Abstract
Increasing evidence suggests an important role for light in regulation of aging and longevity. UV radiation is a mutagen that can promote aging and decrease longevity. In contrast, NIR light has shown protective effects in animal disease models. In invertebrates, visible light can shorten or extend lifespan, depending on the intensity and wavelength composition. Visible light also impacts human health, including retina function, sleep, cancer and psychiatric disorders. Possible mechanisms of visible light include: controlling circadian rhythms, inducing oxidative stress, and acting through the retina to affect neuronal circuits and systems. Changes in artificial lighting (e.g., LEDs) may have implications for human health. It will be important to further explore the mechanisms of how light affects aging and longevity, and how light affects human health.
Keywords: aging, circadian rhythms, light, phototherapy, mitochondria, oxidative stress
1. Effects of invisible light on aging and longevity
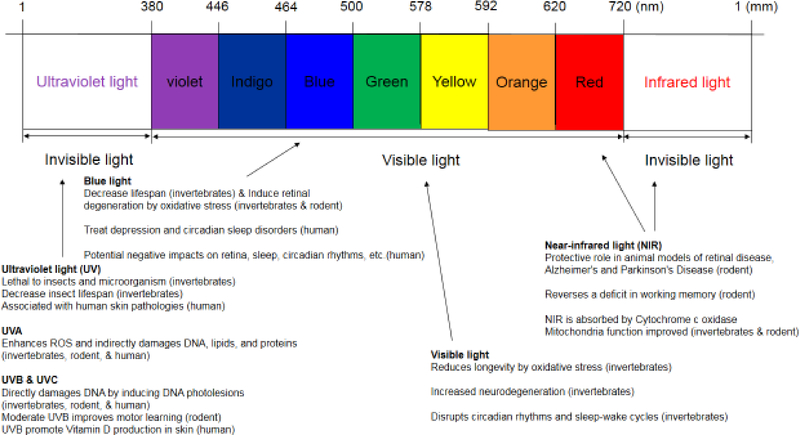
Light is an important environmental factor that affects living organisms, including regulation of circadian rhythms, metabolic rate and growth [Northrop 1925, Tucker, Petitclerc et al. 1984, Sheeba, Sharma et al. 2000, Vinogradova, Anisimov et al. 2009]. The wavelength of visible light is between 380 to 720 nm. Invisible light is divided into two types: infrared light with wavelength greater than 720 nm; and ultraviolet (UV) light with wavelength less than 380nm (Figure 1).
Figure 1:
The wavelengths of visible light, ultraviolet light, infrared light, and their effects on aging. As indicated in the figure, the wavelength of visible light is between 380 to 720 nm; ultraviolet light is less than 380 nm; infrared light is greater than 720 nm. The effects on aging of ultraviolet light (UV), near-infrared light (NIR), blue light, and visible light are listed briefly in the figure, and explained in further detail in the text.
1.1. UV light and aging
The effects of UV light on organisms have been extensively studied and well-reviewed [Amaro-Ortiz, Yan et al. 2014, Sanches Silveira and Myaki Pedroso 2014, Lapierre, Kumsta et al. 2015]. UV light is mainly found to be harmful, but has shown beneficial effects in recent studies when used at moderate dose.
In non-mammalian model organisms, researches on UV showed negative effects. UVB and UVC directly damage DNA, by inducing the formation of DNA photolesions, often resulting in mutations. The two major classes of DNA lesions produced by UVB and UVC are cis-syn cyclobutane pyrimidine dimers and pyrimidine (6–4) pyrimidone photoproducts. The transcriptional status of the sequences and the chromatin environment are important for the repair of these photoproducts[Pfeifer 1997]. The damaging effect of UVA possibly results from type II, oxygen-mediated photodynamic reactions, in which UVA enhances the production of reactive oxygen species (ROS) in the presence of photosensitizing chromophores such as nicotinamide adenine dinucleotide phosphate (NADPH), porphyrins or riboflavin [Dalle Carbonare and Pathak 1992]. In this way, the UVA-generated ROS damages DNA, lipids, and proteins [Santos, Oliveira et al. 2013]. The lethal effects of UV irradiation on insects [Beard 1972] and microorganisms[Reed 2010] are well known, and therefore UV irradiation is used for killing pests and pathogens. There are also reports that UV irradiation decreases adult longevity in insects [Chang-Yu, Zhang et al. 2011]. Administering high-energy UV light to C. elegans triggers an escape behavior, potentially allowing the animal to avoid further UV-mediated cellular damage [Ward, Liu et al. 2008], and also inhibits pharyngeal pumping [Bhatla and Horvitz 2015].
In mammals, UV showed both positive and negative effects. UV radiation is the environmental mutagen associated with the largest percentage of environmentally induced skin pathologies, such as erythema and inflammation, degenerative aging changes, and cancer [Elwood and Jopson 1997]. However, moderate UV exposure could be beneficial for human health. For example, UVB irradiation promotes the generation of vitamin D in the skin [Bouillon 2017]. There is also a recent study showing that moderate UVB exposure on mice improves motor learning and object recognition memory, by elevation of urocanic acid levels in blood and brain, and therefore promoting glutamate biosynthesis and release in specific brain regions [Zhu, Wang et al. 2018].
