, all liver samples were homogenized with ice-cold homogenization buffer, evaluated for total protein content using a bicinchoninic acid (BCA) protein assay kit (Thermo Scientific, Rockford, IL, USA), run on a 4–12% graded SDS polyacrylamide gel and then transferred to a nitrocellulose membrane. CYP2R1 bands were detected using the primary antibody for cyp2r1 (Dr. Li Denver, CO, USA, 1:1,000, RRID:AB_2732819) and secondary goat anti-rabbit AlexaFluor 680 (Rockland, Pottstown, PA, USA; 1:50,000, RRID: AB_1660962). Blots were scanned on an Odyssey (LI-CORE, Lincoln, NE, USA) imaging system. Blots were then stripped and re-blotted with mouse anti-beta actin (LifeSpan Seattle, WA, USA; 1:500 RRID:AB_10944987) and then incubated secondary antibody goat anti-mouse AlexaFluor 680 (Rockland; 1:20,000 RRID:AB_1057546). Band densitometry was assessed using Image Studio Lite (version 5.2.5) software and Cyp2r1 band intensity was normalized to beta-actin.
Ex vivo assay of hepatic 25-hydroxylase activity
Mice were anesthetized with isoflurane, blood was harvested by cardiac puncture, and mice were perfused with PBS. Livers were harvested and 0.7 to 0.8 g of hepatic tissue was homogenized in 3 mL of 0.25M sucrose/PBS. Half of this slurry was added to 1.5 mL of 20mM NADPH, 16 mg/mL ergocalciferol in PBS (experimental sample) and the other half was added to 16 mg/mL ergocalciferol in PBS (negative control sample). This slurry was incubated at 37°C in a rapidly shaking orbital shaker for 4 hours and then heated to 70°C for 10 min before being flash-frozen in liquid nitrogen. These frozen slurries were fully extracted as described below (in - 25(OH)D2/25(OH)D3 and vitamin D2/vitamin D3 in tissue/slurry samples) and we measured levels of ergocalciferol, cholecalciferol, 25(OH)D2, and 25(OH)D3 in the extracts as described above (in - Vitamin D and vitamin D metabolite assays). The measured 25(OH)D2 in the negative control was subtracted from the experimental to determine the enzymatically generated 25(OH)D2. All assays were performed by experimenters blinded to the identity of each sample. Statistical significance was examined by t test or two-way ANOVA as appropriate using Graphpad Prism version 6 software (GraphPad Software, Inc., La Jolla, CA, USA). Linear regression was performed using JMP 11 software.
25(OH)D2/25(OH)D3 and vitamin D2/vitamin D3 in tissue/ slurry samples
All samples were weighed and saponified with methanolic potassium hydroxide,(14) then spiked with deuterated internal standard of each metabolite being measured (d3-Vitamin D3 and d3–25(OH)D2/d3–25(OH)D3). Both deuterated and non-deuterated 25OH neat reagents were purchased from Sigma-Aldrich). After saponification the samples were vortexed and extracted with hexane/methylene chloride. The organic layer was then dried and reconstituted with hexane/methylene chloride and both 25OH and vitamin D were isolated via silica solid-phase extraction (SPE) column.(15) After eluting, the 25(OH) D and vitamin D were then injected onto a HPLC for further purification across a normal phase silica column. After HPLC purification the samples were then dried down in a Savant vacuum dryer. The samples were reconstituted into liquid chromatography–mass spectrometry (LC-MS) grade methanol and LC-MS high purity water, both with 0.1% formic acid, and then injected onto an Agilent 1290 HPLC with an Agilent C18 Poroshell Column coupled to an Agilent 6460 Triple-quad mass spectrometer with electrospray ionization source (ESI) in positive mode and analyzed using Masshunter software.(16,17) Excellent linearity was achieved with both assays with R2 values greater than 0.99. 25(OH)D2/D3 intraassay % coefficient of variation (CV) = 5.0% and interassay %CV = 8.6% with extraction efficiencies of >70%. Vitamin D2/D3 intraassay %CV = 7.9% and interassay %CV = 9.2% with extraction efficiencies of >64%. The limit of quantification (LOQ) for all analytes was 0.5 ng/g, the limit of detection (LOD) for all analytes was 0.1 ng/g.
