Abstract
Background:
Optimal imaging utilization for staging of oropharynx cancer is not well defined.
Methods:
The linked Surveillance, Epidemiology, and End Results (SEER)–Medicare database between 2006 and 2011 was used to compare patient characteristics and hospital region by initial imaging modality used for patients with oropharynx cancer. The primary outcome was 3-year cancer specific survival (CSS). Cox proportional hazard models adjusted for imaging, age, sex, region, education, race, cancer stage, and treatment, which were examined in by backward elimination. We also explored how initial imaging use varied by patient characteristics and hospital region.
Results:
One thousand seven hundred and sixty-five patients underwent initial diagnostic imaging (n = 1,765). Of those, 11.4% (n=202) received CT alone as their initial imaging modality, 5.2% (n=91) underwent MRIs without PET imaging, and 83.3% (n=1,472) of patients’ initial imaging included a PET exam. Overall three-year CSS for the entire population was 63.7%. In the adjusted survival models compared by intial imaging modality, patients who underwent a PET exam had higher survival than CT alone or MRI, respectively (hazard ratio [HR] 1.337; 95% CI 1.001–1.785; P =0.0491) (HR 1.748; 95% CI 1.2–2.545; P = 0.0036).
Conclusions:
Among patients with oropharyngeal cancer, initial staging with PET imaging was associated with improved three-year CSS compared to initial staging with MRI or CT.
Keywords: Oropharyngeal Neoplasms, Neoplasm Staging, Radiology, Survival
Precis:
SEER-Medicare analysis demonstrates an association between initial staging with PET and improved cancer specific survival for patients with cancer of the oropharynx, as compared to MRI-alone initial staging.
Introduction:
In 2017 there were 17,000 new cases of oropharynx cancer OPSCC and over 3,000 deaths from the disease.1 Therapy for OPSCC oropharyngeal squamous cell carcinoma (OPSCC) typically consists of either resection followed by adjuvant radiation with or without chemotherapy or definitive radiation therapy with or without concurrent chemotherapy depending on the stage. However, initial imaging workup to define disease stage is not as well defined. Current guidelines by the National Comprehensive Cancer Network (NCCN) suggest work-up of OPSCC include computerized tomography (CT) with contrast “and/or” magnetic resonance imaging (MRI) with contrast of the neck, however Fludeoxyglucose F 18 positron emission tomography (PET)/CT (PET) is to be utilized ‘as clinically indicated’.2
In 1995, Laubenbacher et al. were one of the first to suggest a role for PET in detection of occult head and neck squamous cell carcinomas.3 While there have been many follow-up studies demonstrating the higher sensitivity and accuracy of PET over CT/MRI imaging to identify sites of involvement by head and neck cancer both at initial staging and during surveillance, the impact on survival has not been established.4–8
To date there have been no prospective randomized controlled trials to evaluate the different imaging modalities at initial staging on cancer specific survival (CSS). A population-based data source such as the Surveillance, Epidemiology, and End Results (SEER)–Medicare database provides an excellent opportunity for comparing the impact of imaging modality differences on OPSCC patient survival. We hypothesize that given the higher sensitivity and accuracy of PET imaging, its utilization will be associated with a higher CSS in patients with OPSCC.
Patients and Methods:
Data source:
The analysis was done using the linked SEER-Medicare dataset. The SEER program collects information from population based cancer registries that cover approximately 28% of the US population.9 The SEER data provides patient specific demographics, tumor characteristics, treatment, overall survival (OS) and CSS. By linking the registry data to Medicare claims, dates of service, payments, procedures and diagnosis codes can also be captured. Diagnoses and procedures are reported with International Classification of Diseases, Ninth Revision, Clinical Modification codes, Current Procedural Terminology (CPT) codes, and the Healthcare Common Procedure Coding System (HCPCS). The database also contains census tract–level socioeconomic measures obtained via the linkage of the patient’s address to census data.
Sample Selection:
The study protocol was approved by the University of Colorado Cancer Center institutional review board. The study sample was comprised of patients with cancer of the oropharynx who received at least one of the three imaging modalities of interest at diagnosis.
