Abstract
Because of the importance of epidermal functions, including stratum corneum hydration and maintenance of permeability barrier homeostasis, in the pathogenesis of a variety of cutaneous and systemic disorders, a wide range of products has been developed to improve epidermal functions. However, the underlying mechanisms whereby certain products, including heparinoid-containing product, are far little understood. In the present study, we assessed the impact of a heparinoid-containing product, Hirudoid® cream, on epidermal permeability barrier function and expression levels of a panel of epidermal mRNA related to formation/maintenance of the permeability barrier in mouse skin. Our results showed that while the baseline levels of transepidermal water rates remained unchanged, treatment with Hirudoid® cream twice daily for 7 days significantly accelerated permeability barrier recovery and increased stratum corneum hydration. In parallel, expression levels of epidermal mRNA for certain differentiation markers-related proteins, lipid synthetic enzymes, keratinocyte proliferation, as well as antimicrobial peptides also increased significantly. Together, these results provide the underlying mechanisms by which topical Hirudoid® cream improves epidermal permeability barrier and antimicrobial function. Because of its benefits for epidermal functions, heparinoid-containing product could be more useful in the management of skin conditions, characterized by abnormal permeability barrier and antimicrobial function.
Keywords: Heparinoid, barrier function, stratum corneum hydration, differentiation
Introduction
Mucopolysaccharide polysulfate, also termed ‘heparinoid’, has long been used to manage a variety of skin conditions. Although heparinoid was first found to exhibit anticoagulant property in 1940s [1,2], heparinoid-containing products are now widely used to treat vascular disorders, such as thrombophlebitis, venous thromboembolism and acute ischemic stroke [3–5]. Studies have also demonstrated benefits of heparinoid-containing products in a number of other dermatoses and epidermal functions. For example, oral administrations of heparinoid is effective in the treatment of oral lichen planus [6,7]. Moreover, topical heparinoid-containing cream not only reduces postoperative ecchymosis and edema [8], but also prevents the development of pressure ulcers [9]. Furthermore, topical applications of a heparinoid-containing product significantly increased stratum corneum hydration in both young and aged humans [10–14], as well as both epidermal growth factor receptor inhibitor- and radiotherapy-induced skin dryness [15–17]. Additionally, a questionnaire survey found that twice-daily applications of a heparinoid cream or lotion for one month markedly improved skin dryness, pruritus and inflammation in subjects with atopic dermatitis, particularly in older patients [18]. Finally, several clinical studies demonstrated that topical Hirudoid® cream improves psoriasis with an efficacy comparable to triamcinolone urea cream [19, 20]. Collectively, this body of evidence suggests that topical heparinoid-containing cream can improve epidermal functions and inhibit inflammation.
Questions Addressed
To determine whether a topical heparinoid-containing product improves epidermal permeability barrier and the underlying mechanisms
Experiment Design
6–8 weeks old C57BL/6J mice were purchased Laboratory Animal Center, Academy of Military Medical Science (Beijing, China), and were fed with mouse diet and water ad libitum in our animal facility. Because Hirudoid® cream is a widely used heparinoid-containing product, we chose Hirudoid®s cream in this study. Hirudoid® cream contains mucopolysaccharide polysulfate (0.3%), thymol, propylene glycol, alcohols adipis lanae, aromatics, methylparaben/propylparaben (E216/E218), etc., made by Mobilat Produktions GmbH, Germany and gifted by Kangzhe Pharmaceuticals Co., China. All primers for qPCR were from Sangon Biotech (Shanghai, China).
Experimental protocols:
All animal procedures were approved by the Animal Study Subcommittee of the Tianjin Medical University, and performed in accordance with their guidelines. Both flanks of 6–8 weeks old C57BL/6J mice were treated topically with Hirudoid® cream twice daily for 7 days. Untreated mice served as normal controls. 18 hours after the last topical treatment, skin samples were collected from both Hirudoid® cream-treated and untreated normal mice for analysis of mRNA expression. Dermis and epidermis was separated by heat separation [21]. The expression levels of mRNA for epidermal antimicrobial peptides, differentiation, proliferation and lipid synthetic enzymes were determined by qPCR.