1.2. NIR and aging
Different from UV, the effect of near-infrared light (NIR) is beneficial. NIR has shown some protective effects in several animal models of retinal disease [Begum, Powner et al. 2013, Calaza, Kam et al. 2015], Alzheimer’s Disease and Parkinson’s Disease [Peoples, Spana et al. 2012, Johnstone, Moro et al. 2015], and can reverse the deficit in working memory in middle-aged mice [Michalikova, Ennaceur et al. 2008], possibly by improving mitochondrial function and ATP production [Begum, Calaza et al. 2015, Calaza, Kam et al. 2015]. Cytochrome c oxidase (COX), which is the terminal enzyme of the respiratory chain on the membrane of mitochondria, is identified as the photoacceptor of the NIR photobiomodulation effects. COX mediates the transfer of electrons from cytochrome c to molecular oxygen, and the reactivity of COX is due to four redox active metal centers: the binuclear CuA, CuB, heme a and heme a3. Analysis of action spectra of living cells in various experimental conditions showed that the peak profiles are similar to absorption spectra of redox metal centers of COX [Karu 2013]. When NIR is absorbed by COX, the redox state of COX changes, to cause the increase in ATP, decrease in both ROS and inflammation, and improved mitochondria function [Begum, Calaza et al. 2015]. NIR elements are produced by older domestic incandescent or halogen lighting (Figure 2, spectrum of LED vs halogen lights), but are not present in fluorescent lighting or LED lighting [Begum, Powner et al. 2013]. The change of artificial lighting may have significant impact on aging and health.
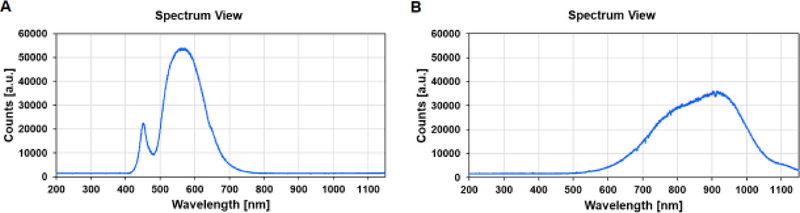
Figure 2:
Wavelength spectrum of LED and halogen lights.
Spectrum views measured by a spectroscope. The x axis represents the wavelength distribution of the light. The y axis “Counts (a.u.)” means arbitrary units of light intensity, which represents the relative intensity of light (relates to watt). A, Spectrum view of white LED light. B, Spectrum view of white halogen light.
In the indoor working environment, and in the animal culture research environment, fluorescent lighting and LED lighting are most common. Humans and research organisms are typically exposed to a mix of spectrum of visible light. In this review, we focus on the effects of visible light on aging and longevity.
2. Effects of visible light on aging and longevity
2.1. Visible light and aging
Visible light from ambient lighting can be toxic and reduce longevity. A recent study showed that visible light can reduce longevity in C. elegans [De Magalhaes Filho, Henriquez et al. 2018]. The effect of light was photon energy dependent, and lifespan was inversely correlated to the time that the C. elegans were exposed [De Magalhaes Filho, Henriquez et al. 2018]. Consistent with the result in C. elegans, our recent study on Drosophila also showed that visible light could reduce longevity [Shen, Zhu et al. 2018]. A previous analysis of different photoperiods showed that life span of Drosophila in constant light (LL) is significantly shorter when compared with alternating light-dark cycles (LD 12:12 h), and constant darkness (DD) [Massie, Aiello et al. 1993]. The recent study in C. elegans is consistent with those observations, showing longest life span under constant darkness (DD) condition but shortest life span under constant light (LL) condition [De Magalhaes Filho, Henriquez et al. 2018]. Further study of the mechanism in C. elegans showed that oxidative stress and unfolded-protein response, but not circadian rhythms, contribute to the reduction of lifespan, as antioxidants feeding could rescue the lifespan reduction caused by visible light [De Magalhaes Filho, Henriquez et al. 2018]. In Drosophila, oxidative stress is also a possible mechanism for the toxic effects of visible light. A previous study of photosensitizers and aging showed that the photosensitizer, methylene blue decreased lifespan, suggesting that photodynamic action, in which the energy of visible light is transferred to the photosensitizer and then in turn transferred to molecular oxygen, may be the mechanism [Massie, Aiello et al. 1993]. In a fly model of Alzheimer’s disease, exposure to dim light of 10 lux at night, which is considered the lower limit of light pollution, increased phosphorylated Tau protein abundance and neurodegeneration, and disrupted circadian rhythms and sleep-wake cycles [Kim, Subramanian et al. 2018]. Studies in rodents showed similar toxic effect of visible light. Rats exposed to 90 days’ continuous bright light from a fluorescent lamp showed increased numbers of neuromelanin-positive neurons, decreased TH-positive neurons in the substantia nigra (SN) region, and reduction of dopamine levels [Romeo, Viaggi et al. 2013]. Consistent with that observation, three months’ fluorescent light exposure to mice caused reduction of dopamine neuron numbers, and reduced levels of dopamine [Romeo, Vitale et al. 2017]. A possible mechanism is that light exposure induced dopamine oxidation which in turn led to increased apoptosis.
