Abstract
Bone tissue engineering utilizes three critical elements – cells, scaffolds, and bioactive factors – to recapitulate the bone tissue microenvironment, inducing the formation of new bone. Recent advances in materials development have enabled the production of scaffolds that more effectively mimic the hierarchical features of bone matrix, ranging from molecular composition to nano/micro-scale biochemical and physical features. This review summarizes recent advances within the field in utilizing these features of native bone to guide the hierarchical design of materials and scaffolds. Biomimetic strategies discussed in this review cover several levels of hierarchical design, including the development of element-doped compositions of hydroxyapatite, the usage of molecular templates for in vitro biomineralization at the nanoscale, the fabrication of biomimetic scaffold architecture at the micro- and nanoscale, and the application of external physical stimuli at the macroscale to regulate bone growth. Developments at each level are discussed with an emphasis on their in vitro and in vivo outcomes in promoting osteogenic tissue development. Ultimately, these hierarchically designed scaffolds can complement or even replace the usage of cells and biological elements, which present clinical and regulatory barriers to translation. As the field progresses ever closer to clinical translation, the creation of viable therapies will thus benefit from further development of hierarchically designed materials and scaffolds.
Keywords: Biomimetic, Hierarchical, Bone, Scaffold, Nanoscale, Architecture
1. Introduction
Biomaterial scaffolds play a critical role in bone tissue engineering by serving as extracellular matrices that can present both chemical and physical cues for cell attachment, proliferation and differentiation towards the development of new bone tissue. An ideal scaffold for bone tissue engineering should be not only osteoconductive to facilitate bone cell attachment, proliferation and migration, but also osteoinductive to stimulate the differentiation of osteoprogenitor cells into osteoblasts [1, 2]. Furthermore, an ideal bone scaffold should promote vascularization to actively support nutrient and oxygen transport for the new-formed bone tissue [3–5]. While the inclusion of cells and biological components like growth factors can often help fulfill these requirements, the selection of scaffold materials and design of scaffold architecture have played a critical and increasingly complex role in promoting bone regeneration by providing mimicry of the native bone matrix. This review thus focuses on the latest advancements in biomimetic scaffold fabrication provided by material selection and structural design in particular.
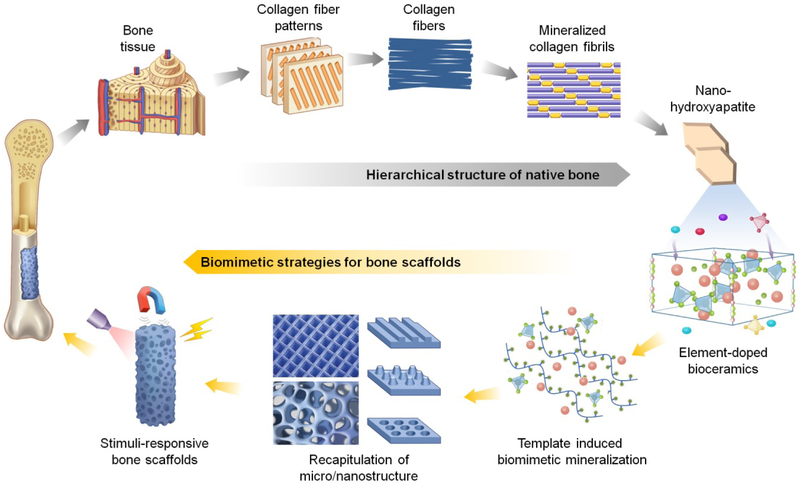
The hierarchical structure of bone matrix has provided important bioinspired motifs for biomaterial and scaffold design. Natural bone consists of compact cortical bone and trabecular cancellous bone, which are composed of densely packed cylindrical osteons and porous network of trabeculae, respectively. Osteons and trabeculae are made up of lamellae with different collagen fiber patterns. The collagen fibers are composed of bundles of mineralized collagen fibrils, and plate-like hydroxyapatite (HA) nanocrystals are deposited in the gaps between collagen molecules within the collagen fibrils (Figure 1) [6]. To recapitulate native chemical and physical cues, tissue engineers have designed scaffolds that are biomimetic at various scales of bone tissue hierarchy, ranging all the way from molecular composition to extracellular micro/nanostructure, as well as response to environmental stimuli.
Figure 1.
The hierarchical structure of bone from macroscale to molecular composition (gray arrows) and the biomimetic construction of bone biomaterials and scaffolds from molecular composition to macroscale (yellow arrows). Inspired by the native features of bone, multi-scaled biomimetic strategies of bone scaffolds have been developed for superior osteogenic properties – these strategies include the usage of element-doped bioceramics, template-induced biomimetic mineralization, recapitulation of extracellular micro/nanostructure, and the development of stimuli-responsive bone scaffolds.
One target for biomimicry is the molecular composition of native bone extracellular matrix, which is comprised of both inorganic components – primarily hydroxyapatite (HA) – as well as organic components – predominantly type-I collagen (COL I). Synthetic HA (Ca10(PO4)6(OH)2) and other bioceramics have thus been widely utilized as bone substitutes due to their good osteoconductivity [7, 8]. Given that trace amounts of foreign ions (CO32−, F−, Mg2+, Zn2+, etc) in biological HA are important to physiological bone health [9], the elemental doping of these ions in synthetic HA or other bioceramics has been pursued to more effectively mimic the native mineral content of bone and thus enhance osteoinduction and other biological functions.
Another target for biomimicry is the organized assembly of HA and collagen within native bone ECM, which is responsible for the strong mechanical properties of native bone [10]. Composite materials of HA and collagen or other natural/synthetic polymers are therefore widely used to improve the inherent brittleness and low fracture toughness of HA, which limit its use under load bearing conditions [11, 12]. However, commonly used composites are simply made by directly mixing HA with polymers, and fail to replicate the nanostructure of mineralized collagen in native bone. On the nanoscale, HA crystals are deposited in the gaps between collagen molecules within the collagen fibrils [13]. Molecular template induced biomineralization has thus been utilized to generate mineralized nanocomposites such as collagen/nano-HA that mimic this organization of bone ECM [14, 15].
Additionally, on both the nanometer and micron scales, one can replicate the unique topological features (such as surface roughness and fiber alignment) and internal pore structure of bone. The ability of tissue engineers to mimic native nano-/microstructure has advanced in particular due to developments in nanotechnology that enable generation of nanoscale structures [16] and advances in 3D printing technologies that allow for the production of sophisticated 3D features [17]. By simulating such nano-/microarchitectural features, one can regulate cell behaviors such as adhesion, migration, proliferation, and differentiation, and thereby influence bone formation [18–20].
Lastly, studies have found that in addition to biochemical cues, environmental physical cues like mechanical, electrical, and magnetic stimuli can affect cell fate and drive bone tissue regeneration [21–24]. For example, bone tissue is piezoelectric and the charges/potentials it generates in response to mechanical activity are capable of enhancing bone growth [21]. External applied electrical stimulation and electromagnetic fields have also been used clinically as a supplement to promote bone healing [22, 25]. New strategies that combine externally applied physical stimuli with the intrinsic cues of material and scaffold systems have thus be used to synergistically regulate bone regeneration.
This review attempts to systematically outline how biomimetic bone scaffolds have been designed at different hierarchical scales – all the way from molecular composition to macroscale – covering approaches that range from biomimetic material synthesis to the fabrication of structural features and the usage of external stimuli to modulate bone growth. In the following sections, we will focus on the latest advances in the aforementioned biomimetic strategies – namely, functional element doping of bioceramics at the level of molecular composition, template induced biomimetic mineralization at the nanoscale, recapitulation of ECM structural features at the micro-/nanoscale, and usage of external physical stimuli with stimuli-responsive scaffolds at the macroscale (Figure 1). The related in vitro cell responses and in vivo bone regeneration produced by these strategies will be highlighted. By summarizing recent advances in these areas, we attempt to point out some of the current key issues and future directions for biomimetic material and scaffold design, in the context of bone tissue engineering.
2. Biomimetic Molecular Composition: Element-Doped Bioceramics
Bioceramics such as HA, β-tricalcium phosphate (β-TCP) and bioactive glass represent a large class of inorganic materials that are widely used in bone tissue engineering [8]. Native biological HA is in fact calcium-deficient carbonated apatite with trace amounts of foreign ions (carbonate, silicate, F−, Cl−, Na+, Mg2+, Fe2+, Zn2+), either incorporated into the apatite crystal lattice or adsorbed on the crystal surface [26]. Inspired by the important role of these ions in bone formation, tissue engineers have investigated various trace ionic doping strategies with synthetic HA, bioactive glass, and other bioceramics in recent years as a method of improving biological performance both in vitro and in vivo [27–29]. In this section, HA is highlighted as a model bioceramic for demonstrating the methodology of element-doping strategies. This section focuses on various anion and cation substituted HA and their enhanced biological properties – such as osteogenesis stimulation, inflammatory suppression, and tumor inhibition – that have broadened the applications of HA in bone tissue engineering.
2.1. Anion substitution
The most biologically relevant anionic substitutions include CO32− for the PO43− or OH− groups, and F− and Cl− for the OH− group. Because carbonates are among the most abundant substituents in bio-apatite and play a vital role in bone metabolism, carbonated HA (CHA) has been synthesized to obtain similar properties to biological HA. CHA has shown superior osteoconductivity compared to pure HA and consists of two types depending on whether it substitutes the CO32− for OH− (type A) or PO43− (type B) – this is effected by selection of processing conditions [30]. Natural bone mineral is in fact a mixture of both types. CO32− incorporation, however, inhibits HA crystal growth, resulting in poorly crystalline structures with increased solubility [31]. In contrast, fluoride substitution increases the crystallinity and crystal size while reducing the solubility of HA [32]. A significant increase of fracture toughness with a consequent decrease of the brittleness index has been observed for F substituted HA (FHA), in comparison with pure HA [33]. It has been reported that FHA can stimulate osteoblast proliferation and differentiation, and that the F− concentration in a HA lattice structure is a critical factor in achieving the optimum balance of cell response and dissolution resistance [34–36]. Chloride substituted HA has also been reported to provide enhanced bioactivity and osteoconductivity [37].
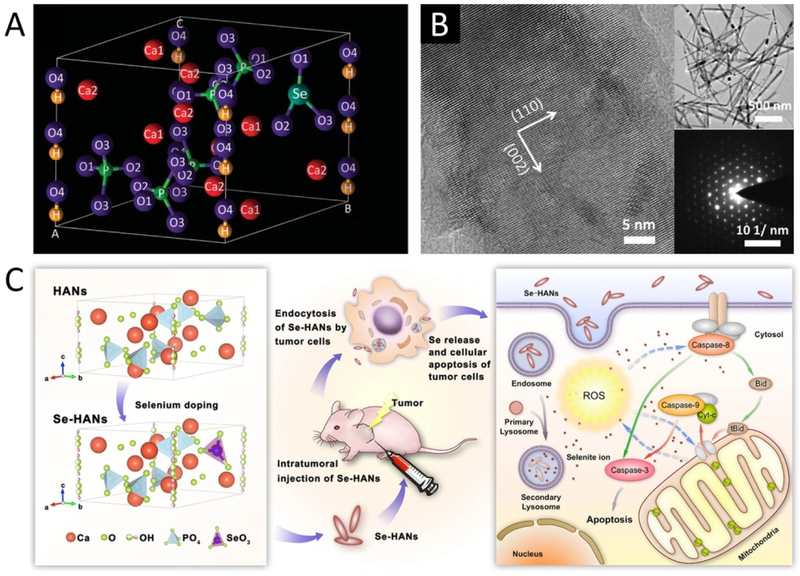
Selenite substituted HA (Se-HA) provides anticarcinogenic properties that are critical to the repair of bone defects caused by bone tumor resection. Se itself has been proven to be a crucial anticarcinogen in the human body, and Se deficiency is highly associated with many health problems [38]. Given that the repair of bone defects caused by bone tumor resection is often hindered by tumor recurrence, biomaterials that combine bone regeneration with tumor-inhibiting ability are essential in these scenarios. This has been achieved through the incorporation of Se in HA nanoparticles (Figure 2). Using a facile co-precipitation process with sodium selenite, needle-like Se-doped HA nanoparticles (Se-HANs) were synthesized and found to simultaneously induce the apoptosis of osteosarcoma cells and support the growth of bone marrow stem cells [39]. The incorporation of Se into the HA lattice was achieved by partially replacing phosphate (PO43−) groups with selenite (SeO32−) groups, and the crystallite size and crystallinity of Se-HANs reduced as the Se/P ratios increased [40]. When the Se/P mass ratio was 3%, Se-HANs exhibited sustainable release of SeO32− ions in PBS solution, with the greatest antitumor effect and biodegradability in a nude mice osteosarcoma model [41]. Subsequent in vitro and in vivo studies found that Se-HANs induced apoptosis of tumor cells by an inherent caspase-dependent apoptosis pathway synergistically orchestrated with the generation of reactive oxygen species [29]. These results indicate that Se-HANs are promising bone graft materials in bone tumor related defects due to their dual functions of not only supporting normal cell growth, but also inhibiting tumor recurrence.
Figure 2.
Se-doped HA nanoparticles. A. Crystal lattice of Se-HA showing the substitution of phosphate ions with selenite ions. B. High resolution transmission electron microscope (HR-TEM) images of Se-HANs. Adapted with permission from [41]. Insets are typical morphology (top) and selected area electron diffraction (SAED, bottom) of the Se-HANs. C. Mechanism of osteosarcoma inhibition with Se-HANs. Adapted with permission from [29]. Copyright 2016 American Chemical Society.
Silicate substituted HA (Si-HA) takes inspiration from the presence of silicon (Si) as an essential trace element in natural bone to create a material with enhanced osteoinductive properties compared to non-substituted HA. Since the 1970s, the role of Si in bone formation has been gradually recognized in the literature [42]. During the earliest stages of bone calcification, Si content increases in the active calcification areas of bone. Its presence increases biomineralization rate and promotes bone formation in native bone, whereas Si deficiency can lead to several bone diseases. Si-substituted HA (Si-HA) has thus been studied in recent years [43, 44]. In a previous work, needle-like Si-HA nanoparticles with Si doping percentage from 0.4 to 1.6 wt% were synthesized by an aqueous precipitation method. Si incorporation is achieved by partially replacing PO43− groups with silicate (SiO44−) groups, which decreases the crystallite size and crystallinity [44]. Si substitution (especially for 0.8 wt% Si) enhanced early osteoblastic cellular attachment [45] and osteogenic differentiation [46] while inhibited the osteoclast differentiation and decreased its resorptive activity in vitro [47]. Si-HA has also shown increased in vivo solubility and bioactivity [48], as well as favourable osteoconductive properties with increased bone ingrowth compared to pure HA [49–51], although it is still not very clear how Si positively influences the biological response of Si substituted CaP materials [52]. On the other hand, recent studies have reported that a Si-containing bioceramic known as akermanite can induce angiogenesis during bone regeneration [53]; however, the angiogenic ability of Si-doped HA has not been verified.
2.2. Cation substitution
Zinc substituted HA (Zn-HA) also takes inspiration from the presence of zinc (Zn) as an essential trace element in bone, and has drawn particular interest due to its ability to inhibit osteoclastic differentiation while promoting osteoblastic activity [54]. Zn deficiency is associated with a variety of bone disorders such as bone growth retardation, low bone density, and osteoporosis [55]. Zn-HA has thus been studied in recent years [56, 57]. Zn incorporation inhibits crystal growth and reduces the crystallinity, lattice parameters, and thermal stability of HA [58]. Zn-HA have shown increased osteoblastic response [59], and reduced cell number and resorption activity of osteoclast-like cells in vitro [60]. Anti-inflammatory and antibacterial effects of Zn-HA have also been reported [61], thus enhancing its potential application as a bone repair material, especially in the form of coatings for bone grafts [57]. Similar antibacterial properties have been found in silver doped HA [62].
Magnesium substituted HA (Mg-HA) has also drawn interest due to the vital role of magnesium (Mg) in stimulating osteoblast proliferation during the early stages of osteogenesis [63]. Mg deficiency impairs bone growth and function, and is thought to be associated with osteoporosis [64]. Similar to Zn substitution, Mg substitution results in decreases to the crystal size, crystallinity, lattice constants, and thermal stability of HA [65]. In vitro studies have found that Mg-HA promotes the proliferation and osteogenic differentiation of BMSCs [66]. Mg-HA has also shown greater osteogenic properties when compared to pure HA control by an in vivo study [67]. In particular, treatment with Mg-HA in an ovine spinal fusion model led to the deposition of new bone tissue with similar bone volume and trabecular number compared to the tissue formed with an autograft treatment [68].
Strontium doped HA (Sr-HA) has also been pursued as a bone tissue engineering material due to Sr’s ability to stimulate bone formation while inhibiting bone resorption in clinical applications [69, 70]. Strontium ranelate, a strontium compound, has been clinically used to treat osteoporosis [71]. Sr-HA has thus provoked an increasing interest in recent years. The substitution of Sr2+ has a strong effect on the crystallinity and lattice parameters of HA, depending on the substitution level. One study found that 3.22 mol% Sr-HA porous microspheres – generated by surfactant-free hydrothermal synthesis – showed optimal proliferation, osteogenic differentiation, and angiogenic factor expression of human osteoblast-like MG63 cells, when compared with a pure HA control [72]. In another study, Sr-HA coating with 3–7% Sr content synthesized by pulsed-laser deposition significantly increased osteogenic differentiation of MG63 cells and reduced proliferation of human osteoclasts [73]. An in vivo study found that a 10 mol% Sr-HA coating obtained by sol-gel methods improved osseointegration of an implant in a rat model [74].