Cholecalciferol and calcidiol administration via oral gavage
Ten-week-old female mice were started on a high-fat diet containing no vitamin D (140080; Tekland 43% from fat [lard and soybean oil]; 4.4 kcal/gm, cholecalciferol concentration 0 IU/g, 2.4% Ca, 1.5% P). After 3 weeks on the diet, mice were administered cholecalciferol (3000 IU/kg) or calcidiol (3000 IU/ kg) via oral gavage. Mice were returned to their home cages and allowed to recover for 3 days before serum was collected by submandibular bleed and serum 25(OH)D3 levels were measured via mass spectrometry.
Results
Mice on a high-fat diet have decreased serum 25(OH)D levels compared to littermates
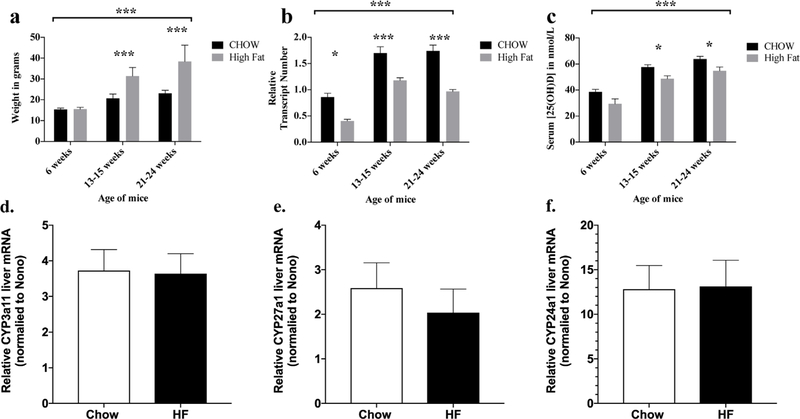
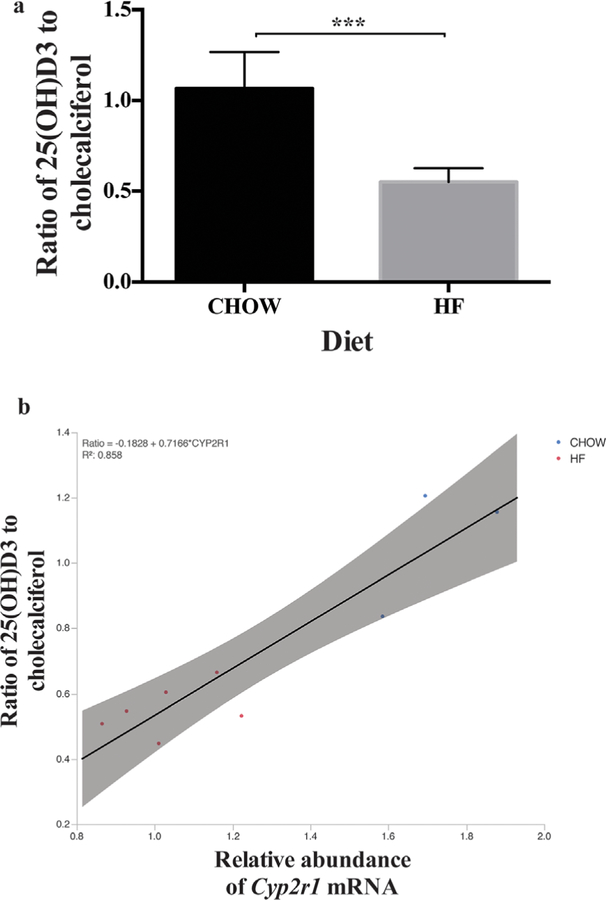
Mice that receive a high-fat diet are significantly heavier than mice that are fed a standard chow diet after 6 weeks of age (Fig. 1A, overall p < 0.001 by two-way ANOVA and groups greater than 6 weeks old were significantly different by Sidak’s post hoc test for all ages greater than 6 weeks with p < 0.001. Serum 25(OH)D3 concentrations were significantly decreased in obese mice relative to lean mice overall (Fig. 1C, p < 0.001 by two-way ANOVA). Post-test analysis corrected for multiple comparisons demonstrated that obese mice had significantly decreased 25(OH)D3 at 13 to 25 weeks old and 21 to 24 weeks old (p < 0.05). By contrast, circulating levels of cholecalciferol were similar in obese and lean mice, whereas the ratio of circulating concentrations of 25(OH)D3 to cholecalciferol, an index of 25-hydroxylase activity, was significantly less in obese mice than in lean mice (Fig. 2A, n ≥ 3 for each group, unpaired t test p < 0.001). These results provide strong evidence of a defect in conversion of parent vitamin D to 25(OH)D.
