Abstract
Background:
The prognostic relevance of human papillomavirus (HPV) status in non-oropharyngeal (OPX) squamous cell cancer (SCC) of the head and neck is controversial. We evaluated the impact of high-risk HPV status on overall survival (OS) in patients with non-OPX SCC using a large database approach.
Methods:
The National Cancer Data Base was queried to identify patients diagnosed from 2004–2014 with SCC of the OPX, hypopharynx (HPX), larynx, and oral cavity (OC) with known HPV status. Survival was estimated using Kaplan-Meier methods; distributions were compared with log-rank tests. Propensity score matching (PSM) and inverse probability of treatment weighing (IPTW) methods were utilized; cohorts were matched on age, sex, Charlson-Deyo score, clinical group stage, treatments received, and anatomic subsite. Propensity analyses were stratified by group stage.
Results:
24,740 patients diagnosed from 2010–2013 were analyzed; 1,085 patients with HPX, 4804 with larynx, 4,018 with OC, and 14,833 with OPX SCC. The proportions of HPV positive cases by site were: 17.7% in HPX, 11% in larynx, 10.6% in OC, and 62.9% in OPX. HPV status was prognostic in multiple un-adjusted and propensity-adjusted non-OPX populations. HPV positivity was associated with superior OS in HPX SCC with hazard ratio (HR) of 0.61 (p<0.001, IPTW), in stage III-IVB laryngeal SCC (HR 0.79, p=0.019, IPTW), and in stage III-IVB OC SCC (HR 0.78, p=0.03, IPTW).
Conclusions:
Positive high-risk HPV status is associated with longer OS in multiple non-oropharynx head and neck populations – hypopharynx, locally-advanced larynx and oral cavity. If prospectively validated, these findings have implications for risk-stratification.
Keywords: Head and neck cancer, larynx, oral cavity, hypopharynx, HPV, p16, national cancer data base, biomarker
PRECIS
We evaluated the prognostic relevance of high-risk HPV status in patients with non oropharyngeal head and neck squamous cell carcinomas using a large database approach given conflicting prior reports. HPV positivity was associated with superior overall survival in 9,907 patients with hypopharynx, and stage III-IVB oral cavity and larynx squamous cell carcinomas using propensity matched and propensity weighed methods.
BACKGROUND
Human papillomavirus (HPV) associated oropharyngeal squamous cell carcinoma (SCC) is an entity clinically distinct from HPV-negative oropharyngeal (OPX) SCC. HPV-associated OPX SCC patients have improved response to therapy, as well as overall survival. Therefore, different treatment options may be appropriate for the patients with HPV-associated OPX SCC1–5. Whether HPV association is relevant to prognosis in mucosal head and neck SCCs outside of the oropharynx is uncertain.
Individual retrospective series of larynx, oral cavity (OC), and hypopharynx (HPX) SCCs have reported favorable survival outcomes in HPV positive cohorts (Table 1)6–8. Multi-site studies have also addressed the question of the impact of HPV status for non-oropharyngeal SCCs that are either p16 or HPV positive 9–11. In contrast, multiple series have found no prognostic difference 12–14; and some series have reported a detrimental association between HPV positivity and survival15–17. To summarize, results have been inconsistent, and interpreting this question has also been complicated by substantial heterogeneity within, and between study cohorts. Variations in sample sizes, geography, method of HPV detection, and other factors may have contributed to inconsistent results. The objective of this study was to evaluate the prognostic value of high-risk HPV status in hypopharyngeal, laryngeal, and oral cavity squamous cell carcinomas using an administrative database approach.
Table 1.
Summary of selected series comparing survival outcomes by HPV status in hypopharynx, larynx, and oral cavity primaries
| Series | Year | N | Primary Site(s) | Population | Relevant Survival Endpoints | Method of Testing |
|---|---|---|---|---|---|---|
| Pooled Primary Sites | ||||||
| Bryant et al. 10 | 2018 | 387 | HPX, LX, OC | Veteran Affairs Hospitals | OS HR 0.41 (p<0.01), CSS HR 0.37 (p=0.018), CM HR 0.46 (p=0.04); using p16 status | p16 and HPV ISH |
| Chung et al.9 | 2014 | 322 | HPX, LX, OC | RTOG 0129, 0234 and 0522 | OS HR 0.57, 5-yr OS 64.4% vs 44.4% (p=0.04), PFS HR 0.63, 5-yr PFS 54.7% vs 34.3% (p=0.01); using p16 status | p16 and HPV ISH |
| Clayman et al.15 | 1994 | 78 | LX, HPX | MD Anderson Cancer Center | Increased risk of death overall (p<0.001), for stage I-II (p=0.025), and stage III-IV (p=0.14), LCR RR 2.4 (p=0.023) | HPV DNA |
| D’Souza et al.14 | 2016 | 845 | HPX, LX, OC | 3 epidemiologic studies GENCAPO (Brazil), CHANCE (US), ARCAGE (Europe) | aHR 0.83 (95% CI 0.60, 1.14) using p16 status, aHR 1.20 (95% CI 0.89, 1.63) using HPV status, and aHR 0.59 (95% CI 0.32, 1.08) using both HPV and p16 status | p16, HPV16 DNA, or both |
| Fakhry et al.12 | 2017 | 621 | LX, OC, NPX | Johns Hopkins Hospital and UCSF | OS HR 0.80 5-year OS 55.7% vs 52% (p =0.26) using p16 status, HR 0.93 5-year OS 61.4% vs 51% (p=0.77) using HPV | p16, and HPV16 DNA |
| Lassen et al.13 | 2014 | 1,294 | HPX (pooled with LX) | DAHANCA trials | OS HR 0.81, 5-year OS 38% vs 35% (p=0.22), EFS HR 1.06, 5-year EFS 28% vs 28% (p=0.73), LRC HR 0.94, 5-year LRC 53% vs 48% (p=0.75) | p16 |
| Salazar et al.11 | 2014 | 158 | HPX, LX, OC | Montefiore Medical Center | p16+ and HPV DNA positive (concordant) non-oropharynx associated with better disease-specific survival (HR 0.04) | P16, HPV DNA, E6/7 mRNA |
| Hypopharynx Primary Site | ||||||
| Dalianis et al.7 | 2015 | 142 | HPX | Karolinska University Hospital | 3-year OS 86% vs 31% (p=0.018), 3-yr DSS 86% vs 43% (p=0.058), using p16 and HPV status | p16 and HPV DNA |
| Larynx Primary Site | ||||||
| Chernock et al.6 | 2013 | 76 | LX | WashU | OS (p=0.058) using p16 status, OS (p=0.045) using p21 status | p16, p21 IHC, HPV DNA |
| Oral Cavity Primary Site | ||||||
| Duray et al.16 | 2012 | 162 | OC | Saint-Pieter and Erasme Hospitals (Brussels) | DFS HR 2.71 (p=0.01); 5-year DFS 40 vs 76% (p=0.007) | HPV DNA |
| Lee et al.17 | 2012 | 173 | OC | Chang Gung Memorial Hospital (Taiwan) | 5-year OS 25% vs 53% (p=0.010), 5-yr DSS 37% vs 68% (p=0.006), 5-yr DFS 38% vs 62% (p=0.037), 5-yr DM 56% vs 19% (p=0.005) | HPV PCR |
| Schwartz et al.8 | 2001 | 254 | Oral SCC | U of Washington | All-cause mortality HR 0.34 (95% CI 0.14, 0.83), disease-specific mortality HR 0.17 (95% CI 0.04, 0.76) | HPV DNA |
METHODS
The National Cancer Date Base (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society, and includes 1,500 accredited cancer program registries which allows for acquisition of information regarding 70% of new cancer diagnoses within the United States 18. The data and statistical methodology are not verified by the Commission on Cancer or the participating hospitals; and are not responsible for the statistical validity or conclusions of this study. Access to de-identified patient data and the corresponding data files was provided to the authors as part of the NCDB Participant Use File program. The current study was exempt from requiring institutional review board approval.