In addition to the aforementioned cations, other metal ions such as copper (Cu2+) and cobalt (Co2+), have been reported to promote angiogenesis by stabilizing hypoxia inducible factor 1 alpha (HIF-1α) and inducing the expression of VEGF in the bone cells, i.e. mimicking hypoxic conditions [75, 76]. The doping of these ions into HA has thus attracted special interest for the promotion of angiogenic tissue development in tandem with osteogenesis. Interestingly, Co-doped HA has been demonstrated to be both pro-angiogenic and pro-osteogenic in an in vitro study, in which the doping of Co2+ induced simultaneous osteogenic differentiation and secretion of VEGF in MG-63 cells [77]. For copper, however, angiogenic capacity has only been reported in Cu-doped bioactive glass [28], and most studies on Cu-doped HA have focused on its antibacterial efficacy [78, 79]. Concerns have been raised due to the potential for oxidative damage to cells causing by these metal ions. Although no cytotoxicity has been found in HA substituted with low levels of Cu2+ or Co2+ [78], their systemic toxicity needs to be further studied before drawing conclusions on potential applications. Other metal ions doped into HA for osteogenic properties include manganese (Mn3+) [80] and iron (Fe2+/Fe3+) [81]. In addition, Fe substituted HA (Fe-HA) magnetic nanoparticles, as discussed later, have recently been utilized for constructing magnetically responsive scaffolds for bone tissue engineering [82, 83].
2.3. Future directions of element-doped bioceramics
Since single ion doping has shown positive results, multi-elemental doping has been considered for further enhancement or even synergetic improvements to HA’s biological properties. In one instance, improved osteogenic and angiogenic properties of HA were observed in vitro by the dual doping of Co2+ and Mg2+ ions [84]. Furthermore, co-substitutions can counteract the previously discussed structural changes that occur during mono-substitution and thus prevent destabilization of the structure and subsequent decomposition during heat treatment [85]. Future work can also investigate the ability of multi-element substitutions to create functionally graded materials that could produce sequential release of multiple ions with different functions for bone regeneration. Inspiration can be taken, for example, from instances where the delivery of an antibiotic and osteoinductive growth factor in a sequential manner has promoted more rapid and efficacious bone tissue repair [86]. Studies also show that bone repair is enhanced by the sequential delivery of angiogenic and osteogenic factors [87], and similar approaches could be taken with the doping and sequential release of angiogenic ions such as Co2+ and one of the various osteogenic cations.
While current studies have shown the improved osteogenic capacity of ion substituted HA, one must note that there are still few in vivo studies, especially in large animal models. Additionally, the mechanism by which the substituted ions promote the biological properties needs further clarification. For example, since the doping ions change the physicochemical properties such as crystal size and surface properties of HA, what is the relationship between these physicochemical changes and the promoted biological responses? Do these effects work in coordination with ion release from HA? Another major concern is that ion release kinetics from HA crystal structures have not been precisely tuned in the literature thus far, and reproducible clinical success hinges on tuning these release kinetics to match the timescale of bone regeneration. So far, only a few studies have characterized in vitro release behavior of these ions from HA [29, 41], while in vivo release profiles are still unknown. In the case of potential co-substitutions involving multiple elements, the above problems would become even more complicated. In spite of these current limitations, the evidence thus far suggests that ion substituted HA show great potential as functional inorganic materials for bone tissue engineering.
3. Nanoscale: Template-Induced Biomimetic Mineralization
As discussed above, bone matrix is a composite of organic and inorganic components that consist primarily of collagen and HA, respectively. Many composite materials for bone tissue engineering have thus been derived from a combination of inorganic materials like bioceramics with natural biomacromolecules such as collagen or synthetic polymers such as polylactic acid (PLA) and poly(ε-caprolactone) (PCL), in order to mimic the native composition of bone. However, many commonly used composites have failed to replicate the nanoscale assembly of mineralized collagen seen in native bone. To truly mimic the native nanostructure of bone, which consists of nanocrystals of HA embedded and aligned within the interstices of collagen fibrils, researchers have thus begun to use organic molecules as instructive templates to induce biomineralization and generate biomimetic nanocomposites that recapitulate the organization of natural bone ECM [88]. The mineralization and production of these composites has thus been effected by the usage of single molecular templates such as collagen or alternatively, multi-molecular templates that comprise multiple biomacromolecules or synthetic polymers.
3.1. Single molecular template
For single template regulated mineralization, a single type of biomolecule acts as a template that provides mineral nucleation sites and regulates the orientation and morphology of crystal growth. The carboxyl, carbonyl, and amino groups of the template molecules initiate the nucleation of calcium phosphate during the assembly process. In co-precipitation methods, the template molecules are often mixed with one or more of the reactive agents – e.g. pre-mineral solutions – that generate HA [88]. For instance, a phosphate solution can be slowly added to a calcium solution containing the premixed protein to generate a composite material. The Ca/P ratio in such reactions is generally maintained at 1.67 as seen in HA. So far, a number of proteins and polysaccharide molecules have been chosen as templates to induce in vitro biomineralization, with the protein templates including collagen, silk, fibronectin and so on, and the polysaccharide templates including hyaluronic acid, chondroitin sulfate, chitosan, sodium alginate, and bacterial cellulose [89].
Collagen is considered to be the most important protein template for regulating bone matrix assembly. About 85–95% of the total proteins in bone are type I collagens. Mineralized collagen fibers have been developed by many research groups for usage as bone graft materials, using the aforementioned co-precipitation methods [90]. Similar to natural bone, the c-axis of HA crystals have been synthesized to run parallel to the long axis of collagen fibers. The derived apatite hybrid materials imitate the composition and the nanostructural characteristics of natural bone. For clinical use, the mineralized collagen fibers are often blended with some biodegradable polymers, such as PLA and PLGA, to produce porous scaffolds with enhanced mechanical strength [91, 92] – some of which have been translated from bench to bedside [93, 94]. Regeneration of full thickness defects of cranial bone was observed in mini pigs, for instance, by implanting intrafibrillar-mineralized collagen loaded with autologous periodontal ligament stem cells [95]. In addition, mineralized collagen can be used for surface modification of scaffolds. In one most recent study, in situ formed mineralized collagen on the surface of 3D printed Ti6Al4V scaffolds significantly promoted the osteogenesis, vascularization, and osteointegration of the scaffolds [96].
Silk fibroin (SF), a natural protein biopolymer with similar fibrous structure to collagen, has also been employed as a template to prepare nano-HA crystals via biomineralization [97]. SF has drawn interest in particular for its extraordinary mechanical properties and relatively low cost as compared to collagen [98]. Shape control of HA nanoparticles generated on SF has been achieved by regulating the nanostructure and the content of the SF template [99, 100]. For example, when using SF nanospheres as a template, the shape of resulting HA crystals can change from pin-like to rice-like with increasing SF concentration [99]. In addition, the usage of an SF template can improve the water dispersibility of HA nanoparticles, by providing a negative charge layer that prevents nanoparticle aggregation [100]. Due to this good dispersibility, mineralized SF-HA nanoparticles are useful as both nanocarrier systems and scaffold additives. In one example, the sustained release of BMP-2 from SF/HA nanocarriers resulted in improved osteogenic differentiation of bone mesenchymal stem cells (BMSCs) in comparison to pure silk and HA nanocarriers [101]. In another case, the addition of SF/HA nanoparticles remarkably increased the stiffness and osteogenic response of scaffold compared to the addition of pure HA aggregates [102].
In addition to the aforementioned natural biomacromolecules, synthetic polymers, such as hydrophilic block copolymers of poly-(vinylpyrrolidone) [103], have been used as templates due to their relatively low cost and highly tunable properties. Biomacromolecule modified graphene oxide [104, 105] and carbon nanotubes [106] have also been used as templates for biomineralization in some preliminary studies.
3.2. Bi/multi-molecular templates
Besides collagen, many other molecules take part in the mineralization process of bone, including mineralization promoters such as osteocalcin (OCN) and osteonectin (ONN), as well as mineralization inhibitors such as citric acid and bone sialoprotein (BSP). Therefore, hybrid materials utilizing a bi/multi-molecular template for mineralization have drawn much attention in recent years, due to their greater similarity in composition to the native bone environment [88, 107].
Since individual molecules can play different roles in promoting or inhibiting the nucleation and growth of HA crystals, the usage of bi/multi-molecular templates provides more parameters in regulating the resultant properties (e.g. size, shape) of the induced HA nanoparticles and ultimately osteogenic cell behavior. Wang et al. [108] investigated the mineralization process regulated by bimolecular templates consisting of collagen and silk fibroin, both of which are promoters of mineralization. Their results suggested that the co-assembly of collagen and silk occurred with ordered secondary structure and regular spatial configuration and played a pivotal role in controlling and regulating HA crystal nucleation and growth. The mineral phase in COL-SF/HA displayed smaller size and more narrow distribution than either single collagen or single silk templates. Further study showed that the mineralized collagen and silk composites improved the in vitro osteogenic differentiation of MSCs, indicating their potential as bone regeneration materials [109].
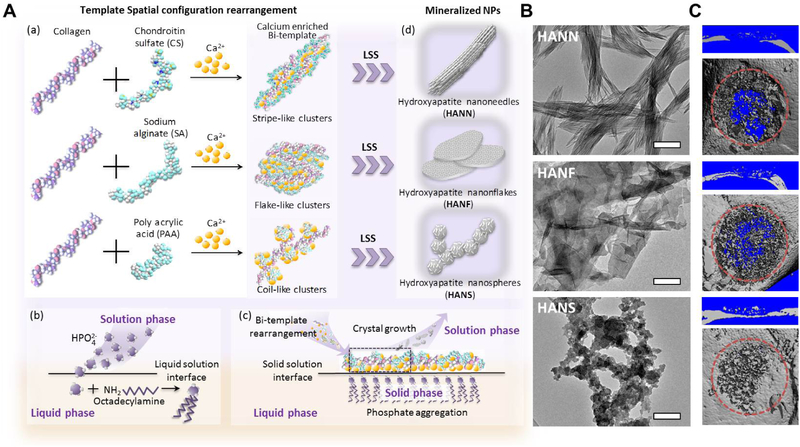
Recent studies have found that a combination of promoter and inhibitor templates can be a promising approach towards regulating the shape of HA nanoparticles, a property with important effects on osteogenesis. Wang et al. [14] designed a novel chimeric molecular template made of silk fibroin and albumin (ALB), which served as mineralization promoters and inhibitors, respectively. The chimeric ALB-SF template induced the production of lower crystallinity HA nanospheres, which were internalized in MSCs at a faster rate and significantly promoted osteoblastic differentiation of MSCs in comparison to HA nanorods generated by the SF template alone. Yang et al. [15] further studied the modulation of bimolecular templates on nanoparticle shape by incorporating collagen with three types of regulating templates – namely, chondroitin sulfate (CS), sodium alginate, and poly acrylic acid (PAA) – to generate HA nano-needles, nano-flakes, and nano-spheres, respectively. The template-induced biomimetic synthesis and shape-control of nanoparticles was realized using a modified liquid-solution-solid (LSS) method (Figure 3A). Due to the different carboxyl content on the molecular chain of regulating templates, the bi-templates form different 3D super-molecule structures that regulate the morphology of mineralized HA nanoparticle in the following LSS reaction (Figure 3B). The nano-spheres enhanced osteogenic potential in vitro by facilitating cellular uptake and autophagy activation, and in vivo studies further confirmed that the spherical nanoparticle (NP) embedded gelatin scaffolds enhanced vascularization and bone formation in a rat cranial defect model (Figure 3C).
Figure 3.
A. Template-induced biomimetic synthesis and shape-control of nanoparticles using a modified liquid-solution-solid (LSS) method. (a) Spatial configuration rearrangement of the bimolecular templates and the resulting Ca2+/template composites. (b) In the LSS method, the phosphate ions from the solution phase react with octadecylamine at the liquid/solution interface to form the solid phase, (c) which is used as a phosphorus source to release PO43− ions into the solution phase and co-precipitate with Ca2+/template composites, (d) resulting in mineralized nanoparticles with different shapes. B. The TEM micrographs of HA nanoneedles, nanoflakes, and nanospheres. C. Micro-CT results showed greater bone formation in the defects implanted with HANS embedded gelatin scaffolds. Adapted with permission from [15].
3.3. Future directions of template-induced biomimetic mineralization
In summary, template-induced biomineralization has been demonstrated to be an effective route to produce nanocomposite biomaterials with improved osteoconductive properties and even osteoinductive properties, showing great potential for bone tissue engineering. Ongoing studies should focus on the mechanisms by which the template regulates mineralization both in native bone and the in vitro biomineralization process, in order to optimize the choice of template molecules and discover more available template molecules. Given that most current studies use natural biomacromolecules as templates for mineralization, one major problem is that the degradation rate of the resulting composite material needs to match with bone tissue regeneration.
As discussed previously, ion doped HA is often superior to non-doped HA in its osteogenic functions. New approaches have thus combined the aforementioned template-induced mineralization processes with this type of elemental substitution [110–112]. In one most recent study, Yang et al. [110] incorporated Si in silk fibroin/collagen templates to synthesize a series of Si doped HA/silk fibroin/collagen composites. Compared with Si-free composites, the doping of Si not only promoted greater adhesion, proliferation, especially osteoblastic differentiation of rat MSCs in vitro, but also significantly improved the repair of a cranial bone defect in rats. Further studies of release kinetics and the affinity of different ions to template molecules like collagen will allow for fine tuning of these osteogenic effects, and may even be used to achieve sequential release of multiple doped ions, as discussed previously.
4. Micro-/Nanoscale: Recapitulation of Physical Structures
The microarchitecture and nanoscale structures found in natural bone are features that tissue engineers may recapitulate in order to stimulate effective tissue growth. These include gross features such as the surface roughness of substrates upon which cells attach and proliferate, as well as distinct structural components such as aligned fibrils of a specific dimension [113]. A wealth of research has demonstrated that MSCs and other bone progenitor cells will more effectively proliferate and differentiate along an osteogenic lineage when these physical cues are properly replicated in tissue engineering scaffolds [113–116]. The following discussion will highlight some of the approaches taken by tissue engineers to reproduce microstructural and nanostructural features on tissue engineering substrates as well as to create required internal porosity within bone scaffolds (Figure 4).
Figure 4.
Illustration of representative micro- and nanostructured surfaces.
4.1. Microstructured surfaces and interfaces
On the micron scale, the extracellular matrix of bone appears primarily as planar lamellae of aligned collagen fibers, with HA deposits distributed amongst the fibers and constituent collagen fibrils. One important microstructural property derived from bone’s heterogeneous, mineralized ECM substrates is surface roughness, which has been shown to promote both adhesion of osteoblasts as well as differentiation of MSCs to an osteogenic lineage [113]. A rough topography is hypothesized to more effectively mimic the mineralized interface encountered by cells adhering to the native bone ECM [114, 117]. Many approaches have been utilized for introducing surface roughness onto scaffolds. Farshid et al., for instance, recently utilized inorganic additives such as boron nitride nanotubes and nanoplatelets to introduce microscale surface roughness into a poly(propylene fumarate) scaffold, resulting in greater collagen deposition and cell attachment by seeded preosteoblasts [118]. Shakir et al. presented another approach in which nano-HA was complexed to chitosan and phenol formaldehyde resins to produce a composite scaffold with enhanced surface roughness from the nano-HA, inducing superior bone regeneration in an in vivo rat calvarium defect model [119].
Another important microstructural feature is the alignment of ECM and bone cells. Natural bone is highly anisotropic due to the longitudinal alignment of collagen fibers, and there is evidence that mesenchymal stem cells will more effectively differentiate to an osteogenic phenotype when confined into such an alignment [115, 120]. Tissue engineers have effected this type of cell alignment by creating parallel, micron scale grooves on substrates such as poly(lactic acid) (PLA), titanium, and HA to allow cells to grow and spontaneously elongate along the direction of these groove alignment [121, 122]. In one study with a calcium phosphate/gelatin composite scaffold, Nadeem et al. found that a groove diameter of 40–50 μm produced optimal osteogenic effects in vitro and notably, greater bone tissue formation and construct integration upon implantation in vivo [113]. It is hypothesized that the alignment of MSCs promotes bone formation by creating physical cues – namely, cell-cell interactions and “contact guidance” – that result in favorable effects such as cytoskeletal alignment and osteogenic gene expression [113, 115]. A more direct approach towards biomimicry, however, is to simply replicate the aligned fibers seen in the native collagen organization of bone. Terranova et al., for instance, investigated the effects of fiber alignment on osteoblast-like cells in an electrospun polystyrene model, and found that parallel fiber alignment produced elongation of cells along fiber direction (Figure 5), in addition to greater cell proliferation independently of other scaffold parameters such as fiber diameter [123]. Sankar et al., in another study, found that mesenchymal stem cells in spheroids would spontaneously migrate and align along the direction of composite PLGA/collagen/HA fibers in a 3D environment [124].
