Abstract
Objectives:
We previously identified CYR61 as an HDAC5-repressed gene in scleroderma (SSc) endothelial cells (ECs). When overexpressed, CYR61 promoted angiogenesis in SSc ECs. In this study we examined the role of CYR61 in fibrosis and determined the mechanisms involved in CYR61-mediated angiogenesis in SSc.
Methods:
Dermal ECs and fibroblasts were isolated from biopsies from healthy subjects or patients with SSc. CYR61 was determined by qPCR, Western blotting, and ELISA. CYR61 was overexpressed using a CYR61 vector or knocked down using siRNA followed by functional and mechanistic studies in fibroblasts and ECs.
Results:
Lower mRNA levels of CYR61 were observed in SSc dermal fibroblasts and ECs compared to healthy controls. In SSc fibroblasts, overexpression of CYR61 led to significant reduction in the expression of profibrotic genes including COL1A1 and ACTA2 and an increase in the expression of matrix degrading genes including MMP1 and MMP3, and pro-angiogenic VEGF. The anti-fibrotic effect of CYR61 was further demonstrated by delay in wound healing, inhibition of gel contraction, inactivation of the TGFβ pathway, and early superoxide production associated with senescence in SSc fibroblasts. In SSc ECs, overexpression of CYR61 led to increased VEGF production. The proangiogenic effects of CYR61 were mediated by signaling through αvβ3 receptors and downstream activation of AMPK, AKT, and the eNOS/NO pathway system.
Conclusions:
We identified CYR61, which is epigenetically regulated by HDAC5, as a potent anti-fibrotic and pro-angiogenic mediator in SSc. Therapeutic intervention to promote CYR61 activity or increase CYR61 levels might be of potential benefit in SSc.
Keywords: Scleroderma, CYR61, fibrosis, angiogenesis, endothelial cells, fibroblasts
Introduction
Systemic sclerosis (scleroderma or SSc) is a connective tissue disease that is characterized by immune activation, vascular abnormalities, and progressive fibrosis in the skin and internal organs. It is a rare disease with a prevalence of 150–300 cases per million in Europe and the United States (1). This disease is associated with significant morbidity and can lead to life threatening complications including pulmonary arterial hypertension, interstitial lung disease, and scleroderma renal crisis. Although the etiology of SSc is still unclear, activation of immune cells and abnormalities of fibroblasts and endothelial cells (ECs) contribute to the disease pathogenesis (2). In addition, dysregulation epigenetic mechanisms in SSc has been implicated in various cell types, and the use of epigenetic modifying drugs has been shown to be beneficial in cells as well as in animal models of SSc (2).
We recently showed that overexpression of the anti-angiogenic histone deacetylase 5 (HDAC5) in SSc ECs contributes to impaired angiogenesis by repressing pro-angiogenic factors (3). We took an unbiased approach to examine genome-wide changes in chromatin accessibility after HDAC5 knockdown in ECs using an assay for transposase-accessible chromatin with sequencing. HDAC5 knockdown led to increased chromatin accessibility, and through bioinformatics analyses and functional assays we identified 3 novel HDAC5-target genes associated with impaired angiogenesis in SSc ECs, including CYR61 (cysteine rich angiogenic inducer 61).
CYR61 is a member of the CCN protein family (CYR61, CTGF, NOV, and WISP1–3), which plays important roles in development, inflammation, tissue repair, and a broad range of pathological processes including fibrosis and cancer (4). CYR61 assumes its diverse functions by its ability to bind different combinations of co-receptors. For example, CYR61 promotes EC proliferation and survival through binding to integrin αvβ3 (5, 6), but enhances fibroblast adhesion and senescence through binding to integrin α6β1 and heparan sulfate proteoglycans (HSPGs) (7, 8). Since this matricellular protein supports angiogenesis (6, 9) and possesses anti-fibrotic properties (8, 10), we hypothesize that CYR61 promotes angiogenesis and inhibits fibrosis in SSc ECs and fibroblasts, respectively. In this study, we characterized the expression and function of CYR61 in diffuse SSc dermal fibroblasts, and further dissected the mechanisms involved in the pro-angiogenic properties of CYR61 in this disease.
Materials and Methods
Patients and Controls.
Two 4mm punch biopsies from the distal forearm of healthy controls and patients with SSc were obtained for fibroblast and EC isolation. Plasma samples from the study participants were also collected to assess plasma CYR61 protein levels. All patients met the ACR/EULAR criteria for the classification of SSc (11). This study was approved by the Institutional Review Board of the University of Michigan and all study participants signed a written informed consent. The demographics and clinical characteristics of the study participants are summarized in Table 1.
Table 1:
SSc patients and healthy controls characteristics.
| SSc (n=62) | Healthy controls (n=40) | |
|---|---|---|
| Age (years) | 55.8 ± 1.7a | 49.5 ± 2.5 |
| Sex | F50/M12 | F31/M9 |
| Diffuse SSc | 41 | N.A. |
| Disease duration (years) | 8.9 ± 1.4 | N.A. |
| Modified Rodnan Skin Score | 12.2 ± 1.3 | N.A. |
| Raynaud’s phenomenon | 56 | N.A. |
| Early disease (< 5yrs) | 37 | N.A. |
| Digital ulcers | 12 | N.A. |
| Teleangectasias | 27 | N.A. |
| Gastrointestinal disease | 47 | N.A. |
| Interstitial lung disease | 29 | N.A. |
| Pulmonary arterial hypertension | 16 | N.A. |
| Renal involvement | 3 | N.A. |
Mean ± SEM
N.A. = Not applicable
Cell culture.
Dermal fibroblasts and ECs were isolated and characterized as previously described (13–15). After digestion ECs were purified using the CD31 MicroBead Kit (Miltenyi Biotech) and grown in EBM-2 media with growth factors (Lonza). Fibroblasts were maintained in RPMI supplemented with 10% fetal bovine serum (FBS). Cells between passage 3 and 6 were used in all experiments.
CYR61 overexpression.