2.2. Blue light and aging
Blue light (400–500 nm, short-wavelength visible light) has shown toxic effects that can contribute to aging. Blue light significantly decreased adult longevity of Drosophila melanogaster [Hori, Shibuya et al. 2014]. The effects of blue light were photon-dose dependent, and flies kept under dark conditions showed the longest mean lifespan. The wavelength of blue light with most toxicity relies on the developmental stage and photon dose [Shibuya, Onodera et al. 2018]. The toxic effects of blue light may be caused by ROS and oxidative stress [Hori, Shibuya et al. 2014, Shibuya, Onodera et al. 2018]. Blue light exposure could induce lipid peroxidation which contributed to retinal degeneration in -6-day-old Drosophila melanogaster. The membranes of photoreceptors in the eye are rich in peroxidation-susceptible polyunsaturated fatty acids, and overexpression of cytochrome b5 (Cyt-b5) prevented lipid peroxidation and retinal degeneration caused by blue light. The possibly mechanism could be that Cyt-b5 enhance the regeneration of antioxidant coenzyme Q in the cells and thus promotes the recycling of vitamins C and E [Chen, Hall et al. 2017].
2.3. Long-wavelength visible light and aging
On the other hand, long-wavelength visible light has shown beneficial effect on aging. Long-term exposure to 670 nm in Drosophila melanogaster increased ATP levels, reduced inflammation, and significantly increased mean lifespan and mobility in old age [Begum, Calaza et al. 2015]. Old flies exposed to 670 nm daily for one week showed significantly improved retinal function, cognitive ability and mobility. The beneficial effect of 670nm light appears to be mediated by improved mitochondria function, as indicated by increased ATP levels, mitochondrial DNA content, cytochrome c oxidase activity, and whole body energy storage [Weinrich, Coyne et al. 2017].
3. Effects of visible light on human health
3.1. Mechanisms for adaptation to light in humans
Human adaptation to ambient light has two mechanisms: image forming (IF) for vision, and non-image forming (NIF). NIF functions are mediated by retinal ganglion cells (RGCs) expressing the photopigment melanopsin, and the action spectrum of melanopsin photopigment peaks around 480 nm blue light [Hatori, Gronfier et al. 2017]. Action spectrum shows which wavelength of light is most effectively used in a physiological reaction, by plotting the rate of the reaction versus input wavelength [Karu 2013]. By testing phototransduction sensitivity to monochromatic stimuli at different wavelengths, the action spectrum of melanopsin was measured with peak sensitivity around 480nm. The action spectrum of RGCs, which contains melanopsin, also peaks around 480 nm, and is consistent with peak sensitivity of NIF photoresponses such as circadian photoentrainment. The action spectrum is different from the excitation spectrum, which shows the relationship of the substance’s radiation wavelength with the external excitation light wavelength. NIF responses vary widely from pupil diameter adjustment to adaptation of the circadian clock. Therefore, NIF is important in aging and age-related disorders, by regulation of circadian rhythmicity.
Since the invention of the incandescent light bulb in the 19th century, halogen light bulbs and fluorescent lamps in the 20th century, and light-emitting diode (LED) in the 21st century, the easy access to light changed human lifestyle. The revolution of LED lighting saves energy, but also delivers a much higher amount of blue light compared to conventional light sources [Hatori, Gronfier et al. 2017].
3.2. The beneficial and harmful effects of blue light
Blue light has negative effects on human health but can also be used to treat human diseases when used at appropriate dose. Moderate blue light may be beneficial to humans and work as an effective “bright light therapy” in treating depression and circadian sleep disorders [Czeisler 2013, Lam, Levitt et al. 2016]. But there are also concerns about the negative effects of blue-rich light on human health, such as potential negative impacts on the retina, sleep, non-visual/circadian biology, cancers, obesity, diabetes, and psychiatric disorders [Stevens, Brainard et al. 2013, Bonmati-Carrion, Arguelles-Prieto et al. 2014].
3.3. The amount of sunlight and human diseases
A lack or increase of sunlight is correlated with certain diseases. For example, while the etiology of multiple sclerosis (MS) is not well known, epidemiologists have observed an increased prevalence of MS in countries at high latitudes with limited sunlight [Ghareghani, Reiter et al. 2018]. There is also a study showing that UV exposure can reduce MS risk [Gallagher, Ilango et al. 2018]. Seasonal affective disorder, a form of depression, shows a high frequency in high latitudes where light availability is decreased and therefore the amplitude and phase of circadian rhythms is blunted. Bright light therapy is a successful treatment to seasonal affective disorder due to limited light availability [Duncan 1996, Gonzalez and Aston-Jones 2008]. Sunlight may associate with suicide, possibly by interacting with serotonin neurotransmission [Vyssoki, Praschak-Rieder et al. 2012, Makris, Reutfors et al. 2016]. Increase in sunlight was found to be inversely related with the age of onset of bipolar disorder [Bauer, Glenn et al. 2014].
