Abstract
High soil carbonate limits crop performance especially in semi-arid or arid climates. To understand how plants adapt to such soils, we explored natural variation in tolerance to soil carbonate in small local populations (demes) of Arabidopsis thaliana growing on soils differing in carbonate content. Reciprocal field-based transplants on soils with elevated carbonate (+C) and without carbonate (−C) over several years revealed that demes native to (+C) soils showed higher fitness than those native to (−C) soils when both were grown together on carbonate-rich soil. This supports the role of soil carbonate as a driving factor for local adaptation. Analyses of contrasting demes revealed key mechanisms associated with these fitness differences. Under controlled conditions, plants from the tolerant deme A1(+C) native to (+C) soil were more resistant to both elevated carbonate and iron-deficiency than plants from the sensitive T6(−C) deme native to (−C) soil. Resistance of A1(+C) to elevated carbonate was associated with higher root extrusion of both protons and coumarin-type phenolics. Tolerant A1(+C) also had better Ca-exclusion than sensitive T6(−C). We conclude that Arabidopsis demes are locally adapted in their native habitat to soils with moderately elevated carbonate. This adaptation is associated with both enhanced iron acquisition and calcium exclusion.
Keywords: Arabidopsis thaliana, calcareous soil, calcium exclusion, coumarin, iron efficiency, local adaptation, proton extrusion
Introduction
Calcareous soils are common under semi-arid and arid climate conditions, where low rainfall and high evapotranspiration favour the accumulation of calcium carbonate and bicarbonate in the upper soil layers (Ruellan, 1973). Calcareous soils have been defined as those between near-to-neutral and alkaline soils (Chesworth, Camps Arbestain, & Macías, 2008). They develop over calcareous parent rock such as limestone and their pH values, usually within the range of 7 to 8.6, largely depend on the solubility of calcite (Bache, 1984). Major constraints for plant performance on these calcareous soils are high concentrations of carbonate, bicarbonate, calcium (Ca), and sometimes also sodium (Na) and boron (B). Moreover, the low availability of essential nutrients, mainly phosphorus (P), iron (Fe), and zinc (Zn) (Ryan et al., 2013), are also main stress factors. Poor soil structure may further hamper root development especially in soils with high carbonate, fine texture, and low organic matter contents (Zolfaghari, Mosaddeghi & Ayoubi, 2016).
These multiple stress factors of calcareous soils limit yield of sensitive crop plants. Unadapted plants, known as calcifuges, do not perform well on calcareous soils mainly because they have low ability to mobilize and use Fe, P, Mn, and/or Zn in efficient ways. Moreover, they have limited capacity to both restrict Ca uptake and compartmentalize high amounts of Ca in their tissues (Hawkesford et al., 2011). Lime-induced chlorosis, mainly due to low bioavailability of Fe, is one of the best-identified symptoms of crop plants that are poorly adapted to calcareous soils. Most affected crops are citrus trees, orchards and vineyards (Tagliavini & Rombolà, 2001; Alcántara, Montilla, Ramírez, García-Molina, & Romera, 2012), but also soya (Aksoy, Maqbool, Tindas. & Caliskan, 2017) and other dicot species (Ozores-Hampton, 2013).
Several mechanisms account for Fe-efficiency in calcicoles, i.e. plants adapted to calcareous soils with high pH (Kruckeberg, 2002). In dicots with strategy I for Fe acquisition, lowering of the pH in the apoplast and the rhizosphere by enhanced H+ exudation favours both the solubility of Fe and the activity of ferric reductase (Marschner & Römheld, 1994). In A. thaliana FIT is the major transcription factor regulating these responses to Fe-deficiency (Colangelo & Guerinot, 2004). Moreover, enhanced root exudation of organic compounds with high binding affinity for Fe is an important feature for increasing Fe availability. Citrate and, especially under high pH conditions, catechol-type phenolics and flavins are playing major roles (Santi & Schmidt, 2009; Cesco et al., 2010, Rodríguez-Celma et al., 2013; Sisó-Terraza et al., 2016b).
Breeding efforts for controlling lime-induced chlorosis revealed the polygenetic character of tolerance to calcareous soils (Bert et al., 2013). Exploration of the natural genetic variation in relation to environmental variables can provide important clues for understanding the polygenetic control of stress tolerance mechanisms in plants (Lasky et al., 2014). This knowledge is fundamental for the development of efficient selection strategies in crop breeding programs (Bandillo et al., 2017). Natural populations of the genetic model plant Arabidopsis thaliana are widely distributed, showing a strong population structure. This natural genetic variation provides an excellent tool for studying mechanisms involved in local adaptation (Smith & Laitinen, 2015). Common garden experiments performed in largely distant locations (e.g. Fournier-Level et al., 2011; Ågren & Schemske, 2012) revealed that not only climate but also geography determines plant fitness as a proxy for local adaptation in A. thaliana.
Obviously, tolerance to extreme climate factors is a main driver for local adaptation, survival, and plant distribution (Heegard 2002; Reyer et al., 2013). However, other factors such as soil salinity (Busoms et al., 2015 & 2018), shade (Callaghan & Pigliucci, 2002) or herbivory (Arany et al., 2009) may also play a crucial role, especially on a small scale. The exploration of natural variation in response to contrasting factors on a small geographic scale may be advantageous because of less interference from climate and other confounding geographic and genetic factors. Less attention has been paid to soil factors both in species distribution models (Buri et al., 2017) and in local adaptation studies where the relevance of soil factors may be species dependent (Macel et al., 2007). Salinity, as one of the most important soil-derived stress factors limiting crop yield, has received most consideration (Cabot, Sibole, Barceló & Poschenrieder, 2014). In fact, studies relating the degree of salinity in the natural habitat of A. thaliana populations to salt tolerance assessed in common gardens and laboratory studies indicate that salinity is a factor for divergent selection both on a large, trans-European (Baxter et al., 2010) and a small (30 km distance) geographic scale (Busoms et al., 2015). These studies provided valuable information regarding the genetic basis of salt tolerance (Busoms et al., 2018). To our best knowledge, local adaptation of natural populations of A. thaliana to other soil factors has not been studied so far.
During a survey of A. thaliana demes in a relatively small area of the NE Catalonia using a species distribution model, we identified geology as a relevant variable determining the presence of A. thaliana in this region (Busoms et al., 2015). The model predicted that the species exclusively occurs on soils developed on granite, granitoid, granodiorite, or hornfels (Busoms, 2015). Such soils have acid to near neutral pH and no, or only low, carbonate contents. In NE Catalonia we were unable to detect A. thaliana on limestone. This contrasts to the fact that in the UK, at Calver, a limestone accession Cal-0 has been reported (https://www.arabidopsis.org/servlets/TairObject?type=species_variant&id=72). The apparent preference of A. thaliana for acid soils, but their presence also on soils with moderately alkaline pH, raises the questions of local adaptation to this relatively narrow range of different soil pH, and the underlying mechanisms. Here, we explore these questions using both common gardens on carbonate-rich and non-carbonate soil and physiological approaches characterizing responses to Fe-deficiency and bicarbonate in contrasting demes.
Materials and Methods
Field sampling and analysis of A. thaliana and corresponding soils
Plants of 24 A. thaliana demes previously described by Busoms et al. (2015) were collected from their natural habitats during 3 years (2013–2015), along with seeds and underlying soil. Three independent soil analyses per site and year were performed. pH, water-holding capacity, and texture were measured using fresh soil following the methods described by Carter & Gregorich (2006). Total soil organic matter was analysed according to Black (1965). Soil carbonate content was determined with the acetic acid dissolution method (Loeppert & Suarez, 1996). The characterization of the elemental composition of soils and the leaf ionome were performed following the procedures described by Busoms et al., (2015). According to the carbonate concentrations, the pH values, and the organic matter contents of the soils, the A. thaliana demes were grouped into three categories: low, medium and high value of the corresponding parameters. Three demes from each group (total number 9) were selected for the field common garden experiments.
Common garden experiments
To detect local adaptation to soil carbonate, common garden experiments were conducted at two field sites with contrasting soil carbonate levels (Supplementary Table 1): Les Planes (LP: 42° 03ʹ 45.1ʺN; 2° 32ʹ 46.6ʺE) as a representative for carbonate-rich soil (+C) and Santa Coloma de Farners (SCF: 41° 50ʹ 41.04ʺN;2 40ʹ 36.13ʺE) as a representative for low-carbonate soil (−C). The same common garden design was reproduced at both sites. The common garden occupied a surface of 2 × 6 metres at each site. Each garden was covered with a shading mesh that reduced 70 % light incidence on sunny days and 50 % on cloudy days. This was required as in their natural habitat these demes always grow on North-oriented sites in the shadow of other plants.
Each common garden was divided into 10 plots of 90 × 90 cm (Table S2). Each plot was subdivided into 9 squares of 30 × 30cm; one for each of the 9 selected demes, distributed randomly. In each square 10 seeds of an individual deme were sown. This design was repeated in each of the 10 plots. The entire common garden experiments were repeated over three years (2013–2015). The position of the entire common garden was moved a few meters every year, in order to avoid contamination by seeds from the former year.