Overexpression of CYR61 in ECs was performed as previously described (3). ECs were transfected with 1.65 μg of CYR61 (Origene; control vector pCMV6-XL4) and lipofectamine 2000 (3.3 μL, Invitrogen) for 24 hours in T12.5 flasks. 5 hours after transfection, culture media were changed to allow cells to grow in EGM supplemented with bovine brain extract (Lonza). To overexpress CYR61 in dermal fibroblasts, 1 μg of CYR61 and 1 μL of lipofectamine 2000 were used to transfect the cells in a 12 well plate for a total of 48 hours.
CYR61 knockdown.
CYR61 expression was knocked down using CYR61 small interfering RNA (siRNA) (ON-TARGETplus siRNA, Dharmacon), while a nontargeted siRNA (Dharmacon) was used as a control. Fibroblasts isolated from healthy controls were transfected with 350 nM of siRNA using TransIT‐TKO transfection reagent (Mirus Bio) for 48 hours before mRNA were collected.
Western blots.
After obtaining cell lysates from cultured cells, proteins were separated by SDS-PAGE and electroblotted onto nitrocellulose membranes. Antibodies used include phosphor-TGFβ receptor II (pTGFβRII, Thermo Scientific), CYR61, TGFβRII, p21, and p16 (all from Abcam), pRB, RB, TGFβ, pSMAD2, pSMAD3, SMAD2/3, p53, pp38, p38, phosphor-endothelial nitric oxide synthase (peNOS), eNOS, pAKT, AKT, phosphor-AMP-activated protein kinase (pAMPK), and AMPK (all from Cell Signaling). β-actin was used as a loading control (Sigma Aldrich). Band quantification was performed using ImageJ (16).
mRNA extraction and qRT-PCR.
Total RNA from ECs and fibroblasts was isolated using Direct-zol™ RNA MiniPrep Kit (Zymo Research). Verso cDNA synthesis kit was used to prepare cDNA (Thermo Scientific). Primers for human CYR61, COL1A1, ACTA2, PPARG, MMP1, MMP3, TGFB, TGFBR2, VEGF, FGF2, CDK1A, and β-actin along with Power SYBR Green PCR master mix (Applied Biosystems) were applied for qPCR, which was run by a ViiA™ 7 Real-Time PCR System. Primers used were KiCqStart® SYBR® Green Primers from Sigma or QuantiTect Primer Assays from Qiagen.
Enzyme-linked immunosorbent assay (ELISA).
After transfection with CYR61 or control vectors, cell culture media were changed into RPMI (for fibroblasts) or EBM-2 (for ECs) with 0.1% FBS and cultured overnight. The levels of CYR61, MMP1, MMP3, VEGF and bFGF in cell culture supernatants were measured using ELISA kits from R&D systems. Absorbance in each well was read using a microplate reader at 450 nm.
β-galactosidase measurement.
To examine the effect of CYR61 on cell senescence, we measured β-galactosidase using the Senescence β-Galactosidase Staining Kit from Cell Signaling.
Gel contraction and cell migration assays.
The procedure for gel contraction was conducted as previously described (17). Forty-eight hours after CYR61 overexpression or knockdown, dermal fibroblasts were suspended in culture media at 2×106 cells/mL. The Cell Contraction Assay kit (Cell Biolabs) was used for gel contraction. The area of the gel was analyzed using ImageJ (16). To evaluate the effect of CYR61 on cell migration, we performed cell migration assays using SSc fibroblasts transfected with control or CYR61 vectors. Transfected cells were grown to confluence and a wound gap was created. Culture media were replaced with RPMI with 0.1% FBS, and pictures were taken using EVOS XL Core Cell Imaging System (Life Technologies) at 0 hours and 48-hours post-scratch. Quantification of the gap difference was done using ImageJ (16).
Immunofluorescence staining.
Cells grown in 8-well chambers were fixed in 4% formalin and blocked. They were then probed with anti-ki67 antibodies (Abcam) or anti-human p21 antibodies (Abcam). Alexa Fluoro antibodies (Invitrogen) were subsequently used. The nuclei were stained using 4’,6-diamidino-2-phenylindole (DAPI, Invitrogen). Ki67- or p21- positive cells were counted using ImageJ (16). To measure superoxide levels, dihydroethidium (DHE, Invitrogen) was used. After fixation, DHE (10 μM) was added to CYR61- or control-transfected fibroblasts for 15 min, and then stained with DAPI. Fluorescence was detected using an Olympus FV-500 confocal microscope and photographs were taken at 400x.
Nitrite/Nitrate measurement.
To examine whether increased nitric oxide (NO) was present after CYR61 overexpression, nitrite and nitrate levels in cell culture supernatant, both stable metabolites for NO, were measured using the OxiSelect in vitro nitric oxide (Nitrite/Nitrate) Assay Kit from Cell Biolabs.
Matrigel tube formation assay.
In our previous study we showed that CYR61 overexpression in diffuse SSc ECs resulted in increased tube formation (3). To examine whether this effect is mediated through the integrin αvβ3 and its downstream pathways, we pre-treated CYR61-overexpressing SSc ECs with neutralizing antibodies to αvβ3 (10 μg/ml) or inhibitors for the AMPK (compound c, 10 μM) or AKT (LY297002, 20 μM) pathways before performing Matrigel tube formation assays. Treated SSc ECs were suspended in EBM-2 with 0.1% fetal bovine serum and plated in 8-well Lab-Tek chambers coated with growth factor reduced Matrigel (BD Biosciences). Cells were fixed and stained after 6 hours of incubation. Quantitation of the tubes formed by ECs was performed by the Angiogenesis Analyzer function in ImageJ (16). Pictures of each well were taken using EVOS XL Core Cell Imaging System (Life Technologies).
Statistical analysis.
Results were expressed as mean ± S.D. To determine the differences between the groups, Student’s t-test, paired t-test or one-way ANOVA was performed using GraphPad Prism version 6 (GraphPad Software, Inc). P-values of less than 0.05 were considered statistically significant.
Results
CYR61 expression in SSc.