4. Possible mechanisms of light effects on aging
4.1. Circadian rhythm disturbance
Changes in circadian rhythms may explain the effect of light on aging. One mechanism of the light function is to act as a zeitgeber (time giver), to control the circadian rhythms, which are endogenous rhythms of about 24 hours. With circadian rhythms, organisms can synchronize their physiology with the daily light-dark phase change of Earth’s rotation. Although light is a zeitgeber to circadian rhythms, in constant darkness (free running conditions), the rhythms still exist and can be reset by exposure to external signals including light. In humans, night time exposure to light could cause disturbances in circadian rhythms. Night shift work or chronic jet lag experience may have a negative effect on obesity, diabetes, and cancer incidence [Haus and Smolensky 2013, Zelinski, Deibel et al. 2014, West and Bechtold 2015]. In addition, disruption of the circadian clock may accelerate physiological aging. In mice, the null mutation in the core clock gene Bmal1 (homolog of cyc) significantly shortened lifespan [Kondratov, Kondratova et al. 2006]. Disruption of the core clock genes has also been shown to affect the lifespan of Drosophila. In per mutants, Drosophila lifespan was reduced [Klarsfeld and Rouyer 1998]. In cyc-null male but not female flies, shortened lifespan was also observed [Hendricks, Lu et al. 2003]. Overexpression of tim in peripheral tissues extended lifespan under ad libitum conditions, by increasing fat metabolism [Katewa, Akagi et al. 2016]. In addition, per-null mutants also showed impaired health span, as indicated by reduced oxidative stress resistance and climbing ability, higher oxidatively damaged proteins and lipids [Krishnan, Kretzschmar et al. 2009].
4.2. Oxidative stress induction by light
Another mechanism of light is to cause oxidative stress which is circadian rhythm-independent. Light was found to drive the expression of a large number of non-circadian genes. These genes are not true circadian genes because they do not continue rhythmic cycle changes in constant darkness (free running conditions) [van der Linden, Beverly et al. 2010]. As discussed above, a recent study in C. elegans showed that the reduction of lifespan by visible light is due to oxidative stress, and antioxidants can rescue the shortened lifespan [De Magalhaes Filho, Henriquez et al. 2018]. Other studies also support that light induces oxidative stress and damages lipids, proteins and DNA [Kuse, Ogawa et al. 2014, Chen, Hall et al. 2017]. Mammalian hepatocytes under visible light exposure showed photodamage, such as inactivation of catalase and inactivation of lysosomal enzymes [Cheng and Packer 1979]. Even light sources with limited UV or blue light (short wavelength) components could cause significant oxidative stress. Incandescent light (which lacks UV and most of the blue wavelengths) can produce pyrimidine dimers in DNA [Ciarrocchi, Sutherland et al. 1985]. Three hours of fluorescent light at 1000 lux can induce significant DNA strand breaks in mammalian cells [Bradley, Erickson et al. 1978].
4.3. Signal transduction through the visual system
Finally, light may function through the retina to affect neuronal circuits and systems. Blue light (short wavelength) has the highest photon energy in the visible spectrum, and can cause retinal damage, called “blue light hazard” [Buczylko, Saari et al. 1996, Maeda, Maeda et al. 2009, Chen, Okano et al. 2012, Maeda, Golczak et al. 2012, Zhong, Kawaguchi et al. 2012]. A study in mice showed that blocking blue light protects the retina from light damage [Narimatsu, Ozawa et al. 2014]. The photoreceptor chromophore retinal can enter the circulation, and therefore, retinal can confer light sensitivity to both visual and non-visual cells. Retinal can induce distortions in PIP2, cause oxidative stress, and cause increase in cytosolic calcium levels [Ratnayake, Payton et al. 2018]. In aged flies, phototaxis and oscillation of electroretinograms (ERGs) are greatly reduced [Ueda, Woods et al. 2018]. In the aging process, the damage of the retina by light could be cumulative, and lead to a decline in neuronal excitability and synaptic transmission.
5. Discussion and Conclusions
Light exposure is an environmental factor that significantly impacts aging and longevity. Light intensity, spectral components, and duration of exposure, are all important factors to consider when conducting animal experiments. Light conditions should be carefully controlled to avoid potential conflicts between different studies.