Functional studies:
Following twice-daily treatments with Hirudoid® cream for 7 days, epidermal biophysical properties were measured using respective probe connected to an MPA5 physiology monitor (Courage+Khazaka electronic GmbH, Cologne, Germany) [22]. Permeability barrier recovery rates were assessed 2 and 4 hours after acute barrier disruption with tape-stripping [22].
Q-PCR for mRNA expression:
Total RNA was isolated from mouse skin, treated as described above, using TRI Reagent (Sigma). First strand cDNA was synthesized from 1μg of total RNA with the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). The real-time PCR contained 20 ng of reversed transcribed total RNA, 450 nM forward and reverse primers, and 10 μl of 2× LightCycler 480 SYBR Green I Master in a final volume of 20 μl in 96-well plates using Mx3000P™ Real-time PCR System (Stratagene, La Jolla, CA). Quantification was performed by the comparative CT method with mouse GADPH used for normalization. Primers sequences are listed in supplemental Table 1. Relative expression of the mRNAs compared to mRNA in normal mice was calculated. Data are expressed as percentage of untreated normal control (setting normal controls as 100%) [22].
Statistics:
GraphPad Prism 5 software was used for all statistical analyses. Mann Whitney test was used to determine significances between the treated and untreated group. Data are expressed as mean ± SEM.
Results
Topical heparinoid-containing cream enhances epidermal permeability barrier homeostasis
Because clinical studies showed that topical heparinoid improved stratum corneum hydration in humans [10–17], we first assessed whether topical heparinoid-containing product, Hirudoid® cream, produces comparable benefits in mice. Indeed, twice-daily applications of Hirudoid® cream significantly elevated the levels of stratum corneum hydration, while neither baseline transepidermal water loss rates (TEWL) nor skin surface pH differed in Hirudoid® cream-treated versus untreated controls (Fig. 1a). We next determined whether topical Hirudoid® cream improves permeability barrier homeostasis, assessed as changes in the kinetics of permeability barrier recovery following 7-day treatments with Hirudoid® cream. As shown in Fig 1b, twice-daily treatments with Hirudoid® cream accelerated permeability barrier recovery after acute barrier disruption. These results demonstrate that topical applications of Hirudoid® cream improve both basal stratum corneum hydration and permeability barrier homeostasis.
Figure 1. Topical Heparinoid-containing cream accelerates permeability barrier recovery.
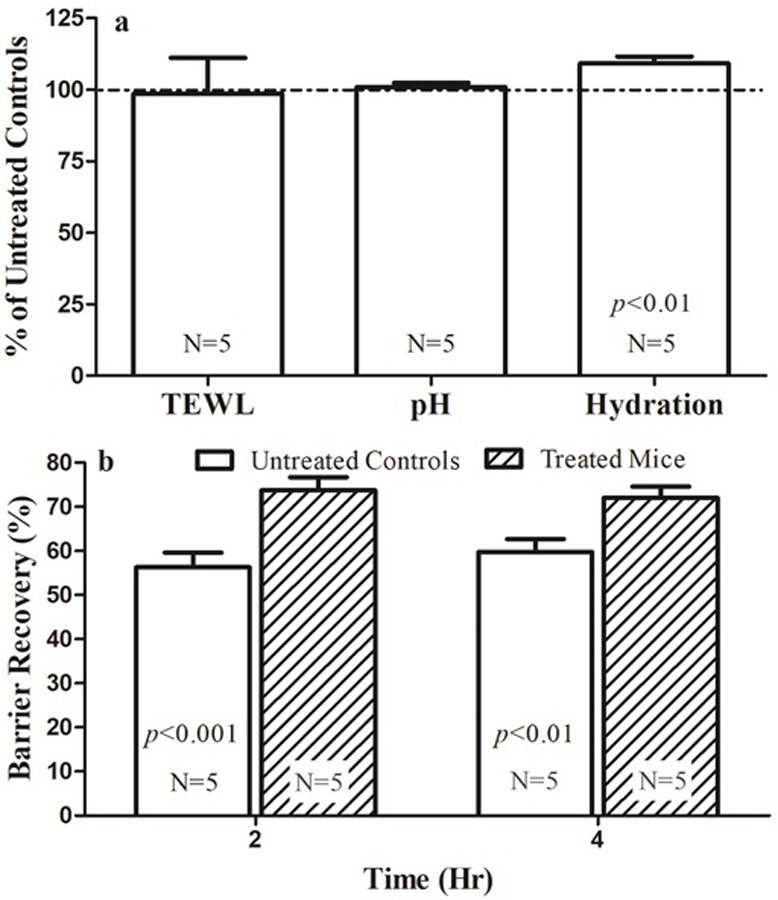
Figure 1a. Changes in basal epidermal function; Figure 1b. Barrier recovery rates. Data are normalized to untreated normal controls, setting controls as 100%. N=9 for all. Significances are indicated in the Figures.