Figure 5.
Pre-osteoblast cells cultured on aligned polystyrene microfibers will attach to single fibers and adopt a stretched morphology along the fiber direction (A, B, C). Cells cultured on randomly distributed fibers will attach to multiple fibers and adopt a wider shape (D, E, F). Adapted with permission from [123].
A similar approach, not related to cell alignment, has been to generate “micro-pits” that imitate the resorption pits containing osteoclasts in native bone tissue – by doing so, physical cues indicative of a bone remodeling state are presented to MSCs [125, 126]. Wilkinson et al. generated pits in poly(ε-caprolactone) of diameters between 20–40 μm and depth of 200 nm, and found that the larger pit diameters in this range produced greater bone nodule formation [125]. Further, micro-pits with specific shapes have been shown to alter cell differentiation on 2D substrates. For instance, rectangles with relatively higher aspect ratio produced greater osteogenic differentiation of MSCs [127].
4.2. Nanostructured surfaces and interfaces
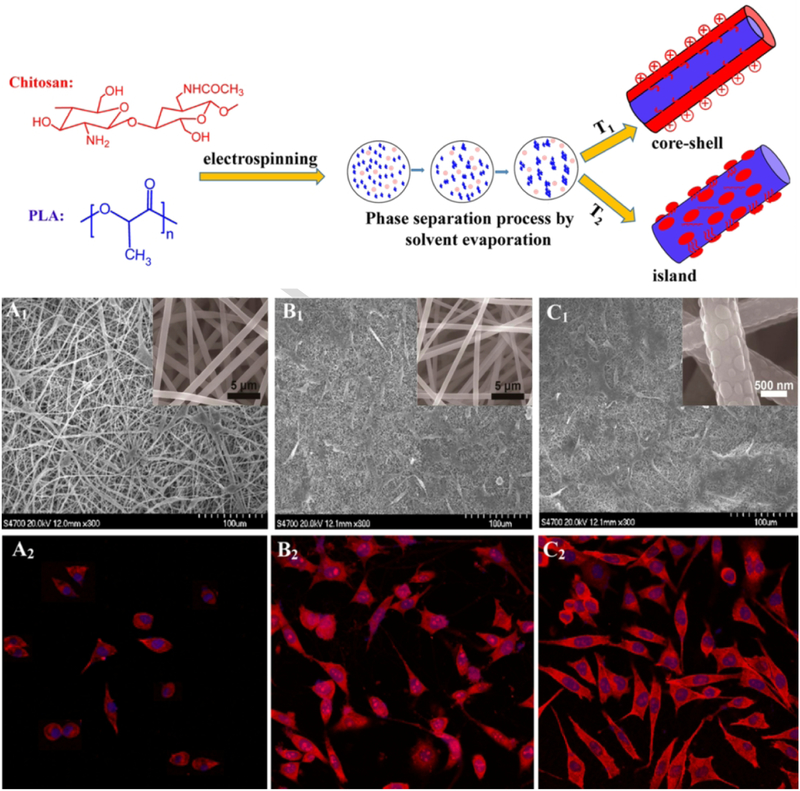
On the nanoscale, the collagen fibers in bone ECM are composed of aligned fibrils with rod or needle-like HA deposits scattered across their surface; these fibrils can be further broken down into their constituent tropocollagen chains. Bone tissue engineering at the nanoscale may thus benefit from mimicry of the fibrillar organization of collagen and/or the HA deposits. As discussed previously, cell and ECM alignment are crucial aspects of bone tissue function and formation – and so, aligned nanofibers are often produced by electrospinning and other techniques to provide enhanced osteogenesis via contact guidance [128, 129]. Nanofibers produced for bone tissue engineering will often incorporate HA or similar calcium phosphate components, taking inspiration from the mineral component localized around native collagen fibrils [128–131]. Similarly to bone tissue engineering at the microscale, surface topography is an important feature to take into account [19]. Given that the HA deposits that produce surface roughness in native bone have dimensions in the nanoscale, evidence indicates that developing surfaces with “nano-island” topography can prove even more beneficial to inducing osteogenesis than simply producing roughness at the microscale [132]. In one recent application, Xu et al. utilized phase separation effects during electrospinning to produce 100–200 nm wide chitosan “islands” on the surface of poly(lactic acid) nanofibers [133]. They found that the presence of these islands produced not only greater cell spreading by seeded preosteoblast cells, but also enhanced mineral deposition and osteogenic activity by these cells (Figure 6).
Figure 6.
Generation of core-shell and island composite fibers of chitosan and PLA (top). Compared to pure PLA fibers (A1, A2), core-shell (B1, B2) and especially island-decorated (C1, C2) fibers of PLA/chitosan effect greater cell attachment and spreading by cultured preosteoblasts. Adapted with permission from [133]. Copyright 2017 American Chemical Society.
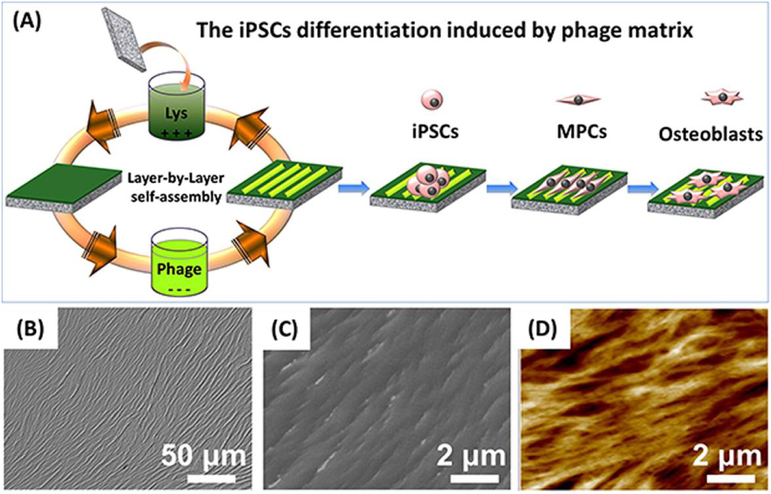
Lim et al., in an earlier study, investigated the effects of island heights ranging from 11–85 nm on polystyrene/polybromostyrene substrates, and discovered in their model that a smaller island height produced greater cell adhesion and spreading by osteoblasts as well as heightened alkaline phosphatase activity – indicating the benefits of scaling down the dimensions of topographical features [132]. Other nanotopography, such as nanopits and nanogrooves, can also affect osteoblastic differentiation [131]. For instance, a study demonstrated the use of nanoscale disorder of 120-nm-diameter, 100-nm-deep nanopits to stimulate human MSCs to produce bone mineral in vitro [134]. Wang et al. [19] found that in the absence of osteogenic supplements, highly ordered ridge/groove nanotopography assembled from phage nanofibers directed the osteoblastic differentiation of induced pluripotent stem cells (iPSCs) due to nanotopography-induced cell elongation (Figure 7). The same conclusion was drawn in studies using rat MSCs [135].
Figure 7.
Ordered surface nanotopography assembled from phage nanofibers directed the osteoblastic differentiation of iPSCs. (A) The surface nanotopography was fabricated by a layer-by-layer electrostatic self-assembly method. The glass substrate was alternately immersed into the cationic poly-L-lysine solution and anionic phage nanofiber solution. (B-D) The bright-field, SEM and AFM image of the resultant ridge/groove nanotopography. Adapted with permission from [19].
In addition to producing nanoscale scaffold features, one can simultaneously present the chemical cues seen at the nanoscale of bone tissue, such as HA itself, to provide synergistic effects towards bone formation. The usage of ceramic nanoparticles is one facile approach towards the simultaneous presentation of physical and chemical cues [136–138]. Hickey et al., in one example, doped poly(L-lactic acid) (PLLA) sheets with HA and magnesium oxide (MgO) nanoparticles to produce a nano-roughened surface topography with osteoconductive chemical moieties (Figure 8) [139]. Interestingly, the gradual degradation of MgO nanoparticles improved proliferation of osteoblasts on these composite substrates [139]. Additionally, given that the HA deposits in native bone are often shaped like rods or needles, it may be more beneficial to fabricate these nanoparticle additives in similar morphologies – this is exemplified in one case by Zhou et al., who electrospun collagen fibers with needle-like HA nanoparticles deposited in parallel [140].
Figure 8.
Topographical profiles – taken via atomic force microscopy – of plain PLLA sheets (A,B) and sheets doped with varying weight percentages of HA or MgO (C-F). Measurements of root mean squared surface roughness indicated that 20% MgO produced the highest roughness at the nanoscale, providing a highly advantageous surface topography for osteoblast proliferation and mineral deposition. Adapted with permission from [139].
4.3. 3D micro/nano-porous structures
A critical requirement for any bone tissue engineering scaffold is to have an interconnected 3D pore structure that allows for cell migration as well as the transport of nutrients and waste [141]. Comprehensive studies have been performed on the effect of average pore size – which can often range from as small as 20 μm to as large as 1500 μm – on osteogenic tissue growth, but no optimal pore size has been definitively established [20, 142]. For instance, studies have found that constructs with average pore size greater than 300 μm can facilitate capillary ingrowth to support bone tissue formation, but may also simultaneously have an inhibitory effect on ossification [20]. However, as a general trend, smaller pore sizes will promote initial cell adhesion due to higher substrate surface area, while larger pores will enable greater cellular infiltration from surrounding tissue – which is critical for tissue maintenance due to the importance of vascular ingrowth [20, 116]. In terms of overall porosity, a high percent porosity of around 80–90% is often desired to enable effective cell migration and proliferation throughout the scaffold [143]. For this reason, nanofiber scaffolds are particularly advantageous, as their notably smaller fiber diameter enables the fabrication of highly porous scaffolds that also present high surface area for the attachment and proliferation of cells [130, 133, 143]. However, nanofibers created by electrospinning tend to produce scaffolds with reduced average pore size compared to larger fiber scaffolds, resulting in decreased cell penetration depth [122].
With regards to the control of porosity and average pore size, 3D printing technologies such as fused deposition modeling, stereolithography and selective laser sintering have enabled the production of scaffolds with greater spatial fidelity and resolution than traditional scaffold fabrication methods [144–146] (Figure 9). 3D printed PCL-TCP and other composite polymer-ceramic scaffolds have been investigated extensively in vitro, and have also been translated into in vivo applications in recent years [147–149]. Hierarchical 3D printed scaffolds have also been constructed by filling the macropores with other bioactive ingredients to improve osteogenesis or angiogenesis [5]. An additional advantage of 3D printing technology is the ability to design scaffolds with precise pore size gradients, which can more effectively simulate the physical cues for bone growth given that native bone contains four levels of varying pore size [150].
Figure 9.
Controllable porous structure generated by 3D printing techniques. A. Fused deposition modeling and the resulting porous glass structure. Adapted with permission from [144]. B. Stereolithography and the resulting porous scaffold. Adapted with permission from [145]. C. Microsphere-based selective laser sintering technique and the resulting macroporous scaffold with internal micropores. Adapted with permission from [146].
Another important factor to consider when designing interconnected, macroporous scaffolds is the surface topography along pore surfaces. One approach of note is the generation of substrate texture by introducing small “mesopores” – in the diameter range of 2–50 nm – in the material that will be used to fabricate a macroporous 3D scaffold [151]. These mesopores additionally provide increased surface area and pore volume that can enhance bioactivity and mineral deposition [152]. Sol-gel derived bioactive glasses, in particular, adopt such a mesoporous texture spontaneously during the sol-gel process, and are well suited for bone applications due to not only their inherently textured topography, but also their bioactivity as ceramics [151, 153]. Accordingly, mesoporous bioactive glasses (MBG) have been found to possess superior osteoconductive properties compared to traditional bioactive glasses and other ceramic materials [152, 154]. Further, the 3D cubic mesoporous structure can be tailored for a size-matched entrapment of BMP-2 within mesopores for sustained release and preserved bioactivity in a hierarchical macro/micro/meso-porous bioactive glass scaffold (Figure 10) [18]. Excitingly, in vivo studies have demonstrated that BMP-2 loaded trimodal porous MBG scaffolds produce mature lamellar bone formation and complete bridging in a model, compared to the incomplete defect bridging produced by bimodal macro/micro-porous scaffolds [18]. The ability of MBG scaffolds to produce sustained release of bioactive factors in situ, as demonstrated both in vitro and in vivo, thus shows strong clinical potential for the induction of localized bone development.
Figure 10.
Mesoporous bioactive glass scaffolds presenting a trimodal porous structure: macropores (200–500 μm) with high interconnectivity, micropores (0.5–5 μm) uniformly embedded in macropore walls, and mesopores with an ordered texture (diameter of 7.5 nm) and potential for entrapment of growth factors. Adapted with permission from [18].
The shape and size of mesopores – and thus the eventual scaffold pore texture – can also be tuned by varying parameters during bioactive glass formation, such as ionic composition and surfactant usage during sol-gel formation [155]. One study, for instance, found that decreasing the relative weight percent of CaO induced a transition in mesoporous structure from 3D cubic to 2D hexagonal [156]. Further research will elucidate how different sizes and morphologies of mesoporous texture may physically stimulate and induce osteogenesis in adhered cells.
4.4. Future directions of physical structure fabrication
As discussed above, various micro- and nanoscale physical structures found in native bone matrix have been replicated on tissue engineering substrates. An area of significant future interest will be the fabrication of these features in 3D and, furthermore, their spatial patterning to repair bone defects that often include other tissue phenotypes at the defect site. Clinical repair of the osteochondral unit, for instance, requires simultaneous repair of bone, cartilage, and the bone-to-cartilage transition, which presents a spatial gradation of surface roughness and other physical features [12]. Additionally, while the effects of surface patterning and cell alignment in 2D have been investigated extensively [113, 115], less is known about how these factors affect cell behavior in 3D. Models with 3D micropatterns are thus needed to elucidate how in vivo microstructural features may affect cell differentiation. Recently, Bao et al. developed an exemplary model by creating 3D microniches of cubical, cylindrical, triangular prismatic, and cuboid shapes within hyaluronic acid hydrogels to study the effects of these 3D volumetric shapes on encapsulated mesenchymal stem cells [157]. In this case, both microniche volume and aspect ratio were found to affect a variety of cell behaviors including osteogenic differentiation and cytoskeletal organization [157].
Ultimately, 3D printing systems that can deposit multiple material formulations offer strong potential for the 3D patterning of roughness and other key physical features [158]. A range of strategies has been used to introduce both micro- and nano- scale topographical features to 3D porous scaffolds – often by using micro-/nanoscale additives during the 3D printing process [130, 133, 159]. Selective laser sintering, for instance, has been used to co-print traditional bone materials such as ceramics with nanoscale additives such as low-dimensional nanomaterial (LDNs) [160–163]. These LDNs include carbon nanotubes, graphene oxide, and boron nitride nanotubes, which can introduce nanoscale features while producing marked improvements in overall scaffold mechanical properties even at low concentrations [161, 162], resulting in significantly greater de novo bone formation in vivo [163]. Future research can apply these novel material formulations with layer-by-layer printing strategies to build scaffolds with varying topography, biochemical composition, and cell directionality across the construct – i.e. in a gradient – to more effectively mimic the distinct tissue layers of bone or heterogeneous tissues containing bone such as ligaments or the osteochondral unit [164, 165].
5. Macroscale: Physical Stimuli-Responsive Bone Scaffolds
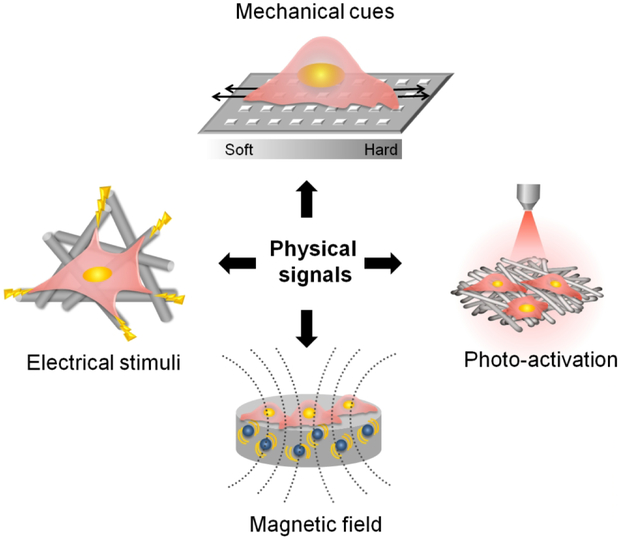
Conventional strategies of tissue engineering typically combine cells, scaffolds and chemical stimuli – such as growth factors – to regenerate or repair injured tissue. Recent studies have found that, in addition to these traditional cues, external physical cues like mechanical, electrical, and magnetic stimuli can affect cell fate and biological processes involved in bone regeneration [24, 166, 167]. This principle has thus been employed to achieve co-regulation of bone repair through the synergistic effects of an external physical stimulus and the internal cues of the scaffold, especially the stimuli-responsive properties (Figure 11).
Figure 11.
Illustration of physical signals (mechanical, electrical, magnetic, and photic stimuli) and intrinsic cues of scaffolds synergistically affect cellular behavior.