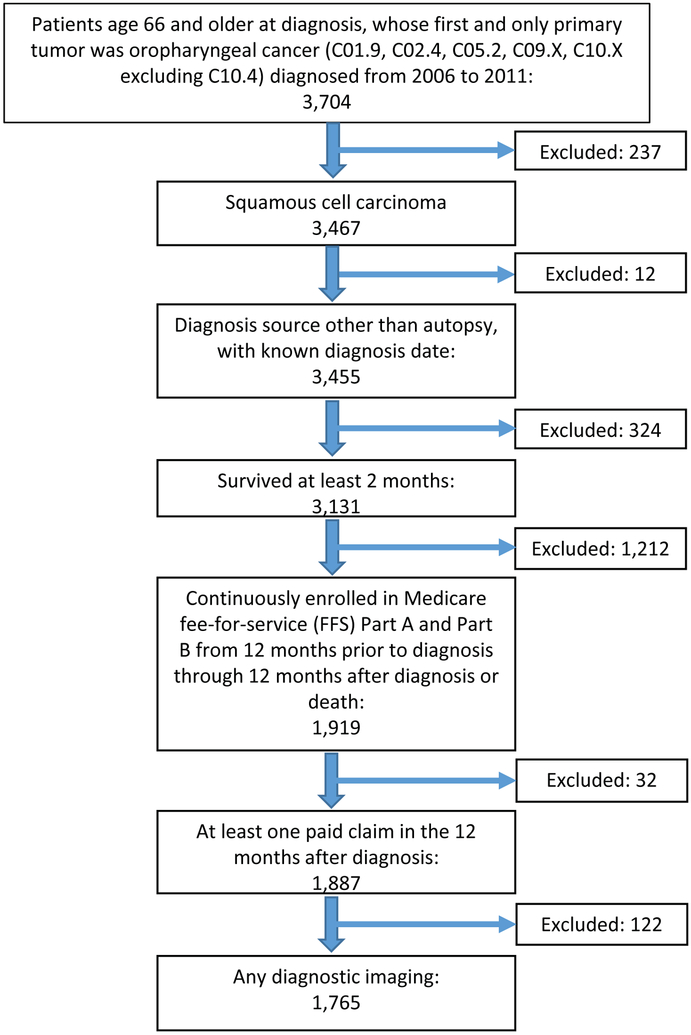
Figure 1 demonstrates that we identified 3,467 patients age 66 and older whose first and only tumor was OPSCC (ICD-O-3 topographic codes C01.9, C02.4, C05.2, C09.X, and C10.X excluding C10.4) diagnosed from 2006 to 2011. Given changes in therapeutic options at the time, 2006 was chosen as our starting point, with the plan to examine a 5 year period. Our analysis included all stages, with stage analysis based on American Joint Committee on Cancer TNM categories. Patients who were diagnosed by autopsy, had unknown diagnosis dates, or survived less than two months were excluded (N = 324). The two month survival exclusion was intentionally selected, given the poor survival outcome associated with OPSCC, if we had selected a longer time period our sample may have been biased with a healthier population. To ensure complete claims history, we only included patients continuously enrolled in fee-for-service Medicare Parts A and B for 12 months before and 12 months after the month of diagnosis (or until death if it occurred within 12 months) (N = 1,919). In addition, patients with no paid claims during the 12-month observation period were excluded (N = 32), leaving 1,887 patients for whom we had complete claims data.
Figure 1:
Consort Diagram
Lastly, we required that patients had undergone imaging within the diagnosis period (defined below). This resulted in a study sample of 1,765 patients (Figure 1). Patients with unknown stage at diagnosis were excluded from survival analyses (N = 252).
Outcomes:
An initial analysis was conducted to identify patient characteristics (demographics and comorbidities) associated with utilization of PET imaging for staging of newly diagnosed OPSCC. The diagnostic period was defined as three months prior to diagnosis through the earlier of two possible end points: two months after the month of diagnosis, or 30 days after the initiation of therapy. Imaging procedures of interest were identified using HCPCS and CPT codes, and included CT of the face or neck, MRI of the neck, and PET (site not specified). Based on the imaging utilization in the diagnostic period, patients were sorted into three groups, Any PET imaging, MRI utilization without PET, or CT alone.
For survival analysis, we limited the sample to patients with known stage (N = 1,513) and evaluated CSS and OS, truncated at three years. CSS was determined using SEER dates of death, which are reported through December 2011 and include cause of death. OS analysis was performed using Medicare-reported dates of death, which extended through December 2013. Survival time was measured from the month of diagnosis through death, disenrollment from Medicare, or the end of the respective reporting period. Patients with less than three years of follow-up time were censored if they did not experience the event. Patients surviving more than three years were censored at 36 months.
Total spending for diagnostic imaging was evaluated and defined as the sum of Medicare payments, patient deductibles and copays, as well as payments made by any other primary payers as reported on the Medicare claims. We included claims from the Medicare Provider Analysis and Review, Outpatient, National Claims History Physician/Supplier, Durable Medical Equipment, Home Health, and Hospice files.
Covariates:
In all multivariate analyses, we adjusted for year of diagnosis, patient sex, age at diagnosis, race/ethnicity, marital status, SEER registry geographic region, population density (metropolitan vs. non-metropolitan), census tract percent below poverty, census tract-level education, and whether the patient visited a teaching hospital. We used Medicare claims from the year before diagnosis to calculate the Charlson Comorbidity Index values according to the National Cancer Institute’s adaptation of the algorithm described by Klabunde et al.10 In survival analyses, we also controlled for AJCC 6th edition stage group, and treatment initiated within six months of diagnosis, including chemotherapy, radiation therapy, and surgery.
Statistical Analysis:
Imaging modality:
Chi-square tests were used to assess univariate associations between categorical variables and imaging modalities at diagnosis. Multivariate polytomous logistic regression models were used to assess the association between patient characteristics and the receipt of different types of imaging.