Fig. 1.
(A) Weight is increased on a high-fat diet by 13 weeks on diet. Mice were started on a high-fat diet at weaning at 3 weeks of age. Overall groups were significantly different (*** p < 0.001 by two-way ANOVA), subgroups were different at 13 to 15 weeks and 21 to 24 weeks of age (Sidak’s multiple comparison posttests, *** p < 0.001 for each). (B) Relative Cyp2r1 liver mRNA abundance is decreased on high-fat diet. Relative abundance of Cyp2r1 mRNA and Nono mRNA were determined by digital droplet quantitative PCR, and Cyp2r1 was normalized to Nono abundance in each sample. Overall groups were different (*** p < 0.001 by two-way ANOVA), subgroups were different at 6, 13 to 15, and 21 to 24 weeks of age (Sidak’s multiple comparison post-tests, * p < 0.05 at 6 weeks, *** p < 0.001 at 13 to 15 and 21 to 24 weeks). (C) Serum 25(OH)D is decreased in parallel with Cyp2R1 mRNA abundance on high-fat diet. Serum 25(OH)D was determined by LC/MS/MS. Overall groups were different (* p < 0.01 by two-way ANOVA), subgroups were different at 13 to 15 and 21 to 24 weeks of age (Sidak’s multiple comparison post-tests, * p < 0.05 at 6 weeks, 13 to 15 weeks, and 21 to 24 weeks). (D) Relative liver Cyp3a11 abundance was not decreased on a high-fat diet. mRNA abundance was determined by digital droplet quantitative PCR and normalized to Nono. Transcript levels did not differ significantly between chow and high-fat diet fat groups (p > 0.05 by t test). (E) Relative liver Cyp27a1 abundance was not decreased on a high-fat diet. mRNA abundance was determined by digital droplet quantitative PCR and normalized to Nono. Transcript levels did not differ significantly between chow and high-fat diet fat groups (p > 0.05 by t test). (F) Relative liver Cyp24a1 abundance was not decreased on a high-fat diet. mRNA abundance was determined by digital droplet quantitative PCR and normalized to Nono. Transcript levels did not differ significantly between chow and high-fat diet fat groups (p > 0.05 by t test).
Fig. 2.
(A) Ratio of 25(OH)D3 to cholecalciferol is increased in lean mice relative to obese mice. The ratio of 25(OH)D to cholecalciferols is increased in lean mice relative to obese mice (n ≥ 3 for each group, all mice 21 to 24 weeks of age, *** p < 0.001 by t test). (B) The ratio of 25(OH) D3 to cholecalciferol correlates with liver Cyp2r1 mRNA abundance. Ratio = –0.1828 + 0.7166* CYP2R1, R2 = 0.858 (n ≥ 3 for each group, all mice 21 to 24 weeks of age, *** p < 0.001 by t test). Red = obese mice on high fat diet; Blue = lean mice on CHOW diet.