5.1. Mechanically mediated bone scaffolds
Bone is a mechanically sensitive tissue, and the influence of mechanical forces on the structural development and remodeling of bone has been long acknowledged in the field [168]. Mechanical stimulation, in particular, can influence cell behaviors by mechanotransduction, which is crucial for maintaining bone tissue homeostasis [169]. In addition, native bone has a piezoelectric property that can convert mechanical force into electrical signals, thereby enhancing bone growth [21].
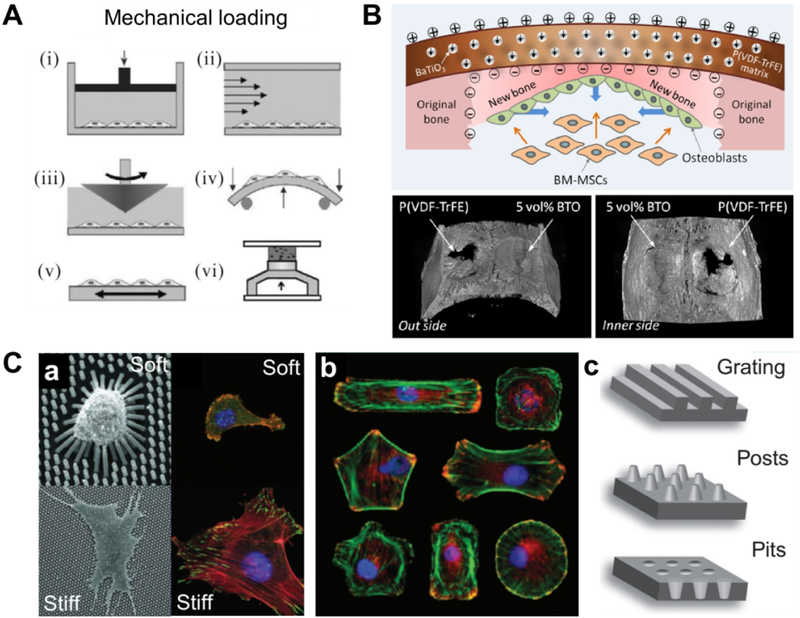
External mechanical forces (Figure 12A) have been demonstrated to be a potent regulator of MSC differentiation in vitro. For instance, a recent study reported that in vitro mechanical stimulation generated highly mineralized bone grafts. PCL-TCP scaffolds seeded with MSCs were cyclically compressed in a bioreactor for a period of 4 weeks for 4 hours per day. Cyclic loading treatment resulted in upregulation of osteogenic gene expression and greater calcium deposition relative to static controls [170]. In another study, dynamic mechanical stimulation regulated human MSC differentiation and led to heightened expression of collagen I, as well as greater mineral deposit formation in the bone layer of a multilayered osteochondral hydrogel [171]. Furthermore, the usage of external mechanical stimuli can work in tandem with the inherent physical cues of a scaffold. For example, aligned or unaligned nanofibers generate different effects on stem cell differentiation when subjected to tensile loading [172]. Scaffolds with anisotropic mechanical properties will thus produce different cell responses under different directions of applied external force, a critical concern for scaffolds that are transplanted in vivo in load-bearing areas.
Figure 12.
A. Methods used to apply mechanical loading to cell and tissue cultures: (i) hydrostatic pressure; (ii, iii) fluid shear stress; (iv) bending; (v) tension; (vi) compression. Adapted with permission from [166]. B. Illustration of biomimetic electric microenvironment created by piezoelectric BaTiO3 nanoparticles/P(VDF-TrFE) composite membranes to enhance bone defect repair. Representative micro-CT images show bone defect repair in rat calvarial models after implantation of polarized nanomembranes with 5 vol % BTO content and polarized neat P(VDF-TrFE) membranes at 12 weeks post implantation. Adapted with permission from [176]. C. Mechanical cues created from substrates with different features, such as (a) stiffness, (b) micropatterning and (c) nanotopography. Adapted with permission from [177].
On another note, external mechanical force can be converted into electrical signals through piezoelectric materials, which have gained increasing attention for bone tissue engineering applications [173]. Among these, poly(vinylidene fluoride) (PVDF) and its copolymers have been the most studied for their high piezoelectric activity and demonstrated osteogenic ability both in vitro and in vivo [174–176]. Piezoelectric ceramics, such as barium titanate (BaTiO3), have also been applied for bone repair. In a recent study, piezoelectric poly(vinylidene fluoridetrifluoroethylene) (P(VDF-TrFE)) membranes doped with BaTiO3 nanoparticles (BTO NP) were capable of restoring the electrical microenvironment around an injury site and enhancing bone regeneration in critical-sized calvarial defects in rats (Figure 12B) [176]. However, a major concern of the piezoelectric materials explored to date is their non-degradability, which limited clinical potential. Future studies should thus focus on the development of piezoelectric materials with tailored biodegradation properties.
In addition to external mechanical cues, recent studies have highlighted the critical role of internal forces – resulting from cell–matrix interactions – on MSC function [166]. The internal forces can be generated passively by the scaffold providing instructive mechanical signals through its stiffness or other mechanical properties (Figure 12C) to promote cell differentiation [177, 178]. Stiffer matrices have been found to be more highly osteogenic, under both 2D [179] and 3D conditions [180]. These findings have sparked great interest in stiffness mediated osteogenic differentiation and provide insight into the design of bone scaffolds, and especially hydrogels [181–185]. For example, Chatterjee et al. [183] examined the differentiation of osteoblasts in a poly(ethylene glycol) hydrogel within a compressive moduli range of 10 kPa to 300 kPa. The results suggested that stiffer hydrogels (compressive moduli of ~225 kPa and higher) enhanced osteogenesis. Recently, a scaffold with thermoresponsive ‘stiffness memory’ features has been fabricated and demonstrated to be able to regulate in vitro osteogenesis and chondrogenesis of human MSCs [186]. While it is known that the responses of osteoblasts to stiffness and substrate topography are related to cell mechanotransduction, [187], the exact mechanisms are an ongoing area of study.
In summary, mechanical stimulation of cells, whether externally applied or internally generated by scaffolds, can be utilized as a critical factor in the regulation of bone regeneration.
5.2. Electrically conductive bone scaffolds
Electrical stimulation therapy has been used in the clinic as a supplement to promote fracture healing and enhance spinal fusion, since the bioelectrical properties of bone were discovered in the 1950s [22, 188, 189]. Recent in vitro studies have shown that electrical stimulation can promote bone cells to migrate, proliferate, and differentiate at the site of stimulation [190–193]. One possible mechanism of electrically stimulated osteogenesis involves the up-regulation of intracellular calcium concentration primarily through the electrically stimulated activation of voltage-gated Ca2+ channels [194], which subsequently modulates osteogenesis via calmodulin pathways [195]. Inspired by the positive effects of electrical stimulation on bone healing, tissue engineers have developed conductive materials that can propagate electrical signals to the defect site for the acceleration of bone regeneration. Unlike the previously discussed piezoelectric materials, conductive materials require an externally applied power source to produce electrical signals.
Due to their versatility and tailorable properties, conductive synthetic polymers have received special attention in bone tissue engineering [196]. Various conductive substrates including porous scaffolds, coatings, and films have been designed by incorporating biocompatible conductive polymers, such as polypyrrole (PPY) [197–201], polyaniline (PANI) [202, 203], and poly(3,4-ethylenedioxythiophene) (PEDOT) [204, 205]. Increasing evidence has shown that electrically stimulated osteogenesis can occur on scaffolds fabricated from these materials. For example, Hardy et al. reported enhanced osteogenic differentiation of human MSCs cultured on PPY-silk scaffolds with electrical stimulation [206]. Another study by Zhang et al. found significantly higher osteogenic gene expression, ALP activity, and calcium deposition of MSCs cultured on conductive PPY/PCL scaffold subjected to 200 μA of direct current for 4 h/day for 21 days. The authors validated the mechanisms by which electrical stimulation regulated cell behavior, and found that electrical stimulation induced activation of voltage-gated ion channels – in particular, calcium channels – that lead to ion fluxes which would eventually regulate MSCs’ function via calmodulin pathways [197]. Enhanced osteogenic differentiation of MSCs was also observed on conductive PANI substrates, when exposed to pulsed electric field (Figure 13) [207]. In addition, the delivery of ES through conductive polymers have shown potential ability for vascularization, for example, human umbilical vein endothelial cells stimulated with 200–400 mV/cm on PANI-coated PCL fibers exhibited highly enhanced viability and adhesion compared to PCL controls [208].
Figure 13.
Schematic of the system used for generating pulsed electric field stimulation to hMSCs cultured on ECM coated conducting PANI substrates, depicting the temporal progression of osteogenic differentiation. Adapted with permission from [207]. Copyright 2015 American Chemical Society.
However, one of the greatest drawbacks of conducting polymers is the nondegradability, restricting their biomedical applications. As shown in the above studies, polymer blends and composites with some degradable polymers have been commonly used to balance the conductivity and degradability of conductive scaffolds. Recently, the synthesis of degradable and conductive copolymers containing conductive oligomers and other degradable polymers has been presented as an alternative solution [209, 210].
Carbon nanomaterials have also attracted much attention due to their electrical conductivity [211]. Recently, carbon nanomaterials have been utilized as additives to fabricate electrically conductive scaffolds with promising applications in bone regeneration. For example, graphene can significantly improve the conductivity of biopolymers, even at low loading content. Addition of 3 wt % graphene to UV cross-linked poly(trimethylene carbonate), for instance, resulted in significantly enhanced electrical conductivity of the polymer and upregulated osteogenic markers in adipose-derived MSCs when placed under regular electrical stimulation [212]. Carbon nanotubes can also be blended with polymers to fabricate conductive electrospun nanofibers [213]. These conductive nanomaterials are nonbiodegradable, and their long-term effects in vivo need further clarification.
Some in vivo studies have shown that these conductive scaffolds exhibit low levels of immunogenicity, suggesting their potential for electrically stimulated bone regeneration in the clinic [214]. However, there are limited in vivo studies showing the osteogenic effects of conductive bone scaffolds, partly due to it is relatively difficult to applied an external electrical stimulation after the scaffold are implanted in vivo. Future study may attempt to combine different electroactive systems, such as previously discussed piezoelectrics and conductive materials, to eliminate the need for external electrical stimulation device.
5.3. Magnetically responsive bone scaffolds
Magnetic stimulation therapy by pulsed electromagnetic fields (EMF) has been used to supplement bone healing in the clinic treatment of bone nonunion or delayed union for several years, albeit on a limited basis [25]. Animal studies have also shown that magnetic stimulation generated from static magnetic fields (SMF) or EMF can promote both bone healing and bone integration to the grafts [215, 216]. At the cellular level, in vitro studies have supported these findings by showing that SMF and EMF enhance osteoblast differentiation [217–219]. Although the underlying biological and biophysical mechanisms of these responses remain elusive, it is hypothesized that magnetic actuation induces the deformation of the cell membrane and reorganization of the cytoskeleton due to the response of intracellular and extracellular water as a diamagnetic fluid, thus activating mechanotransduction signal pathways that enhance osteoblast differentiation [169]. Other possible mechanisms proposed in the literature involve calcium-related osteogenic pathways that may be influenced by ion flow across cell membranes produced by electromagnetic fields [220]. Thus, magnetically responsive scaffolds have attracted increasing interest in recent years for bone tissue engineering.
Magnetic tissue engineering scaffolds have built from these inherent biological responses by incorporating magnetic nanoparticles (MNPs) into various matrices, using techniques such as direct immersion in ferro-fluid [221], electrospinning [222], and 3D printing [223]. The most commonly used MNPs are iron oxide nanoparticles, such as Fe3O4 and Fe2O3 [224, 225]. Fe doped HA nanoparticles can also be used as MNPs [83]. Magnetic bone scaffolds have shown promise for promoting bone regeneration [221, 223, 226]. The incorporation of MNPs in PCL nanofiber scaffolds, for instance, was demonstrated to not only promote the osteogenic differentiation of rat MSCs in vitro but also to enhance substantial blood vessel formation and bone regenerative ability in vivo in rats [227]. Nevertheless, the underlying mechanisms about the magnetic scaffolds stimulating cell and tissue response remain largely unknown. One possible reason is that the incorporated MNPs improve some physical properties, such as the hydrophilicity, degradation rate, mechanical properties, and apatite forming ability of scaffolds, thereby promoting cell adhesion and bone formation. A recent study revealed that the infiltration of MNPs can enhance the adsorption of particular proteins, thereby activated the MAPK/ERK signaling pathway, resulting in promoted cell proliferation [221]. Another potential reason is that the incorporated MNPs may generate internal magnetic field that influence the cell behaviors.
In addition to simply presenting MNP-incorporated magnetic scaffolds, an external magnetic field can be used to facilitate the fixation of magnetic scaffolds [229] and more importantly, regulate cell behavior towords osteogenesis and angiogenesis [230]. Yun et al. [228] studied the effects of an external SMF applied to magnetic PCL/MNP scaffolds on osteoblast differentiation and bone formation and found that the externally applied SMF not only promoted in vitro osteoblastic differentiation, but also significantly enhanced new bone formation in mouse calvarium defects, compared to the magnetic scaffolds alone (Figure 14). Another in vivo study found similar effects of externally applied SMF in repairing rabbit lumbar defects implanted with magnetic nanofibrous PLA/HA/MNPs scaffolds [226]. Additionally, external magnetic fields can exert a direct effect on angiogenesis. For example, an in vitro study demonstrated that an externally applied alternating magnetic field promoted vessel-like organization of endothelial cells in magnetically responsive alginate scaffolds [231]. Although the underlying mechanisms remain elusive in terms of how external magnetic fields stimulate osteogenesis and angiogenesis, it is hypothesized that the magnetic fields induce micro-deformation of magnetically responsive scaffolds, which generates bending/stretching forces that may mechanically stimulate cells [230, 232]. This type of micro-deformation can be tuned by the applied magnetic intensity, concentration of MNPs, and the rigidity of the scaffolds. Further investigation of these parameters will help researchers more accurately create the stimulatory microenvironments for promotion of osteogenic and angiogenic tissue development.
Figure 14.
A. Schematic showing the combination of magnetic cues from internal magnetic nanoparticles and an externally applied static magnetic field (SMF). B. μCT and H&E-stained images showing enhanced bone regeneration in the mouse calvarial defect in SMF stimulated PCL-MNP scaffolds after 6 weeks of implantation. Adapted with permission from [228].
Other applications explored for magnetic scaffolds in bone tissue engineering have exploited their magnetic heating properties, which can be beneficial for bone tumor ablation after the resection of these tumors [223]. In addition, one most recent study used magnetic field alignment of BMP-2 loaded MNPs to pattern the BMP-2 gradients in a hydrogel scaffold, which spatially directed the in vitro osteogenesis of human MSCs to generate osteochondral tissue constructs [233].
Despite these encouraging results, there are still safety concerns with magnetic bone scaffolds, mainly with the cytotoxicity and bioaccumulation of MNPs. Although these above in vitro and in vivo studies have shown that certain magnetic scaffolds have good biocompatibility and do not cause inflammatory reactions, further long-term in vivo studies are needed to confirm their safety.
5.4. Photoactivated bone scaffolds
Recently, photothermal effects have been utilized to stimulate bone regeneration. Yanagi et al. [234] reported that carbon nanotube-based photothermal stress – triggered by near-infrared (NIR) light – upregulated expression of osteogenic genes in preosteoblasts. This resulted in enhanced bone regeneration in critical-sized calvarial defects, when compared with unstimulated controls [234]. Another recent study found that, under near-infrared light irradiation, the incorporation of black phosphorus nanosheets into PLGA scaffolds up-regulated the expression of heat shock proteins, and promote in vitro and in vivo osteogenesis [235] (Figure 15).
Figure 15.
a) Schematic diagram of the preparation of BPs@PLGA membranes. BPs: black phosphorus nanosheet. b) Schematic diagram of rat tibia implantation and NIR irradiation. c) Infrared thermographic maps showing the notations of the highest temperature in irradiated areas. d) Micro-CT reconstruction of regenerated bone tissue in the defect area. Adapted with permission from [235].