There is no uniform measurement unit for intensity of light in research papers. Lux, photons, and Watts are all used in published research studies. The commonly used “lux” indicates the intensity of light perceived by human eyes, which are most sensitive to yellow and green light, even if the radiant energy of each color is equal. Therefore, lux can not accurately indicate the absolute energy of light, such as high energy blue light. For example, use of blue light in Seasonal Affective Disorder (SAD) therapy according to protocols which use lux to determine the light intensity, would be an over-dose [Anderson, Glod et al. 2009]. Photon flux(photons/cm2/sec) is another unit for light measurement. The photochemical reaction of photosynthesis is closely related to the number of absorbed light photons. For example, a photon of blue light has about twice the energy of a red light photon, but the photons of these two colors have the same effect in photosynthesis. Therefore, photon flux is commonly used to describe photosynthetically active radiation (400–700 nm) [Thimijan 1983]. Watt reflects the total absolute radiated power of all electromagnetic radiation (including infrared, ultraviolet, and visible light) passing through a certain area per unit time. Therefore, watt would be the unit to make comparisons between different studies easier, although the intensities with different units can be converted by equations [Enezi, Revell et al. 2011]. It would be helpful to form a consensus on standard lighting conditions for aging experiments in the future.
It will also be important to further explore the mechanisms of how light affects aging and longevity. With the emergence of new lighting technologies, especially light-emitting diode (LED), future research needs to focus on the impact of different light sources on human health and disease. The future of research on light and aging could be:
What are the effects of light of various wavelengths and intensities on aging and longevity?
How do circadian rhythms interact with the effect of light on aging?
What are the detailed mechanisms of oxidative stress in response to light? How could the harmful effects be prevented?
What is the role of the retina in the different effects of light?
Does the microbiome correlate with the effects of light at specific wavelengths?
What light interventions can be developed to help treat aging-related diseases, such as AD and PD?
Would any dietary component (e.g. protein) or dietary supplement (e.g. photo-sensitizing substance) enhance the beneficial effect or reduce the toxic effect of light?
In conclusion, light is a ubiquitous and important regulator that affects aging. Further research on the detailed mechanisms of light would be helpful for researchers to set optimal consensus lighting conditions for research, and to develop noninvasive lighting therapies for aging-related diseases.
Highlights.
Light is important in regulation of aging and longevity.
Visible light may control circadian rhythms, induce oxidative stress, or act through the retina.
Changes in artificial lighting may have significant impact on aging and health.
It will be important to explore the mechanisms of how light affects aging and health.
Acknowledgements
The authors declare no conflicts of interest. This work was supported by grants to J.S. (National Natural Science Foundation of China, 31500970) and to J.T. (NIH/NIA AG057741).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaro-Ortiz A, Yan B and D’Orazio JA (2014). “Ultraviolet radiation, aging and the skin: prevention of damage by topical cAMP manipulation.” Molecules 19(5): 6202–6219. DOI: 10.3390/molecules19056202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JL, Glod CA, Dai J, et al. (2009). “Lux vs. wavelength in light treatment of Seasonal Affective Disorder.” Acta Psychiatr Scand 120(3): 203–212. DOI: 10.1111/j.1600-0447.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- Bauer M, Glenn T, Alda M, et al. (2014). “Relationship between sunlight and the age of onset of bipolar disorder: an international multisite study.” J Affect Disord 167: 104–111. DOI: 10.1016/j.jad.2014.05.032. [DOI] [PubMed] [Google Scholar]
- Beard RL (1972). “Lethal action of UV irradiation on insects.” J Econ Entomol 65(3): 650–654. [DOI] [PubMed] [Google Scholar]
