Abstract
Purpose:
Cardiac native-T1 times have correlated to extracellular volume fraction in patients with confirmed fibrosis. However, whether other factors that can occur either alongside or independently of fibrosis including increased intracellular water volume, altered magnetization transfer (MT), or glycation of hemoglobin, lengthen T1 times in the absence of fibrosis remains unclear. The current study examined whether native-T1 times are elevated in hypertrophic diabetics with elevated hemoglobin A1C (HbA1c) without diffuse fibrosis.
Methods
Native-T1 times were quantified in 27 diabetic and 10 healthy adults using a modified Look-Locker imaging (MOLLI) sequence at 1.5T. The MT ratio (MTR) was quantified using dual flip angle cine balanced steady-state free precession. Gadolinium (0.2 mmol/kg Gd-DTPA) was administered as a bolus and post-contrast T1-times were quantified after 15 minutes. Means were compared using a two-tailed student’s t-test, while correlations were assessed using Pearson’s correlations.
Results
While left ventricular volumes, ejection fraction, and cardiac output were similar between groups, left ventricular mass and mass-to-volume ratio (MVR) were significantly higher in diabetic adults. Mean ECV (0.25±0.02 Healthy vs. 0.25±0.03 Diabetic, P=0.47) and MTR (125±16 % Healthy vs. 125±9 % Diabetic, P=0.97) were similar, however native-T1 times were significantly higher in diabetics (1016±21ms Healthy vs. 1056±31ms Diabetic, P=0.00051). Global native-T1 times correlated with MVR (ρ=0.43, P=0.008) and plasma HbA1c levels (ρ=0.43, P=0.0088) but not ECV (ρ=0.06, P=0.73). Septal native-T1 times correlated with septal wall thickness (ρ=0.50, P=0.001).
Conclusion
In diabetic adults with normal ECV values, elevated native-T1 times may reflect increased intracellular water volume and changes secondary to increased hemoglobin glycation.
Keywords: Diabetes, T1 relaxation time, hypertrophy, HbA1c
1. Introduction
Methods for quantifying the development of diffuse interstitial fibrosis in the setting of non-ischemic heart disease have been undergoing intensive development over the last decade. Quantification of extracellular volume fraction (ECV) through mapping of myocardial T1-relaxation times before and after administration of gadolinium-based contrast agents has demonstrated a strongly correlation with collagen volume fraction as determined by histology [1,2]. In addition, identification of diffuse fibrosis has been shown by several studies to be associated with increased long-term risk of heart failure, diastolic dysfunction, and fatal arrhythmias [3]. The ability to reliably and repeatedly quantify diffuse fibrotic burden in high-risk patient populations can potentially enable earlier intervention. However, even with recent concerns surrounding acute administration of gadolinium contrast agents receding [4,5], concerns remain regarding long-term deposition in deep brain regions following repeated exposure [6].
In response to concerns of gadolinium toxicity, non-contrast or gadolinium-free techniques for myocardial tissue characterization have been increasingly explored in recent years These techniques include mapping of native-T1 relaxation times [7,8], T1ρ times [9], and changes in magnetization transfer (MT) contrast [10,11]. A common attribute of each technique is that elevated T1/T1ρ times and loss of MT contrast occur in fibrotic tissue due to increases in the relative fraction of mobile protons within the extracellular water pool. Importantly, in nonischemic pathologies including hypertrophic (HCM) and hypertensive cardiomyopathy, and patient populations including those with diabetes or end-stage renal disease, diffuse fibrosis often occurs concomitantly with cardiomyocyte hypertrophy. However, most studies examining changes in relaxation times have selected patient populations in which diffuse fibrosis and hypertrophy are concomitant and control populations display neither attribute. Subsequently, whether expansion of the intracellular free water pool within hypertrophied cardiomyocytes, corresponding changes in MT, and disease-related changes in levels of plasma mediators of iron homeostasis combine to lengthen relaxation times independent of changes in ECV is commonly overlooked.
Heart failure among adults with type II diabetes is initially characterized by the development of ventricular hypertrophy and/or diffuse fibrosis, increased inflammation and formation of advanced glycation end-products [12,13]. Importantly, while the development of LV hypertrophy is consistent, the development of diffuse fibrosis is heterogeneous among diabetic patients [[12],[13],[14],[15]]. For example, two recent large studies reported significantly higher [16] and lower [17] ECV values among diabetic patients despite consistent hypertrophy in comparison to healthy controls. In this study we recruited diabetic adults and healthy controls for cardiac magnetic resonance (CMR) tissue characterization. Our initial goal was to examine the correlation between changes in ECV and changes in native T1-relaxation times and MT contrast. However, when all diabetic patients demonstrated normal ECV values, we observed hypertrophy and hemoglobin glycosylation-dependent changes in native-T1 relaxation times without changes in MT contrast.
2. Methods
2.1. Patient selection, ethics, consent and permissions
Twenty-seven diabetic adults were recruited from within the Endocrinology Clinic at the University of Kentucky Medical Center. Ten healthy non-diabetic controls were recruited from within the community. Exclusion criteria included renal failure, prior myocardial infarction or other diagnosed cardiomyopathy. The research protocol was approved by our institutional review board (IRB 12-0795-F3R) and informed consent was obtained from all subjects for participation and publication of findings. Demographic characteristics are summarized in Table 1. Hematocrit and HbA1c were measured at the time of imaging. One healthy control subject was unable to complete the study due to an adverse reaction to gadolinium.
Table 1.