Patients were extracted from 9 individual user files and assorted into 4 primary sites – hypopharynx, larynx, oral cavity and oropharynx. Classification of primary site was defined according to the International Classification of Disease for Oncology, 3rd edition (ICD-O-3) topography codes. Hypopharynx primary was defined by codes C129 through C140. Larynx primary consisted of C320–323, C328 and C329. Oral cavity primary was defined by C003-C009, C020-C023, C030-C031, C039-C041, C048–052, C060-C062, and C068-C069. Oropharyngeal SCCs were included for reference values and comparison of the relative prognostic effect of HPV status. OPX primaries were defined by codes C100-C109, C090–091, C098–099, C019, and C024. Patients with primary diagnosis codes which could not be unambiguously classified into a primary site were excluded. Notable examples were: C028 (overlapping tumor of the tongue), C029 (tongue not otherwise specified [NOS]), C058 (overlapping palate), C059 (palate NOS), C000-C002 (external lip).
Patients with unknown HPV status, non-invasive behavior, distant metastatic disease, non-squamous cell carcinoma histology, unknown vital status or survival time, unknown clinical group stage, prior malignancy, and those who received all treatment outside of the reporting facility were excluded. Squamous cell carcinomas were defined by morphology codes 8052, 8070–8078, 8083, 8084. After relevant inclusion/exclusion criteria were applied 24,740 patients, including 9,907 patients with non-oropharyngeal SCCs were included for analysis. Demographic factors including sex, age and year of diagnosis, race, median income, insurance status, census region, and treatment facility type were examined. Clinicopathologic characteristics including primary subsite, HPV status, American Joint Committee on Cancer (AJCC) group staging, Charlson-Deyo comorbidity score, surgical margin status, extranodal extension, and interventions received during the initial course of treatment were also assessed. HPV positive (HPV+) was defined as having HPV high-risk type 16, type 18, both 16 and 18, or another high-risk type (site-specific factor codes 20 through 60). HPV negative (HPV-) were patients who were negative for high-risk types, both high-risk and low-risk type, or positive only for low-risk types (site-specific factor codes 0 and 10).
To minimize bias selection bias, 2 propensity methods were used. Propensity score models allow one to analyze data from an observation study to reflect results one would expect from a clinical trial, by balancing on a propensity score. Propensity scores are derived from a multivariable logistic regression model predicting probability of HPV status fit as a function of key prognostic factors. HPV groups are then either matched by the propensity score derived from that model to create balanced groups, or observations are re-weighted based on the propensity score itself. Traditional propensity score matching was performed using 1:1 matching without replacement using a greedy 5:1 digit match algorithm19. The inverse probability of treatment weighting (IPTW) method was also used 20. Given the anticipated differences in proportion of HPV positive vs negative cases in non-oropharyngeal SCCs, an IPTW method was used to capture information from patients who would otherwise be discarded by 1:1 matching.
Variables used for propensity analyses were: age at diagnosis as a continuous variable, sex, Charlson-Deyo comorbidity score (0 vs 1–2), clinical group stage, treatment with surgery, radiation therapy, chemotherapy, and anatomic subsite when applicable (e.g. glottic vs supraglottic larynx, oral tongue vs non-oral tongue, base of tongue vs tonsil vs other). In addition to the primary analysis, a cohort of patients with missing HPV status was compared to patients with known HPV status using propensity score matching, employing the same characteristics.
The length of median follow-up was calculated using the median overall survival value of those alive at last follow-up. Overall survival (OS) was defined as time from diagnosis to death or last follow-up, where those alive at last follow-up are censored at time of their last follow-up. Two-year, 5-year and median OS were estimated using the Kaplan-Meier method, and survival distributions were compared via the log-rank test. Univariate Cox proportional hazards models were used to analyze the association between high-risk HPV status and the risk of death. Cox models were fit based on propensity score matched, and re-weighted HPV groups to estimate adjusted hazard ratios. Balance between matched groups was check using mean standardized differences. Analyses were stratified by AJCC 7th edition clinical group stage I-II vs III-IVB, with exception of hypopharynx because of sample size limitations. Statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC); the significance level was 0.05.