Survival:
Univariate survival analysis was performed using the Kaplan-Meier to obtain survival estimates, and using unadjusted Cox proportional hazards models to estimate hazard ratios (HRs). Multivariate survival analysis was performed using Cox proportional hazards regression analysis to estimate adjusted HRs for three-year CSS and OS. The final multivariate models were selected using backward elimination to remove covariates not reaching the p<.05 significance level. The proportional hazards assumption was validated using Schoenfeld residuals. All statistical analyses were performed with SAS 9.4 (SAS Institute, Cary NC) and evaluated at a significance level of p < .05.
Results:
Population Characteristics:
Table 1 reports the sample characteristics. The median follow-up of continuous Medicare FFS coverage was 32 months. Of the 1,765 patients with any imaging during the diagnosis period, 202 (11%) received only CT imaging prior to therapy, 1,472 (83%) were staged with PET and 91 (5%) received an MRI with or without CT imaging, but no PET imaging.
Table 1:
Frequencies by PET with and without other Imaging
| Overall | PET | MRI +/− CT | CT Alone | p-value Comparing Any MRI without PET to Any PET |
p-value Comparing CT Alone to Any PET |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | ||||
| All Patients | 1765 | . | 1472 | . | 91 | . | 202 | . | . | . | |
| Age at Diagnosis | 66 to 69 | 600 | 33.99 | 505 | 34.31 | 27 | 29.67 | 68 | 33.66 | 0.3843 | 0.9232 |
| 70 to 74 | 543 | 30.76 | 456 | 30.98 | 26 | 28.57 | 61 | 30.20 | . | . | |
| 75 and Older | 622 | 35.24 | 511 | 34.71 | 38 | 41.76 | 73 | 36.14 | . | . | |
| Sex | Female | 436 | 24.70 | 330 | 22.42 | 38 | 41.76 | 68 | 33.66 | <.0001 | 0.0004 |
| Male | 1329 | 75.30 | 1142 | 77.58 | 53 | 58.24 | 134 | 66.34 | . | . | |
| Race/Ethnicity Category | White NH | 1488 | 84.31 | 1248 | 84.78 | 72 | 79.12 | 168 | 83.17 | 0.1480 | 0.5513 |
| Non-White or Hispanic | 277 | 15.69 | 224 | 15.22 | 19 | 20.88 | 34 | 16.83 | . | . | |
| Marital Status Category | Non-Married | 833 | 47.20 | 663 | 45.04 | 47 | 51.65 | 123 | 60.89 | 0.2192 | <.0001 |
| Married or Partnered | 932 | 52.80 | 809 | 54.96 | 44 | 48.35 | 79 | 39.11 | . | . | |
| Patient Region at Diagnosis | East | 345 | 19.55 | 291 | 19.77 | 43 | 21.29 | <.0001 | 0.7259 | ||
| Midwest | 156 | 8.84 | 132 | 8.97 | 20 | 9.90 | . | . | |||
| South | 503 | 28.50 | 426 | 28.94 | 15 | 16.48 | 62 | 30.69 | . | . | |
| West | 761 | 43.12 | 623 | 42.32 | 61 | 67.03 | 77 | 38.12 | . | . | |
| Patient Location | Metropolitan | 1445 | 81.87 | 1205 | 81.86 | 77 | 84.62 | 163 | 80.69 | 0.5067 | 0.6870 |
| Non-Metropolitan | 320 | 18.13 | 267 | 18.14 | 14 | 15.38 | 39 | 19.31 | . | . | |
| Poverty Level of Census Tract | Less than Median Level | 881 | 50.00 | 754 | 51.33 | 48 | 52.75 | 79 | 39.11 | 0.7926 | 0.0011 |
| Higher than Median Level | 881 | 50.00 | 715 | 48.67 | 43 | 47.25 | 123 | 60.89 | . | . | |
| Residents in Census Tract with High School Education | Less than Median Level | 881 | 50.00 | 759 | 51.67 | 43 | 47.25 | 79 | 39.11 | 0.4135 | 0.0008 |
| Higher than Median Level | 881 | 50.00 | 710 | 48.33 | 48 | 52.75 | 123 | 60.89 | . | . | |
| Charlson Comorbidity Index Category | 0 | 894 | 50.65 | 756 | 51.36 | 39 | 42.86 | 99 | 49.01 | 0.2886 | 0.4294 |