Two weeks after germination, the number of established seedlings was recorded. Then seedlings in each square were thinned out, leaving two seedlings remaining in each square to prevent confounding effects of establishment success and competition intensity. The remaining seedlings were monitored until fruit maturation. Rosette diameter of each plant was measured every week for 2 months. The number of siliques per plant as a proxy for fitness were count on 10 plants for each deme per site at the fruit maturation stage (Busoms et al., 2015; Postma & Agren, 2016). The other 10 plants for each deme per site were harvested in April of 2013 and 2014 to analyse their leaf ionome using ICP-MS (Busoms et al., 2015). During the 3 months of each field experiment, minimum and maximum temperatures, precipitation and soil composition were monitored.
The effect of local adaptation of A. thaliana demes based on the number of produced siliques was assessed using a modified version of the sympatric vs allopatric (SA) test (Blanquart, Kaltz, Nuismer, & Gandon, 2013). Briefly, we used a generalised linear mixed effects model with a Poisson distribution and log link function to model the mean variance relationship of these count data. We included habitat, deme, and a sympatric vs allopatric indicator variable as fixed effects. Deme x habitat interaction was used as a random effect to account for non-independence between individual plants within the deme in each habitat. All statistical analyses were conducted using the R statistical environment (R Core Team, 2013) and mixed models were fitted using the lme4 package (Bates, Mächler, Bolker, & Walker, 2015).
Potted soil experiments
Potted soil experiments were performed to test whether deme differences in response to soil carbonate revealed in common garden field experiments were reproducible under controlled-environmental conditions in the lab. For this purpose, two contrasting demes were used: A1(+C) a deme native to soil with moderate carbonate content (+C) and T6(−C), native to a siliceous soil with low carbonate (−C). Seeds were collected in the field from multiple plants of each deme during 2015. After a standard cold stratification treatment, seeds were sown in pots filled with either siliceous, non-carbonate (−C) soil from SCF or carbonate-rich soil (+C) from LP. Plants were watered to field capacity twice per week with distilled water. Measures of growth (diameter of rosette) were taken weekly during a month, and samples for genotyping and leaf ionomic analysis were harvested the same day. Additionally, T6(−C) and A1(+C) were grown on universal substrate (Compo Sana Semilleros, Compo Expert, Barcelona, Spain). After 21 days from sowing, half of the plants were treated twice a week with 15 mM NaHCO3; the rest received distilled water. Rosette diameter was measured every week. All pot experiments were performed under controlled-environment conditions in a growth chamber (Conviron CMP5090, Canada), at 21ºC, 70% relative humidity and a photosynthetic photon flux density of 220 µmol m−2 s−1 photosynthetic active radiation with a photoperiod of 8 h light/16 h darkness.
Hydroponic experiments
Hydroponics were used to test whether local adaptation to soil carbonate in A. thaliana is related to differential responses to Fe-deficiency. This cultivation method allows easier analysis of root traits than soil culture. The two contrasting A. thaliana demes, A1(+C) and T6(−C), were grown in nutrient solution under the same controlled-environment conditions as indicated above. After seed germination, the seedlings were pre-cultured and grown as indicated by Sisó-Terraza et al. (2016b). Briefly, seeds were sown in 0.2 ml tubes containing 0.6 % agar prepared in nutrient solution ¼ Hoagland with a final pH of 5.5. The pH of the nutrient solution was adjusted prior to adding the agar and before autoclaving; taking into account that autoclaving decreases pH of the mixture by 0.5 units. Iron was added as 45 µM Fe(III)-EDTA. After 8 days from germination, the bottom of the tubes containing seedlings was cut off and the tubes were placed in opaque 300 ml plastic boxes (pipette tip racks; Starlab, Hamburg, Germany), containing aerated half-strength Hoagland nutrient solution, pH 5.5, supplemented with 20µM Fe(III)-EDTA. Plants were grown for 11 d. After that, plants were transferred to half-strength Hoagland nutrient solution containing 0 or 20µM Fe(III)-EDDHA (Sequestrene, Syngenta, Madrid). Solutions were buffered at pH 5.5 (with 5mM MES) or at 7.5 (with 5mM HEPES) to maintain a stable pH during the whole treatment period (14 days). Nutrient solutions were renewed weekly. Pots without plants, containing only aerated nutrient solution (with and without Fe) were also placed in the growth chamber. These samples were later used as blanks for root exudate analyses. Germination and plant growth took place in the growth chamber (Conviron CMP5090, Canada) under the conditions indicated above. Two batches of plants (12 plants per batch) were grown and analysed.
At day 14, leaf chlorophyll concentrations were measured with a SPAD device (CCM300, Opti-Sciences, Hudson, USA) on three different leaves of three plants per treatment. Activity assays of both root ferric chelate reductase (FCR) and leaf superoxide dismutase (SOD) (see below) were performed using fresh plant material. For the analysis of phenolic compounds in roots, harvested roots were rinsed with distilled water, immediately frozen in liquid N2, and stored at −20ºC until extraction of phenolic compounds. After plant harvest the nutrient solution pH was measured, the solutions were filtered through filter paper (Whatman grade 1; Merck, Darmstadt Germany), frozen and stored at −20ºC until extraction of phenolic compounds
Phenolics in root extracts and root exudates
To test a potential role of phenolics in the differential adaptation of both A. thaliana demes, phenolic compounds were extracted from roots and nutrient solutions as described by Sisó-Terraza et al., (2016b), with the following modifications: first, extraction was carried out without adding internal standards (IS) to identify relevant compounds. This extract was also used to check for the presence of the compounds used as IS and other endogenous isobaric compounds that may co-elute with them, since in both cases there would be analytical interferences in the quantification process. The root extraction was then carried out adding the following three IS compounds: artemicapin C, a methylenedioxy-coumarin, for quantification of the coumarins scopoletin, fraxetin, isofraxidin and fraxinol; esculin, the glucoside form of the coumarin esculetin, for quantification of coumarin glycosides; and the lignan matairesinol, for quantification of coumarinolignans. In the root extracts the concentrations of IS were 3, 4, and 3μM for artemicapin C, esculin and matairesinol, respectively. In the nutrient solutions extracts, only artemicapin C and matairesinol at 8 and 15 µM, respectively, were used as IS. The extracts were analysed for phenolic compounds using an Alliance 2795 HPLC system (Waters, Mildford, MA, USA) coupled to a UV/VIS detector (Waters PDA 2996) and time-of-flight mass spectrometer (MS-TOF; MicrOTOF, Bruker Daltonics, Bremen, Germany).
Effects of exogenous coumarin and co-culture experiments
To explore the possibility that root exudates of carbonate tolerant deme A1 are able to improve Fe acquisition by the carbonate sensitive T6, plants were pre-grown in hydroponics in 100 mL plastic pots as described above. Then uniform seedlings were transferred to half-strength Hoagland nutrient solution with low Fe concentration (5µM) in the form of Fe(III)-EDDHA (Sequestrene, Syngenta). Solutions were buffered at 7.5 (with 5mM HEPES) to maintain a stable pH Solutions were renewed once a week. Two contrasting A. thaliana demes, A1(+C) and T6(−C), were grown either mixed (one T6 plant together with one A1 plants) or alone (2 plants of either deme per pot) as a control. At day 14, leaf chlorophyll concentrations were measured with a SPAD device (CCM300, Opti-Sciences) on three different leaves of three plants per treatment and leaves of plants were used for ionomic analysis.
To see whether coumarins may be responsible for improved Fe acquisition, experiments with exogenous supply of fraxetin were performed. Plants of T6 (−C) were pre-grown in hydroponics as described above. Uniform seedlings were transferred for 7 d to half-strength Hoagland nutrient solution without iron. Solutions were buffered at 7.5 (with 5mM HEPES) to maintain a stable pH during the whole treatment period (14 days). After 7 d plants were transferred for 14d to half-strength Hoagland nutrient solution containing 5µM Fe(III)-EDDHA (Sequestrene, Syngenta) and supplemented or not with 100µM of fraxetin (98%; Sigma Aldrich). At day 14, leaf chlorophyll concentrations were measured with a SPAD device (CCM300, Opti-Sciences) on three different leaves of three plants per treatment and leaves of plants were used for ionomic analysis.
Root-induced pH changes, root ferric chelate reductase activity and leaf superoxide dismutase activity
Plants were pre-grown in hydroponics as described above. Then uniform seedlings were transferred to unbuffered half-strength Hoagland nutrient solution with an initial pH of 7.0 containing 5 or 20 µM Fe(III)-EDDHA (Sequestrene, Syngenta). The root induced changes of pH values in the nutrient solution were measured after 7 days.
Root ferric chelate reducing (FCR) capacity was assessed according to Lucena et al. (2007) using an assay solution containing macronutrients, 100 µM Fe(III)-EDTA, and 300 µM ferrozine (pH 5.0); absorbance measurements were made at 562 nm in a Shimadzu UV-2450 (Shimadzu Corp., Tokyo, Japan). To reveal possible deme differences in enzyme adaptation to higher pH, additional analyses were performed using ferrozine solutions at pH 6.0, 7.0, and 8.0.
Possible differences between demes in their ability to protect leaves against the oxidative stress imposed by Fe deficiency were visualized by analysing the leaves’ superoxide dismutase activity (SOD). For the assay, 0.05 g samples of fresh leaves were ground in 2 mL of 0.1 M cool phosphate buffer (pH 6.8) on ice bath (Kar & Mishra, 1976). The crude extract was centrifuged at 15,000 × g for 15 min at 4°C. The supernatant was used for SOD activity assays. The protein concentration of the supernatant was measured with a NANODROP-2000 spectrophotometer (Thermo Fischer, Waltham, MA, USA). The superoxide anion scavenging activity of plant extracts was determined with the WST (2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt) reduction method, using a SOD assay kit (Kit-WST, Dojindo Lab, Kumamoto, Japan).