RESULTS
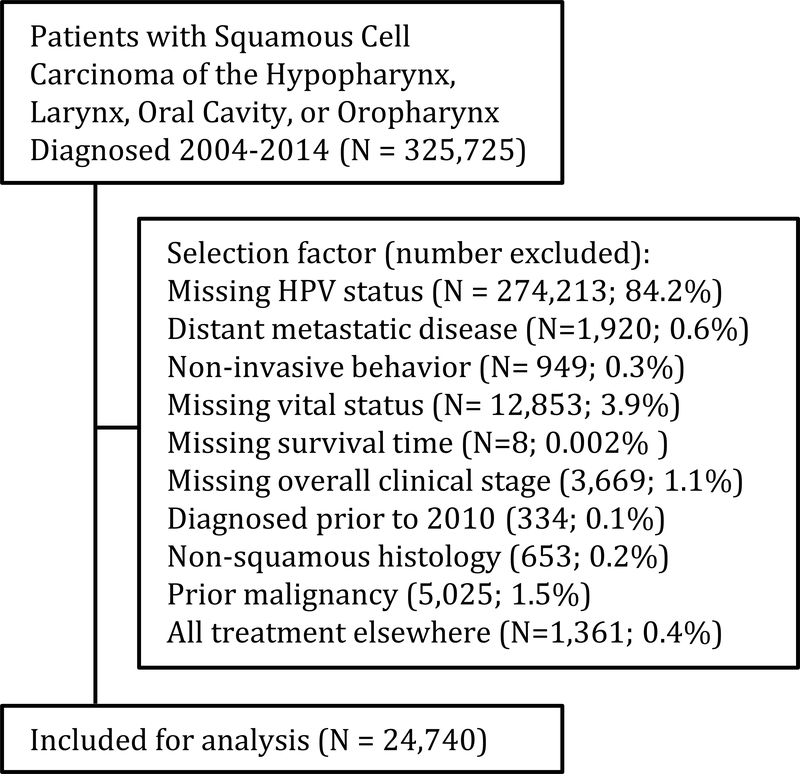
Our methodology identified 325,725 patients with mucosal head and neck squamous cell carcinomas diagnosed between 2004–2014 which included 206,332 patients with non-oropharyngeal primaries (Figure 1). After relevant inclusion/exclusion criteria, 24,740 patients diagnosed with head and neck mucosal SCCs between 2010 and 2013 were included for analysis (Table 2). This included 9,907 patients with non-oropharyngeal SCCs: 1,085 with hypopharynx, 4,804 with larynx and 4,018 with oral cavity SCCs. The availability of HPV status in the un-selected population varied by primary site. The rate of available HPV status data ranged from 9.7% to 12.9% in non-oropharyngeal primary sites. By comparison, high risk HPV status availability for oropharyngeal patients was 24.6% (29,409 of 119,393). The rate of high-risk HPV positivity for those patients tested varied by primary site; 17.7% of patients with hypopharyngeal SCCs were high-risk HPV positive, as were 11% and 10.6% for those with laryngeal and oral cavity SCCs. These values were contrasted by the 62.9% of OPX patients who were high-risk HPV positive. Median follow-up length ranged 2.3 to 2.7 years when estimated by primary site. As expected, utilization of the 3 major treatment modalities varied substantially by primary site. Other patient characteristics are summarized in Table 2. Patients with laryngeal and oral cavity SCCs who were HPV+ were more likely to have poorly differentiated tumors; and patients with hypopharyngeal SCCs also had higher proportion of poorly differentiated tumors in the HPV+ group (47.9% vs 40.3%), not reaching statistical significance (Table S1).
Figure 1.
Selection diagram detailing the relevant inclusion/exclusion factors identifying the analysis population
Table 2.
Comparison of patient and disease characteristics stratified by primary site
| Characteristic | Hypopharynx (N=1,085) | Larynx (N=4,804) | Oral Cavity (N=4,018) | Oropharynx (N=14,833) |
|---|---|---|---|---|
| Median Age at Diagnosis (SD) | 61 (10.48) | 62 (11.4) | 60 (13.53) | 58 (9.55) |
| Median Length of follow-up | 2.3 | 2.5 | 2.5 | 2.7 |
| HPV Data Available (non-missing HPV status/all patients queried) | 12.9% (2,921/22,705) | 9.7% (10,210/105,593) | 11.5% (8,972/78,034) | 24.6% (29,409/119,393) |
| HPV Status | ||||
| Positive | 192 (17.7%) | 530 (11%) | 426 (10.6%) | 9332 (62.9%) |
| Negative | 893 (82.3%) | 4274 (89%) | 3592 (89.4%) | 5501 (37.1%) |
| Sex | ||||
| Male | 884 (81.5%) | 3716 (77.4%) | 2448 (60.9%) | 12325 (83.1%) |
| Female | 201 (18.5%) | 1088 (22.6%) | 1570 (39.1%) | 2508 (16.9%) |
| Charlson-Deyo Score | ||||
| 0 | 795 (73.3%) | 3415 (71.1%) | 3099 (77.1%) | 12359 (83.3%) |
| 1+ | 290 (26.7%) | 1389 (28.9%) | 919 (22.9%) | 2474 (16.7%) |
| Year of Diagnosis | ||||
| 2010 | 109 (10%) | 539 (11.2%) | 528 (13.1%) | 2085 (14.1%) |
| 2011 | 268 (24.7%) | 1105 (23%) | 939 (23.4%) | 3329 (22.4%) |
| 2012 | 315 (29%) | 1490 (31%) | 1220 (30.4%) | 4317 (29.1%) |
| 2013 | 393 (36.2%) | 1670 (34.8%) | 1331 (33.1%) | 5102 (34.4%) |
| Clinical Group Stage | ||||
| I | 60 (5.5%) | 1402 (29.2%) | 1232 (30.7%) | 607 (4.1%) |
| II | 105 (9.7%) | 769 (16%) | 833 (20.7%) | 1123 (7.6%) |
| III | 223 (20.6%) | 1085 (22.6%) | 498 (12.4%) | 2910 (19.6%) |
| IV | 28 (2.6%) | 68 (1.4%) | 77 (1.9%) | 361 (2.4%) |
| IVA | 555 (51.2%) | 1365 (28.4%) | 1266 (31.5%) | 8853 (59.7%) |
| IVB | 113 (10.4%) | 114 (2.4%) | 110 (2.7%) | 966 (6.5%) |
| Grade | ||||
| Well Differentiated | 38 (4.6%) | 567 (14.7%) | 857 (23.3%) | 457 (4.1%) |
| Moderately Diff. | 442 (53.8%) | 2382 (61.8%) | 2194 (59.7%) | 4814 (42.9%) |
| Poorly Diff. | 342 (41.6%) | 903 (23.4) | 627 (17%) | 5946 (53%) |
| Surgery at Primary Site | ||||