| 1 | 434 | 24.59 | 362 | 24.59 | 26 | 28.57 | 46 | 22.77 | . | . | |
| 2 or more | 437 | 24.76 | 354 | 24.05 | 26 | 28.57 | 57 | 28.22 | . | . | |
| Teaching Hospital | No or Unknown | 719 | 40.74 | 599 | 40.69 | 35 | 38.46 | 85 | 42.08 | 0.6740 | 0.7070 |
| Yes | 1046 | 59.26 | 873 | 59.31 | 56 | 61.54 | 117 | 57.92 | . | . | |
| DAJCC Stage Group 6th Ed | Stage 0-I | 81 | 4.59 | 46 | 3.13 | 14 | 15.38 | 21 | 10.40 | <.0001 | <.0001 |
| Stage II-III | 429 | 24.31 | 351 | 23.85 | 20 | 21.98 | 58 | 28.71 | . | . | |
| Stage IV | 1003 | 56.83 | 871 | 59.17 | 43 | 47.25 | 89 | 44.06 | . | . | |
| Unknown Stage | 252 | 14.28 | 204 | 13.86 | 14 | 15.38 | 34 | 16.83 | . | . | |
| Chemotherapy | No | 489 | 27.71 | 333 | 22.62 | 43 | 47.25 | 113 | 55.94 | <.0001 | <.0001 |
| Yes | 1276 | 72.29 | 1139 | 77.38 | 48 | 52.75 | 89 | 44.06 | . | . | |
| Radiation | No | 245 | 13.88 | 147 | 9.99 | 26 | 28.57 | 72 | 35.64 | <.0001 | <.0001 |
| Yes | 1520 | 86.12 | 1325 | 90.01 | 65 | 71.43 | 130 | 64.36 | . | . | |
| Surgery | No | 1195 | 67.71 | 1023 | 69.50 | 52 | 57.14 | 120 | 59.41 | 0.0136 | 0.0039 |
| Yes | 570 | 32.29 | 449 | 30.50 | 39 | 42.86 | 82 | 40.59 | . | . | |
Imaging Utilization:
Multivariate logistic regression analysis found that females were significantly more likely to receive an MRI (OR 2.534; 95% CI 1.600–4.013; P = <0.001) or CT alone (OR 1.554; 95% CI 1.115–2.165; P = 0.0093), than PET based staging at diagnosis. The east, midwest or south regions were much more likely to get a MRI without a PET, as compared to the western United States, which often obtained both (East - OR 0.282; 95% CI 0.139–0.569; P = 0.0004) (Midwest - OR 0.222; 95% CI 0.077–0.644; P = 0.0056) (South - OR 0.306; 95% CI 0.165–0.567; P = 0.002). Patients in census tracts with lower levels of education were found to undergo MRI staging without PET at a higher rate (OR 1.919; 95% CI 1.139–3.232; P = 0.0143). The non-married/partnered patients were found to have a higher rate of CT alone imaging as compared to PET (OR 1.584; 95% CI 1.154–2.174; P = 0.0044).
Survival Outcomes:
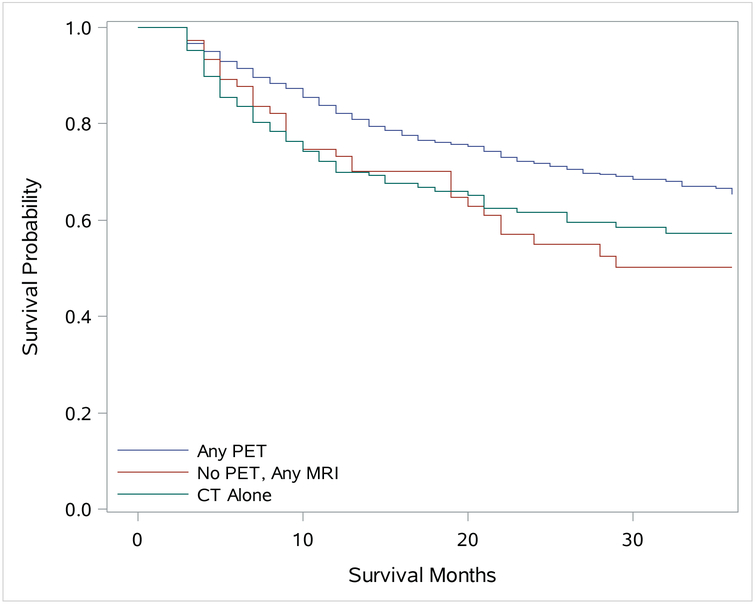
Multivariate analysis, controlling for stage and treatment, of CSS with Cox proportional hazards model found patients who had been staged with PET imaging did significantly better than patients with MRI or CT. The CT alone patients had a hazard ratio (HR) of 1.337 (95% CI 1.001–1.785; P =0.0491), while the MRI patients had a HR of 1.748 (95% CI 1.2–2.545; P = 0.0036). Additional predictors of decreased CSS in our analysis included patient age (75 and older compared to 66 to 69) (HR, 1.92; 95% CI 1.494–2.476; P = <0.0001), female gender (HR, 1.66; 95% CI 1.345–2.049; P = <0.0001), and Charlson Comorbidity Index Score of 2 or more (HR, 1.587; 95% CI 1.262–1.994; P = 0.0001). While non-married patients were less likely to undergo PET imaging, they did not have a significantly worse CSS (HR, 1.187; 95% CI 0.964–1.462; P = 0.1063). Patients within census tracts with an increased percentage of residents having high school level education or less, trended toward poorer outcomes (HR, 1.253; 95% CI 0.997–1.575; P = 0.0532). In addition, as expected, more advanced staged OPSCC patients had poorer CSS. While it was not statistically significant, the western region did have the highest CSS of the four regions at 3 years (67.3%).
