Abstract
Photodynamic therapy (PDT) is a clinical ablation modality to treat cancers and other diseases. PDT involves administration of a photosensitizer, followed by irradiation of target tissue with light. As many photosensitizers are small and hydrophobic, solubilization approaches and nanoscale delivery vehicles have been extensively explored. Liposomes and lipid-based formulations have been used for the past 30 years, and in some cases have been developed into well-defined commercial PDT products. This review provides an overview of common liposomal formulation strategies for photosensitizers for PDT and also photothermal therapy. Furthermore, research efforts have examined the impact of co-loading therapeutic cargo along with photosensitizers within liposomes. Additional recent approaches including imaging, overcoming hypoxia, upconversion and activatable liposomal formulations are discussed.
Keywords: Liposomes, Photosensitizers, Photodynamic therapy, Photothermal therapy
1. Introduction
Photodynamic therapy (PDT) is used for local disease ablation, and involves administration or application of a photosensitizer (PS) and delivery of light. PS are small molecules that absorb visible or near infrared (NIR) light energy, undergo intersystem crossing, and transfer energy to molecular oxygen, thereby creating singlet oxygen, a potent reactive oxygen species (ROS) [1]. When a PS absorbs photons of light, it gets transformed from the electronic ground state into a short-lived excited singlet state which may proceed to a relatively long-lived electronically excited triplet state [2]. During the PS excited triplet state, the PS can undergo reactions in two ways to produce ROS: it can form radicals by transferring electrons to a biological molecules to produce ROS (Type I reactions); or the PS can produce ROS by transferring energy directly to molecular oxygen, to create reactive singlet oxygen (Type II reactions) [3]. Type II reactions based on singlet oxygen are considered the major mechanism for PDT. Singlet oxygen is cytotoxic and reacts with nearby cellular macromolecules and organelles, leading to cell damage and cell death that can occur via specific defined pathways such as apoptosis, autophagy, necrosis, or paraptosis [4].
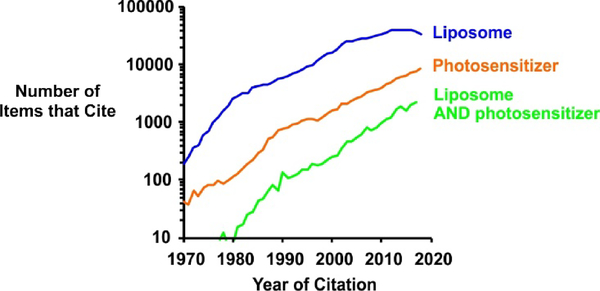
PDT requires the presence of multiple components to be effective in ablating the target tissue, namely the PS itself, a sufficient light dose of appropriate wavelength, and molecular oxygen. The selective delivery of PS at target sites for effective tumor destruction is a challenge. Delivery vehicles and solubilization approaches have been widely explored for PS [5]. Liposomes are a prominent PS delivery vehicles, given their biocompatibility and ability to encapsulate PS in their hydrophobic bilayer [6]. As shown in Figure 1, the number of published items in the Google Scholar database that includes the term liposomes, or photosensitizers within their full text has increased substantially over the past few decades.
Figure 1: Increasing research interest in liposomes and photosensitizers.
The number of items in the Google Scholar database that reference liposomes, photosensitizers and the combination anywhere within their full text were assessed. The search terms for “Liposome” were [“liposome OR liposomes”]; for “Photosensitizer” were [photosensitizer OR photosensitizers AND “photodynamic OR PDT”][3] and for “Liposome AND photosensitizer were [photosensitizer OR photosensitizers AND “photodynamic OR PDT” AND “liposome OR liposomes”]. The search years were from 1970 till 2018.
Liposomes are lipid-based vesicles formed from a lipid bilayer that encloses an aqueous core and can comprise one (unilamellar) or more (multilamellar) concentric bilayers. The structure of liposomes enables them to encapsulate both hydrophobic and hydrophilic compounds including PS [7, 8]. Liposomes used for drug delivery are typically designed with the goal of solubilizing or retaining the therapeutic compounds to release them in target tissues [9, 10]. Factors such as physicochemical properties of drugs and lipid compositions, as well as properties of the target tissue play important roles in maintaining stability of the liposomes and drug release.