Study population demographics and plasma measurements. The two study groups have statistical differences in age, BMI, and HbA1c. Data are presented as mean ± SD
| Variable | Healthy Controls | Diabetic Patients | |
|---|---|---|---|
| Age (years) | 40 ± 12 | 53 ± 11 | p < 0.01 |
| BMI (kg/m2) | 24.5 ± 5.1 | 36.0 ± 8.7 | p < 0.01 |
| Female | 7 (58.33%) | 19 (67.86%) | p = 0.76 |
| HbA1c (%) | 5.4 ± 0.3 | 8.3 ± 1.4 | p < 0.01 |
| Hematocrit (%) | 40.49 ± 2.4 | 40.6 ± 3.5 | p = 0.85 |
| Heart Rate (bpm) | 60.0 ± 6.5 | 72.1 ± 11.5 | p < 0.01 |
2.2. Human Cardiac MRI protocol
CMR was performed on a 1.5 T Siemens MAGNETOM Aera scanner (Siemens Medical Imaging Solutions, Erlanger, Germany) using an 18-channel body coil and 12-channel spine coil. First, a short-axis stack of bSSFP cine images were obtained with prospective ECG triggering to cover the entire left ventricle (TE = 1.2 ms TR = 3.2 ms, bandwidth = 930 Hz, field of view = 260 × 260 mm, slice thickness = 8 mm, flip angle = 50°, 256 × 256 matrix, GRAPPA 2). Acquisition time for each cine bSSFP image set was ~17s, depending on heart rate. Next, pairs of differentially MT-weighted cine bSSFP images and modified Look-Locker imaging (MOLLI) images were acquired in series at each slice location moving from base to apex. For differential MT-weighting, pairs of bSSFP cine images were acquired with excitation flip angles of 5° (proton density reference) and 45° (MT) during end expiratory breath-holds as described in Stromp et al. [10]. All sequence parameters excluding flip angle were identical to those used for scout imaging. MOLLI was performing using a 5(3)3 sequence design with additional parameters of TE = 1.1 ms, TR = 2.7 ms, flip angle = 35°, bandwidth = 1085 Hz, field of view = 272 × 272 mm, slice thickness = 8 mm, 256 × 256 matrix with 66% phase resolution, partial Fourier transform 7/8, GRAPPA 2. Afterwards, gadolinium (0.2 mmol/kg Gd-DTPA) was administered intravenously as a bolus (2 ml/s) and after 15 min post-contrast MOLLI (4(1)3(1)2, TE:1.1 ms, TR:2.7 ms, flip angle:35°, field of view:272 × 272 mm, GRAPPA 2), images were obtained in the same slice positions as pre-contrast images. The scan time for MOLLI was ~15s depending on heart rate.
2.3. Image analysis
MT contrast was quantified by calculating the normalized change in signal between images as (ΔS/So)i=[(S45-S5)/S5]i, where S45 and S5 represent the signal intensity for 45° and 5° excitations, respectively, for each cardiac phase (i). For each patient ΔS/So maps from three end-diastolic phases without cardiac motion were averaged together to reduce random noise yielding a single map of the magnetization transfer ratio (MTR). Maps of T1 relaxation times were automatically reconstructed online using non-rigid body correction. The left ventricular myocardium was manually segmented on each short axis slice using a custom-designed MATLAB script (MATLAB 2016a, MathWorks, Nattick MA). The mean MTR, pre-contrast and post-contrast T1 values were calculated, and the extracellular volume fraction was calculated as ECV = (ΔR1myocardium/ΔR1b1ood)*(1-hematocrit), where ΔR1b1ood was calculated from one mid-ventricular slice. Cardiomyocyte cell volume was estimated as described by Storz et al. [17] as (1-ECV)*LVmass/1.05. The average septal values of MTR, native-T1 and ECV were calculated only in two mid-ventricular slices. Average values for septal MTR, native-T1 and ECV were also calculated for a basal and an apical slice. MTR maps were prepared using a median filter with a 3×2 kernel and color scheme that was previously used to emulate late gadolinium enhancement as described in Stromp et al. [10]. Maps of T1-relaxation times were exported from Siemens image viewing software using the commercial standard colormap.
Separately, left ventricular structure and function were assessed in Argus (Siemens Healthcare, Erlangen, Germany). Left ventricular end diastolic volume (EDV), end systolic volume (ESV), and myocardial mass were all calculated after manual segmentation of endo- and epicardial borders. Ejection fraction (EF) and stroke volume were calculated following identification of peak systole. Cardiac output was then calculated as stroke volume multiplied by heart rate at the time of imaging. Septal wall thickness was measured at the mid-ventricle. Finally, left ventricular mass was normalized to both body mass (LVMI) and end diastolic volume (MVR).
2.4. Statistics
Numeric data are summarized as mean ± SD. Statistical analysis was performed in Microsoft Excel. Differences in parameters of left ventricular structure and function, MTR, T1 times and ECV between control and diabetic adults were assessed using a two-tailed student’s t-test. In order to determine correlations between MTR, heart rate, native-T1 relaxation times, and extracellular volume fraction were measured using Pearson’s correlations. Multivariable regression analysis was performed to analyze the effects of heart rate, mass to volume ratio and HbA1c on native-T1 times. Resulting coefficients were corrected using Shapley-Owen analysis. Statistical significance in pairwise comparisons was defined as p < 0.05.
3. Results
3.1. Demographics, plasma markers, and ventricular structure and function
Demographic and descriptive data are found in Table 1. Diabetic patients were significantly older than healthy controls, however all patients fell within a consistent age quintile metric for assessment of cardiovascular risk. Body mass index and HbA1c values were significantly higher among diabetic adults. All diabetic patients demonstrated HbA1c values above 6.5% at the time of imaging. Hematocrit values were similar between the two groups (Table 1). A trend towards decreased left ventricular volumes was observed in diabetic adults (Table 2), however stroke volume, cardiac output, and ejection fraction were all similar between diabetic and healthy adults (Table 2). While septal wall thickness, myocardial mass, and the ventricular mass-to-volume ratio were all significantly elevated in diabetic adults compared to healthy controls (Table 2), left ventricular mass indexed to body mass was not significantly different between groups (Table 2).
Table 2.