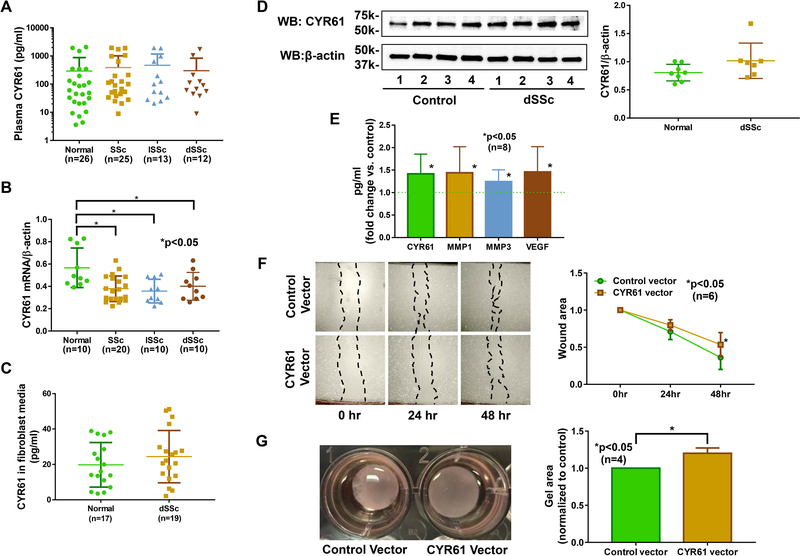
Since CYR61 is secreted and detected in blood, we first measured CYR61 levels in plasma collected from healthy controls and SSc patients. We observed no differences in CYR61 levels between normal and SSc patients, including after stratifying patients into diffuse cutaneous SSc (dSSc) or limited cutaneous SSc (lSSc) (Figure 1A). In dermal fibroblasts, there was significant downregulation of CYR61 mRNA levels in both dSSc and lSSc fibroblasts compared to normal fibroblasts (Figure 1B). Protein levels of CYR61 were measured in dSSc fibroblasts and were found to be similar to control fibroblasts, as indicated by levels of secreted CYR61 in supernatants or CYR61 protein levels in cell lysates (Figure 1C and 1D).
Figure 1. CYR61 is anti-fibrotic in diffuse cutaneous SSc dermal fibroblasts.
(A) The expression of CYR61 was examined in plasma from healthy controls and SSc patients. There was no difference among the groups. (B) At the mRNA level, CYR61 was lower in both diffuse cutaneous SSc (dSSc) and limited cutaneous SSc (lSSc) dermal fibroblasts compared to normal fibroblasts. (C) CYR61 levels in culture media did not differ between normal and dSSc dermal fibroblasts. (D) Similar CYR61 protein levels were observed in normal and dSSc dermal fibroblasts. (E) Overexpression of CYR61 in dSSc dermal fibroblasts led to significant increases in CYR61, MMP1, MMP3, and VEGF in the culture media. (F) Overexpression of CYR61 in dSSc dermal fibroblasts led to significant wider wound width at 48 hrs compared to cells transfected with control vectors (p<0.05). (G) CYR61 overexpression in dSSc fibroblasts resulted in increase in gel area in a gel contraction assay (p<0.05). n=number of patients. Results are expressed as mean +/− SD and p<0.05 was considered significant.
Antifibrotic properties of CYR61 in SSc.
To evaluate the effect of CYR61 in dermal fibroblasts, we first overexpressed CYR61 in normal fibroblasts. Overexpression of CYR61 led to significant decrease in fibrotic markers including COL1A1 and ACTA2, suggesting that CYR61 is indeed anti-fibrotic in these cells (Supplemental Table 1). We then overexpressed CYR61 in dSSc dermal fibroblasts. Similar to what was observed in normal fibroblasts, increased CYR61 led to significant reduction in COL1A1 and ACTA2 (Table 2). This was accompanied by significant increase in the expression of anti-fibrotic PPARG as well as matrix degrading MMP1 and MMP3. In addition to fibrosis-related genes, CYR61 also led to significant increase in angiogenic factors, such as VEGF and FGF2, in dSSc fibroblasts (Table 2). At the protein level, overexpression of CYR61 in dSSc fibroblasts resulted in releasing significant amounts of CYR61, MMP1, 3, and VEGF into the culture media (Figure 1E), suggesting that CYR61 can act in both autocrine and paracrine fashion. To further confirm the role of CYR61 in dermal fibroblasts, we knocked down CYR61 in normal fibroblasts and showed that downregulation of CYR61 led to a pro-fibrotic phenotype (Supplemental Table 2), as indicated by elevated levels of COL1A1 and ACTA2 and decreased MMP1 and MMP3.
Table 2:
The mRNA expression levels of selected target genes after CYR61 overexpression in dSSc dermal fibroblasts. Data are shown as fold change versus control-transfected cells (mean +/− S.D).
| Gene | Expression fold change vs. control vector after 48 hr transfection (n=10 patients) | p-value |
|---|---|---|
| CYR61 | 213 ± 233 | 0.002 |
| COL1A1 | 0.73 ± 0.12 | 0.002 |
| ACTA2 | 0.75 ± 0.34 | 0.04 |
| PPARG | 1.34 ± 0.49 | 0.01 |
| MMP1 | 3.70 ± 5.32 | 0.002 |
| MMP3 | 2.40 ± 1.62 | 0.004 |
| FGF2 | 1.42 ± 0.53 | 0.01 |
| VEGF | 1.45 ± 0.98 | 0.03 |
To examine the anti-fibrotic effect of CYR61 in SSc fibroblasts, we performed two functional assays: a scratch wound assay and a gel contraction assay. Overexpression of CYR61 resulted in slower migration of dSSc fibroblasts (Figure 1F) and reduced gel contraction (Figure 1G). In addition, CYR61 knockdown in normal dermal fibroblasts resulted in increased gel contraction (Supplemental Figure 1), further supporting anti-fibrotic properties of CYR61 in SSc.
CYR61 induces senescence in SSc fibroblasts.