| Yes | 267 (24.6%) | 1787 (37.3%) | 3530 (87.9%) | 5847 (39.4%) |
| No | 817 (75.4%) | 3008 (62.7%) | 488 (12.1%) | 8979 (60.6%) |
| Radiation Therapy | ||||
| Yes | 914 (84.6%) | 3767 (78.9%) | 1987 (49.7%) | 13040 (88.2%) |
| No | 166 (15.4%) | 1010 (21.1%) | 2010 (50.3%) | 1744 (11.8%) |
| Chemotherapy | ||||
| Yes | 785 (73.7%) | 2063 (44%) | 1157 (29.6%) | 11148 (76.4%) |
| No | 280 (26.3%) | 2623 (56%) | 2747 (70.4%) | 3453 (23.6%) |
| Race | ||||
| White | 890 (82%) | 3927 (81.7%) | 3524 (87.7%) | 13412 (90.4%) |
| Black | 166 (15.3%) | 701 (14.6%) | 282 (7%) | 1020 (6.9%) |
| Other/Unknown | 29 (2.7%) | 176 (3.7%) | 212 (5.3%) | 401 (2.7%) |
| Median Income | ||||
| Unknown | 25 | 133 | 118 | 526 |
| <30,000 | 184 (17.4%) | 813 (17.4%) | 517 (13.3%) | 1584 (11.1%) |
| 30,000–35,999 | 197 (18.6%) | 996 (21.3%) | 710 (18.2%) | 2330 (16.3%) |
| 36,000–45,999 | 296 (27.9%) | 1325 (28.4%) | 1091 (28%) | 3918 (27.4%) |
| 46,000+ | 383 (36.1%) | 1537 (32.9%) | 1582 (40.6%) | 6475 (45.3%) |
| Insurance Status | ||||
| Not insured/Unknown | 86 (7.9%) | 413 (8.6%) | 297 (7.4%) | 1012 (6.8%) |
| Private | 376 (34.7%) | 1644 (34.2%) | 1702 (42.4%) | 8424 (56.8%) |
| Medicaid/Medicare/Other Gov’t | 623 (57.4%) | 2747 (57.2%) | 2019 (50.2%) | 5397 (36.4%) |
| Facility Type | ||||
| Community Cancer Program | 194 (18%) | 923 (19.6%) | 574 (15.1%) | 2618 (17.9%) |
| Comprehensive Community Cancer Program | 388 (36.1%) | 1694 (36%) | 994 (26.2%) | 4687 (32.1%) |
| Academic/Research Program | 493 (45.9%) | 2091 (44.4%) | 2225 (58.7%) | 7295 (50%) |
| Integrated Network Cancer Program | ||||
| Facility Location | ||||
| Northeast | 267 (24.8%) | 1150 (24.4%) | 825 (21.8%) | 3101 (21.2%) |
| South | 424 (39.4%) | 1843 (39.1%) | 1359 (35.8%) | 5174 (35.4%) |
| Midwest | 231 (21.5%) | 1110 (23.4%) | 983 (25.9%) | 3772 (25.8%) |
| West | 153 (14.2%) | 615 (13.1%) | 626 (16.5%) | 2553 (17.5%) |
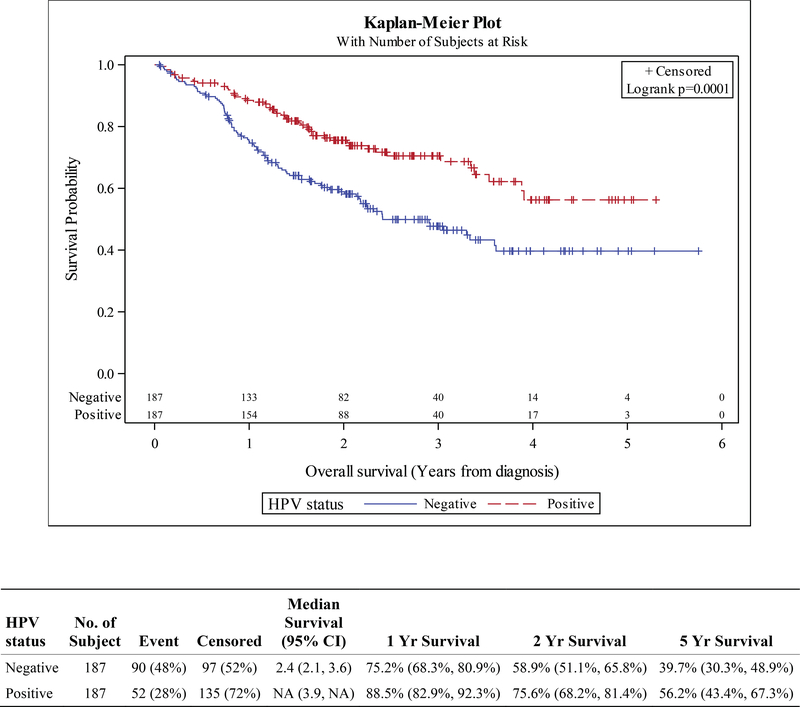
High-risk HPV status was prognostic in multiple non-oropharyngeal sites and subgroups when stratified by clinical stage. HPV positive status was associated with superior survival in patients with stage I-IVB hypopharyngeal SCC; the hazard ratio (HR) was 0.54 (HR p<0.001). Kaplan-Meier survival curves for the overall (unmatched) cohort show 1-year OS rates of 88.2% vs 74.4% (HPV+ vs HPV-). Corresponding 2- and 5-year OS were 75.5% vs 59.7% and 54.3% vs 33.6% (HPV+ vs HPV-, Figure S1). HPV positivity was associated with longer overall survival with traditional propensity score matching as well as IPTW methods. For PSM, overall survival HR was 0.52 (HR p<0.001); actuarial OS rates were 88.5% vs 75.2% at 1 year, 75.6% vs 58.9% at 2 years, and 56.2% vs 39.7% at 5 years comparing HPV+ vs HPV- (Figure 2). Using an IPTW technique, the HR was 0.61 (HR p<0.001).
Figure 2.
Kaplan-Meier curves for overall survival in propensity-matched cohorts of patients with hypopharynx squamous cell carcinoma, stratified by HPV status
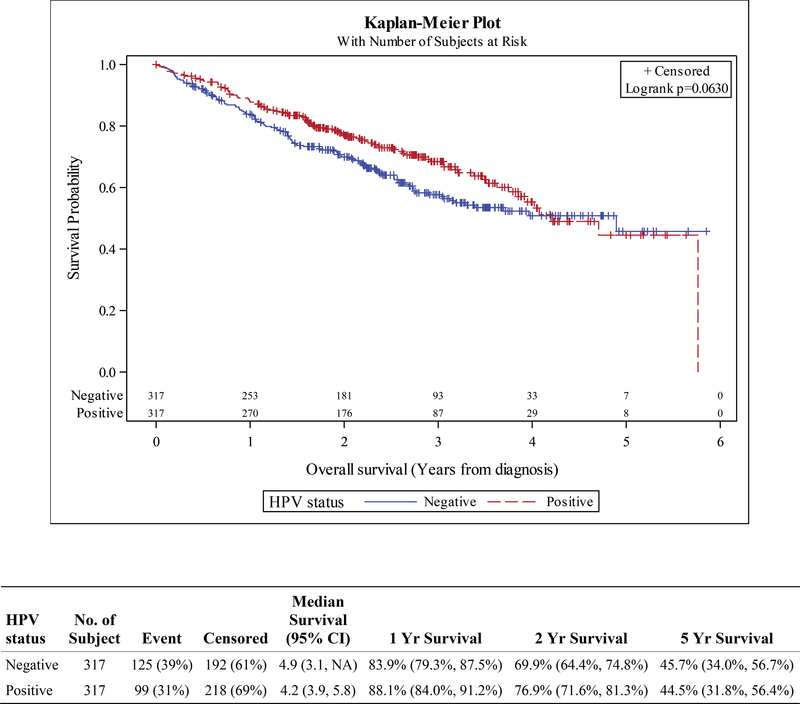
HPV status was also prognostic in the locally advanced laryngeal SCC cohort. For patients with stage III-IVB disease, HPV+ status was associated with superior OS, HR 0.72 (HR p=0.002). 1- and 2-year, and 5-year OS in the unadjusted cohort were 87.8% vs 80.9%, 76.4% vs 66.4%, and 44.3% vs 42.7% (HPV+ vs HPV-, Figure S2). The prognostic value in the stage III-IVB cohort remained significant after adjustment with propensity methods using IPTW and trended towards significance using traditional PSM; IPTW HR 0.79 (HR p=0.019), PSM HR 0.78 (HR p=0.066). OS by matched HPV status at 1-year was 88.1% vs 83.9%, at 2 years 76.9% vs 69.9%, and at 5 years 44.5% vs 45.7% (HPV+ vs HPV-, Figure 3). HPV status did not appear prognostic in those with early stage laryngeal SCC (Figures S3, S4).