The 3 year OS was better for the patients who received a PET exam (56.8%; 95% CI 54.0–59.6%), followed by patients who had a MRI without PET imaging (50.1%; 95% CI 38.4–60.75%), with the lowest survival percentage being the patients who were only imaged with CT alone (47.3% 95% CI 39.5–54.6%). The Cox proportional hazards model for OS, which includes treatment variables, was not significant with the CT alone patients having a HR of 1.213 (95% CI 0.955–1.542; P = 0.114) or the MRI imaged patients HR 1.365 (95% CI 0.977–1.908; P= 0.0683). As expected patients 75 years and older had poorer OS (HR 1.951; 95% CI 1.602–2.377; P = <0.0001). Female (HR 1.229; 95% CI 1.025–1.473; P = 0.0259), non-married patients (HR 1.325; 95% CI 1.127–1.557; P = 0.0006) and patients from census tracts with lower levels of education (HR 1.328; 95% CI 1.136–1.552; P = 0.0004) also showed a significantly lower OS. In addition, patients with a higher Charlson Comorbidity Score and advanced staged disease all had poorer OS.
Discussion:
The therapy of head and neck carcinomas is an interdisciplinary challenge. Correct staging gives important information on the extent of the tumor, lymph node status, and distant metastasis.11 The therapy based on such results has a significant impact on management, prognosis, and long term morbidity. Radiographic imaging plays a significant role in the initial staging and therapeutic management planning for untreated head and neck squamous cell cancer. Several imaging modalities are recommended as part of the initial evaluation of head and neck tumors, including MRI, CT, or PET scan. Current guidelines from the NCCN suggest that patients with OPSCC should be imaged with, “CT with contrast and/or MRI with contrast of the neck,” with PET being recommended, “as clinically indicated”.2
Our study is one of the most comprehensive population based studies to directly evaluate how the modality of imaging used for initial staging impacts CSS. Our findings demonstrate that patients whose initial staging includes a PET have longer CSS than those imaged with either MRI or CT alone. While prior single center studies have shown no diagnostic performance difference in local staging of oral and oropharyngeal cancer when comparing different imaging modalities, these studies did not evaluate survival.12–14 Our study demonstrates a significant difference in CSS based on initial imaging. These findings are consistent with a prior study which reported that PET can improve staging of head and neck cancers, particularly as it relates to radiation therapy planning.15
There are multiple explanations for the improved survival outcomes with the use of PET over CT or MRI based staging in oropharyngeal cancers. Various authors have studied the optimal imaging modality to be used for staging before locoregional treatment. MRI has been reported to have a sensitivity and specificity of 36% and 94% respectively16 while the corresponding values for PET-CT scans have been reported to be 79 and 86% at initial staging.16–18 This idea is again supported by a prior study of patients with head and neck cancer from an unknown primary in which PET found unrecognized disease in 27% more patients, when compared to CT and MRI.19 In a disease where the pattern of spread tends to be lymphatic, for both CT and MRI, which provides structural information at a high spatial resolution,20 suspicion for nodal disease is often based on size, which does not necessarily reflect the physiological activity within. One study comparing individual patients’ PET, CT/MRI and surgical results and found PET had a sensitivity 9% higher than CT/MRI for disease detection.21 Since nodal status significantly impacts survival, accurate staging is important for appropriate therapy.22 The inclusion of nodal disease within a radiation treatment field may mean the difference in a complete response and disease recurrence. It is this improved detection and associated treatment, which we believe results in the improved CSS.
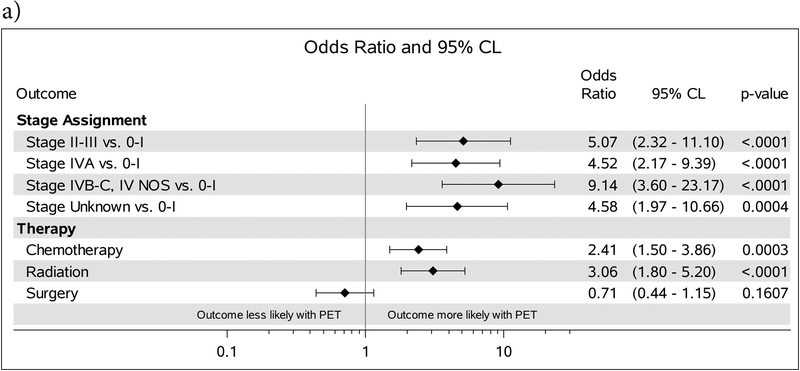
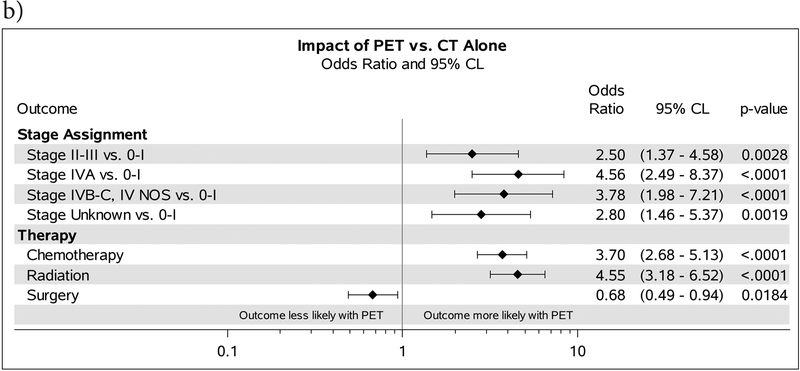
Patients who underwent CT alone initially had a significantly lower CSS. The trend towards improved CSS in favor of PET continued on MVA after controlling for treatment. While it is clear patients who are only imaged by CT alone have a poorer survival, even after controlling for stage and demographics, the true impact may be how PET changes therapy. Our findings show PET is associated with a higher likelihood of chemo and radiation therapy. This has become increasingly important in the era where the vision is shifting towards treatment deintensification with consideration for elimination of elective nodal coverage.23 Many head and neck cancer patients are treated with definitive radiation and sensitive tools that allow for detection of nodal disease becomes critical to prevent marginal misses.