Statistical analysis
Data from common garden field experiments are means ± standard errors (SE) from three years. If not otherwise stated, results from lab experiments are means ± SE from three biological replicates. Statistical difference was assessed using analysis of variance ANOVA followed by Tukey HSD test. Data were checked for normal distribution. If necessary, data were log-transformed prior to ANOVA. The statistical treatment of data for testing local adaptation is given above at the end of the section describing the common garden experiments.
Results
Ranking of fitness in common gardens according to soil properties in the native habitat
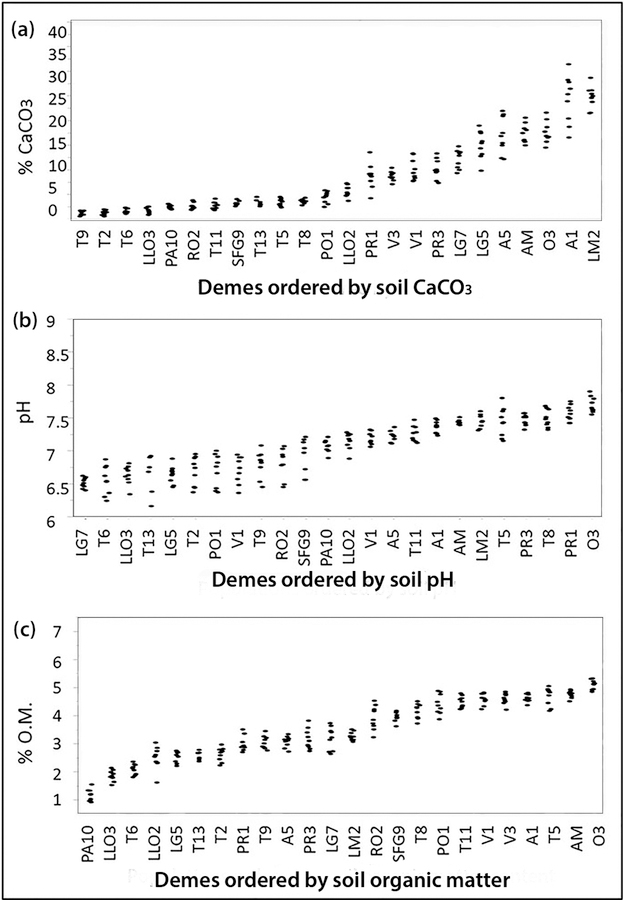
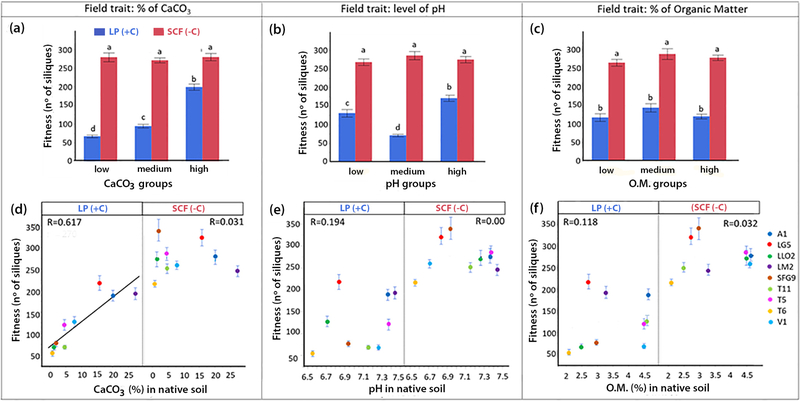
The selection of contrasting A. thaliana demes for testing local adaptation in reciprocal transplants was based on the ranking of 24 demes according to the increasing values of selected soil properties analysed over a 3-years period in the native habitats (Figure 1). The soil factors pH, soil carbonate, and soil organic matter as a proxy for soil fertility were used as selection criterions. For each soil factor the 24 demes were classified into three equally sized groups (8 demes per group) corresponding to the lowest, medium or highest values of each soil factor in the native habitat of the demes. Three demes of each group (9 demes in total) were used for the common garden experiments established on calcareous limestone soil (+C) at Les Planes (LP) and on siliceous soil with only traces of carbonate (−C) at Santa Coloma de Farners (SCF). Soil properties at both field experimental sites are given in Table S1. Figure 2 shows the results of the common garden experiments from both experimental sites. Fitness values are presented as number of siliques produced by the different demes grouped according to increasing values of soil properties in their native habitats. All demes performed better on the granitoid- derived (−C) soil at SCF than on the (+C) soil at LP. Cultivation on the (−C) soil at SCF of the demes from habitats differing in soil pH, soil carbonate, and soil organic matter did not reveal fitness differences among the groups (Figure 2a–c). Therefore, on (−C) soil no correlation between fitness of the demes and the properties of the soils from their original habitat could be established (Figure 2d–f). Contrastingly, common gardens on the (+C) soil clearly revealed fitness differences for demes grouped according to increasing soil carbonate content at the original habitat (Figure 2a). The high-carbonate group performed best, followed by the medium-carbonate group; lowest fitness was observed in the demes from habitats with no or very low carbonate content (Figure 2a). Significant group/field site interactions were observed in the case of grouping plants according to soil carbonate and soil pH. A statistically significant linear relationship between fitness on the calcareous soil at LP and the soil carbonate content in the original habitat of the demes was found (Figure 2d). On the calcareous soil at LP fitness differences were also observed among groups according to the soil pH at their original habitats; accordingly, demes native to soils with the highest pH performed best (Figure 2b). However, fitness was more closely related to soil carbonate than to soil pH (Figure 2e). There was no relationship between demes’ fitness in the common gardens and the soil organic matter at their native habitats (Figure 2f). Based on these results, grouping according to increasing carbonate at the original habitat was used as selection criterion for further analyses.
Figure 1.
Twenty–four Arabidopsis thaliana demes ordered according to the increasing values of soil carbonate, soil pH, or soil organic matter (O.M.) contents in their natural habitat. Three soil samples per habitat and year were analysed; (n = 216).
Figure 2.
Fitness (number of siliques) of A. thaliana demes at both common garden experimental sites, Santa Coloma de Farners (SCF) with carbonate-poor, siliceous soil (−C) (red) and Les Planes (LP) with carbonate-rich calcareous soil (+C) (blue). Demes were classified into three groups according to low, medium, or high soil parameter values in their natural habitat. Classifications by soil CaCO3 (a); by soil pH (b), and by soil organic matter content (c). For each field trait, histograms labelled with different letters are statistically different (n = 540; p<0.05; ANOVA; Tukey HSD). Correlations between fitness (number of siliques) at both sites, LP (+C) (n = 270) and SCF (−C) (n = 270) and order of demes ranked according to increasing soil parameters in their natural habitat: soil carbonate content (d), soil pH (e), and soil organic matter content (f).All bars denote SE.
Leaf ionomics and chlorophyll content
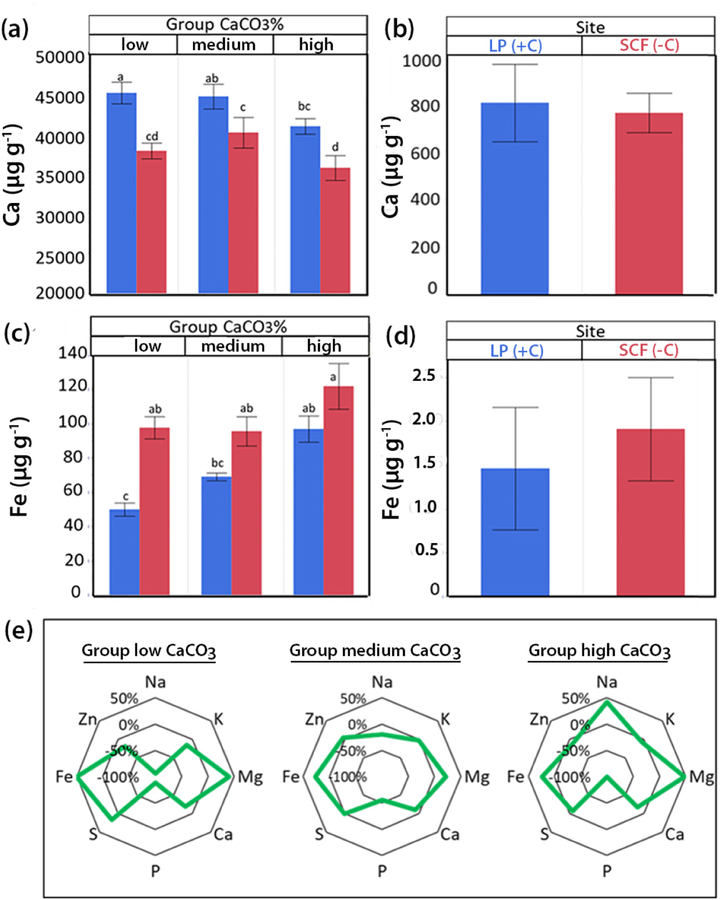
Ionomic analyses revealed significantly (p<0.05) higher leaf Ca concentrations in plants on limestone at LP than on siliceous soil at SCF; no site/group interaction was observed in the two-way ANOVA (Figure 3). Values of extractable soil Ca at both sites were similar (Figure 3b). Leaves from demes of the low-carbonate group had lower Fe (Figure 3c) concentrations than those from the high-carbonate group; factors site/group interactions were statistically significant (p<0.05). Extractable soil Fe concentrations at both common-garden sites fluctuated over the years and were not statistically different (Figure 3d). The low soil Na concentrations were also quite similar at both sites (LP, 57 ± 11 µg g−1; SCF, 58 ± 7 µg g−1). Contrastingly, leaf Na concentrations were distinctively lower in plants from the low-carbonate group (group 1) at SCF. Better Na exclusion in these group-1 plants is in line with the higher Na concentrations in their native soils (106 µg g−1) than in soils of habitats of either the medium carbonate group (71 µg g−1) or the high carbonate group (49 µg g−1). Nonetheless, leaf Na concentrations were lower in the high-carbonate group at LP (Figure 3e). In none of the assessed demes Na concentrations in the leaf dry weights exceeded 0.08 %, a value far below the critical toxicity level in glycophytes (around 0.15%, according to Bergmann, 1993).