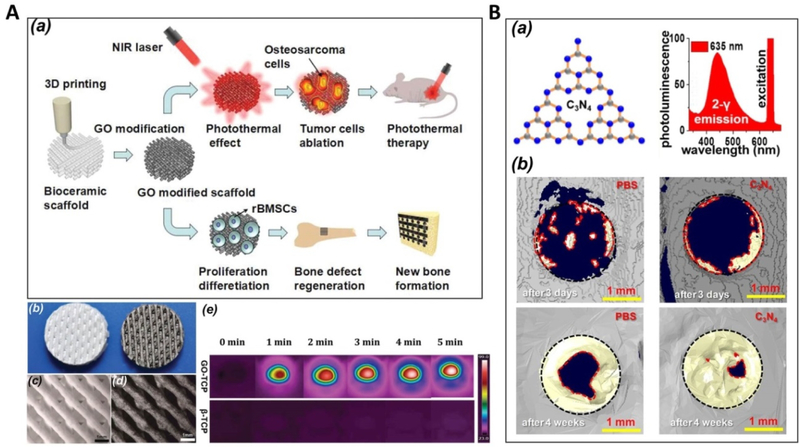
Scaffolds with photothermal effects have also been developed to address the dual challenges of bone regeneration and inhibition of tumor recurrence involved in the repair of bone defects caused by bone tumor resection. Ma et al. [236] prepared such a bifunctional graphene oxide (GO)-modified β-TCP (GO-TCP) composite scaffold with strong photothermal effects when exposed to irradiation from a 808 nm near infrared laser (Figure 16A). The irradiation of scaffolds induced more than 90% of cell death for osteosarcoma cells in vitro, and effectively inhibited tumor growth in mice. Compared to β-TCP scaffolds, the GO-TCP scaffolds produced greater osteogenic differentiation of rabbit MSCs in vitro and significantly promoted new bone formation in rabbit calvarial defects. In another similar study [237], a 3D-printed bioceramic scaffold was modified with self-assembled Ca-P/polydopamine nanolayers. Taking advantage of the photothermal effects of polydopamine, the scaffolds effectively induced tumor cell death in vitro, and significantly inhibited tumor growth in mice. Owing to the nanostructured surface, the scaffold also notably promoted the formation of new bone in a rabbit model even under photothermal treatment. A recent study found that the incorporation of elements (Cu, Fe, Mn and Co) into the bioactive glass-ceramics endowed the scaffolds with photothermal properties, providing a new method for the preparation of bifunctional scaffolds for photothermal tumor therapy and bone regeneration [238]. Similar bifunctional scaffolds have also been available by introducing CuFeSe2 nanocrystals into bioactive glass scaffolds [239].
Figure 16.
A. (a) Schematic illustration for the bifuctional GO-TCP scaffolds. b) Photograph of 3D printed pure β-TCP (left) and GO-TCP (right) scaffolds; microscopic images of pure c) β-TCP and d) GO-TCP scaffolds; (e) GO-TCP scaffolds show a distinct photothermal effect under 808 nm laser irradiation. Adapted with permission from [236]. B. (a) C3N4 sheets and the two-photon fluorescence spectra for the C3N4 sheets dispersed in water for red-light excitation. (b) Comparison of phosphate-buffered saline (PBS) and C3N4 sheets for the enhancement in repairing critical size cranial bone defect under red light in vivo: 3D μ-CT analysis of bone regeneration after 3 days or 4 weeks. Regenerated bone is indicated with a yellow color. Adapted with permission from [240].
In addition to the aforementioned photothermal effects, a recent study reported that, carbon nitride (C3N4) sheets lead to remarkable cell proliferation and osteogenic differentiation through two-photon activation. As a result, highly effective bone regeneration under red light was achieved in a mouse model (91% recovery after 4 weeks) (Figure 16B). The results were attributed to the deep penetration strength of red light into tissue and the resulting cell stimulation by enhanced photocurrent upon two-photon excitation of C3N4 sheets [240].
5.5. Future directions of stimuli-responsive scaffolds
In summary, the reviewed studies in this section have demonstrated the strong potential of mechanically, electrically, magnetically, and optically responsive scaffolds in bone tissue engineering. Due to relatively few in vivo or clinical studies, there is still a long way to go in terms of determining how to apply these stimuli safely and effectively in the human body. Mechanistic in vitro studies, as well, are required to achieve a deeper understanding of the stimulatory effects on cells before these therapies can be translated from the bench to the bedside. Future studies can thus pursue these mechanistic studies with model osteogenic tissue systems or bioreactor-based models where precise stimulation conditions can be studied with physically responsive, cell-seeded scaffolds. These externally applied stimuli can thus act as a powerful complement and additional “fourth element” to the three traditional components of bone tissue engineering.
6. Conclusions and Future Perspective
Natural bone has complex hierarchical architectures spanning from the nanoscale to the macroscale, producing unique combinations of high mechanical strength and distinct biological properties. As discussed previously, tissue engineers have focused many of their efforts for the reconstruction of bone tissue on biomimetic approaches that recapitulate the native tissue microenvironment. This review introduces the latest advances in four different biomimetic strategies, namely functional elemental doping, template induced biomimetic mineralization, micro/nano-structural design, and usage of external physical stimulatory cues as the “fourth element” of tissue engineering to promote bone regeneration. Although there are still difficulties in mimicking the structure and properties of bone tissue synthetically, these advances have shown that bioinspired, hierarchically designed bone scaffolds created from a combination of chemical modification, structural design, and external physical stimulation have great potential for bone repair. In particular, these developments indicate that in vivo bone regeneration may be achieved by internal cues from the scaffold with external physical stimuli, thereby reducing the dependence of bone tissue engineering on purely biological cues such as live cells and growth factors. Such advances will make scaffold-based bone tissue engineering therapies safer, more convenient, and more cost-effective, all of which are crucial requirements for clinical translation. More discussion on clinical translation of bone tissue engineering research can be found in a few recent reviews [241–243].
In future research, attention should be delegated to the mechanisms of interaction between implanted scaffolds and the microenvironment at bone defect sites. Recent biological research has given insight into the various biological and physical microenvironmental factors involved in bone repair and remodeling [244–247], and more investigation is needed to elucidate how these factors interact with implanted scaffolds to affect pathways related to bone regeneration. For example, bone scaffolds have recently been designed to utilize host macrophages to increase vascularization of tissue-engineered bone [248], indicating the importance of incorporating the immunomodulatory function into the design of biomaterials and scaffolds for bone regeneration [249, 250]. Furthermore, given that different types of bone defects have individual microenvironmental characteristics over the course of tissue repair, biomaterials must be “intelligent” to respond to these particular features accordingly. For instance, while transforming growth factor β1 (TGF-β1) may promote osteoinductive signaling in most cases, a high concentration of active TGF-β1 in subchondral bone leads to pathological changes that promote osteoarthritis [251, 252]. Inhibition of this process may thus be beneficial for the treatment of osteoarthritis-related bone defects. In other cases, when treating bone defects caused by tumor resection, material design must meet the requirements of both bone repair and tumor inhibition simultaneously. Additionally, the future design of bone scaffolds should consider the repair requirements of other multiple tissue types, such as periosteum [253], nerves [254], etc., in order to achieve the integrity of bone regeneration, both structurally and functionally. To this end, tissue engineers must consider the design of heterogeneous scaffolds which can replicate the biochemical and physical features of the native extracellular matrix of multiple tissue types, which will require precise spatiotemporal control of biochemical cues such as growth factors [255, 256].
In short, bone tissue engineering will continue to benefit from the development of novel materials and scaffold designs that take inspiration from the hierarchical features of native bone, as well as further understanding of the in vivo mechanisms that underlie the promotion of bone regeneration by multi-scale biological and physical cues.
Acknowledgements
This work was funded by grants from the National Natural Science Foundation of China (31430029, 81801850 and 31870960), the National Key Research and Development Program of China (2018YFC1105701, 2017YFC1103900), and the National Institutes of Health of the United States (R01 AR068073, R01 CA180279, and P41 EB023833).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Fong ELS, Watson BM, Kasper FK, Mikos AG. Building Bridges: Leveraging Interdisciplinary Collaborations in the Development of Biomaterials to Meet Clinical Needs. Adv Mater. 2012;24:4995–5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bose S, Roy M, Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012;30:546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Santos MI, Reis RL. Vascularization in bone tissue engineering: physiology, current strategies, major hurdles and future challenges. Macromol Biosci. 2010;10:12–27. [DOI] [PubMed] [Google Scholar]
- [4].Nguyen BNB, Moriarty RA, Kamalitdinov T, Etheridge JM, Fisher JP. Collagen hydrogel scaffold promotes mesenchymal stem cell and endothelial cell coculture for bone tissue engineering. J Biomed Mater Res Part A. 2017;105:1123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang J, Yang M, Zhu Y, Wang L, Tomsia AP, Mao C. Phage nanofibers induce vascularized osteogenesis in 3D printed bone scaffolds. Adv Mater. 2014;26:4961–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zimmermann EA, Busse B, Ritchie RO. The fracture mechanics of human bone: influence of disease and treatment. BoneKEy reports. 2015;4:743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhou H, Lee J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011;7:2769–81. [DOI] [PubMed] [Google Scholar]
- [8].Fernandez de Grado G, Keller L, Idoux-Gillet Y, Wagner Q, Musset AM, Benkirane-Jessel N, et al. Bone substitutes: a review of their characteristics, clinical use, and perspectives for large bone defects management. Journal of tissue engineering. 2018;9:2041731418776819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zofkova I, Nemcikova P, Matucha P. Trace elements and bone health. Clin Chem Lab Med. 2013;51:1555–61. [DOI] [PubMed] [Google Scholar]
- [10].Nair AK, Gautieri A, Chang SW, Buehler MJ. Molecular mechanics of mineralized collagen fibrils in bone. Nat Commun. 2013;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Stevens MM. Biomaterials for bone tissue engineering. Mater Today. 2008;11:18–25. [Google Scholar]
- [12].Gao C, Peng S, Feng P, Shuai C. Bone biomaterials and interactions with stem cells. Bone Res. 2017;5:17059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Reznikov N, Shahar R, Weiner S. Bone hierarchical structure in three dimensions. Acta Biomater. 2014;10:3815–26. [DOI] [PubMed] [Google Scholar]
- [14].Wang J, Yang G, Wang Y, Du Y, Liu H, Zhu Y, et al. Chimeric Protein Template-Induced Shape Control of Bone Mineral Nanoparticles and Its Impact on Mesenchymal Stem Cell Fate. Biomacromolecules. 2015;16:1987–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yang G, Liu H, Hu X, Chen Z, Friis TE, Wang J, et al. Bio-inspired hybrid nanoparticles promote vascularized bone regeneration in a morphology-dependent manner. Nanoscale. 2017;9:5794–805. [DOI] [PubMed] [Google Scholar]
- [16].Saiz E, Zimmermann EA, Lee JS, Wegst UG, Tomsia AP. Perspectives on the role of nanotechnology in bone tissue engineering. Dent Mater. 2013;29:103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bose S, Vahabzadeh S, Bandyopadhyay A. Bone tissue engineering using 3D printing. Mater Today. 2013;16:496–504. [Google Scholar]
- [18].Tang W, Lin D, Yu YM, Niu HY, Guo H, Yuan Y, et al. Bioinspired trimodal macro/micro/nano-porous scaffolds loading rhBMP-2 for complete regeneration of critical size bone defect. Acta Biomater. 2016;32:309–23. [DOI] [PubMed] [Google Scholar]
- [19].Wang J, Wang L, Yang M, Zhu Y, Tomsia A, Mao C. Untangling the effects of peptide sequences and nanotopographies in a biomimetic niche for directed differentiation of iPSCs by assemblies of genetically engineered viral nanofibers. Nano Lett. 2014;14:6850–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Murphy CM, Haugh MG, O’Brien FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials. 2010;31:461–6. [DOI] [PubMed] [Google Scholar]
- [21].Marino A, Becker RO. Piezoelectric effect and growth control in bone. Nature. 1970;228:473–4. [DOI] [PubMed] [Google Scholar]
- [22].Goldstein C, Sprague S, Petrisor BA. Electrical Stimulation for Fracture Healing: Current Evidence. J Orthop Trauma. 2010;24:S62–S5. [DOI] [PubMed] [Google Scholar]
- [23].Ross CL, Siriwardane M, Almeida-Porada G, Porada CD, Brink P, Christ GJ, et al. The effect of low-frequency electromagnetic field on human bone marrow stem/progenitor cell differentiation. Stem Cell Res. 2015;15:96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang J, Ding C, Ren L, Zhou Y, Shang P. The effects of static magnetic fields on bone. Prog Biophys Mol Biol. 2014;114:146–52. [DOI] [PubMed] [Google Scholar]
- [25].Assiotis A, Sachinis NP, Chalidis BE. Pulsed electromagnetic fields for the treatment of tibial delayed unions and nonunions. A prospective clinical study and review of the literature. J Orthop Surg Res. 2012;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Boanini E, Gazzano M, Bigi A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010;6:1882–94. [DOI] [PubMed] [Google Scholar]
- [27].Wu C, Zhou Y, Lin C, Chang J, Xiao Y. Strontium-containing mesoporous bioactive glass scaffolds with improved osteogenic/cementogenic differentiation of periodontal ligament cells for periodontal tissue engineering. Acta Biomater. 2012;8:3805–15. [DOI] [PubMed] [Google Scholar]
- [28].Wu C, Zhou Y, Xu M, Han P, Chen L, Chang J, et al. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials. 2013;34:422–33. [DOI] [PubMed] [Google Scholar]
- [29].Wang Y, Wang J, Hao H, Cai M, Wang S, Ma J, et al. In Vitro and in Vivo Mechanism of Bone Tumor Inhibition by Selenium-Doped Bone Mineral Nanoparticles. ACS Nano. 2016;10:9927–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Garskaite E, Gross K-A, Yang S-W, Yang TC-K, Yang J-C, Kareiva A. Effect of processing conditions on the crystallinity and structure of carbonated calcium hydroxyapatite (CHAp). CrystEngComm. 2014;16:3950. [Google Scholar]
- [31].Wang LJ, Nancollas GH. Calcium Orthophosphates: Crystallization and Dissolution. Chem Rev. 2008;108:4628–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kim HW, Li LH, Koh YH, Knowles JC, Kim HE. Sol-gel preparation and properties of fluoride-substituted hydroxyapatite powders. J Am Ceram Soc. 2004;87:1939–44. [Google Scholar]
- [33].Bianco A, Cacciotti I, Lombardi M, Montanaro L, Bemporad E, Sebastiani M. F-substituted hydroxyapatite nanopowders: Thermal stability, sintering behaviour and mechanical properties. Ceram Int. 2010;36:313–22. [Google Scholar]
- [34].Cheng K, Weng W, Wang H, Zhang S. In vitro behavior of osteoblast-like cells on fluoridated hydroxyapatite coatings. Biomaterials. 2005;26:6288–95. [DOI] [PubMed] [Google Scholar]
- [35].Wang Y, Zhang S, Zeng X, Ma LL, Weng W, Yan W, et al. Osteoblastic cell response on fluoridated hydroxyapatite coatings. Acta Biomater. 2007;3:191–7. [DOI] [PubMed] [Google Scholar]
- [36].Wang J, Chao Y, Wan Q, Zhu Z, Yu H. Fluoridated hydroxyapatite coatings on titanium obtained by electrochemical deposition. Acta Biomater. 2009;5:1798–807. [DOI] [PubMed] [Google Scholar]
- [37].Cho JS, Yoo DS, Chung YC, Rhee SH. Enhanced bioactivity and osteoconductivity of hydroxyapatite through chloride substitution. J Biomed Mater Res Part A. 2014;102:455–69. [DOI] [PubMed] [Google Scholar]