Females and non-married patients all received a statistically significant lower rate of PET imaging. One important question this study raises, is why females are significantly less likely to receive a PET at diagnosis. Men are known to utilize healthcare less compared to women, prior to a cancer diagnosis, however following a diagnosis of cancer their utilization increases to equal that of women.24 Prior studies have found women are less likely to undergo imaging during presentation of bladder cancer.25 This is in contrast to a larger study which found men were more likely to not undergo staging, as compared to women.26 Our study found women from all sites were more likely to have an MRI or CT than a PET exam, which given the impact on CSS may result in a gender survival disparity. A prior matched-pair study evaluating survival disparities between men and women with head and neck cancer found no advantages for women as compared to men, but did not evaluate imaging utilization.27 Given the prior research in combination with our study, further assessment for the role of imaging utilization on gender outcome differences should be assessed. In addition, non-married patients, both men and women, have been shown to be diagnosed with more advanced cancers and have poorer outcomes.28–30 A prior SEER based study examining multiple cancers found married patients were more likely to undergo staging, even after controlling for age, sex and race. There are multiple theories for why this difference exists: disease detected at earlier stage, high socioeconomic status, or better social support; while the reason is still unknown, it is a factor physicians need to consider when counseling their patients.
Our study also demonstrates significant PET utilization differences across the country, with the western region utilizing both PET and MRI at higher rate than the east, midwest or south. A prior city based imaging utilization analysis which found the highest utilization of PET in the south, Atlanta, and the lowest in the west, Seattle.31 However, it does fit the increased utilization the western region has shown on multiple prior SEER based studies.32–34 This difference in utilization did not result in significant OS or CSS differences between the regions, though the west did have the highest CSS of the four. While these differences may be due to practice patterns, training experiences or patient preference, further study is warranted to explore the potential causes as to why such significant utilization differences exist.
Patients 75 years old and older were also significantly less likely to receive PET imaging. While prior studies have shown utilization decreases with patient age,32, 34 it is important to note that older patients have an increasingly poorer cancer survival.35, 36 While these patients may be choosing not to undergo PET imaging, ageism may also be playing an unintentional role. It should be noted that the Charlson Comorbidity Index Score of 2 or more was also associated with decreased PET utilization and comorbidities may be contributing to the difference we see, however the HR for patients 75 and older was 1.92 and only 1.587 for patients with a Charlson Comorbidity Index Score of 2 or more. Prior publications have described an ‘age issue’ regarding cancer care and our findings show as chronological age increases, patients are undergoing less imaging.
Limitations of this study include the population, as it is based on the Medicare fee-for-service population and those 66 years old and older; therefore, the application to younger OPSCC patients requires further study. The data also does not include prognostic factors such as HPV or smoking status. Given the increasing prevalence of HPV associated OPSCC, even in the elderly population, further clarification on whether there is effect modification between the HPV positive and negative populations is warranted.1 While our multivariable analysis included numerous factors such as treatment type, age, race, gender, etc., the data set is not complete and it is possible that factors, which are not included, could impact the results. PET usage was associated with more advanced disease and while prior studies have demonstrated increased sensitivity and specificity for disease detection it is not clear whether patients with suspected higher stage disease received PET imaging or if patients who receive PET imaging are diagnosed with more advanced disease. Treatment noncompliance may also have a role given the age of the patient population. Additional outcome measurements are not included in the data, which limits the focus to survival.
Overall the data presented in this study support the idea that PET imaging may have a significant impact on patient outcomes. Findings from this SEER-Medicare analysis demonstrates that patients who are initially staged with PET have improved outcomes as compared to those staged with CT alone or MRI with or without CT. This analysis also highlights significantly fewer women are being staged with PET exams. Our data suggests the need for further prospsective research to evaluate if CT or MRI should be considered adequate for initial staging of OPSCC.
Figure 2:
Kaplan-Meier Curve of 3 Year Cancer Specific Survival
Figure 3:
A. ORs comparing stage assignment and receipt of therapy for patients receiving PET vs. those who were imaged with MRI. B. Forest plot comparing outcomes and the OR for patients who received PET vs those who were imaged with CT alone. ORs comparing stage assignment are adjusted for demographic characteristics. ORs comparing receipt of therapy are adjusted for demographic characteristics and stage at diagnosis.