Parameters of left ventricular structure and global function. Data are presented as mean ± SD.
| Healthy Controls | Diabetic Patients | ||
|---|---|---|---|
| End Diastolic Volume (ml) | 91.6 ± 22.5 | 78.6 ± 20.4 | P = 0.09 |
| End Systolic Volume (ml) | 34.2 ± 12.9 | 27.1 ± 11.9 | P = 0.11 |
| Stroke Volume (ml) | 57.5 ± 12.1 | 51.5 ± 13.6 | P = 0.21 |
| Cardiac Output (L/min) | 3.6 ± 1.1 | 3.7 ± 1.0 | P = 0.81 |
| Ejection Fraction (%) | 67 ± 8 | 66 ± 10 | P = 0.37 |
| Septal Wall Thickness (cm) | 0.89 ± 0.13 | 1.22 ± 0.22 | p < 0.01 |
| Left Ventricular Mass (g) | 98.5 ± 29.9 | 126.5 ± 26.5 | p < 0.01 |
| Mass to Volume Ratio (g/ml) | 1.12 ± 0.46 | 1.68 ± 0.49 | p < 0.01 |
| Left Ventricular Mass Index (g/kg) | 1.46 ± 0.39 | 1.26 ± 0.35 | P = 0.16 |
| Cell Volume (mL) | 69.9 ± 22.1 | 90.2 ± 19.3 | P = 0.007 |
3.2. MR tissue characterization
Measured ECV values were not significantly different between diabetic and healthy adults (0.26 ± 0.02 Healthy vs. 0.25 ± 0.03 Diabetic, P = 0.47) as shown in Figure 1. In addition, mean ECV values were below 0.30 in all individuals studied. While measurements of MTR (125 ± 16 % Healthy vs. 125 ± 9 % Diabetic, P = 0.97) were similar in healthy and diabetic adults (Figure 1), native-T1 times were significantly elevated in diabetic patients (1016 ± 23 ms Healthy vs. 1050 ± 45 ms Diabetic, P = 0.02). Post-injection myocardial T1 times (449 ± 26 ms Healthy vs. 423 ± 32 ms Diabetic, P = 0.04) were significantly higher in healthy adults (Figure 1). Representative mid-ventricular maps of native T1-relaxation times and MTR values are shown in Figure 2 for a healthy control, and two diabetic adults: one with a mean native-T1 time within the healthy range and one with significantly heightened mean native-T1. Estimated cellular volume was significantly higher in diabetic adults compared to healthy controls as shown in Table 2. In addition, average values of septal MTR, native-T1 and ECV for a basal and an apical slice of the myocardium are shown in Table 3. Calculations of blood T1 values were found to be significantly higher in healthy adults compared to diabetic patients for post-contrast (288 ± 31 ms Healthy vs. 253 ± 35 ms Diabetic, P = 0.013), but not for pre-contrast (1670 ± 69 ms Healthy vs. 1627 ± 69 ms Diabetic, P = 0.085).
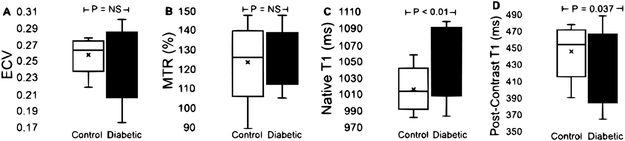
Fig. 1. Box-whisker plots of mean global measurements in healthy controls and diabetic adults.
(A) Extracellular volume fraction (ECV) was similar between healthy adults (control; minimum = 0.22, median = 0.26, maximum = 0.28) and diabetic adults (minimum = 0.18, median = 0.25, maximum = 0.30). (B) In parallel, the magnetization transfer ratio (MTR) was statistically similar between healthy (minimum = 89.7%, median = 124.5%, maximum = 147.6%) and diabetic adults (minimum = 105.4%, median = 125.5%, maximum = 147.6%). (C) While native T1-relaxation times were significantly longer among diabetic adults (minimum = 984 ms, median = 1056 ms, maximum = 1098 ms) in comparison to healthy controls (minimum = 983 ms, median = 1013 ms, maximum = 1058 ms), (D) post-contrast T1 times were shorter in diabetics (423 ± 32 ms) vs. healthy controls (449 ± 26 ms).
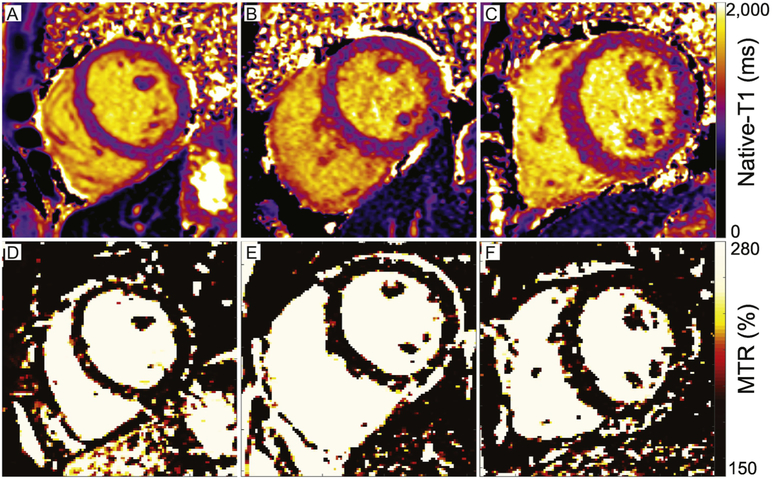
Figure 2. Representative maps of native-T1 and MTR in healthy and diabetic adults.
Maps of native-T1 relaxation times in (A) a healthy control and (B) a diabetic adult with normal left ventricular structural parameters demonstrate similar global mean T1 relaxation times (A: 1014 ms, B: 1023 ms). (C) In contrast, elevated native-T1 relaxation times within the septum, anterior wall, and inferior right ventricular insertion area of a diabetic adult with increased septal wall thickness (1.4 cm) and elevated mass-to-volume ratio (1.4 mg/ml) result in a longer mean left ventricular native-T1 time (C: 1095ms) compared to either A or B. The mean ECV was similar across groups (A: 0.26 A.U., B: 0.24 A.U., C: 0.27 A.U.) and within the range of literature values for healthy tissue. (D-E) Corresponding maps of the magnetization transfer ratio demonstrate similar mean global values across all individuals (A: 134%, B: 127%, C: 124%) that are consistent with literature values from healthy individuals (see reference 10).
Table 3.