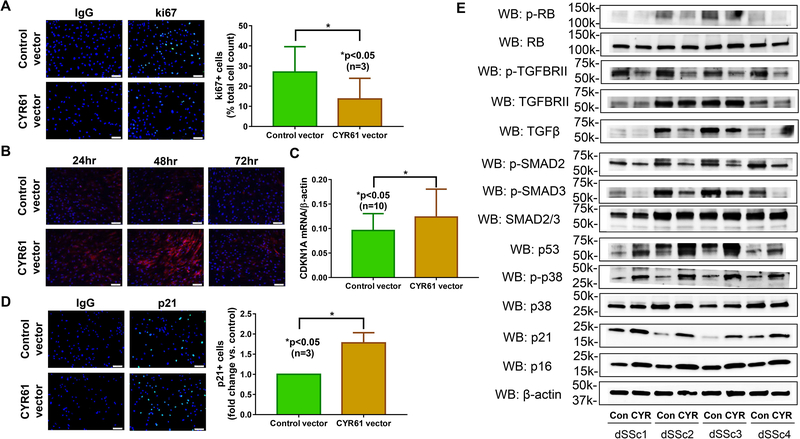
After confirming the anti-fibrotic functional role of CYR61 in dSSc fibroblasts, we examined possible mechanism involved. We hypothesize that CYR61-overexpressing SSc fibroblasts are converted from extracellular matrix (ECM)-producing myofibroblasts into ECM-degrading senescent cells as CYR61 has been shown to trigger senescence by increasing reactive oxygen species (ROS) and activating the p38 pathway through α6β1 integrins and HSPGs (8, 10, 18). We first examined whether CYR61 affects cell proliferation, using the proliferation marker ki67. As shown in Figure 2A, dSSc fibroblasts transfected with CYR61 showed a significant reduction in ki67 staining indicating decreased proliferation. We then measured superoxide production after CYR61 overexpression. We found that superoxide levels increased after 24 hrs of CYR61 overexpression, reaching a peak after 48 hrs (Figure 2B). At 72 hrs post-transfection there was no difference in superoxide levels between control and CYR61-transfected cells. These results suggest that superoxide production is an early event in the CYR61-triggered senescence pathway. We then measured p21 expression, which is a marker for cell senescence. Both mRNA and protein levels of p21 were significantly elevated in CYR61-overexpressing dSSc fibroblasts (Figure 2C, 2D, and 2E). To further dissect the mechanism of CYR61-mediated fibroblast senescence, we examined whether different senescence pathways were involved. As we observed an increase in ROS production (Figure 2B), and ROS can activate stress-activated kinases such as MAPKs and downstream p38 MAPK (19), we first determined whether p38 MAPK was activated after CYR61 overexpression in dSSc dermal fibroblasts. Increased levels of phosphorylated p38 MAPK was indeed observed in CYR61-overexpressing cells (Figure 2E). Because p38 MAPK activation results in increased transcriptional activity of p53 as well as upregulation of p21 and p16, we further examined their expression in CYR61-overexpressing cells. Similar to phosphorylated p38 MAPK, increased accumulation of p53, p21 and p16 was also observed in fibroblasts transfected with the CYR61 vector compared to control (Figure 2E). As all triggers and signaling cascade involved in cell senescence converge on the hypophosphorylated form of Rb, we showed that overexpression of CYR61 in dSSc dermal fibroblasts led to decrease in Rb phosphorylation (Figure 2E). Cell senescence after CYR61 overexpression was also confirmed by β-galactosidase staining (Supplemental Figure 2). These results indicate that the p38 MAPK, p53 and p16/pRb pathways contribute to CYR61-induced fibroblast senescence.
Figure 2. CYR61 induces senescence in diffuse cutaneous SSc dermal fibroblasts.
Overexpression of CYR61 in dSSc dermal fibroblast led to (A) decrease in cell proliferation as shown by ki67 staining; (B) increase of superoxide production which peaked at 48 hrs; (C) and (D) increased p21 mRNA and protein levels. (E) The senescence pathways that were activated by CYR61 in dSSc dermal fibroblasts included p53, p38, p21 and p16, ultimately leading to hypo-phosphorylation of Rb. In addition, the anti-fibrotic effect of CYR61 was also mediated by inactivation of the TGFβ pathway, as indicated by reduced levels of phosphorylated TGFβRII and SMAD2/3. n=number of patients. Results are expressed as mean +/− SD and p<0.05 was considered significant.
CYR61 affects the TGFβ pathway in SSc fibroblasts.
Because TGFβ is the most prominent growth factor in driving fibrosis and collagen production in SSc, we next investigated the effects of CYR61 on TGFβ signaling in control- and CYR61-transfected dSSc dermal fibroblasts. We found that cells overexpressing CYR61 showed attenuated phosphorylation of TGFβRII and smad2/3, and reduced levels of TGFβ. The expression level of total TGFβRII and smad2/3 did not change after CYR61 overexpression (Figure 2E).
Characterizing pro-angiogenic mechanisms of CYR61 in SSc ECs.
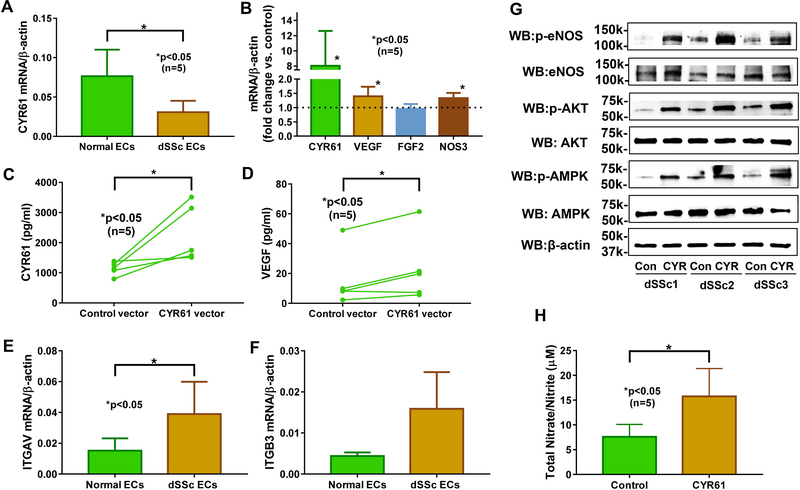
We previously showed that CYR61 was overexpressed after HDAC5 knockdown in ECs, and that it mediated anti-angiogenic effects of HDAC5 in dSSc EC (3). To further confirm its role in SSc angiogenesis, we overexpressed CYR61 in dSSc ECs and showed that this led to increased angiogenic ability of SSc ECs (3). In this study we further dissected the mechanisms involved in the pro-angiogenic properties of CYR61 in SSc. We first determined whether CYR61 expression was altered in dSSc ECs compared to normal ECs and found that CYR61 was lower in dSSc ECs (Figure 3A). We hypothesized that overexpression of CYR61 in SSc ECs leads to increased secretion of CYR61 and other pro-angiogenic factors. We focused on VEGF since it is reported to be a CYR61-regulated gene (20). At the mRNA level, CYR61 overexpression resulted in significant upregulation of VEGF while FGF2, another pro-angiogenic mediator, remained unchanged (Figure 3B). We also measured secreted CYR61 and VEGF in the culture media in control- and CYR61-overexpressing dSSc ECs, and show that overexpression of CYR61 in dSSc ECs increased both CYR61 and VEGF secretion (Figure 3C and 3D).