Figure 3.
Kaplan-Meier curves for overall survival in propensity-matched cohorts of patients with stage III-IVB squamous cell carcinoma of the larynx, stratified by HPV status
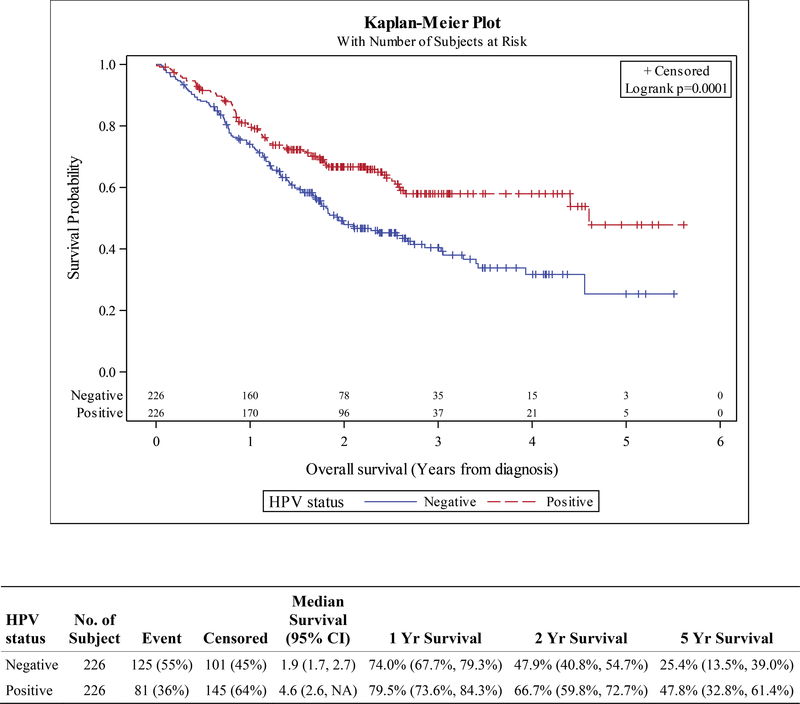
Similarly, high-risk HPV status was prognostic in stage III-IVB oral cavity SCC. In the locally advanced cohort, the unadjusted OS HR was 0.78 (HR p=0.03), 1-year OS was 78.9% vs 76.7%, 2-year OS 66% vs 58.3%, and 5-year OS was 47.4% vs 39.5% (Figure S5). Prognostic significance in the locally advanced cohort remained after adjustment with propensity methods using both IPTW and traditional PSM; PSM HR 0.59 (HR p<0.001), IPTW HR 0.78 (HR p=0.03). Actuarial survival using traditionally matched cohorts at 1, 2 and 5 years was 79.5% vs 74%, 66.7% vs 47.9%, and 47.8% vs 25.4% (HPV+ vs HPV-, Figure 4). HPV status did not appear prognostic in stage I-II oral cavity SCC (Figures S6, S7).
Figure 4.
Kaplan-Meier curves for overall survival in propensity-matched cohorts of patients with stage III-IVB squamous cell carcinoma of the oral cavity, stratified by HPV status
The prognostic impact of high-risk HPV status was also evaluated in oropharyngeal SCCs. As expected, high-risk HPV status was highly significant in both early (stage I-II) and locally advanced (stage III-IVB) settings, using both the unadjusted, and propensity-matched cohorts (with traditional PSM and IPTW). In early stage oropharyngeal SCC, the unadjusted HR was 0.37 (HR p<0.001), PSM HR was 0.42 (HR p<0.001), and IPTW HR was 0.44 (HR p<0.001). For stage III-IVB oropharyngeal SCC the unadjusted HR was 0.33 (HR p<0.001), with HR 0.42 (HR p<0.001) for PSM, and HR 0.41 (HR p<0.001) for IPTW. (Figures S8–S11).
Patients whose HPV status were not available were compared to the primary analysis cohort. For patients with hypopharyngeal SCCs, there was no significant difference in survival outcomes between the 2 cohorts, the HR was 0.90 (HR p = 0.07). The 1-, 2- and 5-year OS were 76.7% vs 76.2%, 62% vs 57.9%, and 36.5% vs 32.2% (HPV known vs HPV unknown, Figure S12). For patients with laryngeal and oral cavity SCCs, there was a significant difference favoring the known HPV status cohort. In the larynx comparison, the HR was 0.89 (HR p = 0.001). The 1-, 2- and 5-year OS were 87.2% vs 85%, 76% vs 72.5%, and 52.2% vs 51.3% (HPV known vs HPV unknown, Figure S13). In the oral cavity cohorts, the HR was 0.88 (HR p < 0.001). The 1-, 2- and 5-year OS were 85.7% vs 83.3%, 72.5% vs 69.6%, and 52.7% vs 52.9% (HPV known vs HPV unknown, Figure S14).
DISCUSSION
This study utilized the National Cancer Data Base, a large administrative database, to evaluate high-risk HPV status as a potential biomarker for SCCs in 3 non-oropharyngeal head and neck primary sites. Prior studies of p16/HPV status in non-oropharyngeal SCCs, have found inconsistent, and at times conflicting results. Geographical variation in the prevalence of HPV co-infection, and differences in p16 testing and classification of its expression may have accounted for some of the observed heterogeneity in previous reports. Importantly, older studies did not routinely address potential imbalances in patient, disease, and treatment characteristics which may have affected prognosis.