MTR, native-T1, and ECV values for a basal and an apical slice of the myocardium. Data are presented as mean ± SD.
| Healthy | Diabetic | ||
|---|---|---|---|
| Basal MTR (%) | 134.5 ± 30.4 | 142.1 ± 32.5 | P = 0.51 |
| Basal T1 (ms) | 1004.2 ± 1043.2 | 1043.2 ± 52.4 | P = 0.03 |
| Basal ECV | 0.24 ± 0.04 | 0.24 ± 0.03 | P = 0.49 |
| Apical MTR (%) | 99.9 ± 12.9 | 88.5 ± 25.8 | P = 0.17 |
| Apical T1 (ms) | 1009.3 ± 33.1 | 1030.3 ± 54.2 | P = 0.24 |
| Apical ECV | 0.26 ± 0.05 | 0.25 ± 0.03 | P = 0.33 |
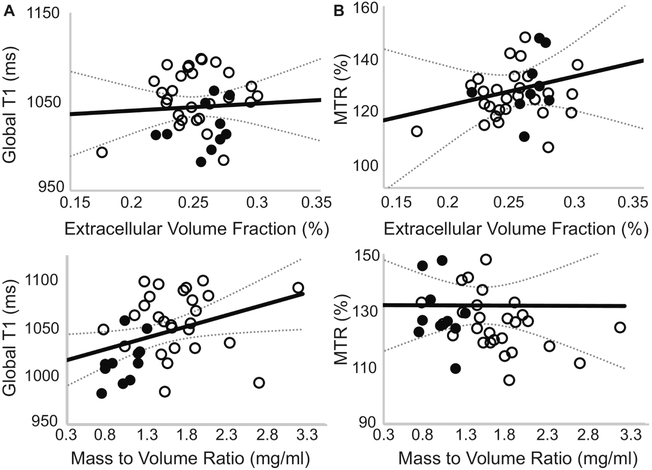
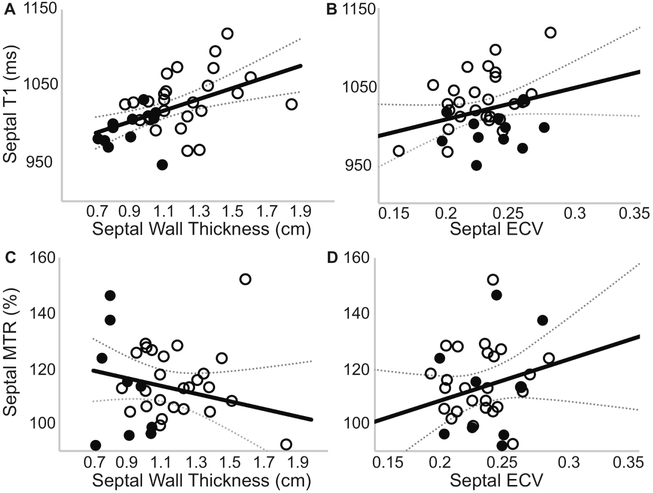
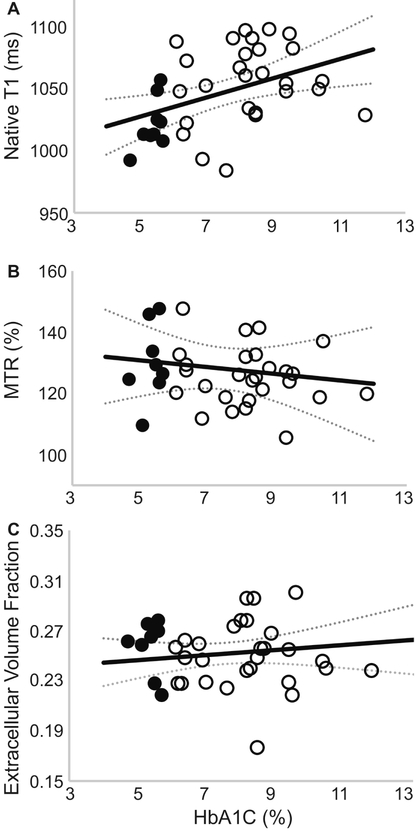
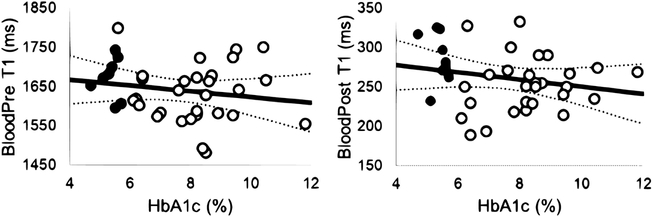
No correlation was observed between average left ventricular ECV and average native-T1 relaxation times or MTR values as shown in Figure 3. However, a significant correlation was observed between global native T1-relaxation times and left ventricular hypertrophy measured as the mass-to-volume ratio (Figure 3), as well as resting heart rate (Pearson’s ρ = 0.52, P = 0.001). In contrast, MTR values were not correlated with changes in the mass-to-volume ratio (Figure 3). Further examination revealed a significant correlation between mid-ventricular septal native T1-relaxation times and corresponding septal wall thickness, even in the absence of a correlation with ECV values as shown in Figure 4. Regional MTR values did not correlate with either septal wall thickness or corresponding regional ECV values (Figure 4). Calculated from ECV and myocardial mass, cellular volume correlated with native-T1 times (Pearson’s ρ = 0.38, P = 0.02) but not MTR (Pearson’s ρ = 0.17, P = 0.32). While neither native-T1 times (Pearson’s ρ = 0.08, P = 0.62) nor MTR (Pearson’s ρ = 0.06, P = 0.74) correlated with hematocrit levels, ECV logically demonstrated a significant correlation with hematocrit (Pearson’s ρ = 0.52, P = 0.0008). Interestingly, myocardial native-T1 times demonstrated a significant correlation with HbA1c levels (Figure 5) that was not observed with either MTR or ECV. However, native-T1 times of blood did not demonstrate a significant correlation with HbA1c (Pearson’s ρ = −0.18, P = 0.29). Additionally, the glycation of hemoglobin did not demonstrate a significant impact on blood T1 for both pre- and post-contrast cases (Figure 6).
Fig. 3. Association of global native-T1 or MTR with global extracellular volume fraction and left ventricular mass to volume ratio.