Hepatic Cyp2r1 mRNA is significantly decreased in obese mice relative to lean mice
We measured mRNA levels of Cyp2r1, the principal 25-hydroxylase, to further examine decreased conversion of cholecalciferol to 25(OH)D in obese mice. Hepatic Cyp2r1 mRNA transcript abundance was significantly decreased in obese mice relative to lean mice overall (Fig. 1B, p < 0.001 by two-way ANOVA). Post-test analysis corrected for multiple comparisons showed significant decreases in relative hepatic Cyp2r1 mRNA transcript abundance in obese relative to lean mice at 6 weeks (p < 0.05), 13 to 15 weeks(p < 0.001), and 21 to 24 weeks of age (p < 0.001). We used linear regression to determine the relationship between the ratio of 25(OH)D3/ cholecalciferol and the normalized Cyp2R1 abundance, and found an R2 value of 0.858 (Fig. 2B, ratio = – 0.1828 + 0.7166* CYP2R1 abundance [R2 = 0.858]), which provides strong evidence that Cyp2R1 transcript abundance can explain 85% of the variation in circulating 25(OH)D3 levels in mice. To examine the possibility that other enzymes involved in 25(OH)D metabolism were altered by high-fat diet and thus contributed to this change, we examined transcript abundance of Cyp3a11, Cyp24a1, and Cyp27a through digital droplet PCR (ddPCR) at 13 weeks of age. There were no significant differences in the relative abundance of liver Cyp3a11 mRNA (Fig. 1D), Cyp27a1 mRNA (Fig. 1E), or Cyp24a1 mRNA (Fig. 1F) with high-fat diet.
Obese mice have significantly decreased CYP2R1 activity in an ex vivo 25-hydroxylase activity assay
We used a modified, non-isotopic assay of 25-hydroxylase activity that we developed to measure CYP2R1 activity in 13-week-old obese and lean mice. Consistent with the reduced abundance of Cyp2r1 transcripts, obese mice had significantly decreased 25-hydroxylase activity compared to their lean litter mates (p < 0.05, Fig. 3).
Fig. 3.
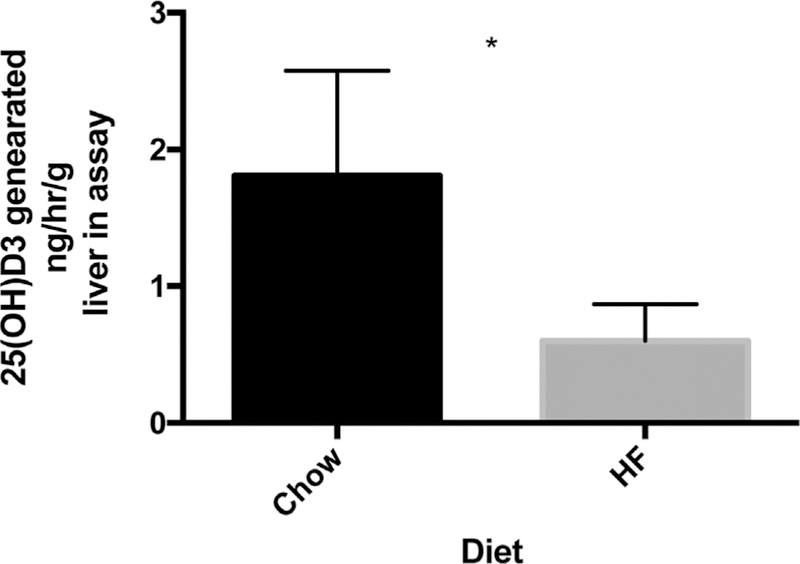
Lean mice have significantly increased hepatic 25-hydroxylase activity relative to obese mice. The ex vivo liver 25-hydroxylase activity of 13-week-old lean mice is greater than that in obese mice (1.8 ± 0.4 ng/ mL/g liver in assay versus 0.6 ± 0.2, n = 5 for each group, all mice 13 weeks of age, * p < 0.05 by t test).