- Begum R, Calaza K, Kam JH, et al. (2015). “Near-infrared light increases ATP, extends lifespan and improves mobility in aged Drosophila melanogaster.” Biol Lett 11(3). DOI: 10.1098/rsbl.2015.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum R, Powner MB, Hudson N, et al. (2013). “Treatment with 670 nm light up regulates cytochrome C oxidase expression and reduces inflammation in an age-related macular degeneration model.” PLoS One 8(2): e57828 DOI: 10.1371/journal.pone.0057828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatla N and Horvitz HR (2015). “Light and hydrogen peroxide inhibit C. elegans Feeding through gustatory receptor orthologs and pharyngeal neurons.” Neuron 85(4): 804–818. DOI: 10.1016/j.neuron.2014.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonmati-Carrion MA, Arguelles-Prieto R, Martinez-Madrid MJ, et al. (2014). “Protecting the melatonin rhythm through circadian healthy light exposure.” Int J Mol Sci 15(12): 23448–23500. DOI: 10.3390/ijms151223448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillon R (2017). “Comparative analysis of nutritional guidelines for vitamin D.” Nat Rev Endocrinol 13(8): 466–479. DOI: 10.1038/nrendo.2017.31. [DOI] [PubMed] [Google Scholar]
- Bradley MO, Erickson LC and Kohn KW (1978). “Non-enzymatic DNA strand breaks induced in mammalian cells by fluorescent light.” Biochim Biophys Acta 520(1): 11–20. [DOI] [PubMed] [Google Scholar]
- Buczylko J, Saari JC, Crouch RK, et al. (1996). “Mechanisms of opsin activation.” J Biol Chem 271(34): 20621–20630. [DOI] [PubMed] [Google Scholar]
- Calaza KC, Kam JH, Hogg C, et al. (2015). “Mitochondrial decline precedes phenotype development in the complement factor H mouse model of retinal degeneration but can be corrected by near infrared light.” Neurobiol Aging 36(10): 2869–2876. DOI: 10.1016/j.neurobiolaging.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Chang-Yu Zhang, Jian-Yu, et al. (2011). “Effects of UV-A exposures on longevity and reproduction in Helicoverpa armigera, and on the development of its F1 generation.” Insect Science 18(6): 697–702. [Google Scholar]
- Chen X, Hall H, Simpson JP, et al. (2017). “Cytochrome b5 protects photoreceptors from light stress-induced lipid peroxidation and retinal degeneration.” NPJ Aging Mech Dis 3: 18 DOI: 10.1038/s41514-017-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Okano K, Maeda T, et al. (2012). “Mechanism of all-trans-retinal toxicity with implications for stargardt disease and age-related macular degeneration.” J Biol Chem 287(7): 5059–5069. DOI: 10.1074/jbc.M111.315432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng LY and Packer L (1979). “Photodamage to hepatocytes by visible light.” FEBS Lett 97(1): 124–128. [DOI] [PubMed] [Google Scholar]
- Ciarrocchi G, Sutherland BM and Sutherland JC (1985). “Incandescent lamps can produce pyrimidine dimers in DNA.” Photochem Photobiol 41(6): 703–705. [DOI] [PubMed] [Google Scholar]
- Czeisler CA (2013). “Perspective: casting light on sleep deficiency.” Nature 497(7450): S13 DOI: 10.1038/497S13a. [DOI] [PubMed] [Google Scholar]
- Dalle Carbonare M and Pathak MA (1992). “Skin photosensitizing agents and the role of reactive oxygen species in photoaging.” J Photochem Photobiol B 14(1–2): 105–124. [DOI] [PubMed] [Google Scholar]
- De Magalhaes Filho CD, Henriquez B, Seah NE, et al. (2018). “Visible light reduces C. elegans longevity.” Nat Commun 9(1): 927 DOI: 10.1038/s41467-018-02934-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan WC Jr. (1996). “Circadian rhythms and the pharmacology of affective illness.” Pharmacol Ther 71(3): 253–312. [DOI] [PubMed] [Google Scholar]
- Elwood JM and Jopson J (1997). “Melanoma and sun exposure: an overview of published studies.” Int J Cancer 73(2): 198–203. [DOI] [PubMed] [Google Scholar]
- Enezi J, Revell V, Brown T, et al. (2011). “A “melanopic” spectral efficiency function predicts the sensitivity of melanopsin photoreceptors to polychromatic lights.” J Biol Rhythms 26(4): 314–323. DOI: 10.1177/0748730411409719. [DOI] [PubMed] [Google Scholar]
- Gallagher LG, Ilango S, Wundes A, et al. (2018). “Lifetime exposure to ultraviolet radiation and the risk of multiple sclerosis in the US radiologic technologists cohort study.” Mult Scler: 1352458518783343 DOI: 10.1177/1352458518783343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghareghani M, Reiter RJ, Zibara K, et al. (2018). “Latitude, Vitamin D, Melatonin, and Gut Microbiota Act in Concert to Initiate Multiple Sclerosis: A New Mechanistic Pathway.” Front Immunol 9: 2484 DOI: 10.3389/fimmu.2018.02484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez MM and Aston-Jones G (2008). “Light deprivation damages monoamine neurons and produces a depressive behavioral phenotype in rats.” Proc Natl Acad Sci U S A 105(12): 4898–4903. DOI: 10.1073/pnas.0703615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Gronfier C, Van Gelder RN, et al. (2017). “Global rise of potential health hazards caused by blue light-induced circadian disruption in modern aging societies.” NPJ Aging Mech Dis 3: 9 DOI: 10.1038/s41514-017-0010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haus EL and Smolensky MH (2013). “Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation.” Sleep Med Rev 17(4): 273–284. DOI: 10.1016/j.smrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Hendricks JC, Lu S, Kume K, et al. (2003). “Gender dimorphism in the role of cycle (BMAL1) in rest, rest regulation, and longevity in Drosophila melanogaster.” J Biol Rhythms 18(1): 12–25. DOI: 10.1177/0748730402239673. [DOI] [PubMed] [Google Scholar]