Healthy controls are represented by filled circles, diabetic adults are represented by empty circles. (A) The average global left ventricular native-T1 time was not correlated with ECV (Pearson’s ρ = 0.06, P = 0.73) derived from pre- and post-contrast T1 times. (B) Similarly, the average left ventricular magnetization transfer ratio (MTR) was not correlated with ECV values (Pearson’s ρ = 0.15, P = 0.38). (C) In contrast, a significant correlation was observed between native-T1 and the left ventricular MVR (Pearson’s ρ = 0.36, P = 0.025). (D) No correlation was observed between the magnetization transfer ratio and the MVR (Pearson’s ρ = 0.07, P = 0.67).
Figure 4. Association of septal native-T1 or MTR values with corresponding extracellular volume fraction and septal wall thickness.
(A) Midventricular septal native-T1 time demonstrates a significant correlation with septal thickness (Pearson’s ρ = 0.50, P = 0.001), (B) but not regional extracellular volume fraction (Pearson’s ρ = 0.28, P = 0.086). (C) No correlation was observed between regional MTR and either septal thickness (Pearson’s ρ = 0.19, P = 0.23) or (D) regional extracellular volume fraction (Pearson’s ρ = 0.23, P = 0.16).
Figure 5. Global average native-T1 relaxation time is strongly correlated to plasma HbA1c at the time of imaging.
(A) A significant correlation (Pearson’s ρ = 0.43, P = 0.0088) was observed between native-T1 relaxation times and corresponding plasma HbA1c measurements. In contrast, neither (B) MTR (Pearson’s ρ = 0.10, P = 0.56) nor (C) ECV (Pearson’s ρ = 0.14, P = 0.41) correlated with plasma HbA1c values.
Figure 6. T1 times of blood are not correlated with HbA1c levels.
Neither (A) pre-contrast (Pearson’s ρ = −0.18, P = 0.29) nor (B) post-contrast (Pearson’s ρ = −0.21, P = 0.20) T1 times of the left ventricular blood pool demonstrated significant correlations with plasma HbA1c values at the time of imaging.
Multivariable regression was used to analyze the relative effects of heart rate, mass to volume ratio, and HbA1C levels on native-T1 times in the myocardium. Following Shapley- Owen correction, heart rate (0.204) and plasma HbA1C levels (0.112) were found to have greater impact on T1 times than mass to volume ratio (0.050). The weighted combination of these predictor variables correlated significantly with native-T1 times (R squared = 0.366, p = 0.002).
4. Discussion
In this study we examined changes in native-T1 relaxation times in diabetic adults without diffuse fibrosis and observed correlations between elevated native-T1 times and broad measures of ventricular hypertrophy, elevated heart rate and plasma HbA1c levels in the presence of preserved magnetization transfer ratio. These findings suggest lengthened native-T1 times of myocardium in the absence of diffuse fibrosis among diabetics may reflect the combined effects of an expanded intracellular water volume in hypertrophied cardiomyocytes and sequalae of increased hemoglobin glycosylation. Importantly, the values of native-T1 times within the diabetic group in this study were consistent with those measured in myocardium that fails to display signal enhancement on late gadolinium-enhanced (LGE) images, but is diffusely fibrotic based on ECV values in patients with HCM [18,19] and hypertension with ventricular hypertrophy [20]. Given that both cardiomyocyte hypertrophy and elevated plasma HbA1c levels are common comorbidities among patients with varying etiologies of non-ischemic cardiomyopathy, the potential impact of these factors on measured T1 times should be taken into consideration as part of further adoption of native-T1 mapping protocols.
Studies of native-T1 relaxation times in patients with ventricular hypertrophy have generally examined patient populations in which hypertrophy and diffuse fibrosis are typically concomitant, such as in hypertrophic cardiomyopathy (HCM), compared to non-hypertrophic control patients. Early studies of HCM patients revealed elevated native-T1 times regardless of segmental LGE status when compared to corresponding healthy control values [[18],[19],[21]]. In a study by Puntman et al. [21], both native-T1 times and ECV were significantly higher in HCM patients by approximately 20% and 50%, respectively, compared to healthy controls. However, when separated by LGE status by Dass et al. [18], HCM patients without gadolinium enhancement demonstrated moderate elevations in native-T1 times that were consistent with those observed in diabetic patients in our study [18]. Similar results were obtained in a more recent study by Kato et al. [19], however neither study reported on ECV values in LGE negative territories. In contrast, Liu et al. reported native-T1 times in line with healthy control values in LGE negative territories of HCM patients; however, they did not report on corresponding ECV or wall thickness measurements [22]. In a recent study of (non-HCM) hypertensive patients by Kuruvilla et al. [20], those with elevated LVMI demonstrated significantly higher ECV and native-T1 times in comparison to both hypertensive patients with normal LVMI and age matched controls. We observed similar elevations in native-T1 times among diabetics with increased left ventricular mass (measured as MVR) to non-HCM patients with heightened LVMI by Kuruvilla et al., despite normal ECV values [23]. Further, Kuruvilla et al. reported a strong correlation between native-T1 and LVMI, and both Dass et al. and Kato et al. reported a correlation between septal wall thickness and segmental T1 times, all among patients with either confirmed or presumed ECV elevations. Interestingly, we also observed significant correlations between native-T1 times and both global (MVR) and regional (septal wall thickness) measures of ventricular hypertrophy, as well as estimated cellular volume, despite all diabetic patients demonstrating ECV values consistent with normal myocardium. These results suggest that in some cases, cardiomyocyte hypertrophy may contribute to lengthened native-T1 times in LGE negative myocardium that is not diffusely fibrotic.