CYP2R1 protein abundance decreases with obesity
To examine if this decrease in CYP2R1 activity in obese mice is due to a decrease in CYP2R1 protein expression, we examined CYP2R1 liver abundance via Western blot. Similar to mRNA we observed a significance decrease in CYP2R1 abundance in mice on a high-fat diet at 13 weeks old (p < 0.01, Western blot in Fig. 4A, quantitation in Fig. 4B).
Fig. 4.
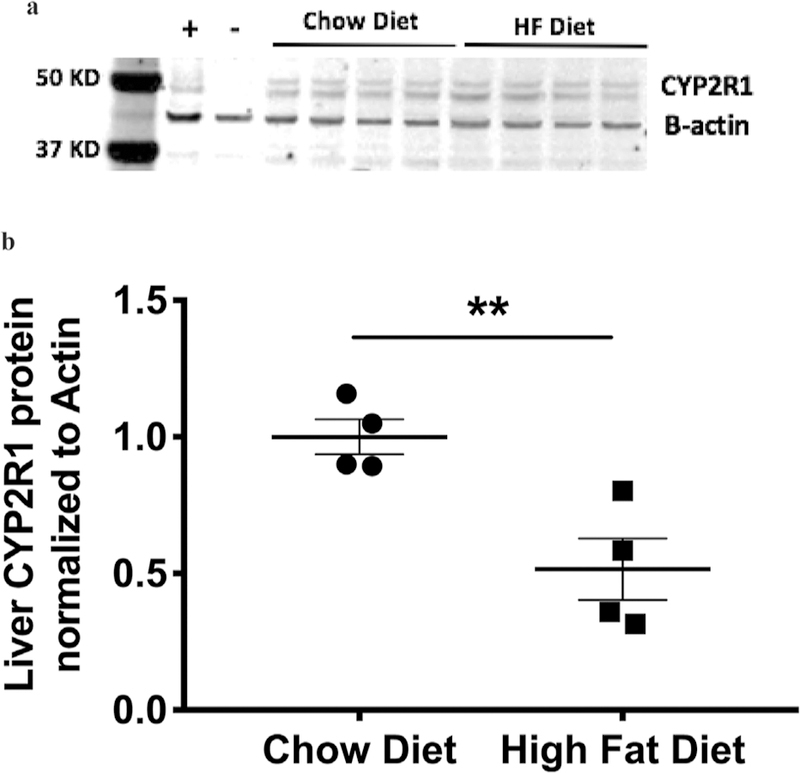
Liver Cyp2r1 protein abundance is decreased with on high-fat diet. (A) Representative Western blot of two diet groups, chow and high fat (n = 4). (B) Quantitation of Cyp2r1 liver protein reveals Cyp2r1 expression is decreased on a high-fat diet at 13 weeks old (t test, p = 0.00978).
Obese mice have significantly increased serum 25(OH)D3 following oral gavage with calcidiol compared to cholecalciferol
In order to test the hypothesis that obese mice would benefit from calcidiol treatment instead of cholecalciferol treatment due to decreased levels of CYP2R1, we administered the identical amounts (3000 IU/kg) of calcidiol or cholecalciferol via oral gavage to mice on a high-fat diet lacking vitamin D. Serum 25(OH)D3 was significantly increased in the calcidiol group (20.6 ng/mL) compared to the cholecalciferol group (37.17 ng/ mL) 3 days post-gavage (p < 0.001, t test, Fig. 5).
Fig. 5.