Topical Hirudoid® cream stimulates expression levels of epidermal mRNA for markers of epidermal structure, function and proliferation in mouse skin
As shown above, topical Hirudoid® cream enhanced permeability barrier homeostasis and stratum corneum hydration. Because both stratum corneum lipids and differentiation marker-related proteins are key determinants of functions [23,24], we next assessed changes in the expression levels of epidermal mRNA for these markers. As shown in Fig. 2a, topical Hirudoid® cream dramatically elevated expression levels of epidermal mRNA for epidermal lipid production and differentiation marker-related proteins, particularly filaggrin and involucrin. Yet, expression levels of PCNA and mouse beta defensin 3 (mBD3) (an analog of human beta defensin 2, HBD2) increased significantly following topical treatments with Hirudoid® cream (Fig 2b). These results indicate that topical heparinoid-containing cream stimulates epidermal differentiation, lipid synthesis, proliferation and antimicrobial defense.
Figure 2. Topical Heparinoid-containing cream regulates expression levels of mRNA in the epidermis.
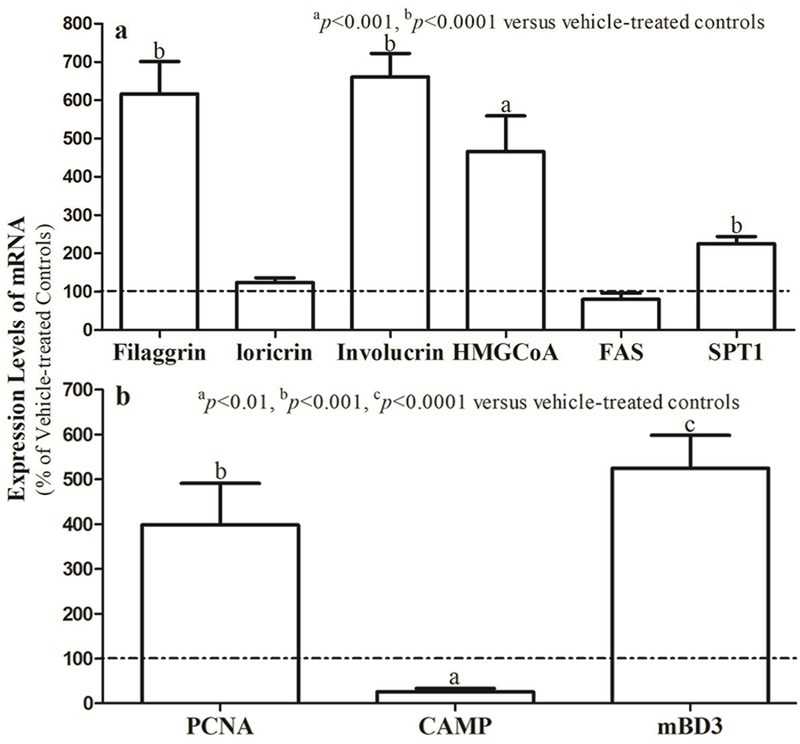
Figure 1a. Expression levels of mRNA for epidermal differentiation marker-related proteins and lipid synthetic enzymes; Figure 1b. Expression levels of mRNA for epidermal proliferation, stratum corneum hydration and antimicrobial defensin. Data are normalized to untreated normal controls, setting controls as 100%. N=9 for all. Significances are indicated in the Figures.