Figure 3.
Ionomics of rosette leaves of A. thaliana demes at both experimental sites, (SCF) with siliceous soil (−C) (red) and Les Planes (LP) with calcareous soil (+C) (blue); demes were classified into three groups according to low, medium, or high soil CaCO3 contents in their natural habitat. Ca concentrations (μg g−1 dry weight) in rosettes (a) and experimental field soil (b); Fe concentrations (μg g−1 dry weight) in rosettes (c) and experimental field soil (d). Values are means from three years ±SE, (n = 30; p<0.05; ANOVA; Tukey HSD). Percent differences for 8 selected elements (green line) (e) between plants grown at SCF (−C) and LP (+C); plants divided into three groups according to the carbonate content in their native soils.
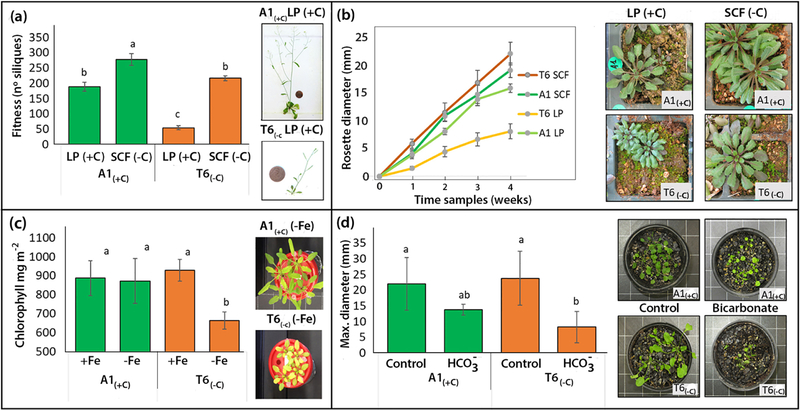
For further physiological studies under laboratory conditions, two extreme demes with distinctive carbonate levels in their native habitat were selected: T6(−C) from the low-carbonate group and A1(+C) from the high-carbonate group. Fitness of both demes in the common garden experiment is shown in Figure 4a. A1(+C) had intrinsically higher fitness than T6(−C) as indicated by a 20 % higher number of siliques on the (−C) site. Cultivation on (+C) soil yielded lower number of siliques in both demes. However, a fitness reduction of 77 % was observed in the sensitive T6(−C) native to (−C) soil, while the fitness reduction in the tolerant A1(+C) was only 31%. The deme/site interactions were statistically significant (p<0.05). These differences were confirmed in the laboratory under controlled environmental conditions with plants growing in pots filled with either (+C) or (−C) soils, using rosette diameter as a phenotype marker (Figure 4b). Increase in rosette diameter was similar in both demes growing on (−C) soil, while on carbonate-rich (+C) soil growth was considerably slower in T6(−C) than in A1(+C) (Figure 4b).
Figure 4.
Common garden lab experiments with A. thaliana demes A1(+C) (green) and T6(−C) (orange) grown on potted calcareous (+C) and siliceous (−C) soil from the common garden experimental fields at LP and SCF, respectively; (a) Fitness (number of siliques); (b) increment of rosette diameter; (c) chlorophyll concentrations (mg m−2); (d) maximum rosette diameter of plants from both demes growing in potting mix weakly irrigated during one month with either 20mM NaHCO3 or distilled water. For all panels, means ±SE are represented. Histogram bars with different letters are statistically different (n = 12; p<0.05; 2-way ANOVA; Tukey HSD).
Although demes native to (−C) and (+C) soils differed in rosette Fe concentrations (Figure 3c), no lime–induced chlorosis was observed in the common gardens neither under field conditions nor in the potted soil experiments. Cultivation in hydroponics using solution without Fe supply was required to reveal differences between A1(+C) and T6(−C) in leaf chlorophyll concentrations. Iron deficiency affected chlorophyll concentrations more in T6(−C) than in A1(+C) (Figure 4c); the deme/treatment interactions were statistically significant. Growing the plants on potting mix irrigated with a 15mM bicarbonate solution also confirmed the differential sensitivity of both demes to bicarbonate, revealing a 36 % and a 67 % decrease in rosette diameter for A1(+C) and T6(−C), respectively (Figure 4d). In the potted soil experiments (Fig. 4 b), but not in the field, dark green colouration of leaves was observed in some plants. These symptoms may indicate P deficiency and was probably favoured by the reduced soil volume in the pots. Under field conditions, leaf P concentrations for plants grown at LP and SCF were 0.69% and 0.38%, respectively. This is within the sufficient range for Brassicaceae (Bergmann, 1993). Comparing the leaf ionome of A1(+C) and T6(−C) grown in pots on soil collected from the common garden sites (Figure 5), it was surprising that on both soils rosettes leaves of T6(−C) had higher cobalt (Co), nickel (Ni) and manganese (Mn) concentrations than those of A1(+C) (Figure 5a–c). Leaves from T6(−C) also accumulated higher Fe concentrations when cultivated on (−C) soil (Figure 5 d). Contrastingly, lower leaf Fe accumulation in T6(−C) compared to A1(+C) was observed on the (+C) soil from LP (Figure 5d). This inhibition of Fe-accumulation in leaves of T6(−C) on the carbonate-rich soil is in line with the results already found under field conditions for plants from the low-carbonate group (Figure 3c).
Figure 5.
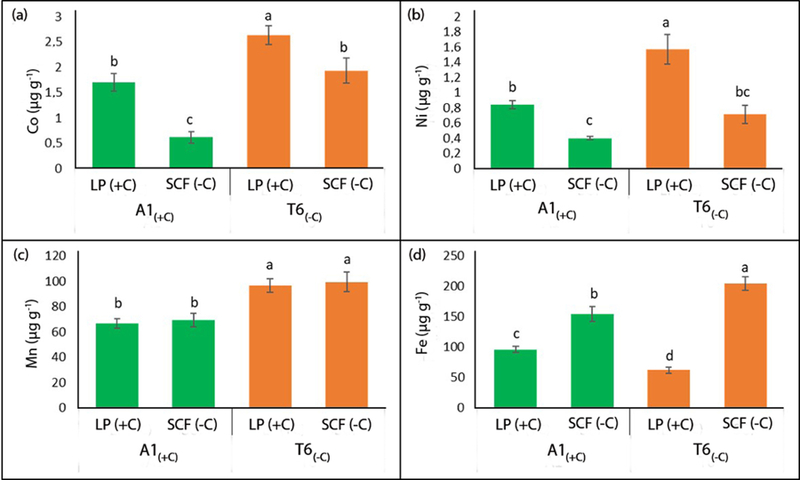
Leaf concentrations (µg g−1 dry weight) of selected micronutrients of A1(+C) and T6(−C) grown on (−C) and (+C) soils from SCF and LP, respectively; (a) Cobalt (Co); (b) Nickel (Ni); (c) Manganese (Mn); (d) Iron (Fe); values are means ±SE (n = 12; p<0.05; 2-way ANOVA; Tukey HSD).
Activities of SOD, FCR and proton extrusion
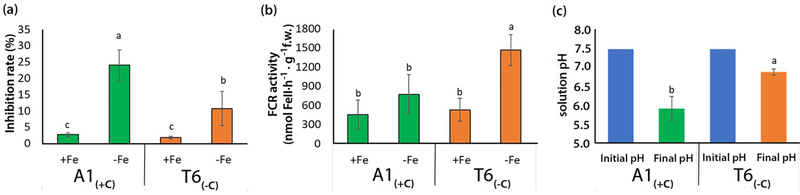
To ascertain deme differences in both the ability of roots to mobilize sparingly soluble Fe forms by acidification of the rhizosphere and to reduce FeIII to FeII, the root-induced pH changes and the root ferric chelate reductase (FCR) activity were analysed. Further, maintenance of SOD activity during Fe deficiency stress is crucial for the preservation of chloroplast integrity (Page et al., 2012). Under Fe deficiency conditions, A1(+C) plants displayed considerably higher increase of leaf SOD activity than T6(−C) plants (Figure 6a); the deme/treatment interaction was statistically significant (p<0.05). Both demes did not differ in FCR activity assayed at the enzyme’s optimal pH of 5.0 in plants grown with adequate Fe supply and exposed to Fe deficiency for 30 minutes. While FCR activity strongly decreased in A1(+C) at pH 6, the activity in T6(−C) was unaffected. At higher pH values, FCR activity strongly decreased in both demes (Figure S1). Exposure of plants to Fe deficient conditions for 14 days strongly enhanced the FCR activity (measured at optimum pH 5.0) much more in T6(−C) than in A1(+C) (Figure 6b). Roots of A1(+C) plants were able to decrease the pH of the unbuffered solution by 1.5 units, from the initial value of 7.5 to close to 6. In contrast, roots of T6(−C) only achieved a decrease of 0.5 pH units (Figure 6c).