- [38].Rayman MP. Selenium and human health. Lancet. 2012;379:1256–68. [DOI] [PubMed] [Google Scholar]
- [39].Wang Y, Ma J, Zhou L, Chen J, Liu Y, Qiu Z, et al. Dual functional selenium-substituted hydroxyapatite. Interface focus. 2012;2:378–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ma J, Wang Y, Zhou L, Zhang S. Preparation and characterization of selenite substituted hydroxyapatite. Mater Sci Eng C Mater Biol Appl. 2013;33:440–5. [DOI] [PubMed] [Google Scholar]
- [41].Wang Y, Hao H, Liu H, Wang Y, Li Y, Yang G, et al. Selenite-Releasing Bone Mineral Nanoparticles Retard Bone Tumor Growth and Improve Healthy Tissue Functions In Vivo. Adv Healthc Mater. 2015;4:1813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Carlisle EM. Silicon: a possible factor in bone calcification. Science. 1970;167:279–80. [DOI] [PubMed] [Google Scholar]
- [43].Qiu Z-Y, Noh I-S, Zhang S-M. Silicate-doped hydroxyapatite and its promotive effect on bone mineralization. Front Mater Sci. 2013;7:40–50. [Google Scholar]
- [44].Qiu ZY, Li G, Zhang YQ, Liu J, Hu W, Ma J, et al. Fine structure analysis and sintering properties of Si-doped hydroxyapatite. Biomed Mater. 2012;7:045009. [DOI] [PubMed] [Google Scholar]
- [45].Guth K, Campion C, Buckland T, Hing KA. Effect of Silicate-Substitution on Attachment and Early Development of Human Osteoblast-Like Cells Seeded on Microporous Hydroxyapatite Discs. Adv Eng Mater. 2010;12:B26–B36. [Google Scholar]
- [46].Honda M, Kikushima K, Kawanobe Y, Konishi T, Mizumoto M, Aizawa M. Enhanced early osteogenic differentiation by silicon-substituted hydroxyapatite ceramics fabricated via ultrasonic spray pyrolysis route. J Mater Sci Mater Med. 2012;23:2923–32. [DOI] [PubMed] [Google Scholar]
- [47].Matesanz MC, Linares J, Lilue I, Sánchez-Salcedo S, Feito MJ, Arcos D, et al. Nanocrystalline silicon substituted hydroxyapatite effects on osteoclast differentiation and resorptive activity. Journal of Materials Chemistry B. 2014;2:2910. [DOI] [PubMed] [Google Scholar]
- [48].Porter AE, Patel N, Skepper JN, Best SM, Bonfield W. Comparison of in vivo dissolution processes in hydroxyapatite and silicon-substituted hydroxyapatite bioceramics. Biomaterials. 2003;24:4609–20. [DOI] [PubMed] [Google Scholar]
- [49].Porter AE, Patel N, Skepper JN, Best SM, Bonfield W. Effect of sintered silicate-substituted hydroxyapatite on remodelling processes at the bone-implant interface. Biomaterials. 2004;25:3303–14. [DOI] [PubMed] [Google Scholar]
- [50].Hing KA, Revell PA, Smith N, Buckland T. Effect of silicon level on rate, quality and progression of bone healing within silicate-substituted porous hydroxyapatite scaffolds. Biomaterials. 2006;27:5014–26. [DOI] [PubMed] [Google Scholar]
- [51].Patel N, Brooks RA, Clarke MT, Lee PMT, Rushton N, Gibson IR, et al. In vivo assessment of hydroxyapatite and silicate-substituted hydroxyapatite granules using an ovine defect model. J Mater Sci-Mater M. 2005;16:429–40. [DOI] [PubMed] [Google Scholar]
- [52].Bohner M Silicon-substituted calcium phosphates - a critical view. Biomaterials. 2009;30:6403–6. [DOI] [PubMed] [Google Scholar]
- [53].Zhai W, Lu H, Chen L, Lin X, Huang Y, Dai K, et al. Silicate bioceramics induce angiogenesis during bone regeneration. Acta Biomater. 2012;8:341–9. [DOI] [PubMed] [Google Scholar]
- [54].Kishi S, Yamaguchi M. Inhibitory effect of zinc compounds on osteoclast-like cell formation in mouse marrow cultures. Biochem Pharmacol. 1994;48:1225–30. [DOI] [PubMed] [Google Scholar]
- [55].Yamaguchi M Role of nutritional zinc in the prevention of osteoporosis. Mol Cell Biochem. 2010;338:241–54. [DOI] [PubMed] [Google Scholar]
- [56].Hu W, Ma J, Wang J, Zhang S. Fine structure study on low concentration zinc substituted hydroxyapatite nanoparticles. Mat Sci Eng C-Mater. 2012;32:2404–10. [Google Scholar]
- [57].Sun G, Ma J, Zhang S. Electrophoretic deposition of zinc-substituted hydroxyapatite coatings. Mater Sci Eng C Mater Biol Appl. 2014;39:67–72. [DOI] [PubMed] [Google Scholar]
- [58].Ren F, Xin R, Ge X, Leng Y. Characterization and structural analysis of zinc-substituted hydroxyapatites. Acta Biomater. 2009;5:3141–9. [DOI] [PubMed] [Google Scholar]
- [59].Thian ES, Konishi T, Kawanobe Y, Lim PN, Choong C, Ho B, et al. Zinc-substituted hydroxyapatite: a biomaterial with enhanced bioactivity and antibacterial properties. J Mater Sci Mater Med. 2013;24:437–45. [DOI] [PubMed] [Google Scholar]
- [60].Shepherd DV, Kauppinen K, Brooks RA, Best SM. An in vitro study into the effect of zinc substituted hydroxyapatite on osteoclast number and activity. J Biomed Mater Res A. 2014;102:4136–41. [DOI] [PubMed] [Google Scholar]
- [61].Velard F, Laurent-Maquin D, Braux J, Guillaume C, Bouthors S, Jallot E, et al. The effect of zinc on hydroxyapatite-mediated activation of human polymorphonuclear neutrophils and bone implant-associated acute inflammation. Biomaterials. 2010;31:2001–9. [DOI] [PubMed] [Google Scholar]
- [62].Samani S, Hossainalipour SM, Tamizifar M, Rezaie HR. In vitro antibacterial evaluation of sol-gel-derived Zn-, Ag-, and (Zn + Ag)-doped hydroxyapatite coatings against methicillin-resistant Staphylococcus aureus. J Biomed Mater Res A. 2013;101:222–30. [DOI] [PubMed] [Google Scholar]
- [63].Ratnayake JTB, Mucalo M, Dias GJ. Substituted hydroxyapatites for bone regeneration: A review of current trends. J Biomed Mater Res B Appl Biomater. 2017;105:1285–99. [DOI] [PubMed] [Google Scholar]
- [64].Rude RK, Gruber HE. Magnesium deficiency and osteoporosis: animal and human observations. J Nutr Biochem. 2004;15:710–6. [DOI] [PubMed] [Google Scholar]
- [65].Ren F, Leng Y, Xin R, Ge X. Synthesis, characterization and ab initio simulation of magnesium-substituted hydroxyapatite. Acta Biomater. 2010;6:2787–96. [DOI] [PubMed] [Google Scholar]
- [66].Xiong XB, Ni XY, Zhou D. Functions of the Mg-HA coating on carbon/carbon composite surface to promote the proliferation and osteogenic differentiation of mBMSCs. Rsc Adv. 2016;6:105056–62. [Google Scholar]
- [67].Landi E, Logroscino G, Proietti L, Tampieri A, Sandri M, Sprio S. Biomimetic Mg-substituted hydroxyapatite: from synthesis to in vivo behaviour. J Mater Sci-Mater M. 2008;19:239–47. [DOI] [PubMed] [Google Scholar]
- [68].Brodano GB, Giavaresi G, Lolli F, Salamanna F, Parrilli A, Martini L, et al. Hydroxyapatite-Based Biomaterials Versus Autologous Bone Graft in Spinal Fusion An In Vivo Animal Study. Spine. 2014;39:E661–E8. [DOI] [PubMed] [Google Scholar]
- [69].Saidak Z, Marie PJ. Strontium signaling: Molecular mechanisms and therapeutic implications in osteoporosis. Pharmacol Ther. 2012;136:216–26. [DOI] [PubMed] [Google Scholar]
- [70].Tat SK, Pelletier JP, Mineau F, Caron J, Martel-Pelletier J. Strontium ranelate inhibits key factors affecting bone remodeling in human osteoarthritic subchondral bone osteoblasts. Bone. 2011;49:559–67. [DOI] [PubMed] [Google Scholar]
- [71].Meunier PJ, Roux C, Ortolani S, Diaz-Curiel M, Compston J, Marquis P, et al. Effects of long-term strontium ranelate treatment on vertebral fracture risk in postmenopausal women with osteoporosis. Osteoporos Int. 2009;20:1663–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Lin K, Liu P, Wei L, Zou Z, Zhang W, Qian Y, et al. Strontium substituted hydroxyapatite porous microspheres: Surfactant-free hydrothermal synthesis, enhanced biological response and sustained drug release. Chem Eng J. 2013;222:49–59. [Google Scholar]
- [73].Capuccini C, Torricelli P, Sima F, Boanini E, Ristoscu C, Bracci B, et al. Strontium-substituted hydroxyapatite coatings synthesized by pulsed-laser deposition: in vitro osteoblast and osteoclast response. Acta Biomater. 2008;4:1885–93. [DOI] [PubMed] [Google Scholar]
- [74].Li Y, Li Q, Zhu S, Luo E, Li J, Feng G, et al. The effect of strontium-substituted hydroxyapatite coating on implant fixation in ovariectomized rats. Biomaterials. 2010;31:9006–14. [DOI] [PubMed] [Google Scholar]
- [75].Sen CK, Khanna S, Venojarvi M, Trikha P, Ellison EC, Hunt TK, et al. Copper-induced vascular endothelial growth factor expression and wound healing. Am J Physiol-Heart C. 2002;282:H1821–H7. [DOI] [PubMed] [Google Scholar]
- [76].Kim KS, Rajagopal V, Gonsalves C, Johnson C, Kalra VK. A novel role of hypoxia-inducible factor in cobalt chloride- and hypoxia-mediated expression of IL-8 chemokine in human endothelial cells. J Immunol. 2006;177:7211–24. [DOI] [PubMed] [Google Scholar]
- [77].Kulanthaivel S, Roy B, Agarwal T, Giri S, Pramanik K, Pal K, et al. Cobalt doped proangiogenic hydroxyapatite for bone tissue engineering application. Mater Sci Eng C Mater Biol Appl. 2016;58:648–58. [DOI] [PubMed] [Google Scholar]
- [78].Huang Y, Zhang X, Zhao R, Mao H, Yan Y, Pang X. Antibacterial efficacy, corrosion resistance, and cytotoxicity studies of copper-substituted carbonated hydroxyapatite coating on titanium substrate. J Mater Sci. 2014;50:1688–700. [Google Scholar]
- [79].Shanmugam S, Gopal B. Copper substituted hydroxyapatite and fluorapatite: Synthesis, characterization and antimicrobial properties. Ceram Int. 2014;40:15655–62. [Google Scholar]
- [80].Huang Y, Ding QQ, Han SG, Yan YJ, Pang XF. Characterisation, corrosion resistance and in vitro bioactivity of manganese-doped hydroxyapatite films electrodeposited on titanium. J Mater Sci-Mater M. 2013;24:1853–64. [DOI] [PubMed] [Google Scholar]
- [81].Panseri S, Cunha C, D’Alessandro T, Sandri M, Giavaresi G, Marcacci M, et al. Intrinsically superparamagnetic Fe-hydroxyapatite nanoparticles positively influence osteoblast-like cell behaviour. J Nanobiotechnol. 2012;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Iafisco M, Sandri M, Panseri S, Delgado-López JM, Gómez-Morales J, Tampieri A. Magnetic Bioactive and Biodegradable Hollow Fe-Doped Hydroxyapatite Coated Poly(l-lactic) Acid Micro-nanospheres. Chem Mater. 2013;25:2610–7. [Google Scholar]
- [83].Gloria A, Russo T, D’Amora U, Zeppetelli S, D’Alessandro T, Sandri M, et al. Magnetic poly(epsilon-caprolactone)/iron-doped hydroxyapatite nanocomposite substrates for advanced bone tissue engineering. Journal of the Royal Society, Interface. 2013;10:20120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kulanthaivel S, Mishra U, Agarwal T, Giri S, Pal K, Pramanik K, et al. Improving the osteogenic and angiogenic properties of synthetic hydroxyapatite by dual doping of bivalent cobalt and magnesium ion. Ceram Int. 2015;41:11323–33. [Google Scholar]
- [85].Aina V, Lusvardi G, Annaz B, Gibson IR, Imrie FE, Malavasi G, et al. Magnesium- and strontium-co-substituted hydroxyapatite: the effects of doped-ions on the structure and chemico-physical properties. J Mater Sci Mater Med. 2012;23:2867–79. [DOI] [PubMed] [Google Scholar]
- [86].Min J, Choi KY, Dreaden EC, Padera RF, Braatz RD, Spector M, et al. Designer Dual Therapy Nanolayered Implant Coatings Eradicate Biofilms and Accelerate Bone Tissue Repair. ACS Nano. 2016;10:4441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Perez RA, Kim JH, Buitrago JO, Wall IB, Kim HW. Novel therapeutic core-shell hydrogel scaffolds with sequential delivery of cobalt and bone morphogenetic protein-2 for synergistic bone regeneration. Acta Biomater. 2015;23:295–308. [DOI] [PubMed] [Google Scholar]
- [88].Ma J, Wang J, Ai X, Zhang S. Biomimetic self-assembly of apatite hybrid materials: from a single molecular template to bi-/multi-molecular templates. Biotechnol Adv. 2014;32:744–60. [DOI] [PubMed] [Google Scholar]
- [89].Bleek K, Taubert A. New developments in polymer-controlled, bioinspired calcium phosphate mineralization from aqueous solution. Acta Biomater. 2013;9:6283–321. [DOI] [PubMed] [Google Scholar]
- [90].Cui F-Z, Li Y, Ge J. Self-assembly of mineralized collagen composites. Mater Sci Eng R-Rep. 2007;57:1–27. [Google Scholar]
- [91].Liao SS, Cui FZ, Zhang W, Feng QL. Hierarchically biomimetic bone scaffold materials: nano-HA/collagen/PLA composite. J Biomed Mater Res B Appl Biomater. 2004;69:158–65. [DOI] [PubMed] [Google Scholar]
- [92].Qiu ZY, Tao CS, Cui H, Wang CM, Cui FZ. High-strength mineralized collagen artificial bone. Front Mater Sci. 2014;8:53–62. [Google Scholar]
- [93].Qiu ZY, Cui Y, Tao CS, Zhang ZQ, Tang PF, Mao KY, et al. Mineralized Collagen: Rationale, Current Status, and Clinical Applications. Materials. 2015;8:4733–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kou JM, Fu TY, Jia XJ, Hou JW, Gao C, Ma YZ, et al. Clinical Observations on Repair of Non-Infected Bone Nonunion by Using Mineralized Collagen Graft. J Biomater Tiss Eng. 2014;4:1107–12. [Google Scholar]
- [95].Zhang C, Yan B, Cui Z, Cui S, Zhang T, Wang X, et al. Bone regeneration in minipigs by intrafibrillarlymineralized collagen loaded with autologous periodontal ligament stem cells. Sci Rep. 2017;7:10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ma L, Wang X, Zhao N, Zhu Y, Qiu Z, Li Q, et al. Integrating 3D Printing and Biomimetic Mineralization for Personalized Enhanced Osteogenesis, Angiogenesis, and Osteointegration. ACS Appl Mater Interfaces. 2018;10:42146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kong XD, Cui FZ, Wang XM, Zhang M, Zhang W. Silk fibroin regulated mineralization of hydroxyapatite nanocrystals. J Cryst Growth. 2004;270:197–202. [Google Scholar]
- [98].Melke J, Midha S, Ghosh S, Ito K, Hofmann S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016;31:1–16. [DOI] [PubMed] [Google Scholar]
- [99].He X, Huang X, Lu Q, Bai S, Zhu H. Nanoscale control of silks for regular hydroxyapatite formation. Progress in Natural Science: Materials International. 2012;22:115–9. [Google Scholar]
- [100].Huang X, Liu X, Liu S, Zhang A, Lu Q, Kaplan DL, et al. Biomineralization regulation by nano-sized features in silk fibroin proteins: synthesis of water-dispersible nano-hydroxyapatite. J Biomed Mater Res B Appl Biomater. 2014;102:1720–9. [DOI] [PubMed] [Google Scholar]
- [101].Ding ZZ, Fan ZH, Huang XW, Bai SM, Song DW, Lu Q, et al. Bioactive natural protein-hydroxyapatite nanocarriers for optimizing osteogenic differentiation of mesenchymal stem cells. Journal Of Materials Chemistry B. 2016;4:3555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Huang X, Bai S, Lu Q, Liu X, Liu S, Zhu H. Osteoinductive-nanoscaled silk/HA composite scaffolds for bone tissue engineering application. J Biomed Mater Res Part B. 2015;103:1402–14. [DOI] [PubMed] [Google Scholar]
- [103].Yao X, Yao H, Li G, Li Y. Biomimetic synthesis of needle-like nano-hydroxyapatite templated by double-hydrophilic block copolymer. J Mater Sci. 2010;45:1930–6. [Google Scholar]
- [104].Wang J, Ouyang Z, Ren Z, Li J, Zhang P, Wei G, et al. Self-assembled peptide nanofibers on graphene oxide as a novel nanohybrid for biomimetic mineralization of hydroxyapatite. Carbon. 2015;89:20–30. [Google Scholar]
- [105].Depan D, Pesacreta TC, Misra RDK. The synergistic effect of a hybrid graphene oxide–chitosan system and biomimetic mineralization on osteoblast functions. Biomater Sci. 2014;2:264–74. [DOI] [PubMed] [Google Scholar]
- [106].Wei G, Zhang J, Xie L, Jandt KD. Biomimetic growth of hydroxyapatite on super water-soluble carbon nanotube-protein hybrid nanofibers. Carbon. 2011;49:2216–26. [Google Scholar]
- [107].Jo BS, Lee Y, Suh JS, Park YS, Lee HJ, Lee JY, et al. A novel calcium-accumulating peptide/gelatin in situ forming hydrogel for enhanced bone regeneration. J Biomed Mater Res Part A. 2018;106:531–42. [DOI] [PubMed] [Google Scholar]
- [108].Wang J, Zhou W, Hu W, Zhou L, Wang S, Zhang S. Collagen/silk fibroin bi-template induced biomimetic bone-like substitutes. J Biomed Mater Res A. 2011;99:327–34. [DOI] [PubMed] [Google Scholar]
- [109].Wang J, Yang Q, Mao C, Zhang S. Osteogenic differentiation of bone marrow mesenchymal stem cells on the collagen/silk fibroin bi-template-induced biomimetic bone substitutes. J Biomed Mater Res A. 2012;100:2929–38. [DOI] [PubMed] [Google Scholar]