Prior studies that examined native-T1 times in diabetic patients used measurement of HbA1c to confirm glycemic status of participants [16,17], but have not examined the impact of HbA1c levels on T1 times. In the current study, we observed a strong correlation between plasma HbA1c levels and myocardial native-T1 times at the time of imaging. There are several potential mechanisms by which elevated plasma HbA1c may contribute to lengthening of myocardial native-T1 times. First, elevated levels of HbA1c have been shown to cause changes in the secondary structure of hemoglobin with substitution of alpha-helices by beta-sheets [24]. Increased beta-sheet content within hemoglobin can contribute to protein unfolding that both alters the transition between spatial conformations and reduces the stability of overall protein structure [24]. Further, structural unfolding of hemoglobin has been shown to cause degradation and displacement of the haeme group, with subsequent impacts on oxygen affinity [25]. In addition, unfolding of hemoglobin can alter the spin state, and thus magnetic moment, of iron atoms through hemichrome formation [26,27]. Importantly, while such processes occur within red blood cells and are not reflected in simple measures of hematocrit, each can lengthen the spin-lattice relaxation time of blood in a manner consistent with reduced hematocrit. However, in this study we did not observe a significant correlation between HbA1c and blood native-T1 times. Alternatively, increased plasma HbA1c is correlated with accumulation of advanced glycosylation end products (AGE) which may alter native-T1 times via two distinct mechanisms. First, the accumulation of AGE is known to alter enzymatic activity involved in serum albumin regulation [26,28], which may contribute to lengthened T1 times. Second, increased tissue accumulation of AGE has been shown to increase underlying inflammation [29,30], which has been repeatedly shown to lengthen myocardial native-T1 times in the absence of diffuse fibrosis [31,32]. However, we were unable to obtain tissue samples to assess the inflammatory status of participants in our study.
Existing CMR tissue characterization studies in diabetic patients have demonstrated inconsistent results. For example, an early study by Wong et al. performed at 1.5T using the MOLLI sequence reported significantly elevated ECV values among diabetic patients compared to age-matched controls, with half of diabetic patients demonstrating ECV values above 30% and greater heterogeneity compared to the healthy group [16]. Unfortunately, Wong et al. did not report native-T1 times in their study. In contrast, in a recent study by Storz et al. performed at 3T using the MOLLI sequence, ECV values were statistically significantly lower in pre-diabetic and diabetic patients compared to healthy controls, although all within the published range of normal values [17]. In contrast, native-T1 times were similar among all groups. Similarly, Levelt et al. observed no difference in resting native-T1 times between diabetic adults and healthy controls, measured at 3T using the ShMOLLI sequence, despite elevated mass-to-volume ratio [33]. However, ECV values were not reported as part of this study [33]. Importantly, we observed a substantially greater difference in indices of ventricular remodeling (e.g. MVR) between diabetic patients and healthy controls than was reported by either Storz et al. or Levelt et al. In addition, HbA1c values were substantially higher among diabetic patients in our study as compared to all three aforementioned studies. With a smaller sample size of diabetic patients who were more hypertrophic and with higher plasma HbA1c levels compared to the larger referenced studies [16,17], it is possible that we observed differences in native-T1 times that were not observed in the prior studies. Unfortunately, neither Levelt et al. nor Storz et al. examined whether a correlation existed between plasma HbA1c levels and native-T1 times despite similar group mean T1 times. However, it is possible that examination of a much larger sample size may fail to confirm correlations between T1, hypertrophy, and HbA1c.
Native-T1 values obtained using the MOLLI sequence are known to demonstrate sensitivity to changes in magnetization transfer [34]. Prior studies have demonstrated changes in MT-weighting in fibrotic and edematous tissue [10,11], but the impact of hypertrophy in the absence of fibrosis was not examined. Measurement of MTR in this study revealed consistent MTR values between healthy controls and diabetic patients, irrespective of degree of ventricular hypertrophy. These findings raise the possibility that while the intracellular water volume increases in hypertrophied cardiomyocytes, the MT between intracellular water and surrounding macromolecules remains consistent. Importantly, preserved MTR in diabetic adults suggests that changes in native-T1 times are independent of MT in this study. Separately, early T1-mapping studies that used MOLLI acquisition schemes demonstrated increased variability in phantom T1 values with increased simulated heart rate [35,36]. While we observed that elevations in T1 values correlated with faster heart rate, multivariable regression demonstrated that elevated heart rate alone is insufficient to explain lengthened T1 times in the absence of diffuse fibrosis. Further, underestimation of T1 at higher heart rates may have caused reported T1 values in diabetic patients to underestimate the true degree of lengthened T1 times, despite the absence of diffuse fibrosis. Finally, intramyocardial fat can bias measured native T1 values depending upon the phase difference between fat and myocardium as described by Kellman et al. [37]. Altered cardiomyocyte fatty acid regulation can lead to the accumulation of intracellular lipid droplets in diabetic subjects [12] which may also contribute to observed changes in T1 times. However, in this study we were unable to obtain and potentially account for measurement of intramyocardial fat content. Importantly, while the impact of cardiomyocyte lipid content on T1 can be both to lengthen or shorten T1 depending on the degree of off-resonance of a given voxel, the magnitude of this effect is typically less than 5% [37].
Several limitations to this study need to be kept in mind. First, we were not able to obtain endocardial biopsies to histologically validate ECV and cardiomyocyte hypertrophy measurements. Second, at the time of data acquisition we did not have access to alternative T1 mapping protocols including saturation recovery-based methods such as ShMOLLI that are considered more precise than MOLLI. In addition, while our selection criteria for both groups stipulated that individuals have no history of heart disease including prior myocardial infarction, we were unable to perform late gadolinium enhancement to confirm such absence. Finally, unlike prior studies of diabetic populations that properly age-matched their control group, our healthy controls were on average a decade younger than diabetic patients. Subsequently, while not the purpose of the study, it is not possible to attribute elevated left ventricular hypertrophy and any subsequent impacts on native-T1 times strictly to diabetic status.
5. Conclusion
In conclusion, this study suggests that both cardiomyocyte hypertrophy and potential impacts of elevated plasma HbA1c levels may contribute to an observation of lengthened myocardial native-T1 times in the absence of underlying diffuse fibrosis. Given that both diabetes and pre-diabetes are common comorbidities among individuals with cardiovascular disease, these factors should be considered alongside native-T1 times when ECV measurement is contraindicated.