Figure 6.
Superoxide dismutase (SOD) activity (inhibition rate %) (a) and root ferric chelate reductase (FCR) activity (nmol Fe(II) h−1 g−1fresh weight) (b) for A. thaliana demes A1(+C) and T6(−C) with adequate (+Fe) or deficient (-Fe) iron supply. Values are means ± SE (n = 12; p< 0.05; 2-way ANOVA; Tukey HSD). Solution pH (c) after one-week cultivation of A1(+C) (green) and T6(−C) (orange) under Fe-deficiency conditions; initial pH: 7.5 (blue). Values with different letters are statistically different (n = 16; p<0.05 comparison of means of final pH values by Student’s t test).
Coumarin exudation by roots
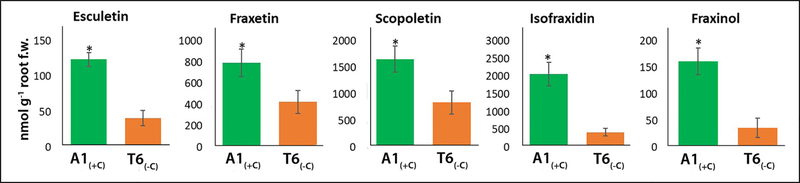
T6(−C) and A1(+C) plants not only differed in the ability to acidify the rhizosphere, but also in the quality (Figure S2) and quantity (Figure 7) of exuded coumarin-type phenolics. When grown without Fe at pH 7.5 plants of both demes produced an array of coumarin-type phenolics including 6 simple coumarins (esculetin, scopoletin fraxetin, isofraxidin, fraxinol, trihidroxymethoxycoumarin), two glycosides of coumarins (scopolin and fraxin) (Figure 7) and 4 coumarinolignans (cleomiscosins A, B, C, and D; Figure S2). The concentrations of all of these phenolic compounds were higher in the nutrient solutions from A1(+C) than in solutions from T6(−C). Isofraxidin, scopoletin and fraxetin were the major coumarins (43, 34 and 17% of the total coumarins, respectively) in the root exudates of A1(+C). In exudates of T6(−C) isofraxidin scopoletin, and fraxetin accounted for 24, 48, and 24% of the total coumarins, respectively. The concentrations of coumarinolignans were similar in the root exudates of both demes (Fig. S2). For root extracts (Figure S1b) both demes present similar profiles, with scopolin being the major compound (62–75%) followed by fraxin (glycoside of fraxetin; 21%) in A1(+C) and by by scopoletin (13%) in T6(−C) (Figure S2).
Figure 7.
Concentrations (nmol g−1 root fresh weight) of coumarins released by the roots into the nutrient solution: esculetin, fraxetin, scopoletin, isofraxidin and fraxinol from plants of A1(+C) and T6(−C) demes grown in buffered nutrient solution without iron at pH 7.5; values are means ± SE; asterisk indicates statistical difference (n = 12; p<0.05; 1-way ANOVA; Tukey HSD).
Exogenous coumarin supply and co-culture experiments
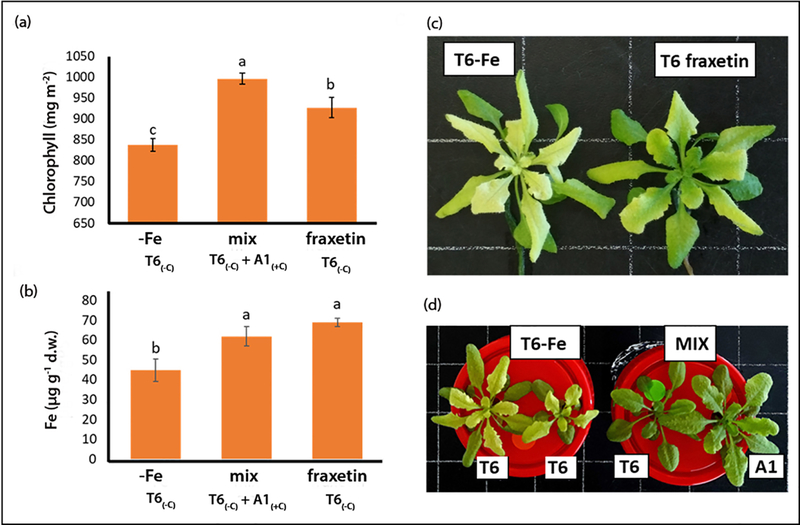
To reveal whether coumarin root exudation may account for the better Fe acquisition, plants from the carbonate sensitive T6(−C) deme were grown in low Fe solution either supplemented with fraxetin or co-cultivated with plants from the carbonate tolerant A1(+C) deme (mixed treatment). The T6(−C) plants cultivated alone under Fe deficient growth conditions without fraxetin supply had low chlorophyll and Fe leaf concentrations (Figure 8a,b). TheT6(−C) phenotype was clearly less chlorotic when these plants were grown in low Fe medium either supplemented with fraxetin (Figure 8c) or in co-culture with the Fe efficient deme A1(+C) (Figure 8d).
Figure 8.
Chlorophyll (a) and Fe concentrations (b) in rosette leaves of carbonate sensitive A. thaliana T6(+C) grown in iron deficient nutrient solution either alone (-Fe) or together with the carbonate tolerant deme A1(+C) (mixed), or alone in solution supplemented with 100 µM fraxetin. Values are means ± SE; values with different letters are statistically different (n = 18; p<0.05; 1-way ANOVA; Tukey HSD). (c) Comparison of rosettes from T6(+C) grown alone in Fe deficient solution (-Fe) (left) and T6(+C) grown alone in Fe deficient solution supplemented with fraxetin (right). (d) Comparison of rosettes from two plants of T6(+C) grown alone in Fe deficient solution (-Fe) (left) with those of one T6(+C) plant grown together with one A1(−C) plant (right).
Discussion
This study addressed three major questions: are there A. thaliana demes locally adapted to calcareous soils? If so, what are the soil factors driving this local adaptation and what are the physiological traits responsible for contrasting tolerance to calcareous soil conditions?
Local adaptation of A. thaliana to calcareous soils
Our survey on the distribution of A. thaliana in NE Catalonia in relation to soil pH and carbonate content localized several demes on soils with slightly alkaline pH and moderate carbonate content (Figure 1). In the reciprocal transplant experiments, all demes performed better on the siliceous soil at SCF than on the carbonate-rich soil at LP (Figure 2a). However, on the carbonate-rich soil demes with higher carbonate concentrations in their native habitats performed much better than demes native to carbonate-poor soils. This deme × habitat interaction pattern for fitness fulfils the local vs. foreign criterion (Kawecki & Ebert, 2004) indicating local adaptation to moderate carbonate concentrations in the soil. This being said, it is also important to note that at the species level A. thaliana would still be classified as a calcifuge species, i.e. it prefers acid to near neutral soils. This is clearly seen by the fitness decline of all assayed demes when cultivated in common gardens on carbonate-rich soil at LP, in comparison to the fitness on siliceous soil at SCF (Figure 2)..
In a local set of A. thaliana accessions from different soil types in Sweden a close relation between FRO2 and root length under Fe deficiency was found (Satbhai et al., 2017). The extreme calcicole Parietaria diffusa also responds to Fe deficiency with enhance root length. In contrast, however, under high carbonate P. diffusa shows shorter roots with cluster root-like morphology. Root exudation of organic acids and phenolics was clearly higher under carbonate exposure than under Fe-deficient conditions (Donnini, De Nisi, Gabotti, Tato & Zocchi, 2012). This supports the view that mechanisms for adaptation to high soil carbonate are not limited to efficient Fe-deficiency responses.
Soil carbonate as an agent of divergent selection
Our second question concerned the agent(s) of divergent selection between the siliceous and limestone habitat. The common garden experiments revealed that the best relation between the fitness of the different demes cultivated on the (+C) soil and the soil parameters from their native habitats was for soil carbonate concentration. The relation between fitness when cultivated on the (+C) soil and soil pH of the native habitats was weaker, and there was no relationship with soil organic matter (Figure 2). This supports our conclusion that soil carbonate is the primary agent of the divergent selection we have identified. This does not exclude soil pH as a secondary selective factor, as soil pH obviously depends on soil carbonate content. However, it has to be taken into account that the relationship between soil carbonate content and soil pH is not linear within the soil pH and carbonate ranges of this study. In soils with pH below 7, carbonate concentrations are low or close to zero (Wang et al., 2015). In addition, the relations between fitness and other soil parameters, such as extractable Fe, Zn or P were not statistically significant (data not shown).