Discussion
Although heparinoid-containing products have been deployed successfully to treat various disorders for over a half century, the basis for their putative benefits remains unknown. To gain insight into this issue, we assessed the transcription of a set genes known to regulate epidermal structure and functions. We demonstrate here that topical treatments with a heparinoid-containing cream dramatically enhanced the basal levels of stratum corneum hydration and the kinetics of permeability barrier recovery. Although the signaling mechanisms by which the heparinoid-containing cream improves epidermal function are unclear, upregulation of the expression levels of epidermal mRNA for several key components associated with epidermal function likely accounts for the improvements in epidermal functions. Because of the importance of filaggrin and ceramides in stratum cornuem hydration [25–28], Hirudoid® cream-induced elevations in expression levels of epidermal mRNA for filaggrin and serine-palmitoyltransferase (SPT) could contribute to the improvements in stratum corneum hydration. In addition, Hirudoid® cream increased the expression levels of epidermal mRNA for lipid synthetic enzymes (HMGCoA reductase and SPT), as well as differentiation marker-related proteins, which could also contribute to the improvements in epidermal permeability barrier function. Our previous studies showed that status of epidermal permeability barrier parallels the expression levels of antimicrobial peptide, including cathelicidin-related antimicrobial peptide (CAMP or LL-37) and mBD3[29], and accordingly we showed here that Hirudoid® cream increased mRNA levels of mBD3, an analog of HBD2, which primarily combats Gram-negative bacteria and Candida [30,31]. LL-37 exerts multiple functions, including antimicrobial property, permeability barrier homeostasis, and proinflammatory ‘alarm’ [32,33]. It is possible that reductions in expression levels of epidermal CAMP mRNA reflect additional anti-inflammatory benefits of heparinoid. Regarding the possible mechanisms by which heparinoid-containing cream increased PCNA expression, it is unclear. However, it has been demonstrated that glycosaminoglyean polysulphate and pentosane polysulphate could increase synthesis of cutaneous hyaluronan [34], while interaction of hyaluronan with its receptor CD44 can stimulate epidermal proliferation and cutaneous wound healing [35,36]. Nevertheless, the present study clearly demonstrate that topical heparinoid-containing cream improves epidermal permeability barrier homeostasis and stratum corneum hydration.
The findings of the present work also suggest possible applications of heparinoid-containing products for other cutaneous disorders. First, a defective epidermal permeability barrier not only provokes production and release of proinflammatory cytokines in the epidermis [37], but also induces inflammatory infiltration in both the epidermis and the dermis [38,39]. Moreover, a key pathogenic role of defective permeability barrier in the development of atopic dermatitis and psoriasis is also well appreciated. Accordingly, improvements in epidermal permeability barrier can prevent and alleviate both atopic dermatitis and psoriasis [40–43]. Pertinently, at least one clinical study demonstrated that topical Hirudoid® cream improves atopic dermatitis [18]. Needless to mention that the benefit of heparinoid-containing cream in epidermal function would expand its clinical utility for the management of skin conditions accompanied and/or driven by a defective epidermal permeability barrier. Second, topical Hirudoid® cream also markedly upregulated expression levels of PCNA, a marker of cell proliferation, in the epidermis, suggesting that topical heparinoid-containing products could benefit cutaneous wound healing and skin conditions accompanied by poor epidermal proliferation, such as chronic glucocorticoid-treated skin and aged skin. Third, heparinoid-containing products could benefit cutaneous infections because of its upregulation of epidermal mBD3 mRNA expression. However, whether heparinoid-containing cream could benefit these dermatoses still require proper clinical trials.