Figure 3. CYR61 promotes angiogenesis in diffuse cutaneous SSc ECs through αvβ3, eNOS, AMPK, and AKT.
(A) CYR61 mRNA levels were significantly lower in dSSc ECs compared to normal ECs. (B) Overexpression of CYR61 in dSSc ECs led to significant increase of CYR61, VEGF, and NOS3. (C-D) Significant elevation of CYR61 and VEGF was observed in culture media from CYR61-overexpressing dSSc ECs. (E-F) ITGAV and ITGB3 mRNA expression levels were elevated in dSSc ECs compared to normal ECs. (G) Significant elevation of phosphorylated eNOS, AKT, and AMPK was observed in CYR61 transfected dSSc ECs. (H) Metabolites of NO, nitrates and nitrites, were increased in culture media collected from CYR61 transfected dSSc ECs. n=number of patients. Results are expressed as mean +/− SD and p<0.05 was considered significant.
To determine whether the receptor for CYR61, αvβ3, is present on ECs, we measured the mRNA levels in both normal and dSSc ECs. There was a significant increase in ITGAV and ITGB3 expression in dSSc ECs compared to normal cells (Figure 3E and 3G). In contrast, ITGAV and ITGB3 levels did not change in CYR61 overexpressing ECs (Supplemental Figure 3).
Since we established that the receptor of CYR61, αvβ3, is present on SSc ECs and overexpression of CYR61 leads to increased CYR61 and VEGF secretion, we then went on to delineate the cellular events that were involved. CYR61 was previously shown to promote angiogenesis in ECs by activating the AMP-activated protein kinase (AMPK) pathway and the AKT/eNOS/NO pathway, both via αvβ3 (21, 22). Therefore, we first measured mRNA levels of NOS3 (encoding eNOS) and found that it was upregulated in CYR61-overexpressing dSSc ECs (Figure 3B). At the protein level the expression of eNOS was variable, however, when we measured phosphorylated eNOS, it was clear that CYR61 leads to eNOS activation (Figure 3G). To examine whether the eNOS-NO cascade is involved in CYR61-mediated angiogenesis, metabolites of NO were measured in culture media after control- and CYR61- overexpression. As expected, activation of eNOS led to significant increase in NO metabolites in CYR61-overexpressing cells (Figure 3H), suggesting that this pathway is involved in CYR61-mediated angiogenesis in SSc ECs. We then examined whether the AMPK and AKT pathways were activated. As shown in Figure 3G, enhanced phosphorylation of AMPK and AKT were observed in CYR61-overexpressing dSSc ECs. Taken together, these results suggest that CYR61 overexpression in dSSc ECs leads to increased excretion of CYR61, which through binding to αvβ3, activates the AMPK/AKT/eNOS pathways to promote angiogenesis.
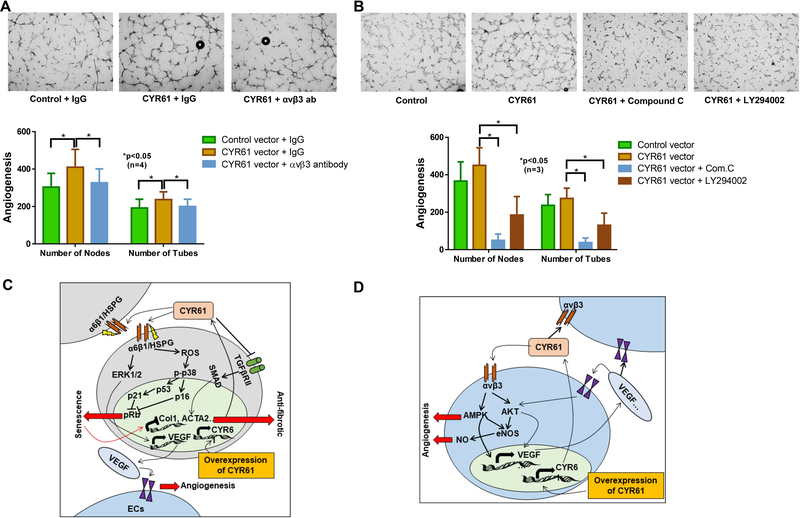
To further confirm the involvement of αvβ3 in CYR61-mediated angiogenesis in SSc ECs, we performed matrigel tube formation assays using CYR61-overexpressing dSSc ECs in the presence or absence of blocking antibodies for αvβ3. The pro-angiogenic property of CYR61 was blocked by the presence of the αvβ3 antibodies compared to the IgG isotype control (Figure 4A). In addition, inhibitors of AMPK (compound c) or AKT (LY294002) pathways also blocked the pro-angiogenic activities of CYR61 (Figure 4B).
Figure 4. Blockade of αvβ3, AMPK, or AKT inhibits the pro-angiogenic property of CYR61.