Acknowledgements
The authors thank Ms. Rebecca Holtkamp for assistance in patient recruitment and monitoring. In addition, we thank Dr. Dean Sherry for discussion of hemoglobin glycosylation chemistry. This work was supported by financial assistance from the American Heart Association National Affiliate (14CRP20380071) and National Institutes of Health (R01HL128592) to MV.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Iles LM, Ellims AH, Llewellyn H, Hare JL, Kaye DM, McLean CA, Taylor AJ. Histological validation of cardiac magnetic resonance analysis of regional and diffuse interstitial myocardial fibrosis. Eur Hear J Cardiovasc Imaging 2015; 16:14–22. 10.1093/ehjci/jeu182. [DOI] [PubMed] [Google Scholar]
- [2].Messroghli DR, Nordmeyer S, Dietrich T, Dirsch O, Kaschina E, Savvatis K, O h-Ici D, Klein C, Berger F, Kuehne T. Assessment of Diffuse Myocardial Fibrosis in Rats Using Small-Animal Look-Locker Inversion Recovery T1 Mapping. Circ Cardiovasc Imaging 2011;4:636–640. [DOI] [PubMed] [Google Scholar]
- [3].Puntmann VO, Carr-White G, Jabbour A, Yu C-Y, Gebker R, Kelle S, Hinojar R, Doltra A, Varma N, Child N, Rogers T, Suna G, Ucar EA, Goodman B, Khan S, Dabir D, Herrmann E, Zeiher AM, Nagel E. T1-Mapping and Outcome in Nonischemic Cardiomyopathy All-Cause Mortality and Heart Failure. J. Amer. Coll. of Cardio 2016;9:40–50. 10.1016/j.jcmg.2015.12.001. [DOI] [PubMed] [Google Scholar]
- [4].Broome DR. Nephrogenic systemic fibrosis associated with gadolinium based contrast agents A summary of the medical literature reporting. Eur J Radiol 2008;66:230–234. 10.1016/j.ejrad.2008.02.011. [DOI] [PubMed] [Google Scholar]
- [5].Bardin T, Richette P. Nephrogenic systemic fibrosis. Curr Opin Rheumatol 2010;22:54–58. 10.1097/BOR.0b013e328333bf3d. [DOI] [PubMed] [Google Scholar]
- [6].Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB. Gadolinium deposition in the brain: summary of evidence and recommendations. www.thelancet.com neurology Pers View Lancet Neurol 2017; 16:564–70. 10.1016/S1474-4422(17)30158-8. [DOI] [PubMed] [Google Scholar]
- [7].Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, Gatehouse PD, Arai AE, Friedrich MG, Neubauer S, Schulz-Menger J, Schelbert EB. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson 2013; 15:92 10.1186/1532-429X-15-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Roujol S, Weingartner S, Foppa M, Chow K, Kawaji K, Ngo LH, Kellman P, Manning WJ, Thompson RB, Nezafat R. Accuracy, precision, and reproducibility of four T1 mapping sequences: a head-to-head comparison of MOLLI, ShMOLLI, SASHA, and SAPPHIRE. Radiology 2014;272:683–689. 10.1148/radiol.14140296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stoffers RH, Madden M, Shahid M, Contijoch F, Solomon J, Pilla JJ, Gorman Iii JH, Gorman RC, Witschey WRT. Assessment of myocardial injury after reperfused infarction by T1ρ cardiovascular magnetic resonance. 10.1186/s12968-017-0332-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stromp TA, Leung SW, Andres KN, Jing L, Fornwalt BK, Charnigo RJ, Sorrell VL, Vandsburger MH. Gadolinium free cardiovascular magnetic resonance with 2-point Cine balanced steady state free precession. J Cardiovasc Magn Reson 2015;17:90 10.1186/s12968-015-0194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Weber OM, Speier P, Scheffler K, Bieri O. Assessment of magnetization transfer effects in myocardial tissue using balanced steady-state free precession (bSSFP) cine MRI. Magn Reson Med 2009;62:699–705. 10.1002/mrm.22053. [DOI] [PubMed] [Google Scholar]
- [12].Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: An update of mechanisms contributing to this clinical entity. Circ Res 2018;122:624–638. 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Marwick TH, Ritchie R, Shaw JE, Kaye D. Implications of Underlying Mechanisms for the Recognition and Management of Diabetic Cardiomyopathy. J Am Coll Cardiol 2018;71:339–351. 10.1016/j.jacc.2017.11.019. [DOI] [PubMed] [Google Scholar]
- [14].Ladeiras-Lopes R, Moreira HT, Bettencourt N, Fontes-Carvalho R, Sampaio F, Ambale-Venkatesh B, Wu C, Liu K, Bertoni AG, Ouyang P, Bluemke DA, Lima JA. Metabolic Syndrome is Associated with Impaired Diastolic Function Independently of Mri-Derived Myocardial Extracellular Volume: the Mesa Study. Diabetes 2018;67:1007–1012. 10.2337/db17-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ng AC, Auger D, Delgado V, van Elderen SG, Bertini M, Siebelink H-M, van der Geest RJ, Bonetti C, van der Velde ET, de Roos A, Smit JW, Leung DY, Bax JJ, Lamb HJ. Association Between Diffuse Myocardial Fibrosis by Cardiac Magnetic Resonance Contrast-Enhanced T 1 Mapping and Subclinical Myocardial Dysfunction in Diabetic Patients A Pilot Study. 2011. 10.1161/CIRCIMAGING.111.965608. [DOI] [PubMed] [Google Scholar]
- [16].Wong TC, Piehler KM, Kang IA, Kadakkal A, Kellman P, Schwartzman DS, Mulukutla SR, Simon MA, Shroff SG, Kuller LH, Schelbert EB. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Hear J 2014;35:657–664. 10.1093/eurheartj/eht193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Storz C, Hetterich H, Lorbeer R, Heber SD, Schafnitzel A, Patscheider H, Auweter S, Zitzelsberger T, Rathmann W, Nikolaou K, Reiser M, Schlett CL, von Knobelsdorff-Brenkenhoff F, Peters A, Schulz-Menger J, Bamberg F. Myocardial tissue characterization by contrast-enhanced cardiac magnetic resonance imaging in subjects with prediabetes, diabetes, and normal controls with preserved ejection fraction from the general population. Eur Hear Journal-Cardiovascular Imaging 2018;19:701–708. 10.1093/ehjci/jex190. [DOI] [PubMed] [Google Scholar]