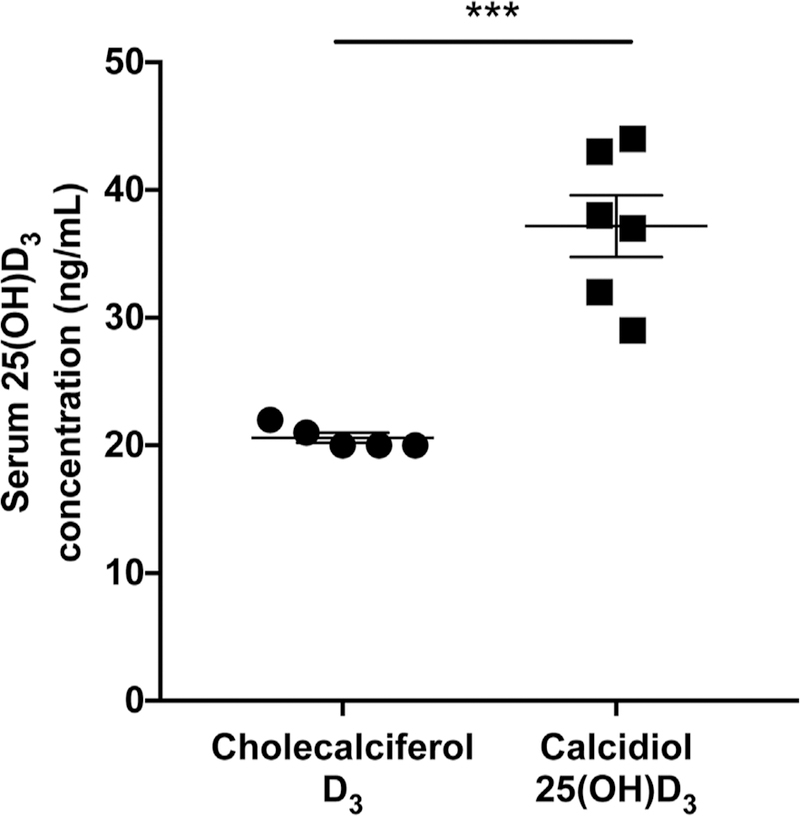
Obese mice orally gavaged with calcidiol have significantly increased serum 25(OH)D3 compared to obese mice gavaged with cholecalciferol. Serum 25(OH)D3 was significantly increased in the calcidiol group (20.6 ng/mL) compared to the cholecalciferol group (37.17 ng/mL) 3 days following gavage (p = 0.0002, t test).
Discussion
Vitamin D homeostasis is altered in obese subjects, who have lower circulating levels of 25(OH)D, even though basal vitamin D2 and D3 serum concentrations are not significantly different than their race, SES, and age-matched lean peers.(5–11,18) Moreover, obese individuals show blunted increases in circulating levels of parent vitamin D after whole-body ultraviolet radiation or a pharmacologic dose of vitamin D2 orally compared to lean individuals.(19) These alterations in vitamin D homeostasis have been considered evidence of sequestration of vitamin D in fat stores, despite experimental work showing that fat stores of vitamin D in the obese are inadequate to explain these alterations.(20) Recently, a volumetric dilutional model has been proposed as an alternative explanation for the variability in serum 25(OH)D concentrations that are characteristic of obese individuals.(10) In the current work we present evidence in support of an additional mechanism for these findings, specifically that levels of CYP2R1, the principal hepatic 25-hydroxylase, are significantly reduced in obese mice.
Recent GWASs have identified the CYP2R1 gene locus as a significant determinant of serum levels of 25(OH)D,(1–3) thus raising the possibility that variations in CYP2R1 enzyme activity might also contribute to interindividual variation in vitamin D homeostasis. Here we report for the first time that obesity secondary to a high-fat diet leads to decreased hepatic abundance of Cyp2r1 mRNA and decreased conversion of cholecalciferols to 25(OH)D. This result suggests the possibility that CYP2R1 is not, as is commonly believed, a typically constitutive gene that is stably expressed without regulation. Previous studies in rats with uremia support our premise that CYP2R1 expression may vary under specific pathophysiological conditions because uremic rats show a decrease in 25hydroxylation activity, as well as decreased relative abundance of Cyp2r1 mRNA.(21) Interestingly, our results show an acute effect of a high-fat diet on liver abundance of Cyp2r1 mRNA as well as a chronic effect of obesity or high-fat diet on Cyp2r1 mRNA abundance. This first effect occurs prior to a significant difference in weight being observed, suggesting that diet-derived fatty acids may directly regulate expression of CYP2R1,(22–24) or that diet-mediated microbiome changes may lead to decreased CYP2R1 expression prior to, or independent of, weight gain as recently described in other high-fat diet–related pathologies.(25,26) The mechanisms of fatty acid mediated changes in CYP abundance are an area of active research, but, regulation of CYP2R1 specifically is not well characterized. To examine the possibilities of fatty acid regulation or inflammatory cytokine regulation in silico we used the ENCODE project transcription factor data and the transcription factor recognition site program TFSEARCH (http://www.cbrc.jp/htbin/nph-tfsearch). This approach did not identify any constitutive androstane receptor (CAR) pregnane X receptor (PXR) or peroxisome proliferator-activated receptor (PPAR) binding sites in the proximal promoter of mouse or human CYP2R1. However, it is possible that other fatty-acid–related transcription factors not well characterized by ENCODE and TFSEARCH would be involved. Several binding sites for the inflammation-related transcription factor NF-k-B are identified by TFSEARCH, supportive of the possibility for microbiome change–related immune-mediated regulation of CYP2R1 expression.