(A) The pro-antigenic activity of CYR61 in promoting tube formation in dSSc ECs was inhibited by αvβ3 blocking antibodies. (B) Pretreating the dSSc ECs with inhibitors for AMPK (compound c) or AKT (LY294002) reduced tube formation significantly after CYR61 overexpression. Angiogenesis was shown by counting the number of nodes or the number of tubes, using ImageJ. (C) CYR61 overexpression in dSSc fibroblasts results in increased release of CYR61 that binds to the α6β1 integrin/HSPG. CYR61 exerts anti-fibrotic properties by two mechanisms: (1) Inducing senescence by ROS production thereby activating p38/p16 or p38/p53/p21 pathways; (2) Attenuating the TGFβ pathway by inactivating the TGFβ/TGFβ receptor II pathway. CYR61 can also increase VEGF release from fibroblasts which can possibly increase the angiogenic potential of ECs. Studies have suggested that CYR61 promotes VEGF production through activating the α6β1 integrin/HSPG-ERK pathway. (D) CYR61 overexpression in dSSc ECs results in increased CYR61 excretion leading to improved angiogenesis by binding αvβ3 receptors and signaling through the AMPK/ AKT/NO pathways. CYR61 can also increase expression and excretion of pro-angiogenic VEGF, possibly through the AKT and AMPK pathways. The pro-angiogenic effect of VEGF is known to be mediated through the AKT and its downstream NO signaling pathway. n=number of patients. Results are expressed as mean +/− SD and p<0.05 was considered significant.
Discussion
In this study we examined the effect of CYR61 on both SSc fibroblasts and ECs. As summarized in Figure 4C, overexpressing CYR61 in SSc fibroblasts attenuates fibrogenesis by inducing cellular senescence and impairing the TGFβ pathway. We showed that overexpressing CYR61 converted SSc fibroblasts from ECM-producing myofibroblasts into ECM-degrading senescent cells. This is supported by a reduction in fibrotic markers and an increase in matrix degrading MMPs after CYR61 was overexpressed in SSc fibroblasts. In addition, the myofibroblast phenotype was inhibited as shown by the functional assays. We also demonstrated that markers for cell proliferation were reduced, key mediators for cell senescence were elevated, and the activation of TGFβRII and downstream Smad pathways were inhibited. We believe sufficient evidence was generated to support that CYR61 is indeed anti-fibrotic in SSc fibroblasts.
CYR61 has been studied extensively in the skin, as it is a negative regulator of collagen homeostasis in fibroblasts, and is substantially elevated in aged and senescent human skin (23, 24). CYR61 has been shown to trigger senescence by increasing ROS and activating the p38 pathway through α6β1 integrins and HSPGs (8, 10, 18). In addition, it downregulates TGFβRII, thereby impairs TGFβ responsiveness in fibroblasts (18, 25). Although in lung or kidney fibrosis models CYR61 blockade leads to improvement of fibrosis (26, 27), in animal models of cutaneous wound healing as well as liver fibrosis, CYR61 was shown to possess anti-fibrotic properties (8, 10, 18). We were able to recapitulate the involvement of p38 MAPK pathway in cell senescence in CYR61-overexpressing SSc fibroblasts. However, instead of downregulating TGFβRII, we showed decreased TGFβ levels, possibly leading to inactivation of TGFβRII and its downstream Smad2/3 pathway in these cells.
Interestingly, another member of the CCN family, CCN2/CTGF (connective tissue growth factor), is also involved in fibrosis. Although CYR61 and CTGF share similar structures, receptors, and downstream signaling pathways, the subtle differences in their sequences alter the surface area and charge, thereby affecting their interactions with binding partners making them functionally unique (28). It is suggested that in wound healing, CYR61 and CTGF act as yin and yang; CTGF promotes myofibroblast proliferation and matrix deposition to provide tissue integrity and promote wound repair when wound occurs, while CYR61 promotes wound resolution by inducing myofibroblast senescence and matrix degradation (8). CTGF, which promotes fibrosis, is elevated in SSc patients and plays key roles in myofibroblast transformation through interaction with the TGFβ pathway (29–31). The imbalance between CTGF and CYR61 could play a major role in promoting fibrosis in SSc.
It has been suggested that CYR61 activates VEGF production in fibroblasts through the α6β1/HSPG/ERK axis (32). We indeed found that CYR61-overexpressing SSc fibroblasts released elevated levels of VEGF (Figure 1E), promping us to speculate that CYR61 can promote EC angiogenesis through fibroblasts (Figure 4C). This is possible, as cross-talk between ECs and fibroblasts in SSc has been documented (33). Although VEGF has limited effect on SSc ECs (the so called VEGF paradox), we showed previously that these cells do respond to higher levels of VEGF (34), which strengthens the argument that overexpressing CYR61 in fibroblasts might increase the angiogenic potential of SSc ECs.
In addition to fibroblasts, the effect of CYR61 in SSc ECs was also examined in this study (Figure 4D). CYR61 overexpression in SSc ECs led to increased excretion of CYR61, which through binding to αvβ3, activated the AMPK/AKT pathways to promote angiogenesis. CYR61 also activated expression of VEGF to further increase its pro-angiogenic potential. Both CYR61 and pro-angiogenic factors can act in an autocrine or a paracrine fashion. The involvement of αvβ3 and the AKT and AMPK pathways in CYR61-mediated angiogenesis was supported by our blocking antibody and inhibitor studies. Our results are consistent with previous reports suggesting the involvement of the AMPK pathway and the AKT/eNOS/NO pathway in CYR61-mediated angiogenesis in ECs (21, 22). The ability of CYR61 to regulate VEGF was also documented (20). In addition, its angiogenic activity was also observed in in vivo rat cornea models and ischemic rabbit hind-limb models (21, 22, 35–39). As CYR61 is also critical for EC survival (6, 40) and increased apoptosis has been reported in SSc ECs (41), lower levels of CYR61 in SSc ECs could contribute to both impaired angiogenesis and increased apoptosis seen in these cells.
Inconsistent results were reported for CYR61 serum levels in SSc (42, 43). Similar to what we observed, Saigusa et al showed that serum CYR61 levels were comparable among healthy controls, dSSc, and lSSc patients (42). They also showed that CYR61 was downregulated in dermal small blood vessels of SSc patients compared to controls, which agrees with what we observed in this study (42).
We previously showed that CYR61 promotes EC tube formation in dSSc ECs (3). In this study, we compiled evidence to indicate that CYR61 is indeed pro-angiogenic and anti-fibrotic in SSc ECs and fibroblasts, respectively. Therefore, our data suggest that upregulating CYR61 in SSc might be of therapeutic potential in this disease. Indeed, CYR61 attenuated two key pathologic mechanisms in SSc: impaired angiogenesis and increased fibrosis. CYR61 also modulates immune responses through macrophages (44, 45), and the possibility that CYR61 inhibits macrophage-driven fibrosis by affecting M2 polarization and monocyte/macrophage migration warrants further investigation.