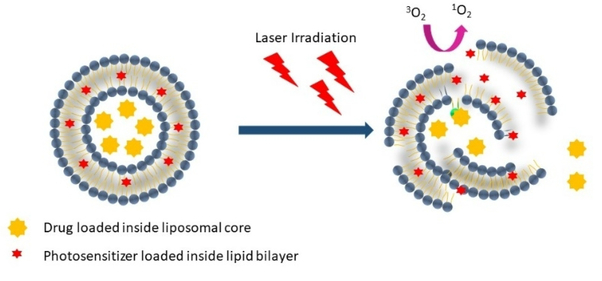
Several PS formulation methods exist. If the PS is suitably hydrophilic, it can be directly dissolved in aqueous buffer, as is the case for Photofrin®. Other PS, such as Temoporin (Foscan®) are dissolved in organic solvent excipients (ethanol and propylene glycol) that can maintain PS solubility in aqueous buffers upon preparation for administration [11]. Other PS, such as HPPH are formulated in mostly aqueous buffers with the assistance of surfactants like TWEEN 80 [12]. While such relatively straightforward approaches offer the major advantage of simplicity, defined nanoparticulate formulations can also be useful PS delivery vehicles for PDT [13]. Liposomes and related liposome-like structures are a preferred choice, as they can solubilize a range of PS, as well as other molecules that can readily be co-loaded [14]. Figure 2 shows how liposomes are versatile in solubilizing PS, and that laser irradiation results in singlet oxygen generation, with possible bilayer destabilization leading to release of other entrapped cargo.
Figure 2: Versatility of liposomal PS.
Liposomes can be formed with PS and other drugs. When irradiated, the PS absorbs the energy and transfers it to molecular oxygen to produce a highly reactive oxygen species (ROS - 1O2) which can disrupt the structure of liposomes to release the cargo encapsulated inside.
Lipophilic drugs and PS encapsulated within the bilayer may interact with plasma proteins causing them to be released before reaching the target tissue. The structure as well as composition of liposomes play an important role in liposomal stability and bioavailability. While liposomes with high concentration of fluid lipid constituents such as egg phosphatidylcholine (PC) are easily destabilized and can entrapped release drugs in circulation, liposomes more saturated phospholipids and cholesterol (CHOL) generally exhibit higher stability in circulation [15].
In 1983 liposomal dipalmitoyl PC formulations of HpD were reported by Giulio Jori and colleagues to be effective for systemically delivering PS to tumors in rats [16]. It has been shown that liposomes can effectively deliver PS to tumors. A human glioma was implanted in the brain of rats and was treated with PDT using a liposomal formulation of Photofrin and accumulation of Photofrin in tumor tissue was higher using the liposomal group [17]. Therefore, liposomal PS formulations not only solubilize PS for administration, but can improve their physiological behavior in vivo.
There are numerous factors to consider in developing liposomal PS formulations. These include formulation stability during storage, and stability in physiological environments such as plasma. Other factors can impact the efficacy of PDT including the photophysical parameters of the PS in the formulation, which may be impacted by PS density. Lipophilic PS tend to aggregate in aqueous media, thereby reducing their photosensitizing efficacy [18] whereas liposomal formulations can decrease PS aggregation, thus retaining their photosensitivity [19]. This review aims to summarize representative strategies involved in designing liposomal formulations of PS for traditional PDT, as well as emerging applications that are conducive to liposome formulations.
2. Liposomes
2.1. Background
Liposomes are widely used and explored for pharmaceutical drug delivery applications [20, 21]. They are typically composed of phospholipids and CHOL, along with the active components. Some liposomes are made by incorporating surface coating components that help in restricting the serum components from binding to the liposome membranes and thus prevent their interaction and simultaneous elimination through phagocytic cells in the reticuloendothelial system and the circulation system. The most commonly used surface coating component used for liposomes at present is polyethylene glycol (PEG). PEGylated liposomes are sometimes referred to as sterically stabilized liposomes, stealth liposomes, or long-circulating liposomes as they usually circulate in blood for extended durations [22].
Liposomes are prepared in various ways depending on the physicochemical properties of the liposomal constituents and the compounds encapsulated inside [23, 24]. The most common ways of preparation include mechanical dispersion, solvent dispersion and detergent removal. Mechanical dispersion methods include techniques such sonication, pressure cell, extrusion, freeze-thawed liposomes, lipid film hydration, micro-emulsification, membrane extrusion and dried reconstituted vesicles [25]. Solvent dispersion methods include techniques like ether injection, ethanol injection and reverse phase evaporation. Detergent removal method is another approach but has the disadvantage is that the detergent cannot be completely removed from the liposomes [24]. Emerging methods for liposome formulation include the use of scalable microfluidic apparatus and the use of supercritical fluid approaches [26].