In summary, topical applications of a heparinoid-containing cream improve epidermal permeability barrier and antimicrobial function, suggesting that heparinoid-containing products could be useful in the management of skin conditions accompanied by xerosis, certain infections, defective epidermal permeability barrier and/or poor proliferation and differentiation.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported in part by China National Natural Science Foundation (NSFC 81573075 and 81301360, LH), the Science Foundation of Tianjin Medical University (2013KY06, LH), and NIH grant (AR061106, PME) administered by the Northern California Institute for Research and Education, with resources from the Research Service, Department of Veterans Affairs Medical Center, San Francisco, California.
Footnotes
Conflicts of interest: None
Statement of Ethics: Animal experiments conform to internationally accepted standards and have been approved by the appropriate institutional review body.
References
- 1.SEIFTER J, BEGANY AJ Studies on the action of a synthetic heparinoid. Am J Med Sci 1948;216:234. [PubMed] [Google Scholar]
- 2.SORENSON CW, SEIFTER J, WRIGHT IS A new synthetic anticoagulant (heparinoid) preliminary report of its action in humans. Bull N Y Acad Med 1949;25:448. [PubMed] [Google Scholar]
- 3.Hirsh J Overview of low molecular weight heparins and heparinoids: basic and clinical aspects. Aust N Z J Med 1992;22:487–95. [PubMed] [Google Scholar]
- 4.Mehta PP, Sagar S, Kakkar VV.Treatment of superficial thrombophlebitis: a randomized, bouble-blind trial of heparinoid cream. Br Med J 1975; 3:614–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams HP Jr, Woolson RF, Biller J, Clarke W. Studies of Org 10172 in patients with acute ischemic stroke. TOAST Study Group. Haemostasis 1992;22:99–103. [DOI] [PubMed] [Google Scholar]
- 6.Femiano F, Scully C. Oral lichen planus: clinical and histological evaluation in an open trial using a low molecular weight heparinoid (sulodexide). Int J Dermatol 2006;45:986–9. [DOI] [PubMed] [Google Scholar]
- 7.Femiano F, Gombos F, Scully C. Oral erosive/ulcerative lichen planus: preliminary findings in an open trial of sulodexide compared with cyclosporine (ciclosporin) therapy. Int J Dermatol 2003;42:308–11. [DOI] [PubMed] [Google Scholar]
- 8.Simsek G, Sari E, Kilic R, Bayar Muluk N. Topical Application of Arnica and Mucopolysaccharide Polysulfate Attenuates Periorbital Edema and Ecchymosis in Open Rhinoplasty: A Randomized Controlled Clinical Study. Plast Reconstr Surg 2016;137:530e–535e. [DOI] [PubMed] [Google Scholar]
- 9.Kuisma I, Tamelander G. Mucopolysaccharide polysulphate cream in the prevention of pressure sores--a double blind study. Ann Clin Res 1987;19:374–7. [PubMed] [Google Scholar]
- 10.O’Goshi KI, Tabata N, Sato Y, Tagami H. Comparative study of the efficacy of various moisturizers on the skin of the ASR miniature swine. Skin Pharmacol Appl Skin Physiol 2000; 13:120–7. [DOI] [PubMed] [Google Scholar]
- 11.Manabe H, Nozawa A, Matsumoto M, Ohtani M. Evaluation of Moisturizing Effect of Heparinoid Ointment (Hirudoid Soft Ointment) Diluted by White Petrolatum (Propeto). Yakugaku Zasshi 2017; 137:763–766. [DOI] [PubMed] [Google Scholar]
- 12.Wanitphakdeedecha R, Eimpunth S, Manuskiatti W.The effects of mucopolysaccharide polysulphate on hydration and elasticity of human skin. Dermatol Res Pract 2011;2011:807906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bagherani N The efficacy of bed bath together with heparinoid-containing moisturizers in treating senile xerosis. Dermatol Ther 2016;29:69. [DOI] [PubMed] [Google Scholar]