Supplementary Material
Acknowledgments
Funding: This work was supported by the Arthritis National Research Foundation and the Scleroderma Foundation to Dr. Tsou. Dr. Khanna is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases grant number K24AR063120 and the National Institute of Allergy and Infectious Diseases grant number UM1AI110557. Dr. Sawalha is supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health grants number R01AI097134 and U19AI110502 and the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health grant number R01AR070148.
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
References
- 1.Barnes J, Mayes MD. Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Curr Opin Rheumatol. 2012;24(2):165–70. [DOI] [PubMed] [Google Scholar]
- 2.Tsou PS, Sawalha AH. Unfolding the pathogenesis of scleroderma through genomics and epigenomics. J Autoimmun. 2017;83:73–94. PMCIDPMC5573604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsou PS, Wren JD, Amin MA, Schiopu E, Fox DA, Khanna D, et al. Histone Deacetylase 5 Is Overexpressed in Scleroderma Endothelial Cells and Impairs Angiogenesis via Repression of Proangiogenic Factors. Arthritis Rheumatol. 2016;68(12):2975–85. PMCIDPMC5125850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krupska I, Bruford EA, Chaqour B. Eyeing the Cyr61/CTGF/NOV (CCN) group of genes in development and diseases: highlights of their structural likenesses and functional dissimilarities. Hum Genomics. 2015;9:24 PMCIDPMC4579636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grote K, Salguero G, Ballmaier M, Dangers M, Drexler H, Schieffer B. The angiogenic factor CCN1 promotes adhesion and migration of circulating CD34+ progenitor cells: potential role in angiogenesis and endothelial regeneration. Blood. 2007;110(3):877–85. [DOI] [PubMed] [Google Scholar]
- 6.Leu SJ, Lam SC, Lau LF. Pro-angiogenic activities of CYR61 (CCN1) mediated through integrins alphavbeta3 and alpha6beta1 in human umbilical vein endothelial cells. J Biol Chem. 2002;277(48):46248–55. [DOI] [PubMed] [Google Scholar]
- 7.Chen CC, Young JL, Monzon RI, Chen N, Todorovic V, Lau LF. Cytotoxicity of TNFalpha is regulated by integrin-mediated matrix signaling. EMBO J. 2007;26(5):1257–67. PMCIDPMC1817641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010;12(7):676–85. PMCIDPmc2919364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kireeva ML, Mo FE, Yang GP, Lau LF. Cyr61, a product of a growth factor-inducible immediate-early gene, promotes cell proliferation, migration, and adhesion. Mol Cell Biol. 1996;16(4):1326–34. PMCIDPmc231116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim KH, Chen CC, Monzon RI, Lau LF. Matricellular protein CCN1 promotes regression of liver fibrosis through induction of cellular senescence in hepatic myofibroblasts. Mol Cell Biol. 2013;33(10):2078–90. PMCIDPmc3647960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. 2013;72(11):1747–55. [DOI] [PubMed] [Google Scholar]
- 12.Assassi S, Swindell WR, Wu M, Tan FD, Khanna D, Furst DE, et al. Dissecting the heterogeneity of skin gene expression patterns in systemic sclerosis. Arthritis Rheumatol. 2015;67(11):3016–26. PMCIDPMC5394431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsou PS, Amin MA, Campbell P, Zakhem G, Balogh B, Edhayan G, et al. Activation of the Thromboxane A2 Receptor by 8-Isoprostane Inhibits the Pro-Angiogenic Effect of Vascular Endothelial Growth Factor in Scleroderma. J Invest Dermatol. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsou PS, Rabquer BJ, Ohara RA, Stinson WA, Campbell PL, Amin MA, et al. Scleroderma dermal microvascular endothelial cells exhibit defective response to pro-angiogenic chemokines. Rheumatology (Oxford). 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He Y, Tsou PS, Khanna D, Sawalha AH. Methyl-CpG-binding protein 2 mediates antifibrotic effects in scleroderma fibroblasts. Annals of the rheumatic diseases. 2018;77(8):1208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He Y, Tsou P-S, Khanna D, Sawalha AH. Methyl-CpG-binding protein 2 mediates antifibrotic effects in scleroderma fibroblasts. Ann Rheum Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borkham-Kamphorst E, Schaffrath C, Van de Leur E, Haas U, Tihaa L, Meurer SK, et al. The anti-fibrotic effects of CCN1/CYR61 in primary portal myofibroblasts are mediated through induction of reactive oxygen species resulting in cellular senescence, apoptosis and attenuated TGF-beta signaling. Biochim Biophys Acta. 2014;1843(5):902–14. [DOI] [PubMed] [Google Scholar]
- 19.Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nature Reviews Molecular Cell Biology. 2014;15:482. [DOI] [PubMed] [Google Scholar]
- 20.Mo FE, Muntean AG, Chen CC, Stolz DB, Watkins SC, Lau LF. CYR61 (CCN1) is essential for placental development and vascular integrity. Mol Cell Biol. 2002;22(24):8709–20. PMCIDPMC139880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park YS, Hwang S, Jin YM, Yu Y, Jung SA, Jung SC, et al. CCN1 secreted by tonsil-derived mesenchymal stem cells promotes endothelial cell angiogenesis via integrin alphav beta3 and AMPK. J Cell Physiol. 2015;230(1):140–9. [DOI] [PubMed] [Google Scholar]
- 22.Hwang S, Lee HJ, Kim G, Won KJ, Park YS, Jo I. CCN1 acutely increases nitric oxide production via integrin alphavbeta3-Akt-S6K-phosphorylation of endothelial nitric oxide synthase at the serine 1177 signaling axis. Free Radic Biol Med. 2015;89:229–40. [DOI] [PubMed] [Google Scholar]
- 23.Quan T, Qin Z, Robichaud P, Voorhees JJ, Fisher GJ. CCN1 contributes to skin connective tissue aging by inducing age-associated secretory phenotype in human skin dermal fibroblasts. J Cell Commun Signal. 2011;5(3):201–7. PMCIDPMC3145867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quan T, Qin Z, Voorhees JJ, Fisher GJ. Cysteine-rich protein 61 (CCN1) mediates replicative senescence-associated aberrant collagen homeostasis in human skin fibroblasts. J Cell Biochem. 2012;113(9):3011–8. [DOI] [PubMed] [Google Scholar]