Ideally, liposomes are expected to stably encapsulate therapeutic drugs in circulation releasing them selectively in target tissue. Factors which affect this include the physicochemical properties of drugs, lipid composition and the target tissue all affect the circulating stability of liposomes and their drug release properties. While hydrophilic drugs can often be stably retained inside the aqueous core of liposomes in circulation, it may be difficult to release them at the target site owing to low membrane permeability. On the other hand, hydrophobic drugs, encapsulated within the bilayer, may be removed from the liposomes through interactions with serum components such as proteins before reaching the target tissue.
2.2. Passively targeted liposomes
Angiogenesis in malignant tumors increase vascular permeability in tumor vessel walls producing fenestrae of a pore size suitable for nanoparticles to enter. Moreover, tumor tissue does not have a functional lymphatic system so the macromolecules, once removed from blood, do not have a lymphatic mechanism to drain back into central circulation. This leads to spontaneous accumulation, retention and extravasation of macromolecules in tumor tissue and this phenomenon is referred to as the enhanced permeability and retention (EPR) effect [27]. Liposomes and other nanoparticles are often designed to exploit this phenomena as they are able to passively accumulate in the tumor tissue at relatively high levels, although drug delivery to solid tumors generally poses a challenge due to owing to high interstitial fluid pressure [28].
2.3. Actively targeted liposomes
Active targeting intends to augment the tumor-selective uptake of liposome encapsulated compounds. The goal is to increase the amount of liposomes accumulating in the tumor that would otherwise accumulate through passive targeting, increasing the therapeutic efficacy of the drug while preventing side-effects by limiting systemic exposure [22]. The basic strategy involved in active targeting is the coupling of targeting moieties to the surface of liposomes enabling the liposomes to selectively bind to specific tissue and cells. These tumor-targeting moieties are either bound directly to the lipophilic anchor by covalent bonds or by a spacer arm or through other mechanisms which enable the attachment of a targeting agent such as an antibody [29]. While this approach has been the focus of significant research, there have been few examples of active-targeting liposomes drastically enhancing tumor drug biodistribution, as there are several physiological barriers present in vivo which limit the ability of targeted liposomes to selectivity bind to cancer cells in solid tumors. It has been reported that antibody targeting of liposomes does not enhance tumor biodistribution, but can enhance uptake of liposomes into target cells, which is a more subtle effect [30]. Liposomal targeting of tumor vasculature is an alternative approach that may be have advantages compared to targeting tumor cells themselves [31].
3. PS formulations
3.1. PS background
Hematoporphyrin Derivative (HpD), which has been commercialized as Photofrin®, is a water-soluble mixture of porphyrin oligomers and was the first PS to be approved clinically for PDT, with approval in 1993 for treating bladder cancer in Canada, and subsequent approvals by the US Food and Drug Administration (FDA) for other solid tumors thereafter [32]. Photofrin® contains a mixture of water soluble but lipophilic dimers and oligomers of 2 to 9 porphyrin units mostly linked by ether bonds and is a first generation PS. Research efforts have subsequently developed second generation PS that are pure compounds with greater absorption in the near infrared (NIR) wavelength. Use of a pure, non-oligomeric, PS simplifies purification and analysis for quality control purposes. PS generally aim for high singlet oxygen quantum yields, and strong light absorption between 600 and 1000 nm, as this is considered the therapeutic window for light penetration, below or above which light does not optimally propagate within tissue [33–35]. Visudyne®, a lyophilized lipid/liposome-based formulation of Verteporfin, also known as benzoporphyrin derivative monoacid (BPD-MA) has these attributes, and was approved by the FDA in 2000 to treat age related macular degeneration (AMD) [36].
PS have been used for PDT to treat a variety of diseases, including cancers, macular degeneration, psoriasis, actinic keratosis, and others [37–39]. For a PDT treatment to be effective, sufficient amount of PS, light and oxygen are essential [1]. PS are typically administered through intravenous injections, or topically for dermatological applications. Following the administration of the PS the target site is illuminated with light of a specific wavelength which matches the absorption of the PS [40, 41]. Light within the red and near infrared region are most commonly used as they provide the best tissue penetration particularly for anticancer applications while other wavelengths in the blue and green range of the spectra are used for dermatological applications [15].