Acknowledgments
Funding Statement: This project was supported by Population Health Shared Resource, University of Colorado Cancer Center, P30CA046934.
Footnotes
Conflict of Interest Statement: The authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
- 1.Zumsteg ZS, Cook-Wiens G, Yoshida E, et al. Incidence of oropharyngeal cancer among elderly patients in the united states. JAMA Oncology. 2016;2: 1617–1623. [DOI] [PubMed] [Google Scholar]
- 2.Network NCC. Head and Neck Cancers - Version 2.2018 - June 20, 2018. Available from URL: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf [accessed September 15, 2018. [Google Scholar]
- 3.Laubenbacher C, Saumweber D, Wagner-Manslau C, et al. Comparison of Fluorine-18-Fluorodeoxyglucose PET, MRI and Endoscopy for Staging Head and Neck Squamous-Cell Carcinomas. Journal of Nuclear Medicine. 1995;36: 1747–1757. [PubMed] [Google Scholar]
- 4.Dibble EH, Lara Alvarez AC, Truong M-T, Mercier G, Cook EF, Subramaniam RM. 18F-FDG Metabolic Tumor Volume and Total Glycolytic Activity of Oral Cavity and Oropharyngeal Squamous Cell Cancer: Adding Value to Clinical Staging. Journal of Nuclear Medicine. 2012;53: 709–715. [DOI] [PubMed] [Google Scholar]
- 5.Sivarajah S, Isaac A, Cooper T, et al. Association of fludeoxyglucose f 18–labeled positron emission tomography and computed tomography with the detection of oropharyngeal cancer recurrence. JAMA Otolaryngology–Head & Neck Surgery. 2018;144: 1037–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krabbe CA, Balink H, Roodenburg JLN, Dol J, de Visscher JGAM. Performance of 18F-FDG PET/contrast-enhanced CT in the staging of squamous cell carcinoma of the oral cavity and oropharynx. International Journal of Oral and Maxillofacial Surgery. 2011;40: 1263–1270. [DOI] [PubMed] [Google Scholar]
- 7.Subramaniam RM, Alluri KC, Tahari AK, Aygun N, Quon H. PET/CT Imaging and Human Papilloma Virus–Positive Oropharyngeal Squamous Cell Cancer: Evolving Clinical Imaging Paradigm. Journal of Nuclear Medicine. 2014;55: 431–438. [DOI] [PubMed] [Google Scholar]
- 8.Subramaniam RM, Truong M, Peller P, Sakai O, Mercier G. Fluorodeoxyglucose–Positron-Emission Tomography Imaging of Head and Neck Squamous Cell Cancer. American Journal of Neuroradiology. 2010;31: 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.L Warren J, N Klabunde C, Schrag D, B Bach P, Riley G. Overview of the SEER-Medicare data - Content, research applications, and generalizability to the United States elderly population. 2002. [DOI] [PubMed] [Google Scholar]
- 10.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. Journal of Clinical Epidemiology. 2000;53: 1258–1267. [DOI] [PubMed] [Google Scholar]
- 11.Kutler DI, Wong RJ, Kraus DH. Functional imaging in head and neck cancer. Curr Oncol Rep. 2005;7: 137–144. [DOI] [PubMed] [Google Scholar]
- 12.Seitz O, Chambron-Pinho N, Middendorp M, et al. 18F-Fluorodeoxyglucose-PET/CT to evaluate tumor, nodal disease, and gross tumor volume of oropharyngeal and oral cavity cancer: comparison with MR imaging and validation with surgical specimen. Neuroradiology. 2009;51: 677–686. [DOI] [PubMed] [Google Scholar]
- 13.Sohn B, Koh YW, Kang WJ, Lee J-H, Shin N-Y, Kim J. Is there an additive value of 18 F-FDG PET-CT to CT/MRI for detecting nodal metastasis in oropharyngeal squamous cell carcinoma patients with palpably negative neck? Acta Radiologica. 2016;57: 1352–1359. [DOI] [PubMed] [Google Scholar]
- 14.GUENZEL T, FRANZEN A, WIEGAND S, et al. The Value of PET Compared to MRI in Malignant Head and Neck Tumors. Anticancer Research. 2013;33: 1141–1146. [PubMed] [Google Scholar]
- 15.Mazzola R, Alongi P, Ricchetti F, et al. 18F-Fluorodeoxyglucose-PET/CT in locally advanced head and neck cancer can influence the stage migration and nodal radiation treatment volumes. La radiologia medica. 2017;122: 952–959. [DOI] [PubMed] [Google Scholar]
- 16.Braams JW, Pruim J, Freling NJM, et al. Detection of Lymph Node Metastases of Squamous-Cell Cancer of the Head and Neck with FDG-PET and MRI. Journal of Nuclear Medicine. 1995;36: 211–216. [PubMed] [Google Scholar]
- 17.Kyzas PA, Evangelou E, Denaxa-Kyza D, Ioannidis JPA. 18F-Fluorodeoxyglucose Positron Emission Tomography to Evaluate Cervical Node Metastases in Patients With Head and Neck Squamous Cell Carcinoma: A Meta-analysis. JNCI: Journal of the National Cancer Institute. 2008;100: 712–720. [DOI] [PubMed] [Google Scholar]