- 25.Quan T, He T, Shao Y, Lin L, Kang S, Voorhees JJ, et al. Elevated cysteine-rich 61 mediates aberrant collagen homeostasis in chronologically aged and photoaged human skin. Am J Pathol. 2006;169(2):482–90. PMCIDPMC1698795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurundkar AR, Kurundkar D, Rangarajan S, Locy ML, Zhou Y, Liu RM, et al. The matricellular protein CCN1 enhances TGF-beta1/SMAD3-dependent profibrotic signaling in fibroblasts and contributes to fibrogenic responses to lung injury. FASEB J. 2016;30(6):2135–50. PMCIDPMC4871800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai CF, Lin SL, Chiang WC, Chen YM, Wu VC, Young GH, et al. Blockade of cysteine-rich protein 61 attenuates renal inflammation and fibrosis after ischemic kidney injury. Am J Physiol Renal Physiol. 2014;307(5):F581–92. [DOI] [PubMed] [Google Scholar]
- 28.Holbourn KP, Acharya KR, Perbal B. The CCN family of proteins: structure–function relationships. Trends in Biochemical Sciences. 2008;33(10):461–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato S, Nagaoka T, Hasegawa M, Tamatani T, Nakanishi T, Takigawa M, et al. Serum levels of connective tissue growth factor are elevated in patients with systemic sclerosis: association with extent of skin sclerosis and severity of pulmonary fibrosis. J Rheumatol. 2000;27(1):149–54. [PubMed] [Google Scholar]
- 30.Holmes AM, Ponticos M, Shi-Wen X, Denton CP, Abraham DJ. Elevated CCN2 expression in scleroderma: a putative role for the TGFbeta accessory receptors TGFbetaRIII and endoglin. J Cell Commun Signal. 2011;5(3):173–7. PMCIDPmc3145876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abraham DJ, Shiwen X, Black CM, Sa S, Xu Y, Leask A. Tumor necrosis factor alpha suppresses the induction of connective tissue growth factor by transforming growth factor-beta in normal and scleroderma fibroblasts. J Biol Chem. 2000;275(20):15220–5. [DOI] [PubMed] [Google Scholar]
- 32.Chen CC, Mo FE, Lau LF. The angiogenic factor Cyr61 activates a genetic program for wound healing in human skin fibroblasts. J Biol Chem. 2001;276(50):47329–37. [DOI] [PubMed] [Google Scholar]
- 33.Serratì S, Chillà A, Laurenzana A, Margheri F, Giannoni E, Magnelli L, et al. Systemic sclerosis endothelial cells recruit and activate dermal fibroblasts by induction of a connective tissue growth factor (CCN2)/transforming growth factor β–dependent mesenchymal-to-mesenchymal transition. Arthritis Rheum. 2013;65(1):258–69. [DOI] [PubMed] [Google Scholar]
- 34.Tsou PS, Rabquer BJ, Ohara RA, Stinson WA, Campbell PL, Amin MA, et al. Scleroderma dermal microvascular endothelial cells exhibit defective response to pro-angiogenic chemokines. Rheumatology (Oxford). 2016;55(4):745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Babic AM, Kireeva ML, Kolesnikova TV, Lau LF. CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci U S A. 1998;95(11):6355–60. PMCIDPMC27701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fataccioli V, Abergel V, Wingertsmann L, Neuville P, Spitz E, Adnot S, et al. Stimulation of angiogenesis by Cyr61 gene: a new therapeutic candidate. Hum Gene Ther. 2002;13(12):1461–70. [DOI] [PubMed] [Google Scholar]
- 37.Hinkel R, Trenkwalder T, Petersen B, Husada W, Gesenhues F, Lee S, et al. MRTF-A controls vessel growth and maturation by increasing the expression of CCN1 and CCN2. Nat Commun. 2014;5:3970. [DOI] [PubMed] [Google Scholar]
- 38.Rayssac A, Neveu C, Pucelle M, Van den Berghe L, Prado-Lourenco L, Arnal JF, et al. IRES-based vector coexpressing FGF2 and Cyr61 provides synergistic and safe therapeutics of lower limb ischemia. Mol Ther. 2009;17(12):2010–9. PMCIDPMC2814383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chintala H, Krupska I, Yan L, Lau L, Grant M, Chaqour B. The matricellular protein CCN1 controls retinal angiogenesis by targeting VEGF, Src homology 2 domain phosphatase-1 and Notch signaling. Development. 2015;142(13):2364–74. PMCIDPMC4510592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Y, Zhang Y, Hui L, Yang H, Yang Y, Wang A, et al. Cysteinerich 61 RNA interference inhibits pathological angiogenesis via the phosphatidylinositol 3kinase/Aktvascular endothelial growth factor signaling pathway in endothelial cells. Molecular medicine reports. 2016;14(5):4321–7. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Kahaleh B. Epigenetic repression of bone morphogenetic protein receptor II expression in scleroderma. J Cell Mol Med. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saigusa R, Asano Y, Taniguchi T, Yamashita T, Takahashi T, Ichimura Y, et al. A possible contribution of endothelial CCN1 downregulation due to Fli1 deficiency to the development of digital ulcers in systemic sclerosis. Exp Dermatol. 2015;24(2):127–32. [DOI] [PubMed] [Google Scholar]
- 43.Lin J, Li N, Chen H, Liu C, Yang B, Ou Q. Serum Cyr61 is associated with clinical disease activity and inflammation in patients with systemic lupus erythematosus. Medicine (Baltimore). 2015;94(19):e834 PMCIDPMC4602578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bai T, Chen CC, Lau LF. Matricellular protein CCN1 activates a proinflammatory genetic program in murine macrophages. J Immunol. 2010;184(6):3223–32. PMCIDPMC2832719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rother M, Krohn S, Kania G, Vanhoutte D, Eisenreich A, Wang X, et al. Matricellular signaling molecule CCN1 attenuates experimental autoimmune myocarditis by acting as a novel immune cell migration modulator. Circulation. 2010;122(25):2688–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.