For any cancer therapeutic modality, targeted destruction of tumor cells is one of the main objectives for successful treatment. There are two factors that aid PDT in selective killing of tumor cells: i) selective accumulation of the PS and ii) confined and accurate delivery of light at the tumor site. The first criteria can be achieved by using PS that selectively accumulate in tumor tissues owing to leakage in blood vessels and lymphatic vessels. The second requirement is met by delivering light at target tissues using selective placement of optical fibers for target irradiation. PDT possesses advantages over other therapeutic methods; it is a fast, minimally-invasive, targeted treatment method, the capability to treat patients with multiple doses without generating scars with minimal side effects
PDT has limitations, such as sunlight sensitivity following treatment, the requirement of an external light source, limited tissue penetration of light, and limited and heterogeneous PS uptake in tumor. Emerging approaches such as Cerenkov radiation (CR)-induced PDT have been proposed to overcome some of these hurdles, and in theory could remove the requirement of an external light source and overcome penetration depth issues. Kotagiri et al. demonstrated a CR-induced PDT technique by activating titanium dioxide (TiO2) as a PS using CR from radionuclides [42]. When transferrin coated TiO2 was co-administered with 64Cu into mice bearing HT1080 xenografts, improvement in survival and tumor ablation was observed. Another study reported magnetic nanoparticles coated with porphyrin molecules and 89Zr radionuclide (89Zr-MNP/TCPP). When 89Zr-MNP/TCPP was administered to mice bearing 4T1 tumors, a magnet was applied to the tumor to enhance drug delivery leading to tumor remission. Cerenkov luminescence (CL) and Cerenkov resonance energy transfer (CRET) enable imaging of the treatment procedure [43]. Liposomes have potential for being used with CR approaches as they can readily load radionuclides and PS.
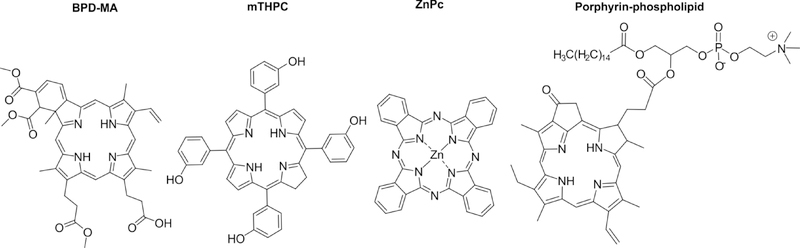
Many PS used in the treatment of cancer have a tetrapyrrole backbone structure. The structure of PS dictates their photophysical and physical properties [44]. Since PS tend to be at least partially hydrophobic in nature, liposomes are well-suited to solubilize or load these compounds within their bilayer. Hydrophobic PS can also be formulated simply in an organic solvent or a surfactant based buffer, whereas liposomes have can serve as an alternative approach. Figure 3 provides structural representation of different PS used in PDT for cancer treatment, including BPD-MA and mTHPC, which are comprised in well-defined commercial liposomal formulations. Zinc phthalocyanine (Zn-Pc) is a phthalocyanine-based PS that has been used in several studies with liposomal formulations. Porphyrin-phospholipid is a PS-lipid conjugate that results in stable and non-exchangeable incorporation into the liposome bilayer.
Figure 3: Chemical structures of PS commonly used in liposomal formulations.
BPD-MA (also known as Verteporfin) is the PS in the Visudyne® liposome formulation. Meso-tetra-hydroxyphenyl-chlorin (mTHPC, also known as Temoporfin and formulated as Foscan®) is the PS in the Foslip® and Fospeg® liposome formulations. ZnPc is the PS in the CGP55847 liposomal formulation. Pyropheophorbide-based porphyrin-phosopholipid is a PS-lipid conjugate that integrates stably in bilayers.
3.2. Liposomal PS formulations
Liposomes are a well-established pharmaceutical carrier and represent an attractive system for delivering PS, as they can solubilize PS and potentially alter their pharmacokinetics and biodistribution, and therefore efficacy and safety [8]. Since liposomes can enhance blood circulation of drugs and enhance delivery to tumor tissue via the EPR effect they have potential to improve the efficacy of PS delivery. However, one consideration is that many bilayer-loader PS will exchange with serum components if administered in the blood, thereby complicating design of liposomes that modulate PS behavior [45].