- 14.Hayama K, Takano Y, Tamura J, Tagami H, Terui T. Effectiveness of a heparinoid-containing moisturiser to treat senile xerosis. Australas J Dermatol 2015;56:36–9. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe S, Nakamura M, Takahashi H, Hara M, Ijichi K, Kawakita D, Morita A. Dermopathy associated with cetuximab and panitumumab: investigation of the usefulness of moisturizers in its management. Clin Cosmet Investig Dermatol 2017;10:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sekiguchi K, Akahane K, Ogita M, Haga C, Ito R, Arai S, Ishida Y, Tsukada Y, Kawamori J. Efficacy of heparinoid moisturizer as a prophylactic agent for radiation dermatitis following radiotherapy after breast-conserving surgery: a randomized controlled trial. Jpn J Clin Oncol 2018;48:450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekiguchi K, Ogita M, Akahane K, Haga C, Ito R, Arai S, Ishida Y, Tsukada Y, Kawamori J. Randomized, prospective assessment of moisturizer efficacy for the treatment of radiation dermatitis following radiotherapy after breast-conserving surgery. Jpn J Clin Oncol 2015;45:1146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawakami T, Soma Y. Questionnaire survey of the efficacy of emollients for adult patients with atopic dermatitis. J Dermatol 2011;38:531–5. [DOI] [PubMed] [Google Scholar]
- 19.Shi H and Peng T. Clinical efficacy of psoriasis capsule combined with heparinoid ointment for the treatment of psoriasis vulgairs. Clin J Dermatol 2014; 43:370–372 [Google Scholar]
- 20.Lei L A study of 34 patients with plaque psoriasis vulgaris treated with Hirudoid® Cream. J Dermatol Venereol 2003; 25:30 [Google Scholar]
- 21.Kassis V, Søndergaard J. Heat-separation of normal human skin for epidermal and dermal prostaglandin analysis. Arch Dermatol Res 1982;273:301–6. [DOI] [PubMed] [Google Scholar]
- 22.Man G, Mauro TM, Zhai Y, Kim PL, Cheung C, Hupe M, Crumrine D, Elias PM, Man MQ Topical hesperidin enhances epidermal function in an aged murine model. J Invest Dermatol 2015; 135: 1184–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elias PM, Feingold KR. Coordinate regulation of epidermal differentiation and barrier homeostasis. Skin Pharmacol Appl Skin Physiol 2001;14:S28–34. [DOI] [PubMed] [Google Scholar]
- 24.Feingold KR, Elias PM. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim Biophys Acta 2014;1841:280–94 [DOI] [PubMed] [Google Scholar]
- 25.Hoste E, Kemperman P, Devos M, Denecker G, Kezic S, Yau N, Gilbert B, Lippens S, De Groote P, Roelandt R, Van Damme P, Gevaert K, Presland RB, Takahara H, Puppels G, Caspers P, Vandenabeele P, Declercq W. Caspase-14 is required for filaggrin degradation to natural moisturizing factors in the skin. J Invest Dermatol 2011;131:2233–41. [DOI] [PubMed] [Google Scholar]
- 26.Pendaries V, Malaisse J, Pellerin L, Le Lamer M, Nachat R, Kezic S, Schmitt AM, Paul C, Poumay Y, Serre G, Simon M. Knockdown of filaggrin in a three-dimensional reconstructed human epidermis impairs keratinocyte differentiation. J Invest Dermatol 2014;134:2938–2946. [DOI] [PubMed] [Google Scholar]
- 27.Jeon S, Cho Y. Epidermal Hydration Is Improved by Enhanced Ceramide Metabolism in Aged C57BL/6J Mice After Dietary Supplementation of Royal Jelly. J Med Food 2015;18:999–1006. [DOI] [PubMed] [Google Scholar]