The current study is the largest to examine patient outcomes in the 3 non-oropharyngeal primary sites by high risk HPV status, finding a significant survival advantage associated with HPV positivity in patients with hypopharyngeal, and locally advanced oral cavity and laryngeal SCCs. However significant, the magnitude of the effect was notably less when compared to that of HPV status in OPX SCC. The results of the current study support 2 other analyses that had previously evaluated p16/HPV status as a prognostic biomarker. These 2 studies evaluated p16 and HPV status in the same 3 non-oropharyngeal primary sites, within the co-operative group setting and the Veteran Affairs (VA) health system 9,10.
Chung et al. used 356 prospectively collected tumor specimens from 3 randomized Radiation Therapy Oncology Group (RTOG) studies. They found positive p16 status was associated with better progression free survival (HR 0.63) and OS (HR 0.56), when the 3 non-oropharyngeal sites were analyzed collectively. When each primary site was examined individually, only in hypopharyngeal SCCs was there seen a significant association between p16 status and survival (HR 0.33 for PFS and OS). The same analysis using HPV detected by in-situ hybridization (ISH) did not find significance, presumably due to sensitivity of ISH testing and smaller sample size. Although the authors adjusted the analysis for age, sex, T and N classification, neither performance status nor the type of treatments received were taken into account. p16+ non-oropharynx patients were fitter than their p16- counterparts (Zubrod 0 in 62.9% vs 46.2%, p=0.02).
Bryant et al. identified 387 patients with locally advanced hypopharyngeal, oral cavity and laryngeal SCCs who had undergone p16 testing and were treated in VA hospitals. Like the RTOG secondary analysis, the authors found p16 status was prognostic with regard to overall survival, cancer specific survival (CSS), and competing mortality (CM) when analyzed collectively. The prognostic effect of p16 status was at least as significant in non-oropharyngeal SCCs compared to OPX SCCs (OS HR 0.41 vs 0.53; CSS HR 0.37 vs 0.5). Notably, the VA study attempted to account for patient, disease, and treatment co-variates using several multi-variable Cox models, which minimized the influence of confounders. Again, limited by sample size, p16 testing of individual non-OPX primary sites did not reach significance. Testing for p16 status itself was important. The authors were able to identify within the VA population a survival difference between non-OPX patients who had p16 testing compared to their untested counterparts. The rate of HPV status testing in the current study was 10.7% overall (range 9.7% to 12.9 by site), comparable to the 8.4% seen in the VA study. The prevalence of HPV positive disease in the current study closely resembles the rate of HPV positivity detected by ISH in the RTOG study; 11.6% in NCDB (range 10.6% to 11.7% by site) vs 9.4% in RTOG (range 5.3% to 14.6% by site). HPV status assessed by p16 IHC among the 2 prior studies closely resembled each other (19.3% in RTOG vs 20.1% in VA studies).
We examined the relevance of high-risk HPV status with the aid of several statistical refinements. Using propensity-weighed and propensity-matched methods, we minimized the impact of confounding co-variates by considering co-morbidity, treatment allocation, subsite where appropriate in addition to traditional patient and disease factors such as age and group stage. Also, primary sites were analyzed separately and stratified by group stage (except in hypopharynx due to sample size constraints), finding the prognostic impact of HPV status appeared to be confined to locally advanced disease for oral cavity and laryngeal SCCs. The study population here captured the majority of newly diagnosed head and neck SCCs, reflecting outcomes in community cancer centers and treatment delivered outside of academic institutions. Thus, the results from this database study can be more readily generalized to the broader population. Likewise, its validity extends outside the predominantly male VA demographic, which is largely driven by classic risk factors for head and neck cancer, and the more selective clinical trial population from the above studies.
There were several important limitations to the current study. Administratively collected cancer registry data was an inherent weakness21. HPV testing was not centrally reviewed, and the method of testing is not pre-specified by the NCDB. HPV testing was presumably performed as part of clinical care and heterogenous with respect to technique. However, classification of HPV status available from the Collaborative Stage Data Set Version 02.05 can be informative. Patients included in the high-risk HPV group tested positive for either type 16, type 18, both, or another high-risk type (including 33, 35, and 20 other types). Low-risk types, such as 6, 11 (and 17 other types) were not erroneously considered part of the HPV-positive cohort.
In addition, the NCDB does not routinely capture information on treatment response, patterns of failure, salvage therapies received after initial diagnosis, and most importantly cause of death. Smoking history is pertinent to prognosis in p16-positive and p16-negative oropharyngeal SCCs and would be expected to affect outcomes in non-oropharyngeal SCCs regardless of p16 status 22. Propensity-score matching/weighing cannot fully eliminate the risk of residual imbalances; it can only minimize the effect of observed confounders, allowing unobserved, and thus unbalanced co-variates to potentially influence outcome23. Though comparable to prior studies, the rate of HPV testing was significantly lower in non-oropharyngeal sites, raising concerns that the tested population is not representative of the entire non-oropharyngeal SCC population.
To ascertain whether the primary cohort of patients with known HPV status was subject to selection bias, a matched cohort of patients with unknown HPV status was used for comparison. Notably, non-missing HPV status was consistently associated with a reduced HR with range of 0.88 – 0.90. Several selection factors may have influenced these results. While the proportion of HPV-positive cases is unknown in this population, the cohort with missing data likely had a lower rate of HPV-positive cases. Cases with unknown HPV status were more likely to be retrieved from earlier years in the database. The rising prevalence of HPV-associated oropharyngeal SCCs has been well-described in the literature, and a similar phenomenon may exist for non-oropharyngeal SCCs. Differences in survival, based on availability of HPV information, were also seen in the VAMC analysis. The differences in that study appeared more significant that those seen here (5-year CSS 70.7% vs 61.9%). Increased likelihood of testing in patients based on demographic characteristics, i.e. young, non-drinkers and non-smokers may have also enriched our primary cohort with HPV-positive cases. Other factors, including the availability of HPV testing, and socioeconomic status may have indirectly contributed to the difference in prognosis.
Primary site ambiguity and associated coding errors are another source of concern. However, the impact of these errors was likely mitigated by several mechanisms. With respect to hypopharynx vs oropharynx ambiguity, those with a hypopharyngeal primary were defined by ICD-O-3 topography codes C129-C132, C138–139, corresponding to the distinct primary subsites: pyriform sinus, post-cricoid region, hypopharyngeal aspect of the aryepiglottic fold, and overlapping lesions of the hypopharynx. Additionally, patients in the NCDB are only coded once for their primary diagnosis, such that any patients with potentially ambiguous primary sites are not duplicated across files. Information contained within the NCDB are subject to numerous data integrity and quality-assurance measures18. Data are abstracted from patient records by Certified Tumor Registrars (CTR), who undergo training specific to cancer registry operations, for which the training and certification have increased over time18,24. NCDB data undergo a battery of data integrity checks, the rigor of which have significantly increased over time; the quality of data originating from large research hospitals and small community centers are no different in terms of completeness or accuracy18,25. To ensure accuracy, internal quality monitoring and data verification are done in advance of annual releases, which are also periodically evaluated by site surveyors from the CoC. Additionally, the data have undergone multiple data-quality reviews26–30.