- [110].Yang Q, Du Y, Wang Y, Wang Z, Ma J, Wang J, et al. Si-doping bone composite based on protein template-mediated assembly for enhancing bone regeneration. Front Mater Sci. 2017;11:106–19. [Google Scholar]
- [111].Zhong Z, Qin J, Ma J. Electrophoretic deposition of biomimetic zinc substituted hydroxyapatite coatings with chitosan and carbon nanotubes on titanium. Ceram Int. 2015;41:8878–84. [Google Scholar]
- [112].Ma J, Qin J. Graphene-like Zinc Substituted Hydroxyapatite. Crystal Growth & Design. 2015;15:1273–9. [Google Scholar]
- [113].Nadeem D, Smith CA, Dalby MJ, Meek RMD, Lin S, Li G, et al. Three-dimensional CaP/gelatin lattice scaffolds with integrated osteoinductive surface topographies for bone tissue engineering. Biofabrication. 2015;7. [DOI] [PubMed] [Google Scholar]
- [114].Boyan BD, Bonewald LF, Paschalis EP, Lohmann CH, Rosser J, Cochran DL, et al. Osteoblast-mediated mineral deposition in culture is dependent on surface microtopography. Calcif Tissue Int. 2002;71:519–29. [DOI] [PubMed] [Google Scholar]
- [115].Ber S, Kose GT, Hasirci V. Bone tissue engineering on patterned collagen films: an in vitro study. Biomaterials. 2005;26:1977–86. [DOI] [PubMed] [Google Scholar]
- [116].Kenar H, Kose GT, Hasirci V. Tissue engineering of bone on micropatterned biodegradable polyester films. Biomaterials. 2006;27:885–95. [DOI] [PubMed] [Google Scholar]
- [117].Henkel J, Woodruff MA, Epari DR, Steck R, Glatt V, Dickinson IC, et al. Bone Regeneration Based on Tissue Engineering Conceptions - A 21st Century Perspective. Bone Res. 2013;1:216–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Farshid B, Lalwani G, Mohammadi MS, Simonsen J, Sitharaman B. Boron nitride nanotubes and nanoplatelets as reinforcing agents of polymeric matrices for bone tissue engineering. J Biomed Mater Res Part B. 2017;105:406–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Shakir M, Jolly R, Khan AA, Ahmed SS, Alam S, Rauf MA, et al. Resol based chitosan/nanohydroxyapatite nanoensemble for effective bone tissue engineering. Carbohydr Polym. 2018;179:317–27. [DOI] [PubMed] [Google Scholar]
- [120].Takano Y, Turner CH, Owan I, Martin RB, Lau ST, Forwood MR, et al. Elastic anisotropy and collagen orientation of osteonal bone are dependent on the mechanical strain distribution. J Orth Res. 1999;17:59–66. [DOI] [PubMed] [Google Scholar]
- [121].Perizzolo D, Lacefield WR, Brunette DM. Interaction between topography and coating in the formation of bone nodules in culture for hydroxyapatite- and titanium-coated micromachined surfaces. J Biomed Mater Res. 2001;56:494–503. [DOI] [PubMed] [Google Scholar]
- [122].Badami AS, Kreke MR, Thompson MS, Riffle JS, Goldstein AS. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials. 2006;27:596–606. [DOI] [PubMed] [Google Scholar]
- [123].Terranova L, Mallet R, Perrot R, Chappard D. Polystyrene scaffolds based on microfibers as a bone substitute; development and in vitro study. Acta Biomater. 2016;29:380–8. [DOI] [PubMed] [Google Scholar]
- [124].Sankar S, Sharma CS, Rath SN. Enhanced osteodifferentiation of MSC spheroids on patterned electrospun fiber mats - An advanced 3D double strategy for bone tissue regeneration. Materials Science & Engineering C-Materials for Biological Applications. 2019;94:703–12. [DOI] [PubMed] [Google Scholar]
- [125].Wilkinson A, Hewitt RN, McNamara LE, McCloy D, Dominic Meek RM, Dalby MJ. Biomimetic microtopography to enhance osteogenesis in vitro. Acta Biomater. 2011;7:2919–25. [DOI] [PubMed] [Google Scholar]
- [126].Cao X Targeting osteoclast-osteoblast communication. Nat Med. 2011;17:1344–6. [DOI] [PubMed] [Google Scholar]
- [127].Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci U S A. 2010;107:4872–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Jose MV, Thomas V, Johnson KT, Dean DR, Nyairo E. Aligned PLGA/HA nanofibrous nanocomposite scaffolds for bone tissue engineering. Acta Biomater. 2009;5:305–15. [DOI] [PubMed] [Google Scholar]
- [129].Anjaneyulu U, Priyadarshini B, Grace AN, Vijayalakshmi U. Fabrication and characterization of Ag doped hydroxyapatite-polyvinyl alcohol composite nanofibers and its in vitro biological evaluations for bone tissue engineering applications. J Sol-Gel Sci Technol. 2017;81:750–61. [Google Scholar]
- [130].Zhang YZ, Venugopal JR, El-Turki A, Ramakrishna S, Su B, Lim CT. Electrospun biomimetic nanocomposite nanofibers of hydroxyapatite/chitosan for bone tissue engineering. Biomaterials. 2008;29:4314–22. [DOI] [PubMed] [Google Scholar]
- [131].Gong T, Xie J, Liao J, Zhang T, Lin S, Lin Y. Nanomaterials and bone regeneration. Bone Res. 2015;3:15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Lim JY, Hansen JC, Siedlecki CA, Runt J, Donahue HJ. Human foetal osteoblastic cell response to polymer-demixed nanotopographic interfaces. Journal of the Royal Society, Interface. 2005;2:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Xu T, Yang H, Yang D, Yu ZZ. Polylactic Acid Nanofiber Scaffold Decorated with Chitosan Islandlike Topography for Bone Tissue Engineering. ACS Appl Mater Interfaces. 2017;9:21094–104. [DOI] [PubMed] [Google Scholar]
- [134].Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007;6:997–1003. [DOI] [PubMed] [Google Scholar]
- [135].Wang J, Wang L, Li X, Mao C. Virus activated artificial ECM induces the osteoblastic differentiation of mesenchymal stem cells without osteogenic supplements. Sci Rep. 2013;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Obata A, Hotta T, Wakita T, Ota Y, Kasuga T. Electrospun microfiber meshes of silicon-doped vaterite/poly(lactic acid) hybrid for guided bone regeneration. Acta Biomater. 2010;6:1248–57. [DOI] [PubMed] [Google Scholar]
- [137].Nejati E, Firouzdor V, Eslaminejad MB, Bagheri F. Needle-like nano hydroxyapatite/poly(L-lactide acid) composite scaffold for bone tissue engineering application. Mat Sci Eng C-Bio S. 2009;29:942–9. [Google Scholar]
- [138].Wang P, Zhao L, Liu J, Weir MD, Zhou X, Xu HH. Bone tissue engineering via nanostructured calcium phosphate biomaterials and stem cells. Bone Res. 2014;2:14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Hickey DJ, Ercan B, Sun LL, Webster TJ. Adding MgO nanoparticles to hydroxyapatite-PLLA nanocomposites for improved bone tissue engineering applications. Acta Biomater. 2015;14:175–84. [DOI] [PubMed] [Google Scholar]
- [140].Zhou YY, Yao HC, Wang JS, Wang DL, Liu Q, Li ZJ. Greener synthesis of electrospun collagen/hydroxyapatite composite fibers with an excellent microstructure for bone tissue engineering. International journal of nanomedicine. 2015;10:3203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Lee EJ, Kasper FK, Mikos AG. Biomaterials for Tissue Engineering. Ann Biomed Eng. 2014;42:323–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Cox SC, Thornby JA, Gibbons GJ, Williams MA, Mallick KK. 3D printing of porous hydroxyapatite scaffolds intended for use in bone tissue engineering applications. Mater Sci Eng C-Mater Biol Appl. 2015;47:237–47. [DOI] [PubMed] [Google Scholar]
- [143].Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:3413–31. [DOI] [PubMed] [Google Scholar]
- [144].Wegst UG, Bai H, Saiz E, Tomsia AP, Ritchie RO. Bioinspired structural materials. Nat Mater. 2015;14:23–36. [DOI] [PubMed] [Google Scholar]
- [145].Elomaa L, Teixeira S, Hakala R, Korhonen H, Grijpma DW, Seppala JV. Preparation of poly(epsilon-caprolactone)-based tissue engineering scaffolds by stereolithography. Acta Biomater. 2011;7:3850–6. [DOI] [PubMed] [Google Scholar]
- [146].Du Y, Liu H, Shuang J, Wang J, Ma J, Zhang S. Microsphere-based selective laser sintering for building macroporous bone scaffolds with controlled microstructure and excellent biocompatibility. Colloids Surf B Biointerfaces. 2015;135:81–9. [DOI] [PubMed] [Google Scholar]
- [147].Babilotte J, Guduric V, Le Nihouannen D, Naveau A, Fricain JC, Catros S. 3D printed polymer-mineral composite biomaterials for bone tissue engineering: Fabrication and characterization. J Biomed Mater Res B Appl Biomater. 2019. [DOI] [PubMed] [Google Scholar]
- [148].Alehosseini M, Golafshan N, Kharaziha M, Fathi M, Edris H. Hemocompatible and Bioactive Heparin-Loaded PCL-α-TCP Fibrous Membranes for Bone Tissue Engineering. Macromol Biosci. 2018;18. [DOI] [PubMed] [Google Scholar]
- [149].Malikmammadov E, Tanir TE, Kiziltay A, Hasirci V, Hasirci N. PCL-TCP wet spun scaffolds carrying antibiotic-loaded microspheres for bone tissue engineering. J Biomat Sci-Polym E. 2018;29:805–24. [DOI] [PubMed] [Google Scholar]
- [150].Bracaglia LG, Smith BT, Watson E, Arumugasaamy N, Mikos AG, Fisher JP. 3D printing for the design and fabrication of polymer-based gradient scaffolds. Acta Biomater. 2017;56:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Jones JR, Ehrenfried LM, Hench LL. Optimising bioactive glass scaffolds for bone tissue engineering. Biomaterials. 2006;27:964–73. [DOI] [PubMed] [Google Scholar]
- [152].Zhang XD, Zeng DL, Li N, Wen J, Jiang XQ, Liu CS, et al. Functionalized mesoporous bioactive glass scaffolds for enhanced bone tissue regeneration. Sci Rep. 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Peter M, Binulal NS, Nair SV, Selvamurugan N, Tamura H, Jayakumar R. Novel biodegradable chitosan-gelatin/nano-bioactive glass ceramic composite scaffolds for alveolar bone tissue engineering. Chem Eng J. 2010;158:353–61. [Google Scholar]
- [154].Zhao SC, Zhang JH, Zhu M, Zhang YD, Liu ZT, Tao CL, et al. Three-dimensional printed strontium-containing mesoporous bioactive glass scaffolds for repairing rat critical-sized calvarial defects. Acta Biomater. 2015;12:270–80. [DOI] [PubMed] [Google Scholar]
- [155].Zhou YH, Shi MC, Jones JR, Chen ZT, Chang J, Wu CT, et al. Strategies to direct vascularisation using mesoporous bioactive glass-based biomaterials for bone regeneration. Int Mater Rev. 2017;62:392–414. [Google Scholar]
- [156].Izquierdo-Barba I, Salinas AJ, Vallet-Regi M. Bioactive Glasses: From Macro to Nano. Int J Appl Glass Sci. 2013;4:149–61. [Google Scholar]
- [157].Bao M, Xie J, Piruska A, Huck WTS. 3D microniches reveal the importance of cell size and shape. Nat Commun. 2017;8:1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–85. [DOI] [PubMed] [Google Scholar]
- [159].Arcaute K, Mann BK, Wicker RB. Stereolithography of three-dimensional bioactive poly(ethylene glycol) constructs with encapsulated cells. Ann Biomed Eng. 2006;34:1429–41. [DOI] [PubMed] [Google Scholar]
- [160].Gao C, Feng P, Peng S, Shuai C. Carbon nanotube, graphene and boron nitride nanotube reinforced bioactive ceramics for bone repair. Acta Biomater. 2017;61:1–20. [DOI] [PubMed] [Google Scholar]
- [161].Shuai C, Feng P, Wu P, Liu Y, Liu X, Lai D, et al. A combined nanostructure constructed by graphene and boron nitride nanotubes reinforces ceramic scaffolds. Chem Eng J. 2017;313:487–97. [Google Scholar]
- [162].Feng P, Peng S, Wu P, Gao C, Huang W, Deng Y, et al. A nano-sandwich construct built with graphene nanosheets and carbon nanotubes enhances mechanical properties of hydroxyapatite-polyetheretherketone scaffolds. Int J Nanomedicine. 2016;11:3487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Peng S, Feng P, Wu P, Huang W, Yang Y, Guo W, et al. Graphene oxide as an interface phase between polyetheretherketone and hydroxyapatite for tissue engineering scaffolds. Sci Rep. 2017;7:46604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Bittner SM, Smith BT, Diaz-Gomez L, Hudgins CD, Melchiorri AJ, Scott DW, et al. Fabrication and mechanical characterization of 3D printed vertical uniform and gradient scaffolds for bone and osteochondral tissue engineering. Acta Biomater. 2019;90:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Diaz-Gomez L, Smith BT, Kontoyiannis PD, Bittner SM, Melchiorri AJ, Mikos AG. Multimaterial Segmented Fiber Printing for Gradient Tissue Engineering. Tissue engineering Part C, Methods. 2019;25:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Hao J, Zhang Y, Jing D, Shen Y, Tang G, Huang S, et al. Mechanobiology of mesenchymal stem cells: Perspective into mechanical induction of MSC fate. Acta Biomater. 2015;20:1–9. [DOI] [PubMed] [Google Scholar]
- [167].Balint R, Cassidy NJ, Cartmell SH. Electrical Stimulation: A Novel Tool for Tissue Engineering. Tissue Eng Part B-Re. 2013;19:48–57. [DOI] [PubMed] [Google Scholar]
- [168].Ozcivici E, Luu YK, Adler B, Qin YX, Rubin J, Judex S, et al. Mechanical signals as anabolic agents in bone. Nat Rev Rheumatol. 2010;6:50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Santos LJ, Reis RL, Gomes ME. Harnessing magnetic-mechano actuation in regenerative medicine and tissue engineering. Trends Biotechnol. 2015;33:471–9. [DOI] [PubMed] [Google Scholar]
- [170].Ravichandran A, Lim J, Chong MSK, Wen F, Liu Y, Pillay YT, et al. In vitro cyclic compressive loads potentiate early osteogenic events in engineered bone tissue. J Biomed Mater Res B Appl Biomater. 2017;105:2366–75. [DOI] [PubMed] [Google Scholar]
- [171].Steinmetz NJ, Aisenbrey EA, Westbrook KK, Qi HJ, Bryant SJ. Mechanical loading regulates human MSC differentiation in a multi-layer hydrogel for osteochondral tissue engineering. Acta Biomater. 2015;21:142–53. [DOI] [PubMed] [Google Scholar]
- [172].Subramony SD, Dargis BR, Castillo M, Azeloglu EU, Tracey MS, Su A, et al. The guidance of stem cell differentiation by substrate alignment and mechanical stimulation. Biomaterials. 2013;34:1942–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Tandon B, Blaker JJ, Cartmell SH. Piezoelectric materials as stimulatory biomedical materials and scaffolds for bone repair. Acta Biomater. 2018. [DOI] [PubMed] [Google Scholar]
- [174].Damaraju SM, Shen Y, Elele E, Khusid B, Eshghinejad A, Li J, et al. Three-dimensional piezoelectric fibrous scaffolds selectively promote mesenchymal stem cell differentiation. Biomaterials. 2017;149:51–62. [DOI] [PubMed] [Google Scholar]
- [175].Ribeiro C, Correia DM, Rodrigues I, Guardão L, Guimarães S, Soares R, et al. In vivo demonstration of the suitability of piezoelectric stimuli for bone reparation. Mater Lett. 2017;209:118–21. [Google Scholar]
- [176].Zhang X, Zhang C, Lin Y, Hu P, Shen Y, Wang K, et al. Nanocomposite Membranes Enhance Bone Regeneration Through Restoring Physiological Electric Microenvironment. ACS Nano. 2016;10:7279–86. [DOI] [PubMed] [Google Scholar]
- [177].Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125:3015–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell stem cell. 2009;5:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. [DOI] [PubMed] [Google Scholar]
- [180].Pek YS, Wan AC, Ying JY. The effect of matrix stiffness on mesenchymal stem cell differentiation in a 3D thixotropic gel. Biomaterials. 2010;31:385–91. [DOI] [PubMed] [Google Scholar]
- [181].Hsieh WT, Liu YS, Lee YH, Rimando MG, Lin KH, Lee OK. Matrix dimensionality and stiffness cooperatively regulate osteogenesis of mesenchymal stromal cells. Acta Biomater. 2016;32:210–22. [DOI] [PubMed] [Google Scholar]
- [182].Li X, Huang Y, Zheng L, Liu H, Niu X, Huang J, et al. Effect of substrate stiffness on the functions of rat bone marrow and adipose tissue derived mesenchymal stem cells in vitro. J Biomed Mater Res A. 2014;102:1092–101. [DOI] [PubMed] [Google Scholar]
- [183].Chatterjee K, Lin-Gibson S, Wallace WE, Parekh SH, Lee YJ, Cicerone MT, et al. The effect of 3D hydrogel scaffold modulus on osteoblast differentiation and mineralization revealed by combinatorial screening. Biomaterials. 2010;31:5051–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Lv H, Li L, Sun M, Zhang Y, Chen L, Rong Y, et al. Mechanism of regulation of stem cell differentiation by matrix stiffness. Stem Cell Res Ther. 2015;6:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Shih YR, Tseng KF, Lai HY, Lin CH, Lee OK. Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. J Bone Miner Res. 2011;26:730–8. [DOI] [PubMed] [Google Scholar]