Limitations to our study include that we only used female mice and that our characterization was limited to mRNA abundance, 25(OH)D to cholecalciferol ratio, and ex vivo activity measurement, but did not include characterization of CYP2R1 protein abundance because commercially available antibodies do not specifically recognize the CYP2R1 protein. Strengths of our study include the determination of and use of the most stable housekeeping mRNA for control (nono), confirmation using another stable housekeeping gene 18S, our finding that a clinical variable (serum 25(OH)D3) changed in our model system in concert with the obesity-mediated change in Cyp2r1 mRNA despite increased intake of cholecalciferol in the high-fat diet group, and a consistent biological difference between groups at several ages.
In conclusion, obese mice had significantly lower serum levels of 25(OH)D3 than lean mice overall, and at all time points. Our studies confirm the work of others showing that vitamin D supplementation is less effective in obese subjects than in lean subjects, and now provides a biological mechanism to explain these observations. Thus, we suggest that it may be more effective to treat vitamin D insufficiency in the obese with calcidiol than with calciferols.
Acknowledgments
The project described was supported by the NIH, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (K08-HD087964-01) and the NIH, National Center for Research Resources (NCRR) and the National Center for Advancing Translational Sciences (NCATS) (UL1TR000003). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosures
All authors state that they have no conflicts of interest to disclose.
References
- 1.Ahn J, Yu K, Stolzenberg-Solomon R, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet 2010;19(13): 2739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 2010;376(9736):180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson D, Holt BJ, Pennell CE, Holt PG, Hart PH, Blackwell JM. Genome-wide association study of vitamin D levels in children: replication in the Western Australian Pregnancy Cohort (Raine) study. Genes Immun 2014;15(8):578–83. [DOI] [PubMed] [Google Scholar]
- 4.Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci U S A 2004;101(20):7711–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samuel L, Borrell LN. The effect of body mass index on optimal vitamin D status in U.S. adults: the National Health and Nutrition Examination Survey 2001–2006. Ann Epidemiol 2013;23(7):409–14. [DOI] [PubMed] [Google Scholar]
- 6.Shinkov A, Borissova AM, Dakovska L, Vlahov J, Kassabova L, Svinarov D. Winter 25-hydroxyvitamin D levels in young urban adults are affected by smoking, body mass index and educational level. Eur J Clin Nutr 2015;69(3):355–60. [DOI] [PubMed] [Google Scholar]
- 7.Turer CB, Lin H, Flores G. Prevalence of vitamin D deficiency among overweight and obese US children. Pediatrics 2013;131(1):e152–61. [DOI] [PubMed] [Google Scholar]
- 8.Bryant GA, Koenigsfeld CF, Lehman NP, Smith HL, Logemann CD, Phillips KT. A retrospective evaluation of response to vitamin D supplementation in obese versus nonobese patients. J Pharm Pract 2015;28(6):543–7. [DOI] [PubMed] [Google Scholar]
- 9.Dhaliwal R, Mikhail M, Feuerman M, Aloia JF. The vitamin D dose response in obesity. Endocr Pract 2014;20(12):1258–64. [DOI] [PubMed] [Google Scholar]