High soil carbonate is associated with multiple soil properties, which have a considerable influence on plant mineral nutrition and growth (Clark & Baligar, 2000). The high bicarbonate concentrations in the soil solution of carbonate-rich soils can influence both pH homeostasis of plant tissues and their organic acid metabolism (Poschenrieder et al., 2018). High soil Ca concentrations and low availability of Fe, Zn and P are the main stress factors on such high carbonate soils (Chesworth et al., 2008). On carbonate-rich soils at LP, leaf Fe concentrations were higher and Ca concentrations lower, in plants with the highest carbonate in their native habitat, when compared to the group of plants coming from the low carbonate habitats (Figure 3). This indicates both more efficient Fe-acquisition and better Ca-exclusion in the carbonate-adapted plants. Some calcifuges have strict requirements for low tissue Ca concentrations and Ca toxicity may occur with leaf Ca of around only 0.2 %. Contrastingly, calcicole plants withstand 4% or even higher leaf Ca concentrations (Jeffries & Willies, 1964). According to a literature review by Broadley et al. (2003), the average leaf Ca concentration in A. thaliana is 3.08 %.
Taking these parameters together, our data do not indicate that cultivation of (−C) demes on (+C) soil causes toxic leaf Ca concentrations. However, the more efficient Ca exclusion in the plants native to habitats with moderately carbonated soils supports mechanisms of local adaptation. Further studies are required to clarify whether the more efficient leaf Ca exclusion in the demes native to moderately carbonated soils is due to reduced transpiration-driven, apoplastic Ca transport or transmembrane Ca transport, lower xylem loading, or related to differences in vacuolar storage in either or both roots and leaves (White & Broadley, 2003).
Better Fe acquisition in carbonate adapted plants
The ability to mobilize Fe from carbonate-rich soil is considered a fundamental trait for plant adaptation to carbonate soils. Here, we assessed if such an adaptive mechanism is involved in the local adaptation to carbonate –rich soils we have observe in A. thaliana. Clear differences in Fe acquisition between carbonate-adapted and non-adapted demes were observed. When plants were grown on our carbonate-rich soil leaf Fe concentrations of around 50 μg g−1 dry weight in our (−C) demes were observed, an Fe concentration indicative of severe Fe deficiency (Li, Kronzucker, & Shi, 2016). Contrastingly, under the same growth conditions (+C) plants had sufficient Fe concentrations of around 100 μg Fe g−1 dry weight (Waters & Troupe, 2012). Whereas, when plants were grown on our low carbonate soils all plants showed sufficient levels of Fe in their leaves. Plants native to (−C) soils therefore appear are unable to mobilize Fe from the carbonate-rich soil at LP, while plants from (+C) habitats have mechanisms allowing the acquisition and translocation of Fe to the leaves even on soils with elevated carbonate.
Multiple mechanisms may cooperate to allow plants to acquire Fe on carbonate-rich soils: acidification of the rhizosphere by root proton exudation, enhanced ferric reductase activity, root exudation of organic acids, flavins and coumarin-type phenolics, and/or efficient Fe translocation to leaves (Ishimaru et al., 2007; Lemanceau, Bauer, Kraemer, & Briat, 2009; Rodríguez-Celma et al., 2013; Waters, Sisó-Terraza et al., 2016a; Waters, Amundsen, & Graef, 2018). To characterize the specific mechanisms allowing naturally selected carbonate tolerance in A. thaliana, two extreme demes, T6(−C) sensitive and A1(+C) tolerant, were further analysed.
A1(+C), native to soil with moderate carbonate content, was much more efficient than T6(−C), the deme from siliceous soil, in both lowering the substrate pH (Figur 6c) and producing coumarin-rich root exudates (Figure 7). Previous studies revealed that feruloyl-CoA 6’-hydroxylase1 (F6’H1)-dependent coumarin exudation is essential for the resistance of A. thaliana to Fe deficiency induced by high pH (Rodríguez-Celma et al., 2013; Schmid et al., 2014; Schmidt et al., 2014). Scopoletin 8-hydroxylase (S8H) an enzyme involved in fraxetin biosynthesis is also strongly induced under Fe-deficiency (Tsai et al., 2018; Rajniak et al., 2018). Under Fe deficiency, roots of A. thaliana Col-0 exudate higher amounts of coumarins (Fourcroy et al., 2014; Schmidt et al., 2014; Sisó-Terraza et al., 2016b; Ziegler, 2017). The type of coumarins released under Fe-deficiency depends on the substrate pH. Under acid pH sideretine is the main compound, while under alkaline conditions the less oxidized fraxetin is predominant (Rajniak et al., 2018). The catechol-type coumarins, like esculetin and fraxetin are able to mobilize Fe from sparingly soluble Fe(III)-oxide at neutral pH (Schmidt et al., 2014; Sisó-Terraza et al., 2016b; Rajniak et al., 2018). It has been proposed that the role of these catechol-type coumarins is to facilitate Fe(III) availability for the FRO2 reductase to generate Fe2+ that will be then transported through IRT1 activity (Fourcroy et al., 2016). Recently, Tsai et al. (2018) showed that root exudation of fraxetin by natural accessions of A. thaliana grown on substrate with sparingly soluble Fe was closely correlated to growth and leaf chlorophyll content. Here we demonstrate that the higher exudation rate of these catechol group-bearing coumarin-type phenolics is related to the soil characteristics in the natural habitat of A. thaliana demes. The relevance of Fe mobilization by root exudates containing coumarin–type phenolics is further validated by our experiments using either exogenous fraxetin supply to the sensitive T6(−C) or co-cultivation of T6(−C) with the tolerant A1(+C) (Fig 8). Both type of treatments enhanced leaf Fe concentrations and chlorophyll contents in the carbonate sensitive T6(−C) when cultivated under conditions of low Fe availability (Figure 8).
Although the exudation of fraxetin can promote Fe reduction at near to neutral pH (Schmid et al., 2014; Sisó et al., 2016b), FCR and IRT1 may still be required for Fe acquisition (Fourcroy et al., 2016). Acidification of the root apoplast is an essential condition for the activity of iron ferric reductase (FRO2) (Susín 1996; Kosegarten et al., 2004). Major differences in AHA2-mediated proton extrusion under Fe-deficiency have been observed in a set of natural accessions of A. thaliana. Strong acidification was associated with high induction of the iron transporter gene IRT1 (Santi & Schmidt, 2009). A relation with the Fe availability of the soils in the natural habitat was assumed, but not established.
Moreover, the ability of roots to acidify the rhizosphere is an important feature of plants that efficiently mobilize soil Fe. The relevance of a plant’s ability to lower the pH of the substrate for enhancing Fe availability may be curtailed in carbonate-rich soil because of the strong buffer capacity of soil carbonate (Schubert, Schubert, & Mengel, 1990). However, a substantial local increase in proton extrusion in root tips may provide hot spots for Fe solubilisation.
Here, we provide evidence of higher proton extrusion in response to Fe deficiency in A. thaliana naturally adapted to soil with moderate carbonate content. Surprisingly, this ability was not accompanied by a higher FCR activity in A1(+C), in comparison to the non-adapted T6(−C) (Figure 6a). The activity of FRO2 is strongly induced by Fe deficiency. However, FCR activity is hampered at alkaline pH (Moog, Kooij, Bruggemann, Schiefelbein, & Kuiper,, 1995; Susín et al., 1996). Our results confirm the strong negative influence of high pH on FCR activity (Figure S1). Curiously, the carbonate sensitive T6(−C) displayed higher FCR activity than the tolerant A1(−C) under both long-term Fe deficiency (14 d; Figure 6a) and short, 30 min deprivation of Fe (Figure S1). This higher FCR activity in T6(−C), however, was poorly effective in Fe-acquisition and is likely a symptom of its low tissue Fe-status rather than an Fe-efficiency mechanisms. The dual control of FRO2 involves both a local induction by the Fe-availability and a systemic regulation by the foliar Fe status (Vert, Briat, & Curie, 2003). Grafting experiments revealed that FRO2 expression in the roots strongly depends on long-distance signalling from the leaves (Kumar et al., 2017). The lower FCR activity in A1(+C) than in T6(−C) under Fe deficiency may reflect the better ability to maintain higher Fe tissue concentrations in A1(+C) (Figure 3c & 5d). In this carbonate tolerant deme, the more intense exudation of catechol-type coumarins like esculetin and fraxetin, can provide a higher pool of available Fe to the root cells. Esculetin and fraxetin can efficiently mobilize Fe from Fe hydroxide precipitates (Schmid et al., 2014; Sisó-Terraza et al., 2016b; Rajniak et al., 2018). Moreover, these coumarins can reduce Fe(III) (FeCl3 and Fe(III)-EDTA) to Fe(II) (Schmidt et al., 2014; Tsai et al., 2018; Rajniak et al., 2018). These abilities were observed at higher rates under neutral than under moderate acidic pH conditions Our results indicate that coumarin exudation may be more relevant for plant adaptation to carbonate soil than a high FCR activity.