HPV infection is not necessarily oncogenic. Acute infection or colonization with high risk HPV types have been detected in small fractions of the healthy population, leading to a bystander effect and/or false positive testing31. Distinguishing between transcriptionally inactive passenger infections from viral-mediated carcinogenesis is key to understanding the role of HPV status in non-oropharyngeal sites. Many have proposed HPV E6/7 mRNA as the gold standard for determining the proportion of patients whose disease is attributable to HPV infection 32,33. Studies examining attributable fractions using multiple test types (HPV DNA, p16, and E6 mRNA) have typically seen rates <5%, though others have found rates similar to those reported here 34–36.
The findings presented here contributes to a growing body of literature describing HPV-associated cancers in sites that have not classically been linked to HPV infection; reports have demonstrated HPV status to be highly prognostic in anal and esophageal cancers37,38. Treatment de-escalation has been the subject of active research for patients with oropharyngeal SCC. Several ongoing and recently completed randomized studies in the co-operative group setting have used induction chemotherapy, trans-oral robotic surgery or definitive chemoradiotherapy strategies. However, hypopharyngeal, oral cavity, and laryngeal SCCs have been excluded by definition in these trials. The results here should encourage prospective validation with centrally reviewed HPV/p16 testing and rigorous classification of the primary site. It also underscores the need for further exploration of a potentially causal link between HPV infection and non-oropharyngeal head and neck SCCs, either by traditional pathways mediated by E6 and E7 oncoproteins, or another mechanism.
Supplementary Material
Figure S1. Kaplan-Meier curves for overall survival in unmatched cohorts of patients with squamous cell carcinoma of the hypopharynx, stratified by HPV status
Figure S2. Kaplan-Meier curves for overall survival in unmatched cohorts of patients with stage III-IVB squamous cell carcinoma of the larynx, stratified by HPV status
Figure S3. Kaplan-Meier curves for overall survival in propensity-matched cohorts of patients with stage I-II squamous cell carcinoma of the larynx, stratified by HPV status
Figure S4. Kaplan-Meier curves for overall survival in unmatched cohorts of patients with stage I-II squamous cell carcinoma of the larynx, stratified by HPV status
Figure S5. Kaplan-Meier curves for overall survival in unmatched cohorts of patients with stage III-IVB squamous cell carcinoma of the oral cavity, stratified by HPV status
Figure S6. Kaplan-Meier curves for overall survival in propensity-matched cohorts of patients with stage I-II squamous cell carcinoma of the oral cavity, stratified by HPV status
Figure S7. Kaplan-Meier curves for overall survival in unmatched cohorts of patients with stage I-II squamous cell carcinoma of the oral cavity, stratified by HPV status
Figure S8. Kaplan-Meier curves for overall survival in propensity-matched cohorts of patients with stage III-IVB squamous cell carcinoma of the oropharynx, stratified by HPV status
Figure S9. Kaplan-Meier curves for overall survival in unmatched cohorts of patients with stage III-IVB squamous cell carcinoma of the oropharynx, stratified by HPV status
Figure S10. Kaplan-Meier curves for overall survival in propensity-matched cohorts of patients with stage I-II squamous cell carcinoma of the oropharynx, stratified by HPV status
Figure S11. Kaplan-Meier curves for overall survival in unmatched cohorts of patients with stage I-II squamous cell carcinoma of the oropharynx, stratified by HPV status
Figure S12. Kaplan-Meier curves for overall survival in matched cohorts of patients with squamous cell carcinoma of the hypopharynx, stratified by availability of HPV data
Figure S13. Kaplan-Meier curves for overall survival in matched cohorts of patients with squamous cell carcinoma of the larynx, stratified by availability of HPV data
Figure S14. Kaplan-Meier curves for overall survival in matched cohorts of patients with squamous cell carcinoma of the oral cavity, stratified by availability of HPV data
Acknowledgments
SOURCES OF FUNDING
Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292.
Footnotes
CONFLICT OF INTEREST
Kristin A. Higgins: Consultant: Astra Zeneca, Varian Medical Systems; advisory boards: Astra Zeneca, Genetech; Industry funded research: RefleXion Medical
REFERENCES
- 1.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–269. [DOI] [PubMed] [Google Scholar]
- 3.Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J Clin Oncol. 2015;33(29):3235–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lassen P, Eriksen JG, Hamilton-Dutoit S, et al. HPV-associated p16-expression and response to hypoxic modification of radiotherapy in head and neck cancer. Radiother Oncol. 2010;94(1):30–35. [DOI] [PubMed] [Google Scholar]
- 5.Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11(8):781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chernock RD, Wang X, Gao G, et al. Detection and significance of human papillomavirus, CDKN2A(p16) and CDKN1A(p21) expression in squamous cell carcinoma of the larynx. Mod Pathol. 2013;26(2):223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalianis T, Grun N, Koch J, et al. Human papillomavirus DNA and p16(INK4a) expression in hypopharyngeal cancer and in relation to clinical outcome, in Stockholm, Sweden. Oral Oncol. 2015;51(9):857–861. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz SR, Yueh B, McDougall JK, Daling JR, Schwartz SM. Human papillomavirus infection and survival in oral squamous cell cancer: a population-based study. Otolaryngol Head Neck Surg. 2001;125(1):1–9. [DOI] [PubMed] [Google Scholar]
- 9.Chung CH, Zhang Q, Kong CS, et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol. 2014;32(35):3930–3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryant AK, Sojourner EJ, Vitzthum LK, et al. Prognostic Role of p16 in Nonoropharyngeal Head and Neck Cancer. J Natl Cancer Inst. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salazar CR, Anayannis N, Smith RV, et al. Combined P16 and human papillomavirus testing predicts head and neck cancer survival. Int J Cancer. 2014;135(10):2404–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fakhry C, Westra WH, Wang SJ, et al. The prognostic role of sex, race, and human papillomavirus in oropharyngeal and nonoropharyngeal head and neck squamous cell cancer. Cancer. 2017;123(9):1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lassen P, Primdahl H, Johansen J, et al. Impact of HPV-associated p16-expression on radiotherapy outcome in advanced oropharynx and non-oropharynx cancer. Radiother Oncol. 2014;113(3):310–316. [DOI] [PubMed] [Google Scholar]
- 14.D’Souza G, Anantharaman D, Gheit T, et al. Effect of HPV on head and neck cancer patient survival, by region and tumor site: A comparison of 1362 cases across three continents. Oral Oncol. 2016;62:20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clayman GL, Stewart MG, Weber RS, el-Naggar AK, Grimm EA. Human papillomavirus in laryngeal and hypopharyngeal carcinomas. Relationship to survival. Arch Otolaryngol Head Neck Surg. 1994;120(7):743–748. [DOI] [PubMed] [Google Scholar]
- 16.Duray A, Descamps G, Decaestecker C, et al. Human papillomavirus DNA strongly correlates with a poorer prognosis in oral cavity carcinoma. Laryngoscope. 2012;122(7):1558–1565. [DOI] [PubMed] [Google Scholar]
- 17.Lee LA, Huang CG, Liao CT, et al. Human papillomavirus-16 infection in advanced oral cavity cancer patients is related to an increased risk of distant metastases and poor survival. PLoS One. 2012;7(7):e40767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med. 2007;26(4):734–753. [DOI] [PubMed] [Google Scholar]
- 20.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winchester DP, Stewart AK, Phillips JL, Ward EE. The national cancer data base: past, present, and future. Ann Surg Oncol. 2010;17(1):4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol. 2012;30(17):2102–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jagsi R, Bekelman JE, Chen A, et al. Considerations for observational research using large data sets in radiation oncology. Int J Radiat Oncol Biol Phys. 2014;90(1):11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Cancer Registrars Association: Certification Exam. http://ncra-usa.org/CTR/Certification-Exam. Accessed 01/22, 2019.