- [18].Dass S, Suttie JJ, Piechnik SK, Ferreira VM, Holloway CJ, Baneijee R, Mahmod M, Cochlin L, Karamitsos TD, Robson MD, Watkins H, Neubauer S. Myocardial tissue characterization using magnetic resonance noncontrast T1 mapping in hypertrophic and dilated cardiomyopathy. Circ Cardiovasc Imaging 2012;5:726–733. 10.1161/CIRCIMAGING.112.976738. [DOI] [PubMed] [Google Scholar]
- [19].Kato S, Nakamori S, Bellm S, Jang J, Basha T, Maron M, Manning WJ, Nezafat R. Myocardial Native T 1 Time in Patients With Hypertrophic Cardiomyopathy. Am J Cardiol 2016;118:1057–1062. 10.1016/j.amjcard.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kuruvilla S, Janardhanan R, Antkowiak P, Keeley EC, Adenaw N, Brooks J, Epstein FH, Christopher Kramer yz M, Salerno M. Increased Extracellular Volume and Altered Mechanics Are Associated With LVH in Hypertensive Heart Disease, Not Hypertension Alone. JACC Cardiovasc Imaging 2015;8:172–180. 10.1016/j.jcmg.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Puntmann VO, Voigt T, Chen Z, Mayr M, Karim R, Rhode K, Pastor A, Carr-White G, Razavi R, Schaeffter T, Nagel E. Native T1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. JACC Cardiovasc Imaging 2013;6:475–484. 10.1016/j.jcmg.2012.08.019. [DOI] [PubMed] [Google Scholar]
- [22].Liu JM, Liu A, Leal J, Mcmillan F, Francis J, Greiser A, Rider OJ, Myerson S, Neubauer S, Ferreira VM, Piechnik SK, Uk S. Measurement of myocardial native T1 in cardiovascular diseases and norm in 1291 subjects. J Cardiovasc Magn Reson 2017; 19 10.1186/s12968-017-0386-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ugander M, Oki AJ, Hsu LY, Kellman P, Greiser A, Aletras AH, Sibley CT, Chen MY, Patricia Bandettini W, Arai AE. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. Eur Heart J 2012;33:1268–1278. 10.1093/eurheartj/ehr481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ye S, Ruan P, Yong J, Shen H, Liao Z, Dong X. The impact of the HbA1c level of type 2 diabetics on the structure of haemoglobin. Sci Rep 2016;6:1–10. 10.1038/srep33352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Goodarzi M, Moosavi-Movahedi AA, Habibi-Rezaei M, Shourian M, Ghourchian H, Ahmad F, Farhadi M, Saboury AA, Sheibani N. Hemoglobin fructation promotes heme degradation through the generation of endogenous reactive oxygen species. Spectrochim Acta - Part A Mol Biomol Spectrosc 2014;130:561–567. 10.1016/j.saa.2014.04.056. [DOI] [PubMed] [Google Scholar]
- [26].Ioannou A, Varotsis C. Modifications of hemoglobin and myoglobin by Maillard reaction products (MRPs). 2017. 10.1371/journal.pone.0188095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sugawara Y, Kadono E, Suzuki A, Yukuta Y, Shibasaki Y, Nishimura N, Kameyama Y, Hirota M, Ishida C, Higuchi N, Haramoto K, Sakai Y, Soda H. Hemichrome formation observed in human haemoglobin A under various buffer conditions. Acta Physiol Scand 2003;179:49–59. 10.1046/j.1365-201X.2003.01142.x. [DOI] [PubMed] [Google Scholar]
- [28].Ahmed N, Dobler D, Dean M, Thornalley PJ. Peptide Mapping Identifies Hotspot Site of Modification in Human Serum Albumin by Methylglyoxal Involved in Ligand Binding and Esterase Activity* Downloaded from. J Biol Chem 2005;280:5724–5732. 10.1074/jbc.M410973200. [DOI] [PubMed] [Google Scholar]
- [29].Fishman S, Sonmez H, Basman C, Singh V, Poretsky L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: a review. Mol Med 2018;24:59 10.1186/s10020-018-0060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nozynski J, Zakliczynski M, Konecka-Mrowka D, Zielinska T, Zakliczynska H, Nikiel B, Mlynarczyk-Liszka J, Mrowka A, Zembala-Nozynska E, Pijet M, Rdzanowska K, Lange D, Przybylski R, Zembala M. Ann Transplant 2012;17:53–61. 10.12659/AOT.883223. [DOI] [PubMed] [Google Scholar]
- [31].Puntmann V, Peker E, Chandrashekhar Y, Nagel E. Circ Res 2016; 119:277–299. 10.1161/CIRCRESAHA.116.307974. [DOI] [PubMed] [Google Scholar]
- [32].Puntmann V, Isted A, Hinojar R, Foote L, Carr-White G, Nagel E. Radiology 2017;285:63–72. 10.1148/radiol.2017162732. [DOI] [PubMed] [Google Scholar]
- [33].Mahmod M, Piechnik SK, Levelt E, Ferreira VM, Francis JM, Lewis A, Pal N, Dass S, Ashrafian H, Neubauer S, Karamitsos TD. Adenosine stress native T1 mapping in severe aortic stenosis: evidence for a role of the intravascular compartment on myocardial T1 values. J Cardiovasc Magn Reson 2014; 16:92 10.1186/s12968-014-0092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Robson MD, Piechnik SK, Tunnicliffe EM, Neubauer S. T1 measurements in the human myocardium: The effects of magnetization transfer on the SASHA and MOLLI sequences. Magn Reson Med 2013;70:664–670. 10.1002/mrm.24867. [DOI] [PubMed] [Google Scholar]
- [35].Raman FS, Kawel-Boehm N, Gai N, Freed M, Han J, Liu C-Y, Lima JA, Bluemke DA, Liu S. Modified look-locker inversion recovery T1 mapping indices: assessment of accuracy and reproducibility between magnetic resonance scanners. J Cardiovasc Magn Reson 2013;15:64 10.1186/1532-429X-15-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med 2004;52 10.1002/mrm.20110. [DOI] [PubMed] [Google Scholar]
- [37].Kellman P, Bandettini WP, Mancini C, Hammer-Hansen S, Hansen MS, Arai AE. Characterization of myocardial T1-mapping bias caused by intramyocardial fat in inversion recovery and saturation recovery techniques. J Cardiovasc Magn Reson 2015; 17:33 10.1186/s12968-015-0136-y. [DOI] [PMC free article] [PubMed] [Google Scholar]