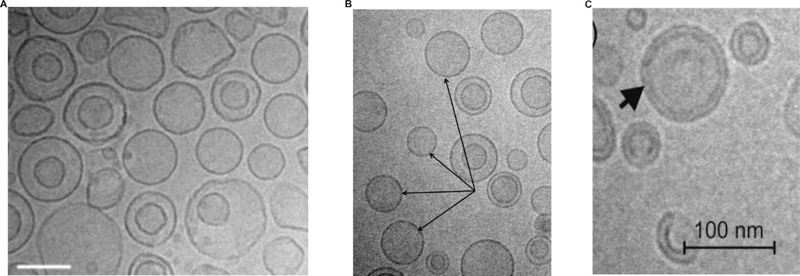
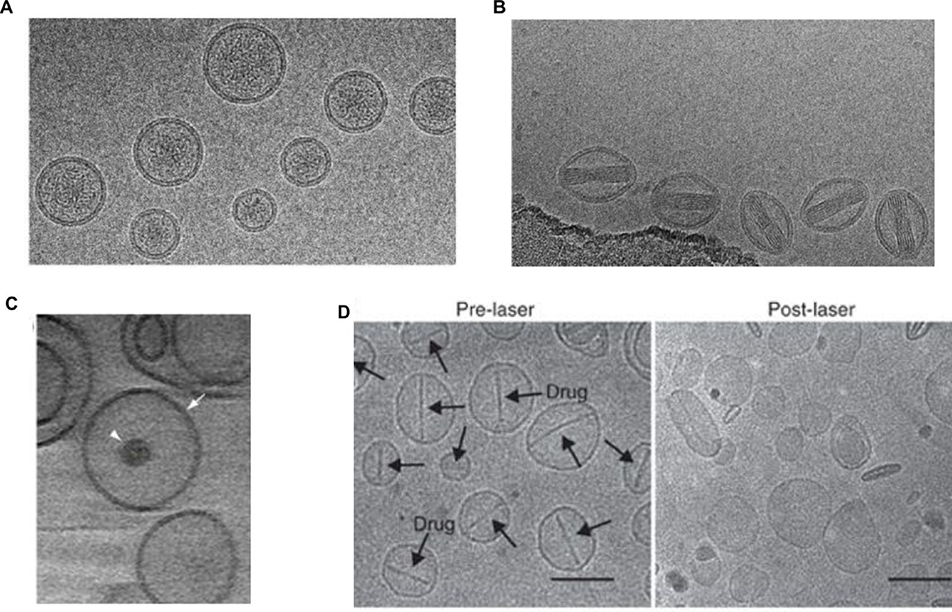
Figure 4 shows cryogenic transmission electron microscopy (cryo TEM) images of Temoporfin encapsulated into different liposome formulations. The hydrophobic PS is located in the bilayer. Generally, PS can be loaded into liposomes without disrupting bilayer integrity and typical liposome morphology.
Figure 4:
Images of Temoporfin-loaded liposomal formulations by cryo TEM. A) Liposomal formulation containing DPPC:DPPG:Temoporfin in mass ratio of 18:2:1.5. (Scale bars, 100 nm) B) Liposomal formulation containing a mixture of phospholipids (NAT8539):Terpenes:Temoporfin in mass ratio of 133:10:1.5 (Scale bars, 200 nm). Arrows show unilamellar vesicles. C) Liposomal formulation containing Temoporfin:Soy bean lecithin lipoid S-75 in mass ratio of 0.225:15 (Scale bars, 100 nm). Reprinted with permission from Ref. [46]; Ref. [47] and Ref. [48].
Table 1 shows a small representation of various liposomal PS formulations described for PDT. A wide range of lipids and formulations parameters is apparent. The vast majority of these formulations make use of phospholipids as the main lipid. CHOL and PEG can be included to enhance formulation stability. A wide range of loading ratios can be observed but typically involve substantial less than 15 % PS, based on the molar percentage of the total combined PS and lipid amounts. At PS ratios higher than this, liposome formation may be inhibited and aggregation can occur. Liposomes are typically formed with the extrusion method, which involves down-sizing the liposome by passing the suspension through a membrane with a well-defined pore size. This extrusion step is effective for early research studies, but is not trivial to scale to commercial levels. Many liposomal formulations also incorporate the PS into the formulation at the time of lipid production, which simplifies manufacturing. This approach is preferable if the PS can be dissolved in the same organic solvent as the lipids prior to introduction of aqueous buffer and extrusion.