- 18.Xu G-Z, Zhu X-D, Li M-Y. Accuracy of whole-body PET and PET-CT in initial M staging of head and neck cancer: A meta-analysis. Head & Neck. 2011;33: 87–94. [DOI] [PubMed] [Google Scholar]
- 19.Rusthoven KE, Koshy M, Paulino AC. The role of fluorodeoxyglucose positron emission tomography in cervical lymph node metastases from an unknown primary tumor. Cancer. 2004;101: 2641–2649. [DOI] [PubMed] [Google Scholar]
- 20.Schöder H, Yeung HWD, Gonen M, Kraus D, Larson SM. Head and Neck Cancer: Clinical Usefulness and Accuracy of PET/CT Image Fusion. Radiology. 2004;231: 65–72. [DOI] [PubMed] [Google Scholar]
- 21.Roh J-L, Yeo N-K, Kim JS, et al. Utility of 2-[18F] fluoro-2-deoxy-d-glucose positron emission tomography and positron emission tomography/computed tomography imaging in the preoperative staging of head and neck squamous cell carcinoma. Oral Oncology. 2007;43: 887–893. [DOI] [PubMed] [Google Scholar]
- 22.Amini A, Jasem J, Jones BL, et al. Predictors of overall survival in human papillomavirus-associated oropharyngeal cancer using the National Cancer Data Base. Oral Oncology. 2016;56: 1–7. [DOI] [PubMed] [Google Scholar]
- 23.Loganadane G, Kelly JR, Lee NC, et al. Incidence of radiographically occult nodal metastases in HPV+ oropharyngeal carcinoma: Implications for reducing elective nodal coverage. Pract Radiat Oncol. 2018;8: 397–403. [DOI] [PubMed] [Google Scholar]
- 24.Burnside C, Hudson T, Williams C, Lawson W, Laiyemo AO. Sex differences in the use of healthcare services among US adults with and without a cancer diagnosis. Turkish journal of urology. 2018;44: 298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohn JA, Vekhter B, Lyttle C, Steinberg GD, Large MC. Sex disparities in diagnosis of bladder cancer after initial presentation with hematuria: A nationwide claims-based investigation. Cancer. 2014;120: 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merrill RM, Sloan A, Anderson AE, Ryker K. Unstaged cancer in the United States: a population-based study. BMC cancer. 2011;11: 402–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts JC, Li G, Reitzel LR, Wei Q, Sturgis EM. No Evidence of Sex-Related Survival Disparities among Head and Neck Cancer Patients Receiving Similar Multidisciplinary Care: A Matched-Pair Analysis. Clinical Cancer Research. 2010: 1078–0432.CCR-1010–0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adekolujo OS, Tadisina S, Koduru U, Gernand J, Smith SJ, Kakarala RR. Impact of Marital Status on Tumor Stage at Diagnosis and on Survival in Male Breast Cancer. American journal of men’s health. 2017;11: 1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osborne C, Ostir GV, Du X, Peek MK, Goodwin JS. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat. 2005;93: 41–47. [DOI] [PubMed] [Google Scholar]
- 30.Rendall MS, Weden MM, Favreault MM, Waldron H. The Protective Effect of Marriage for Survival: A Review and Update. Demography. 2011;48: 481. [DOI] [PubMed] [Google Scholar]
- 31.Parker L, Levin DC, Frangos A, Rao VM. Geographic Variation in the Utilization of Noninvasive Diagnostic Imaging: National Medicare Data, 1998–2007. American Journal of Roentgenology. 2010;194: 1034–1039. [DOI] [PubMed] [Google Scholar]
- 32.Patel MK, Cote ML, Ali-Fehmi R, Buekers T, Munkarah AR, Elshaikh MA. Trends in the Utilization of Adjuvant Vaginal Cuff Brachytherapy and/or External Beam Radiation Treatment in Stage I and II Endometrial Cancer: A Surveillance, Epidemiology, and End-Results Study. International Journal of Radiation Oncology*Biology*Physics. 2012;83: 178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou AH, Chung SY, Patel VR, et al. Do geographic differences or socioeconomic disparities affect survival in sinonasal squamous cell carcinoma? International Forum of Allergy & Rhinology. 2017;7: 1195–1200. [DOI] [PubMed] [Google Scholar]
- 34.Vyas A, Madhavan SS, Sambamoorthi U, et al. Healthcare Utilization and Costs During the Initial Phase of Care Among Elderly Women With Breast Cancer. Journal of the National Comprehensive Cancer Network : JNCCN. 2017;15: 1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quaglia A, Tavilla A, Shack L, et al. The cancer survival gap between elderly and middle-aged patients in Europe is widening. European Journal of Cancer. 2009;45: 1006–1016. [DOI] [PubMed] [Google Scholar]
- 36.De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE-5—a population-based study. The Lancet Oncology. 2014;15: 23–34. [DOI] [PubMed] [Google Scholar]