- 25.Stewart A, Gay E, Patel-Parekh L, Winchester D, Edge S, Ko CJJoCO. Provider feedback improves reporting on quality measures: National profile reports for adjuvant chemotherapy for stage III colon cancer. 2007;25(18_suppl):6572–6572. [Google Scholar]
- 26.Eberle C, Phillips J, Tary P, Menck HJJRM. Quality management in the National Cancer Data Base: a re-abstracting study of the Midwest region. 1997;24:93–97. [Google Scholar]
- 27.Fremgen A, Jessup J, Menck HJJRM. Hospital use of NCDB data to assess quality of cancer patient care. 1995;22:69–71. [Google Scholar]
- 28.Mulnar K, Phillips J, Fritz AJJRM. Quality of oncology data: Findings from the Commission on Cancer PCE study. 2001;28:24–34. [Google Scholar]
- 29.Sylvester M, Blankenship C, Carter A, Douglas L, Stewart AJJRM. Quality control: The American College of Surgeons Commission on Cancer Standards, National Cancer Data Base, and Cancer Liason Program; 2001;28:68–74. [Google Scholar]
- 30.NCDB Public Benchmark Reports. http://oliver.facs.org/BMPub/. Accessed 01/22, 2019.
- 31.Rietbergen MM, Snijders PJ, Beekzada D, et al. Molecular characterization of p16-immunopositive but HPV DNA-negative oropharyngeal carcinomas. Int J Cancer. 2014;134(10):2366–2372. [DOI] [PubMed] [Google Scholar]
- 32.Shi W, Kato H, Perez-Ordonez B, et al. Comparative prognostic value of HPV16 E6 mRNA compared with in situ hybridization for human oropharyngeal squamous carcinoma. J Clin Oncol. 2009;27(36):6213–6221. [DOI] [PubMed] [Google Scholar]
- 33.Jordan RC, Lingen MW, Perez-Ordonez B, et al. Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol. 2012;36(7):945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castellsague X, Alemany L, Quer M, et al. HPV Involvement in Head and Neck Cancers: Comprehensive Assessment of Biomarkers in 3680 Patients. J Natl Cancer Inst. 2016;108(6):djv403. [DOI] [PubMed] [Google Scholar]
- 35.Ndiaye C, Mena M, Alemany L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. The Lancet Oncology. 2014;15(12):1319–1331. [DOI] [PubMed] [Google Scholar]
- 36.Combes JD, Franceschi S. Role of human papillomavirus in non-oropharyngeal head and neck cancers. Oral Oncol. 2014;50(5):370–379. [DOI] [PubMed] [Google Scholar]
- 37.Rajendra S, Xuan W, Merrett N, et al. Survival Rates for Patients With Barrett High-grade Dysplasia and Esophageal Adenocarcinoma With or Without Human Papillomavirus Infection. JAMA Network Open. 2018;1(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun G, Dong X, Tang X, Qu H, Zhang H, Zhao E. The prognostic value of HPV combined p16 status in patients with anal squamous cell carcinoma: a meta-analysis. Oncotarget. 2018;9(8):8081–8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Kaplan-Meier curves for overall survival in unmatched cohorts of patients with squamous cell carcinoma of the hypopharynx, stratified by HPV status
Figure S2. Kaplan-Meier curves for overall survival in unmatched cohorts of patients with stage III-IVB squamous cell carcinoma of the larynx, stratified by HPV status
Figure S3. Kaplan-Meier curves for overall survival in propensity-matched cohorts of patients with stage I-II squamous cell carcinoma of the larynx, stratified by HPV status
Figure S4. Kaplan-Meier curves for overall survival in unmatched cohorts of patients with stage I-II squamous cell carcinoma of the larynx, stratified by HPV status
Figure S5. Kaplan-Meier curves for overall survival in unmatched cohorts of patients with stage III-IVB squamous cell carcinoma of the oral cavity, stratified by HPV status
Figure S6. Kaplan-Meier curves for overall survival in propensity-matched cohorts of patients with stage I-II squamous cell carcinoma of the oral cavity, stratified by HPV status
Figure S7. Kaplan-Meier curves for overall survival in unmatched cohorts of patients with stage I-II squamous cell carcinoma of the oral cavity, stratified by HPV status
Figure S8. Kaplan-Meier curves for overall survival in propensity-matched cohorts of patients with stage III-IVB squamous cell carcinoma of the oropharynx, stratified by HPV status
Figure S9. Kaplan-Meier curves for overall survival in unmatched cohorts of patients with stage III-IVB squamous cell carcinoma of the oropharynx, stratified by HPV status
Figure S10. Kaplan-Meier curves for overall survival in propensity-matched cohorts of patients with stage I-II squamous cell carcinoma of the oropharynx, stratified by HPV status
Figure S11. Kaplan-Meier curves for overall survival in unmatched cohorts of patients with stage I-II squamous cell carcinoma of the oropharynx, stratified by HPV status
Figure S12. Kaplan-Meier curves for overall survival in matched cohorts of patients with squamous cell carcinoma of the hypopharynx, stratified by availability of HPV data
Figure S13. Kaplan-Meier curves for overall survival in matched cohorts of patients with squamous cell carcinoma of the larynx, stratified by availability of HPV data
Figure S14. Kaplan-Meier curves for overall survival in matched cohorts of patients with squamous cell carcinoma of the oral cavity, stratified by availability of HPV data