Table 1:
Representative liposomal PS formulations
| PS Formulation | Lipids | Lipid ratio | PS incorporation | Liposome formation | Size (nm) | Ref |
|---|---|---|---|---|---|---|
| BPD-MA Visudyne® | DMPC:EPG | 3:5 (mol.) | 12.5 mol. % | Lyophilized | 150–300 | [49–51] |
| mTHPC Foslip® | DPPC:DPPG | 9:1 (mass) | 8 mol. % | Lyophilized | 111 PDI: 0.1 | [52] |
| mTHPC Fospeg® | DPPC:DPPG:DSPE-PEG | 9:1:1 (mass) | 8 mol. % | Extrusion | 114 PDI: 0.1 | [52, 53] |
| ZnPc CGP55847® | POPC:DOPS | 9:1 (mass) | 1 mass % | Solvent mix, lypohilization | 70–80 | [54] |
| 5-ALA | Ceramide:CHOL: palmitate: cholesteryl sulfate | 50:28:17:5 (mass) | 1:3 PS:lipid | Reverse phase evaporation | 400 PDI: 0.6 | [55] |
| Bodipy | Egg PC: DOPE | 70:25 (mol.) | 5 mol. % | Extrusion | 107 PDI: 0.03 | [56] |
| BPD-MA | DPPC:CHOL:DPPG | 20:10:5 (mol.) | 0.8 mol. % | Extrusion | 103 | [57] |
| Ce6 | EDOPC:CHOL | 10:5 (mol.) | Post-loading (PS:lipid 1 % vol.) | Extrusion then mixing | 143 | [58] |
| Ge (IV) Pc | POPC:DOPS | 9:1 (mass) | 1 mass % | Solvent mix, lypohilization | - | [59] |
| ICG | DPPC: Soy-PC:CHOL: DSPE-PEG2K | 100:50:30:0.5 (mol.) | Lipid film hydration with 10 mg/mL ICG | Extrusion and gel filtration | 80 | [60] |
| mTHPC | DPPC: DPPG | 18:2 (mass) | 7 mass % | Extrusion | 117 PDI: 0.1 | [46] |
| Photofrin | DPPC | 100 % | 2:1 PS:lipid (mass) | Sonication | - | [61] |
| Porphycene derivatives | DOPC | 100 % | 0.5 mol. % | Sonication and filtration | 110 | [62] |
| PPIX derivative | DOPC | 100 % | 10 mol. % | Sonication and filtration | 140 | [63] |
| TPP derivative | DMPC | 100 % | Post-loading (0.1 mol %) | Extrusion | 160 | [64] |
| ZnPc | HSPC:PE–NH2 | 69:6 (mol.) | 3 mol. % | Extrusion | 85 | [65] |
Abbreviations
Asterisk indicates the pore size used for extrusion of liposomes, rather than the measured liposome diameter.
PDI: Polydispersity index
5-ALA: 5-Aminolevulinic acid; BPD-MA: Benzoporphyrin derivative monoacid ring A, Ce6: Chlorin e6; DiBDP: Dimeric Bodipy; Ge (IV) Pc: Germanium (IV) phthalocyanine; ICG: Indocyanine green; mTHPC: meta-tetrahydroxyphenylchlorin; PPIX: Protoporphyrin IX; ZnPc: Zinc phthalocyanine; TPP: Meso-tetraphenyl porphyrin
CHOL: cholesterol; DMPC: Dimyristoylphosphatidylcholine; DOPC: Dioleoylphosphotidylcholine; DOPE: Dioleoylphosphotidylethanolamine; DPPC: Dipalmitoylphosphatidylcholine; DPPG: Dipalmitoylphosphatidylglycerol; DSPE-PEG: distearoylphosphatidylethanolamine-polyethylene glycol; EDOPC: 1,2-dioleoyl-sn-glycero-3-ethylphosphocholine; POPC: 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine; DMPC: Dimyristoylphosphatidylcholine; OOPS: dioleoyl-phosphatidylserine; DSPC: distearoylphosphatidylcholine; DPPE-mPEG: 1,2-Dipalmitoyl-sn-glycero-3-phosphoethanolamine: Methoxy poly(ethylene glycol); EPG: Egg phosphatidylglycerol; EYPC: L-α-Phosphatidylcholine; DOPC: Dioleoylphosphatidylcholine; DOPS: dioleoylphosphatyidylserine; HSPC: Hydrogenated Soy L-α-Phosphatidylcholine; PE-NH2: 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N (hexanoylamine); POPC: 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine
Various interrelated factors impact the efficacy of PS performance in PDT. Hydrophobic PS tend to aggregate in aqueous media and stronger photodynamic activity is shown by monomeric species [18], so aggregation decreases photosensitizing efficacy and liposomal formulations can significantly decrease PS aggregation. For example, it was found that egg phosphatidylcholine liposomal formulation of lipophilic PS hypocrellin A enabled the PS to be completely monomerized that ultimately enhanced selective uptake in tumors when compared to a suspension of the PS in dimethyl sulfoxide-solubilized saline [19]. Some of the experimental formulations in Table 1 incorporate PEG into the liposome formulation. This can provide formulation stability and in some cases PEGylation has been shown to enhance PS delivery to tumors [66].