- Hori M, Shibuya K, Sato M, et al. (2014). “Lethal effects of short-wavelength visible light on insects.” Sci Rep 4: 7383 DOI: 10.1038/srep07383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone DM, Moro C, Stone J, et al. (2015). “Turning On Lights to Stop Neurodegeneration: The Potential of Near Infrared Light Therapy in Alzheimer’s and Parkinson’s Disease.” Front Neurosci 9: 500 DOI: 10.3389/fnins.2015.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karu TI (2013). “Cellular and Molecular Mechanisms of Photobiomodulation (Low-Power Laser Therapy).” IEEE Journal of Selected Topics in Quantum Electronics 20(2): 143–148. [Google Scholar]
- Katewa SD, Akagi K, Bose N, et al. (2016). “Peripheral Circadian Clocks Mediate Dietary Restriction-Dependent Changes in Lifespan and Fat Metabolism in Drosophila.” Cell Metab 23(1): 143–154. DOI: 10.1016/j.cmet.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Subramanian M, Cho YH, et al. (2018). “Short-term exposure to dim light at night disrupts rhythmic behaviors and causes neurodegeneration in fly models of tauopathy and Alzheimer’s disease.” Biochem Biophys Res Commun 495(2): 1722–1729. DOI: 10.1016/j.bbrc.2017.12.021. [DOI] [PubMed] [Google Scholar]
- Klarsfeld A and Rouyer F (1998). “Effects of circadian mutations and LD periodicity on the life span of Drosophila melanogaster.” J Biol Rhythms 13(6): 471–478. DOI: 10.1177/074873098129000309. [DOI] [PubMed] [Google Scholar]
- Kondratov RV, Kondratova AA, Gorbacheva VY, et al. (2006). “Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock.” Genes Dev 20(14): 1868–1873. DOI: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan N, Kretzschmar D, Rakshit K, et al. (2009). “The circadian clock gene period extends healthspan in aging Drosophila melanogaster.” Aging (Albany NY) 1(11): 937–948. DOI: 10.18632/aging.100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuse Y, Ogawa K, Tsuruma K, et al. (2014). “Damage of photoreceptor-derived cells in culture induced by light emitting diode-derived blue light.” Sci Rep 4: 5223 DOI: 10.1038/srep05223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam RW, Levitt AJ, Levitan RD, et al. (2016). “Efficacy of Bright Light Treatment, Fluoxetine, and the Combination in Patients With Nonseasonal Major Depressive Disorder: A Randomized Clinical Trial.” JAMA Psychiatry 73(1): 56–63. DOI: 10.1001/jamapsychiatry.2015.2235. [DOI] [PubMed] [Google Scholar]
- Lapierre LR, Kumsta C, Sandri M, et al. (2015). “Transcriptional and epigenetic regulation of autophagy in aging.” Autophagy 11(6): 867–880. DOI: 10.1080/15548627.2015.1034410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A, Maeda T, Golczak M, et al. (2009). “Involvement of all-trans-retinal in acute light-induced retinopathy of mice.” J Biol Chem 284(22): 15173–15183. DOI: 10.1074/jbc.M900322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Golczak M and Maeda A (2012). “Retinal photodamage mediated by all-trans-retinal.” Photochem Photobiol 88(6): 1309–1319. DOI: 10.1111/j.1751-1097.2012.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris GD, Reutfors J, Larsson R, et al. (2016). “Serotonergic medication enhances the association between suicide and sunshine.” J Affect Disord 189: 276–281. DOI: 10.1016/j.jad.2015.09.056. [DOI] [PubMed] [Google Scholar]
- Massie HR, Aiello VR and Williams TR (1993). “Influence of photosensitizers and light on the life span of Drosophila.” Mech Ageing Dev 68(1–3): 175–182. [DOI] [PubMed] [Google Scholar]
- Michalikova S, Ennaceur A, van Rensburg R, et al. (2008). “Emotional responses and memory performance of middle-aged CD1 mice in a 3D maze: effects of low infrared light.” Neurobiol Learn Mem 89(4): 480–488. DOI: 10.1016/j.nlm.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Narimatsu T, Ozawa Y, Miyake S, et al. (2014). “Biological effects of blocking blue and other visible light on the mouse retina.” Clin Exp Ophthalmol 42(6): 555–563. DOI: 10.1111/ceo.12253. [DOI] [PubMed] [Google Scholar]
- Northrop JH (1925). “The Influence of the Intensity of Light on the Rate of Growth and Duration of Life of Drosophila.” J Gen Physiol 9(1): 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples C, Spana S, Ashkan K, et al. (2012). “Photobiomodulation enhances nigral dopaminergic cell survival in a chronic MPTP mouse model of Parkinson’s disease.” Parkinsonism Relat Disord 18(5): 469–476. DOI: 10.1016/j.parkreldis.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Pfeifer GP (1997). “Formation and processing of UV photoproducts: effects of DNA sequence and chromatin environment.” Photochem Photobiol 65(2): 270–283. [DOI] [PubMed] [Google Scholar]