- [186].Wu L, Magaz A, Wang T, Liu C, Darbyshire A, Loizidou M, et al. Stiffness memory of indirectly 3D-printed elastomer nanohybrid regulates chondrogenesis and osteogenesis of human mesenchymal stem cells. Biomaterials. 2018;186:64–79. [DOI] [PubMed] [Google Scholar]
- [187].Papachroni KK, Karatzas DN, Papavassiliou KA, Basdra EK, Papavassiliou AG. Mechanotransduction in osteoblast regulation and bone disease. Trends Mol Med. 2009;15:208–16. [DOI] [PubMed] [Google Scholar]
- [188].Gan JC, Glazer PA. Electrical stimulation therapies for spinal fusions: current concepts. Eur Spine J. 2006;15:1301–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol. 2015;11:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Mobini S, Leppik L, Parameswaran VT, Barker JH. In vitro effect of direct current electrical stimulation on rat mesenchymal stem cells. Peerj. 2017;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Wang Y, Cui HT, Wu ZX, Wu NP, Wang ZL, Chen XS, et al. Modulation of Osteogenesis in MC3T3-E1 Cells by Different Frequency Electrical Stimulation. PLoS One. 2016;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Tsai MT, Li WJ, Tuan RS, Chang WH. Modulation of Osteogenesis in Human Mesenchymal Stem Cells by Specific Pulsed Electromagnetic Field Stimulation. J Orth Res. 2009;27:1169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Zhao Z, Watt C, Karystinou A, Roelofs AJ, McCaig CD, Gibson IR, et al. Directed migration of human bone marrow mesenchymal stem cells in a physiological direct current electric field. Eur Cell Mater. 2011;22:344–58. [DOI] [PubMed] [Google Scholar]
- [194].Brighton CT, Wang W, Seldes R, Zhang GH, Pollack SR. Signal transduction in electrically stimulated bone cells. Journal Of Bone And Joint Surgery-American Volume. 2001;83A:1514–23. [DOI] [PubMed] [Google Scholar]
- [195].Zayzafoon M Calcium/calmodulin signaling controls osteoblast growth and differentiation. J Cell Biochem. 2006;97:56–70. [DOI] [PubMed] [Google Scholar]
- [196].Balint R, Cassidy NJ, Cartmell SH. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014;10:2341–53. [DOI] [PubMed] [Google Scholar]
- [197].Zhang J, Li M, Kang ET, Neoh KG. Electrical stimulation of adipose-derived mesenchymal stem cells in conductive scaffolds and the roles of voltage-gated ion channels. Acta Biomater. 2016;32:46–56. [DOI] [PubMed] [Google Scholar]
- [198].Hu WW, Hsu YT, Cheng YC, Li C, Ruaan RC, Chien CC, et al. Electrical stimulation to promote osteogenesis using conductive polypyrrole films. Mater Sci Eng C Mater Biol Appl. 2014;37:28–36. [DOI] [PubMed] [Google Scholar]
- [199].Liu H, Wang R, Chu HK, Sun D. Design and characterization of a conductive nanostructured polypyrrolepolycaprolactone coated magnesium/PLGA composite for tissue engineering scaffolds. J Biomed Mater Res A. 2015;103:2966–73. [DOI] [PubMed] [Google Scholar]
- [200].He Y, Wang S, Mu J, Dai L, Zhang Z, Sun Y, et al. Synthesis of polypyrrole nanowires with positive effect on MC3T3-E1 cell functions through electrical stimulation. Mater Sci Eng C Mater Biol Appl. 2017;71:43–50. [DOI] [PubMed] [Google Scholar]
- [201].Huang ZB, Yin GF, Liao XM, Gu JW. Conducting polypyrrole in tissue engineering applications. Front Mater Sci. 2014;8:39–45. [Google Scholar]
- [202].Rajzer I, Rom M, Menaszek E, Pasierb P. Conductive PANI patterns on electrospun PCL/gelatin scaffolds modified with bioactive particles for bone tissue engineering. Mater Lett. 2015;138:60–3. [Google Scholar]
- [203].Qazi TH, Rai R, Boccaccini AR. Tissue engineering of electrically responsive tissues using polyaniline based polymers: a review. Biomaterials. 2014;35:9068–86. [DOI] [PubMed] [Google Scholar]
- [204].Guex AG, Puetzer JL, Armgarth A, Littmann E, Stavrinidou E, Giannelis EP, et al. Highly porous scaffolds of PEDOT:PSS for bone tissue engineering. Acta Biomater. 2017;62:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Shahini A, Yazdimamaghani M, Walker KJ, Eastman MA, Hatami-Marbini H, Smith BJ, et al. 3D conductive nanocomposite scaffold for bone tissue engineering. Int J Nanomedicine. 2014;9:167–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Hardy JG, Geissler SA, Aguilar D Jr., Villancio-Wolter MK, Mouser DJ, Sukhavasi RC, et al. Instructive Conductive 3D Silk Foam-Based Bone Tissue Scaffolds Enable Electrical Stimulation of Stem Cells for Enhanced Osteogenic Differentiation. Macromol Biosci. 2015;15:1490–6. [DOI] [PubMed] [Google Scholar]
- [207].Thrivikraman G, Lee PS, Hess R, Haenchen V, Basu B, Scharnweber D. Interplay of Substrate Conductivity, Cellular Microenvironment, and Pulsatile Electrical Stimulation toward Osteogenesis of Human Mesenchymal Stem Cells in Vitro. ACS Appl Mater Interfaces. 2015;7:23015–28. [DOI] [PubMed] [Google Scholar]
- [208].Li Y, Li X, Zhao R, Wang C, Qiu F, Sun B, et al. Enhanced adhesion and proliferation of human umbilical vein endothelial cells on conductive PANI-PCL fiber scaffold by electrical stimulation. Mater Sci Eng C Mater Biol Appl. 2017;72:106–12. [DOI] [PubMed] [Google Scholar]
- [209].Xie M, Wang L, Ge J, Guo B, Ma PX. Strong Electroactive Biodegradable Shape Memory Polymer Networks Based on Star-Shaped Polylactide and Aniline Trimer for Bone Tissue Engineering. ACS Appl Mater Interfaces. 2015;7:6772–81. [DOI] [PubMed] [Google Scholar]
- [210].Guo B, Glavas L, Albertsson A-C. Biodegradable and electrically conducting polymers for biomedical applications. Prog Polym Sci. 2013;38:1263–86. [Google Scholar]
- [211].Cha CY, Shin SR, Annabi N, Dokmeci MR, Khademhosseini A. Carbon-Based Nanomaterials: Multifunctional Materials for Biomedical Engineering. ACS Nano. 2013;7:2891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Sayyar S, Bjorninen M, Haimi S, Miettinen S, Gilmore K, Grijpma D, et al. UV Cross-Linkable Graphene/Poly(trimethylene Carbonate) Composites for 3D Printing of Electrically Conductive Scaffolds. ACS Appl Mater Interfaces. 2016;8:31916–25. [DOI] [PubMed] [Google Scholar]
- [213].Shao S, Zhou S, Li L, Li J, Luo C, Wang J, et al. Osteoblast function on electrically conductive electrospun PLA/MWCNTs nanofibers. Biomaterials. 2011;32:2821–33. [DOI] [PubMed] [Google Scholar]
- [214].Hardy JG, Lee JY, Schmidt CE. Biomimetic conducting polymer-based tissue scaffolds. Curr Opin Biotechnol. 2013;24:847–54. [DOI] [PubMed] [Google Scholar]
- [215].Fredericks DC, Nepola JV, Baker JT, Abbott J, Simon B. Effects of pulsed electromagnetic fields on bone healing in a rabbit tibial osteotomy model. J Orthop Trauma. 2000;14:93–100. [DOI] [PubMed] [Google Scholar]
- [216].Puricelli E, Ulbrich LM, Ponzoni D, Filho JJ. Histological analysis of the effects of a static magnetic field on bone healing process in rat femurs. Head Face Med. 2006;2:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Wang J, An YX, Li FJ, Li DM, Jing D, Guo TW, et al. The effects of pulsed electromagnetic field on the functions of osteoblasts on implant surfaces with different topographies. Acta Biomater. 2014;10:975–85. [DOI] [PubMed] [Google Scholar]
- [218].Jansen JHW, van der Jagt OP, Punt BJ, Verhaar JAN, van Leeuwen JPTM, Weinans H, et al. Stimulation of osteogenic differentiation in human osteoprogenitor cells by pulsed electromagnetic fields: an in vitro study. BMC Musculoskel Disord. 2010;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Maredziak M, Smieszek A, Tomaszewski KA, Lewandowski D, Marycz K. The effect of low static magnetic field on osteogenic and adipogenic differentiation potential of human adipose stromal/stem cells. J Magn Magn Mater. 2016;398:235–45. [Google Scholar]
- [220].Petecchia L, Sbrana F, Utzeri R, Vercellino M, Usai C, Visai L, et al. Electro-magnetic field promotes osteogenic differentiation of BM-hMSCs through a selective action on Ca2+-related mechanisms. Sci Rep. 2015;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Zhu Y, Yang Q, Yang M, Zhan X, Lan F, He J, et al. Protein Corona of Magnetic Hydroxyapatite Scaffold Improves Cell Proliferation via Activation of Mitogen-Activated Protein Kinase Signaling Pathway. ACS Nano. 2017;11:3690–704. [DOI] [PubMed] [Google Scholar]
- [222].Cai Q, Shi Y, Shan D, Jia W, Duan S, Deng X, et al. Osteogenic differentiation of MC3T3-E1 cells on poly(L-lactide)/Fe3O4 nanofibers with static magnetic field exposure. Mater Sci Eng C Mater Biol Appl. 2015;55:166–73. [DOI] [PubMed] [Google Scholar]
- [223].Zhang JH, Zhao SC, Zhu M, Zhu YF, Zhang YD, Liu ZT, et al. 3D-printed magnetic Fe3O4/MBG/PCL composite scaffolds with multifunctionality of bone regeneration, local anticancer drug delivery and hyperthermia. Journal Of Materials Chemistry B. 2014;2:7583–95. [DOI] [PubMed] [Google Scholar]
- [224].Li X, Wei J, Aifantis KE, Fan Y, Feng Q, Cui FZ, et al. Current investigations into magnetic nanoparticles for biomedical applications. J Biomed Mater Res A. 2016;104:1285–96. [DOI] [PubMed] [Google Scholar]
- [225].Nguyen DH, Lee JS, Choi JH, Park KM, Lee Y, Park KD. Hierarchical self-assembly of magnetic nanoclusters for theranostics: Tunable size, enhanced magnetic resonance imagability, and controlled and targeted drug delivery. Acta Biomater. 2016;35:109–17. [DOI] [PubMed] [Google Scholar]
- [226].Meng J, Xiao B, Zhang Y, Liu J, Xue H, Lei J, et al. Super-paramagnetic responsive nanofibrous scaffolds under static magnetic field enhance osteogenesis for bone repair in vivo. Sci Rep. 2013;3:2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Singh RK, Patel KD, Lee JH, Lee EJ, Kim JH, Kim TH, et al. Potential of magnetic nanofiber scaffolds with mechanical and biological properties applicable for bone regeneration. PLoS One. 2014;9:e91584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Yun HM, Ahn SJ, Park KR, Kim MJ, Kim JJ, Jin GZ, et al. Magnetic nanocomposite scaffolds combined with static magnetic field in the stimulation of osteoblastic differentiation and bone formation. Biomaterials. 2016;85:88–98. [DOI] [PubMed] [Google Scholar]
- [229].Panseri S, Russo A, Sartori M, Giavaresi G, Sandri M, Fini M, et al. Modifying bone scaffold architecture in vivo with permanent magnets to facilitate fixation of magnetic scaffolds. Bone. 2013;56:432–9. [DOI] [PubMed] [Google Scholar]
- [230].Xu H-Y, Gu N. Magnetic responsive scaffolds and magnetic fields in bone repair and regeneration. Front Mater Sci. 2014;8:20–31. [Google Scholar]
- [231].Sapir Y, Cohen S, Friedman G, Polyak B. The promotion of in vitro vessel-like organization of endothelial cells in magnetically responsive alginate scaffolds. Biomaterials. 2012;33:4100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Sapir-Lekhovitser Y, Rotenberg MY, Jopp J, Friedman G, Polyak B, Cohen S. Magnetically actuated tissue engineered scaffold: insights into mechanism of physical stimulation. Nanoscale. 2016;8:3386–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Li C, Armstrong JP, Pence IJ, Kit-Anan W, Puetzer JL, Correia Carreira S, et al. Glycosylated superparamagnetic nanoparticle gradients for osteochondral tissue engineering. Biomaterials. 2018;176:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Yanagi T, Kajiya H, Kawaguchi M, Kido H, Fukushima T. Photothermal stress triggered by near infrared-irradiated carbon nanotubes promotes bone deposition in rat calvarial defects. J Biomater Appl. 2015;29:1109–18. [DOI] [PubMed] [Google Scholar]
- [235].Tong L, Liao Q, Zhao Y, Huang H, Gao A, Zhang W, et al. Near-infrared light control of bone regeneration with biodegradable photothermal osteoimplant. Biomaterials. 2019;193:1–11. [DOI] [PubMed] [Google Scholar]
- [236].Ma H, Jiang C, Zhai D, Luo Y, Chen Y, Lv F, et al. A Bifunctional Biomaterial with Photothermal Effect for Tumor Therapy and Bone Regeneration. Adv Funct Mater. 2016;26:1197–208. [Google Scholar]
- [237].Ma H, Luo J, Sun Z, Xia L, Shi M, Liu M, et al. 3D printing of biomaterials with mussel-inspired nanostructures for tumor therapy and tissue regeneration. Biomaterials. 2016;111:138–48. [DOI] [PubMed] [Google Scholar]
- [238].Liu Y, Li T, Ma H, Zhai D, Deng C, Wang J, et al. 3D-printed scaffolds with bioactive elements-induced photothermal effect for bone tumor therapy. Acta Biomater. 2018;73:531–46. [DOI] [PubMed] [Google Scholar]
- [239].Dang W, Li T, Li B, Ma H, Zhai D, Wang X, et al. A bifunctional scaffold with CuFeSe2 nanocrystals for tumor therapy and bone reconstruction. Biomaterials. 2018;160:92–106. [DOI] [PubMed] [Google Scholar]
- [240].Tiwari JN, Seo YK, Yoon T, Lee WG, Cho WJ, Yousuf M, et al. Accelerated Bone Regeneration by Two-Photon Photoactivated Carbon Nitride Nanosheets. ACS Nano. 2017;11:742–51. [DOI] [PubMed] [Google Scholar]
- [241].Woodruff MA, Lange C, Reichert J, Berner A, Chen F, Fratzl P, et al. Bone tissue engineering: from bench to bedside. Mater Today. 2012;15:430–5. [Google Scholar]
- [242].Roddy E, DeBaun MR, Daoud-Gray A, Yang YP, Gardner MJ. Treatment of critical-sized bone defects: clinical and tissue engineering perspectives. Eur J Orthop Surg Traumatol. 2018;28:351–62. [DOI] [PubMed] [Google Scholar]
- [243].Hoffman T, Khademhosseini A, Langer RS. Chasing the Paradigm: Clinical Translation of 25 Years of Tissue Engineering. Tissue Eng Part A. 2019;25:679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Xian L, Wu X, Pang L, Lou M, Rosen CJ, Qiu T, et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med. 2012;18:1095–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Wu M, Chen G, Li YP. TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4:16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Crane JL, Cao X. Bone marrow mesenchymal stem cells and TGF-beta signaling in bone remodeling. J Clin Invest. 2014;124:466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Xie H, Cui Z, Wang L, Xia Z, Hu Y, Xian L, et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med. 2014;20:1270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Li T, Peng M, Yang Z, Zhou X, Deng Y, Jiang C, et al. 3D-printed IFN-gamma-loading calcium silicate-beta-tricalcium phosphate scaffold sequentially activates M1 and M2 polarization of macrophages to promote vascularization of tissue engineering bone. Acta Biomater. 2018;71:96–107. [DOI] [PubMed] [Google Scholar]
- [249].Sridharan R, Cameron AR, Kelly DJ, Kearney CJ, O’Brien FJ. Biomaterial based modulation of macrophage polarization: a review and suggested design principles. Mater Today. 2015;18:313–25. [Google Scholar]
- [250].Chen Z, Klein T, Murray RZ, Crawford R, Chang J, Wu C, et al. Osteoimmunomodulation for the development of advanced bone biomaterials. Mater Today. 2016;19:304–21. [Google Scholar]
- [251].Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, et al. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19:704–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Shen J, Li S, Chen D. TGF-beta signaling and the development of osteoarthritis. Bone Res. 2014;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Baldwin JG, Wagner F, Martine LC, Holzapfel BM, Theodoropoulos C, Bas O, et al. Periosteum tissue engineering in an orthotopic in vivo platform. Biomaterials. 2017;121:193–204. [DOI] [PubMed] [Google Scholar]
- [254].Marrella A, Lee TY, Lee DH, Karuthedom S, Syla D, Chawla A, et al. Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Mater Today. 2018;21:362–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Bittner SM, Guo JL, Mikos AG. Spatiotemporal control of growth factors in three-dimensional printed scaffolds. Bioprinting. 2018;12:e00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Koons GL, Mikos AG. Progress in three-dimensional printing with growth factors. J Controlled Release. 2019;295:50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]