3.3. Commercial liposomal PS
3.3.1. Visudyne®
Visudyne® was the first and only clinically-approved liposomal PS formulation. It was developed and manufactured by QLT in Vancouver, with a distribution agreement with Novartis and eventual sale of the drug to Valeant Pharmaceuticals. Visudyne® is a lyophilized formulation of DMPC: EPG in a molar ratio of 3:5 with BPD-MA PS within the liposomes with a PS:lipid ratio of 1:8 [49–51]. Ascorbyl palmitate and butylated hydroxytoluene are included as excipients. It has been used clinically for the treatment of subfoveal choroidal neovascularization (CNV) due to acute-macular degeneration (AMD), pathologic myopia and ocular histoplasmosis syndrome (OHS) [67, 68]. Apart from AMD, PDT with this formulation has been also used for the treatment of other ailments like polypoidal choroidal vasculopathy, central serous chorioretinopathy and choroidal hemangioma [67]. BPD-MA, developed from protoporphyrin IX (PpIX) dimethyl ester [69], possesses several advantageous properties of a PS [70] like chemical stability, efficient singlet oxygen generation, strong absorption of red light at 692 nm and fast extravasation from body resulting in reduced skin photosensitivity [67]. However, the hydrophobic nature of BPD-MA makes it prone to self-aggregate in aqueous media [71]. Thus liposomes were considered as a carrier for the intravenous delivery of the lipophilic PS. Visudyne has been explored for anti-cancer applications. Ritcher et al. observed that the liposomal formulation of BPD-MA allows better uptake of the PS into tumor tissues and neovasculature [72].
3.3.2. Foslip® and Fospeg®
Foslip® and Fospeg® are liposomal formulations of the PS 5,10,15,20-tetrakis(3-hydroxyphenyl)-chlorin or Temoporfin (mTHPC, which is available as Foscan®) manufactured by Biolitec. mTHPC is considered one of the most potent currently available PS. It is a pure compound with high absorption of red light (652 nm) [73]. It was approved by the European Medicines Agency for the palliative treatment of advanced head and neck squamous cell carcinoma [74, 75]. For PDT using Foscan®, a time interval of 4 days is usually maintained between drug administration and illumination [73]. mTHPC does not have good water solubility so liposomes were considered as a favorable delivery system [76]. The first liposomal formulation of mTHPC with the trade name Foslip® was made with DPPC, DPPG and mTHPC (DPPC:DPPG in mass ratio of 9:1) with mTHPC loaded in PS:lipid ratio of 1:12 [52]. Later, stealth liposomes encapsulated with mTHPC were prepared to increase their circulation time in bloodstream, thus formulating the PEGylated formulation, trade name Fospeg® [46]. This was prepared by adding DSPE-PEG to the previous formulation such that the mass ratio of lipids was DPPC:DPPG:DSPE-PEG was 9:1:1 and the PS loading ratio was 1:13 PS:lipid [52]. Reddi’s group also found that PEG layer can affect the cytotoxicity and accumulation of liposomes in cells [77]. In another study, when liposomal formulations of temoporfin, Foslip® and Fospeg®, were compared by injecting a 0.15 mg/kg PS dose in mice with tumor model HT29 with light dose of 10 J/cm2, Fospeg® had higher efficacy compared to Foslip® [53].
3.3.3. CGP55847
CGP55847 is a lyophilized Zn-Pc formulation developed by Ciba-Geigy (which would become Novartis). It is formed with controlled solvent injection, followed by buffer exchange into a lactose solution and eventual lyophilization with a composition of ZnPc:POPC:DOPS of 1:90:10 (mass ratio) and liposome size of 70–80 nm [54]. When administered to mice bearing METH-A tumors at doses of 0.125 mg/kg, potent tumor cures were achieved with light doses 150–180 J/cm2 of 671 nm laser irradiation [78]. CGP55847 has been shown to accumulate minimally in muscle tissue, but well in Ehrlich carcinomas and B16 melanomas, especially in necrotic areas [79]. CGP55847 underwent phase I/II human clinical trials in Switzerland in patients suffering from squamous cell carcinoma of the upper aerodigestive tract in the 1990s [80], but further development apparently did not proceed thereafter.