Lower leaf Fe concentrations in T6(−C) than in A1(+C) may not only be due to lower Fe uptake, but also to impaired Fe root to shoot transport in T6(−C). A higher shoot accumulation of Co, Ni and Mn is consistently related to a low Fe nutritional status in A. thaliana accessions (Baxter et al., 2008). Here, we found that on the carbonate-rich soil the sensitive T6(−C) accumulated higher Co, Ni and Mn, but lower Fe leaf concentrations than the tolerant A1(+C). This seems not a consequence of a concentration effect due to growth inhibition, yet higher Co and Mn leaf concentrations in T6(−C) were also observed under the optimal growth conditions on the siliceous soil. Our results indicate that the more efficient Fe acquisition strategy in A1(+C) is less affected by interference from other divalent cations of transition metals than in T6(−C) (Figure 5).
Conclusions
We can conclude from this study that under the Mediterranean climate in NE Spain soil carbonate content is a strong factor for divergent selection in A. thaliana. Although this species performs better on siliceous substrate, some demes can grow and reproduce on soils with moderate carbonate contents. Better Fe-acquisition in A thaliana deme A1(+C) native to soil with moderate carbonate content is related to higher exudation rates of both protons and coumarin–type phenolics leading to enhanced Fe mobilization from the soil, coupled with better Ca-exclusion. These mechanisms are likely to be key features responsible for the local adaptation of A. thaliana to moderate carbonate soils. Higher SOD activity may further contribute to better maintenance of chloroplast integrity in the adapted deme. Interestingly, enhanced FCR activity does not appear to play a role in this evolved tolerance to moderate carbonate soils in A. thaliana.
Supplementary Material
ACKNOWLEDGEMENTS
This work was funded by the Spanish MINECO projects BFU2013–42839-R, BFU2016–75176-R & AGL2016–75226-R. DES funded by US National Institutes of Health (grant 2R01GM078536 and 2P4ES007373–19A1) and BBSRC (grants BB/L000113/1). We thank the ECAF Santa Coloma de Farners for providing field sites and technical support. J.T. and L.P. were supported by PhD fellowships PIF2013 and PIF2017 from UAB. A.L.-V. was supported by a MINECO-FPI contract (BES-2014–070986).
Footnotes
The authors declare no conflicts of interests.
REFERENCES
- Ågren J & Schemske DW. (2012). Reciprocal transplants demonstrate strong adaptive differentiation of the model organism Arabidopsis thaliana in its native range. New Phytologist, 194, 1112–1122. [DOI] [PubMed] [Google Scholar]
- Aksoy E, Maqbool A, Tindas I & Caliskan S (2017). Soybean: A new frontier in understanding iron deficiency tolerance mechanisms in plants. Plant and Soil, 418, 37–44. [Google Scholar]
- Alcántara E, Montilla I, Ramírez P, García-Molina P & Romera FJ (2012). Evaluation of quince clones for tolerance to iron chlorosis on calcareous soil under field conditions. Scientia Horticulturae, 138, 50–54. [Google Scholar]
- Arany AM, de Jong TJ & van der Meijden E. (2009). Herbivory and local genetic differentiation in natural populations of Arabidopsis thaliana (Brasicaceae). Plant Ecology 201, 651–659. [Google Scholar]
- Bache BW (1984). The role of calcium buffering soils. Plant Cell and Environment, 7, 391–395. [Google Scholar]
- Bandillo NB, Anderson JE, Kantar MB, Stupar RM, Specht JE, Graef GL & Lorenz AJ (2017). Dissecting the genetic basis of local adaptation in soybean. Scientific Reports, 7, 17195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker BM, & Walker SC (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67, 1–48. [Google Scholar]
- Baxter IR, Vitek O, Lahner B, Muthukumar B, Borghi M, Morrissey J, … & Salt DE (2008). The leaf ionome as a multivariable system to detect a plant’s physiological status. Proceedings of the National Academy of Sciences, 33: 12081–12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter I, Brazelton JN, Yu D, Huang YS, Lahner B, Yakubova E, …& Salt DE (2010). A coastal cline in sodium accumulation in Arabidopsis thaliana is driven by natural variation of the sodium transporter AtHKT1;1. PLoS Genetics, 6, e1001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann W (1993) Ernährungsstörungen bei Kulturpflanzen 3rd ed. Gustav Fischer, Jena. [Google Scholar]
- Bert PF, Bordenave L, Donnart M, Hévin C, Ollat N & Decroocq S (2013). Mapping genetic loci for tolerance to lime-induced iron deficiency chlorosis in grapevine rootstocks Vitis sp. Theoretical and Applied Genetics, 2, 451–473. [DOI] [PubMed] [Google Scholar]
- Black CA (1965). Operator variation. In Methods of Soil Analysis Part1. Physical and Mineralogical Properties Including Statistics of Measurement and Sampling. Agronomy Monograph 9.1 pp 50–53. American Society of Agronomy American Society of Agronomy, Inc.; USA. [Google Scholar]
- Blanquart F, Kaltz O, Nuismer SL, & Gandon S (2013). A practical guide to measuring local adaptation. Ecology Letters, 16, 1195–1205. [DOI] [PubMed] [Google Scholar]
- Broadley MR, Bowen HC, Cotterill HL, Hammond JP, Meacham MC, Mead A, & White PJ (2003). Variation in the shoot calcium content of angiosperms. Journal of Experimental Botany, 54, 1431–1446. [DOI] [PubMed] [Google Scholar]
- Buri A, Cianfrani C, Pinto-Figueroa E, Spangenberg JE, Adatte T, Guisan A& Pradervand J-N (2017). Soil factors improve predictions pf plant species distribution in mountain environment. Progress in Physical Geography, 41, 703–722. [Google Scholar]
- Busoms S, 2015. Local adaptation of wild populations of Arabidopsis thaliana to coastal and inland habitats in Catalonia Ph.D. Thesis Universitat Autònoma de Barcelona & University of Aberdeen. [Google Scholar]
- Busoms S, Terés J, Huan XY, Bomblies K, Danku J, Douglas A, …, Salt DE (2015). Salinity is an agent of divergent selection driving local adaptation of Arabidopsis thaliana to coastal habitats. Plant Physiology, 168, 915–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busoms S, Paajanen P, Marburger S, Bray S, Huang X-Y, Poschenieder C, …, Salt DE (2018) Fluctuating selection on migrant adaptive sodium transporter alleles in coastal Arabidopsis thaliana. Proceedings of the National Academy of Sciences, 115, 12443–12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabot C, Sibole JV, Barceló J & Poschenrieder C (2014). Lessons from crop plants struggling with salinity. Plant Science, 226, 2–13. [DOI] [PubMed] [Google Scholar]
- Callaghan HS, Pigliucci M (2002). Shade-induced plasticity and its ecological significance in wild populations of Arabidopsis thaliana. Ecology 83, 1965–1980. [Google Scholar]
- Carter MR & Gregorich EG (2006). Soil Sampling and Methods of Analysis, 2nd ed. Taylor and Francis, CRC Press, Boca Raton, Florida, USA. [Google Scholar]
- Cesco S, Neumann G, Tomasi N, Pinton R & Weisskopf L (2010) Release of plant-borne flavonoids ino the rhizosphere and their role in plant nutrition. Plant and Soil 329, 1–25. [Google Scholar]
- Chesworth W, Camps Arbestain M. & Macías F (2008). Calcareous Soils. In Encyclopedia of Soil Science ed Chesworth W, pp 77–79 Encyclopaedia of Earth Sciences Series. Springer, Dordrecht. [Google Scholar]
- Clark RB & Baligar VC (2000) Acidic and alkaline soil constraints on plant mineral nutrition. In Plant-Environment Interactions ed. Wilkinson RE, pp 133–177. Marcel Dekker, New York [Google Scholar]
- Colangelo EP, Guerinot ML (2004) The essential basic helix-loop-helix protein FIT1 is required for the iron deficiency response. Plant Cell 16, 3400–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnini S, De Nisi P, Gabotti D, Tato L & Zocchi G (2012) Adaptive strategies of Parietaria diffusa (M. & K.) to calcareous habitat with limited iron availability. Plant Cell and Environment 35, 1171–1184. [DOI] [PubMed] [Google Scholar]
- Fourcroy P, Sisó-Terraza P, Sudre D, Savirón M, Reyt G, Gaymard F, …, & Briat J-B (2014). Involvement of the ABCG37 transporter in secretion of scopoletin and derivatives by Arabidopsis roots in response to iron deficiency. New Phytologist, 201, 155–167. [DOI] [PubMed] [Google Scholar]
- Fournier-Level A, Korte A, Cooper MD, Nordborg M, Schmitt J & Wilczek AM (2011). A map of local adaptation in Arabidopsis thaliana. Science, 334: 86–89. [DOI] [PubMed] [Google Scholar]
- Hawkesford M, Horst W, Kichey T, Lambers H, Schjoerring J, Møller IS & White P (2011). Functions of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants 3rd edn., pp. 135–189 Academic Press, London, [Google Scholar]
- Heegaard E (2002) A model of alpine species distribution in relation to snowmelt time and altitude. Journal of Vegetation Science, 13, 493–504. [Google Scholar]
- Ishimaru Y, Kim S, Tsukamoto T, Oki H, Kobayashi T, Watanabe S, …, Nishizawa NK (2006). Mutational reconstructed ferric chelate reductase confers enhanced tolerance in rice to iron deficiency. Proceedings of the National Academy of Science, USA, 104, 7373–7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffries RL & Willies AJ (1964). Studies on the calcicole-calcifuge habit: II. The influence of calcium on the growth and establishment of four species in soil and sand culture. Journal of Ecology, 52, 691–707. [Google Scholar]