- 10.Drincic A, Fuller E, Heaney RP, Armas LA. 25-Hydroxyvitamin D response to graded vitamin D(3) supplementation among obese adults. J Clin Endocrinol Metab 2013;98(12):4845–51. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher JC, Yalamanchili V, Smith LM. The effect of vitamin D supplementation on serum 25(OH)D in thin and obese women. J Steroid Biochem Mol Biol 2013;136:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irizar A, Barnett CR, Flatt PR, Ioannides C. Defective expression of cytochrome P450 proteins in the liver of the genetically obese Zucker rat. Eur J Pharmacol 1995;293(4):385–93. [DOI] [PubMed] [Google Scholar]
- 13.Watson AM, Poloyac SM, Howard G, Blouin RA. Effect of leptin on cytochrome P-450, conjugation, and antioxidant enzymes in the ob/ ob mouse. Drug Metab Dispos 1999;27(6):695–700. [PubMed] [Google Scholar]
- 14.Roseland JM, Patterson KY, Andrews KW, et al. Interlaboratory trial for measurement of vitamin D and 25-hydroxyvitamin D [25(OH) D] in foods and a dietary supplement using liquid chromatography–mass spectrometry. J Agric Food Chem 2016;64(16): 3167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larson-Meyer DE, Ingold BC, Fensterseifer SR, et al. Sun exposure in pigs increases the vitamin D nutritional quality of pork. PLoS One 2017;12(11):e0187877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richards Z, Batai K, Farhat R, et al. Prostatic compensation of the vitamin D axis in African American men. JCI Insight 2017;2(2): e91054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makowski AJ, Rathmacher JA, Horst RL, Sempos CT. Simplified 25-hydroxyvitamin D standardization and optimization in dried blood spots by LC-MS/MS. J AOAC Int 2017;100(5):1328–36. [DOI] [PubMed] [Google Scholar]
- 18.Pathak K, Soares MJ, Calton EK, Zhao Y, Hallett J. Vitamin D supplementation and body weight status: a systematic review and meta-analysis of randomized controlled trials. Obes Rev 2014; 15(6):528–37. [DOI] [PubMed] [Google Scholar]
- 19.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 2000; 72(3):690–3. [DOI] [PubMed] [Google Scholar]
- 20.Pramyothin P, Biancuzzo RM, Lu Z, Hess DT, Apovian CM, Holick MF. Vitamin D in adipose tissue and serum 25-hydroxyvitamin D after roux-en-Y gastric bypass. Obesity (Silver Spring) 2011;19(11): 2228–34. [DOI] [PubMed] [Google Scholar]
- 21.Michaud J, Naud J, Ouimet D, et al. Reduced hepatic synthesis of calcidiol in uremia. J Am Soc Nephrol 2010;21(9):1488–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarke SD. Polyunsaturated fatty acid regulation of gene transcription: a molecular mechanism to improve the metabolic syndrome. J Nutr 2001;131(4):1129–32. [DOI] [PubMed] [Google Scholar]
- 23.Finn RD, Henderson CJ, Scott CL, Wolf CR. Unsaturated fatty acid regulation of cytochrome P450 expression via a CAR-dependent pathway. Biochem J 2009;417(1):43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosen H, Reshef A, Maeda N, et al. Markedly reduced bile acid synthesis but maintained levels of cholesterol and vitamin D metabolites in mice with disrupted sterol 27-hydroxylase gene. J Biol Chem 1998;273(24):14805–12. [DOI] [PubMed] [Google Scholar]
- 25.Bruce-Keller AJ, Salbaum JM, Luo M, et al. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatr 2015;77(7):607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulz MD, Atay C, Heringer J, et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature 2014;514(7523):508–12. [DOI] [PMC free article] [PubMed] [Google Scholar]