- 28.Simpson E, Böhling A, Bielfeldt S, Bosc C, Kerrouche N. Improvement of skin barrier function in atopic dermatitis patients with a new moisturizer containing a ceramide precursor. J Dermatolog Treat 2013;24:122–5. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Martin M, Martin-Ezquerra G, Man MQ, Hupe M, Youm JK, Mackenzie DS, Cho S, Trullas C, Holleran WM, Radek KA, Elias PM. Expression of epidermal CAMP changes in parallel with permeability barrier status. J Invest Dermatol 2011;131:2263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joly S, Maze C, McCray PB Jr, Guthmiller JM. Human beta-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. J Clin Microbiol 2004;42:1024–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schröder JM, Harder J. Human beta-defensin-2. Int J Biochem Cell Biol 1999;3:645–51. [DOI] [PubMed] [Google Scholar]
- 32.Li N, Yamasaki K, Saito R, Fukushi-Takahashi S, Shimada-Omori R, Asano M, Aiba S. Alarmin function of cathelicidin antimicrobial peptide LL37 through IL-36γ induction in human epidermal keratinocytes. J Immunol 2014;193:5140–8. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi T, Kulkarni NN, Lee EY, Zhang LJ, Wong GCL, Gallo RL. Cathelicidin promotes inflammation by enabling binding of self-RNA to cell surface scavenger receptors. Sci Rep 2018; 8:4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Francis DJ, Hutadilok N, Kongtawelert P, Ghosh P. Pentosan polysulphate and glycosaminoglycan polysulphate stimulate the synthesis of hyaluronan in vivo. Rheumatol Int 1993;13:61–4. [DOI] [PubMed] [Google Scholar]
- 35.Bourguignon LY, Ramez M, Gilad E, Singleton PA, Man MQ, Crumrine DA, Elias PM, Feingold KR. Hyaluronan-CD44 interaction stimulates keratinocyte differentiation, lamellar body formation/secretion, and permeability barrier homeostasis. J Invest Dermatol 2006;126:1356–65. [DOI] [PubMed] [Google Scholar]
- 36.Bourguignon LY, Wong G, Xia W, Man MQ, Holleran WM, Elias PM. Selective matrix (hyaluronan) interaction with CD44 and RhoGTPase signaling promotes keratinocyte functions and overcomes age-related epidermal dysfunction. J Dermatol Sci 2013;72:32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elias PM, Wood LC, Feingold KR. Epidermal pathogenesis of inflammatory dermatoses. Am J Contact Dermat 1999;10:119–26. [PubMed] [Google Scholar]
- 38.Proksch E, Brasch J, Sterry W. Integrity of the permeability barrier regulates epidermal Langerhans cell density. Br J Dermatol 1996;134:630–8. [PubMed] [Google Scholar]
- 39.Lin TK, Man MQ, Santiago JL, Park K, Roelandt T, Oda Y, Hupe M, Crumrine D, Lee HJ, Gschwandtner M, Thyssen JP, Trullas C, Tschachler E, Feingold KR, Elias PM. Topical antihistamines display potent anti-inflammatory activity linked in part to enhanced permeability barrier function. J Invest Dermatol 2013;133:469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Man MQ, Ye L, Hu LZ, Jeong S, Lv CZ, Elias PM. Improvements in Epidermal Function Prevent Relapse of Psoriasis: A Self-Controlled Study. Clin Exp Dermatol 2019. January 4. doi: 10.1111/ced.13888 [DOI] [PubMed]
- 41.Simpson EL, Chalmers JR, Hanifin JM, Thomas KS, Cork MJ, McLean WH, Brown SJ, Chen Z, Chen Y, Williams HC. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol 2014;134:818–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chalmers JR, Haines RH, Mitchell EJ, Thomas KS, Brown SJ, Ridd M, Lawton S, Simpson EL, Cork MJ, Sach TH, Bradshaw LE, Montgomery AA, Boyle RJ, Williams HC. Effectiveness and cost-effectiveness of daily all-over-body application of emollient during the first year of life for preventing atopic eczema in high-risk children (The BEEP trial): protocol for a randomised controlled trial. Trials 2017;18:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Angelova-Fischer I, Neufang G, Jung K, Fischer TW, Zillikens D. A randomized, investigator-blinded efficacy assessment study of stand-alone emollient use in mild to moderately severe atopic dermatitis flares. J Eur Acad Dermatol Venereol 2014;28:S9–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