- Kar M & Mishra D (1976). Catalase, peroxidase and polyphenoloxidase activities during rice leaf senescence. Plant Physiology, 57, 315–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawecki T & Ebert D (2004). Conceptual issues in local adaptation. Ecology Letters, 7, 1225–1241. [Google Scholar]
- Kosegarten H, Hoffmann B, Rroco E, Grolig F, Glusenkamp KH & Mengel K (2004). Apoplastic pH and Fe-III reduction in young sunflower Helianthus annuus roots. Physiologia Planarum, 122, 95–106. [Google Scholar]
- Kruckeberg AR (2002). Geology and Plant Life. In The Effects of Landforms and Rock Types on Plants; Chapter 5; pp. 103–228.University of Washington Press, Seattle,WA. [Google Scholar]
- Kumar RK, Chu H-H, Abundis C, Vasques K, Rodriguez DC, Chia J-C, …, & Vatamaniuk VK (2017). Iron-nicotianamine transporters are required for proper long-distance iron signaling. Plant Physiology, 175, 1254–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky JR, Des Marais DL, Lowry DB, Povoltska I, McKay JK, Richards JH, …, & Juenger TE (2014). Natural variation in abiotic stress responsive gene expression and local adaptation to climate in Arabidopsis thaliana. Molecular Biology and Evolution 31: 2283–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemanceau P, Bauer P, Kraemer S & Briat J-F (2009). Iron dynamics in the rhizosphere as a case study for analyzing interactions between soils, plans and microbes. Plant and Soil, 321, 513–535. [Google Scholar]
- Li G, Kronzucker HJ & Shi W (2016). The response of the root apex in plant adaptation to iron heterogeneity in soil. Frontiers in Plant Science, 7, 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeppert RH & Suarez DL (1996). Carbonate and gypsum, In Methods of Soil Analysis, part 3. Methods eds Sparks DL, Page AL pp 437–474. SSSA Book Series no. 5. Soil Science Society of America and American Society of Agronomy, Madison, Wisconsin, USA. [Google Scholar]
- Lucena C, Romera FJ, Rojas CL, García MJ, Alcántara E, & Pérez-Vicente R (2007). Bicarbonate blocks the expression of several genes involved in the physiological responses to Fe deficiency of Strategy I plants. Functional Plant Biology,34, 1002–1009. [DOI] [PubMed] [Google Scholar]
- Macel M, Lawson CS, Mortimer SR, Smilauerova M, Bischoff A, Crémieux L … & Steinger T (2007). Climate vs. soil factors in local adapatation of two common plant species. Ecology, 88, 424–433. [DOI] [PubMed] [Google Scholar]
- Marschner H, Römheld V (1994) Strategies of plants for acquisition of iron. Plant and Soil 165, 261–274. [Google Scholar]
- Moog PR, Kooij TAW, Bruggemann W, Schiefelbein JW & Kuiper PJC (1995). Responses to iron deficiency in Arabidopsis thaliana: The Turbo iron reductase does not depend on the formation of root hairs and transfer cells. Planta, 195, 505–513. [DOI] [PubMed] [Google Scholar]
- Ozores-Hampton M (2013). Effective strategies to correct iron deficiency in Florida Vegetable crops. HortTechnology, 23, 548–552. [Google Scholar]
- Page MD, Allen MD, Kropat J, Urzica EI, Karpowicz SJ, Hsieh SI, …, & Merchant SS (2012) Fe sparing and Fe recycling contribute to increased superoxide dismutase capacity in iron-starved Chlamydomonas reinhardtii. Plant Cell 24, 2649–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poschenrieder C, Fernández JA, Rubio L, Pérez L, Terés J, & Barceló J (2018). Transport and use of bicarbonate in plants: current knowledge and challenges ahead. International Journal of Molecular Sciences, 19, 1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma FM& Agren J (2016). Early life stage contributes strongly to local adaptation in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA, 113, 7590–7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2013). R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria: URL http://www.R-project.org/. [Google Scholar]
- Rajniak J, Giehl RFH, Chang E, Murgia I, von Wirén N, Sattely ES (2018). Biosynthesis of redox-active metabolites in response to iron deficiency in plants. Nature Chemical Biology 14, 442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyer C, Leuzinger S, Ramming A, Bartholomeus RP, Bonfante A, de Lorenzi F … Pereira M (2013). A plant’s perspective of extremes: terrestrial plant responses to changing climatic variability. Global Change Biology, 19, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Celma J, Lin WD, Fu GM, Abadía J, López-Millán AF, & Schmidt W (2013). Mutually exclusive alterations in secondary metabolism are critical for the uptake of insoluble iron compounds by Arabidopsis and Medicago truncatula. Plant Physiology, 162, 1473–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruellan A (1973). Morphology and distribution of calcareous soils in the Mediterranean and desert regions. In Calcareous soils. FAO Soils Bulletin, 21, 7–16. [Google Scholar]
- Ryan J, Rashid A, Torren J, Yau SK, Ibrikci H, Sommer R & Erenoglu EB (2013). Micronutrient constraints to crop production in the Middle East-West Asia region: significance, research, and management. Advances in Agronomy, 122, 1–84. [Google Scholar]
- Santi S & Schmidt W (2009). Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytologist, 183, 1072–1084. [DOI] [PubMed] [Google Scholar]
- Satbhai S, Setzer C, Feynschlag F, Slovak R, Kerdaffrec E & Busch W (2017) Natural allelic variation of FRO2 modulates Arabidopsis root growth under iron deficiency. Nature Comunications 8, 15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid NB, Giehl RFH, Döll S, Mock H-P, Strehmel N, Scheel D, …, & von Wirén N (2014). Feruloyl-CoA 69-hydroxylase1-dependent coumarins mediate iron acquisition from alkaline substrates in Arabidopsis. Plant Physiology, 164, 160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Günther C, Weber M, Spörlein C, Loscher S, Böttcher C, Schobert R, & Clemens S (2014). Metabolome analysis of Arabidopsis thaliana roots identifies a key metabolic pathway for iron acquisition. PLoS One, 9, e102444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert S, Schubert E & Mengel K (1990). Effect of low pH of the root medium on proton release, growth, and nutrient uptake of field beans Vicia faba. Plant and Soil, 124, 239–244. [Google Scholar]
- Sisó-Terraza P, Ríos JJ, Abadía J, Abadía A & Álvarez-Fernández A(2016a). Flavins secreted by roots of iron-deficient Beta vulgaris enable mining of ferric oxide via reductive mechanisms. New Phytologist, 209, 733–745 [DOI] [PubMed] [Google Scholar]
- Sisó-Terraza P, Luis-Villarroya A, Fourcroy P, Briat J-F, Abadía A, Gaymard F, …, & Álvarez-Fernández A (2016b). Accumulation and secretion of coumarinolignans and other coumarins in Arabidopsis thaliana roots in response to iron deficiency at high pH. Frontiers in Plant Science, 7, 1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LM & Laitinen RAE (2015). Use of natural variation in Arabidopsis thaliana to study adaptation. In Molecular Mechanisms in Plant Adaptation ed. Laitinen R, pp 31–59. John Wiley and Sons, Inc., Malden. [Google Scholar]
- Susín S, Abadía A, González-Reyes JA, Lucena JJ & Abadía J (1996). The pH requirement for in vivo activity of the iron –deficiency-induced “turbo” ferric chelate reductase. Plant Physiology, 110, 111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliavini M & Rombolà AD (2001). Iron deficiency and chlorosis in orchard and vineyard ecosystems. European Journal of Agronomy, 15, 71–92. [Google Scholar]
- Tsai H-H, Rodríguez-Celma J, Lan P, Wu Y-C, Vélez-Berúdez IC, & Schmidt W (2018). Scopoletin 8-Hydroxylase-mediated fraxetin production is crucial for iron mobilization. Plant Physiology 177, 194–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vert GA, Briat J-F & Curie C (2003). Dual regulation of the Arabidopsis high‐affinity root iron uptake system by local and long‐distance signals. Plant Physiology, 132, 796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Li W, Yang Z, Chen Y, Shao W & Ji J (2015). An invisible soil acidification: Critical role of soil carbonate and its impact on heavy metal bioavailability. Scientific Reports, 5, 12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters BM & Troupe GC (2012). Natural variation in iron use efficiency and mineral remobilization in cucumber Cucumis sativus. Plant and Soil, 352, 185–197. [Google Scholar]
- Waters BM, Amundsen K, & Graef G (2018). Gene expression profiling of iron deficiency chlorosis sensitive and tolerant soybean indicates key roles for phenylpropanoids under alkalinity stress. Frontiers in Plant Science, 9, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ & Broadley MR (2003). Calcium in plants. Annals of Botany, 92, 487–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler J, Schmidt S, Strehmel N, Scheel D, & Abel S (2017). Arabidopsis transporter ABCG37/PDR9 contributes primarily highly oxygenated coumarins to root exudation. Scientific Reports, 7, 3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolfaghari Z, Mosaddeghi MR & Ayoubi S (2016). Relationships of soil shrinkage parameters and indices with intrinsic soil properties and environmental variables in calcareous soils. Geoderma, 77, 23–34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.