- Ratnayake K, Payton JL, Lakmal OH, et al. (2018). “Blue light excited retinal intercepts cellular signaling.” Sci Rep 8(1): 10207 DOI: 10.1038/s41598-018-28254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed NG (2010). “The history of ultraviolet germicidal irradiation for air disinfection.” Public Health Rep 125(1): 15–27. DOI: 10.1177/003335491012500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo S, Viaggi C, Di Camillo D, et al. (2013). “Bright light exposure reduces TH-positive dopamine neurons: implications of light pollution in Parkinson’s disease epidemiology.” Sci Rep 3: 1395 DOI: 10.1038/srep01395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo S, Vitale F, Viaggi C, et al. (2017). “Fluorescent light induces neurodegeneration in the rodent nigrostriatal system but near infrared LED light does not.” Brain Res 1662: 87–101. DOI: 10.1016/j.brainres.2017.02.026. [DOI] [PubMed] [Google Scholar]
- Sanches Silveira JE and Myaki Pedroso DM (2014). “UV light and skin aging.” Rev Environ Health 29(3): 243–254. DOI: 10.1515/reveh-2014-0058. [DOI] [PubMed] [Google Scholar]
- Santos AL, Oliveira V, Baptista I, et al. (2013). “Wavelength dependence of biological damage induced by UV radiation on bacteria.” Arch Microbiol 195(1): 63–74. DOI: 10.1007/s00203-012-0847-5. [DOI] [PubMed] [Google Scholar]
- Sheeba V, Sharma VK, Shubha K, et al. (2000). “The effect of different light regimes on adult life span in Drosophila melanogaster is partly mediated through reproductive output.” J Biol Rhythms 15(5): 380–392. DOI: 10.1177/074873000129001477. [DOI] [PubMed] [Google Scholar]
- Shen J, Zhu X, Gu Y, et al. (2018). “Toxic effect of visible light on Drosophila lifespan depending upon diet protein content.” J Gerontol A Biol Sci Med Sci. DOI: 10.1093/gerona/gly042. [DOI] [PubMed] [Google Scholar]
- Shibuya K, Onodera S and Hori M (2018). “Toxic wavelength of blue light changes as insects grow.” PLoS One 13(6): e0199266 DOI: 10.1371/journal.pone.0199266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RG, Brainard GC, Blask DE, et al. (2013). “Adverse health effects of nighttime lighting: comments on American Medical Association policy statement.” Am J Prev Med 45(3): 343–346. DOI: 10.1016/j.amepre.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Thimijan RW (1983). “Photometric, radiometric, and quantum light units of measure-a review of procedures for interconversion.” Hortscience 18: 818–822. [Google Scholar]
- Tucker HA, Petitclerc D and Zinn SA (1984). “The influence of photoperiod on body weight gain, body composition, nutrient intake and hormone secretion.” J Anim Sci 59(6): 1610–1620. [DOI] [PubMed] [Google Scholar]
- Ueda A, Woods S, McElree I, et al. (2018). “Two novel forms of ERG oscillation in Drosophila: age and activity dependence.” J Neurogenet 32(2): 118–126. DOI: 10.1080/01677063.2018.1461866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden AM, Beverly M, Kadener S, et al. (2010). “Genome-wide analysis of light- and temperature-entrained circadian transcripts in Caenorhabditis elegans.” PLoS Biol 8(10): e1000503 DOI: 10.1371/journal.pbio.1000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova IA, Anisimov VN, Bukalev AV, et al. (2009). “Circadian disruption induced by light-at-night accelerates aging and promotes tumorigenesis in rats.” Aging (Albany NY) 1(10): 855–865. DOI: 10.18632/aging.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyssoki B, Praschak-Rieder N, Sonneck G, et al. (2012). “Effects of sunshine on suicide rates.” Compr Psychiatry 53(5): 535–539. DOI: 10.1016/j.comppsych.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Ward A, Liu J, Feng Z, et al. (2008). “Light-sensitive neurons and channels mediate phototaxis in C. elegans.” Nat Neurosci 11(8): 916–922. DOI: 10.1038/nn.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrich TW, Coyne A, Salt TE, et al. (2017). “Improving mitochondrial function significantly reduces metabolic, visual, motor and cognitive decline in aged Drosophila melanogaster.” Neurobiol Aging 60: 34–43. DOI: 10.1016/j.neurobiolaging.2017.08.016. [DOI] [PubMed] [Google Scholar]
- West AC and Bechtold DA (2015). “The cost of circadian desynchrony: Evidence, insights and open questions.” Bioessays 37(7): 777–788. DOI: 10.1002/bies.201400173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinski EL, Deibel SH and McDonald RJ (2014). “The trouble with circadian clock dysfunction: multiple deleterious effects on the brain and body.” Neurosci Biobehav Rev 40: 80–101. DOI: 10.1016/j.neubiorev.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Zhong M, Kawaguchi R, Kassai M, et al. (2012). “Retina, retinol, retinal and the natural history of vitamin A as a light sensor.” Nutrients 4(12): 2069–2096. DOI: 10.3390/nu4122069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Wang N, Yao L, et al. (2018). “Moderate UV Exposure Enhances Learning and Memory by Promoting a Novel Glutamate Biosynthetic Pathway in the Brain.” Cell 173(7): 1716–1727 e1717. DOI: 10.1016/j.cell.2018.04.014. [DOI] [PubMed] [Google Scholar]