3.4. Other liposomal PS formulations
3.4.1. Porphyrins and chlorins
Porphyrins and chlorins are amongst the most common type of PS. These contain four pyrrole subunits linked by four methine bridges [81]. Porphyrin and related molecules are known to possess photophysical properties of a good PS, including some absorption in the NIR range and strong singlet oxygen quantum yields. Their property of selective accumulation in tumor tissues have made researchers study their applications as both an imaging agent and a therapeutic agent [82]. However, the hydrophobic nature of porphyrins and porphyrin derivatives lead to forming aggregates in aqueous media [15]. To overcome this, liposomes have been used as an efficient approach for delivery.
Jiang et al. reported unilamellar DPPC liposomes for the encapsulation and delivery of Photofrin. When liposomes loaded with Photofrin or free PS were used for PDT of U87 human glioma athymic nude mice xenografts [17] and 9L rat gliosarcomas [61], liposomal Photofrin showed better accumulation of the drug in tumors and enhanced tumor cell killing. In another study, liposomes formed with DMPC:CHOL:DPPG:Photofrin in molar ratio of 100:100:60:10 were administered (10 mg/kg Photofrin in liposomal form or free) in mice bearing human gastric cancer xenografts followed by irradiation, the amount of cellular destruction by necrosis was significantly high in the liposomal form as compared to free PS [83]. Protoporphyrin IX (PpIX) was functionalized with oleylamine arms entrapped into liposomes made with DOPC (porphyrin:DOPC in molar ratio 1:10) and used for in vitro studies with cancer cells. HeLa and AGS cells were irradiated and the liposomal formulations showed more efficiency in killing cells than free protoporphyrin IX [63]. When PpIX was conjugated to a polyethylene glycol-cholesterol polymer and anchored to POPC liposomes and administered to mice bearing U14 tumor xenografts, longer tumor retention was observed and with irradiation tumor growth inhibition and in vivo fluorescence imaging was enhanced compared to control groups. The presence of CHOL that aided in lipid raft mediated endocytosis along with the PS that enabled light activated enhanced permeability of plasma membrane [84]. Further, several cargo-loaded liposomal formulations of porphyrins have been studied recently. These formulations have been discussed later in the review.
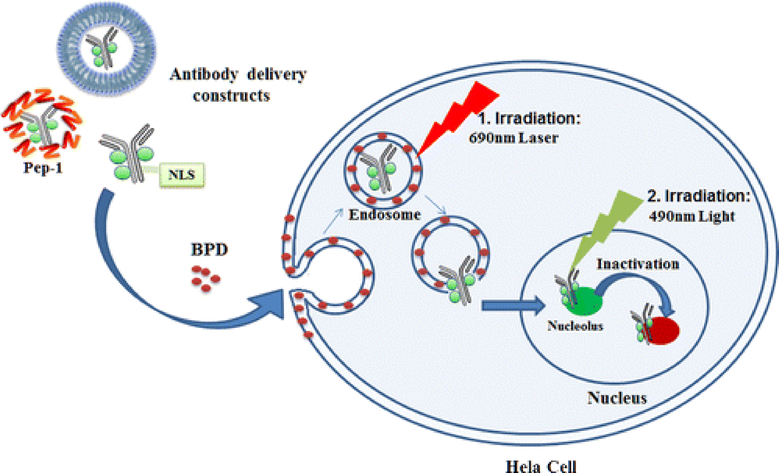
Several liposomal formulations of chlorins have focused on the BPD-MA and chlorin e6 trimethyl ester (Ce6) PS [15]. BPD-MA has not only been used for AMD applications, but has been explored extensively for tumor suppression. In another study, Wang et al. demonstrated phototherapeutic cellular destruction by dual irradiation method using antibody-PS constructs by liposomes encapsulated with BPD. When HeLa cells were treated with antibody constructs (TuBB-9-FITC: antibody TuBB-9 conjugated with photoactivatable compound Fluorescein Isothiocyanate, FITC) encapsulated in liposomes (DPPC: DOTAP: CHOL: PEG2000-DSPE in molar ratio) containing BPD in lipid bilayer followed by irradiation at 690 nm, liposomal destruction due to photosensitive BPD releases antibody constructs. TuBB-9-FITC goes inside nucleus and binds to proliferation associated protein Ki-67. A second light irradiation at 490 nm activates photosensitive molecule FITC to cause light inactivation of protein molecule Ki-67 causing cell damage [85]. Figure 5 illustrates this process.
Figure 5:
Schematic illustration of phototherapeutic cell elimination by targeting proliferation associated protein Ki- 67 with antibody constructs (TuBB-9-FITC) encapsulated in liposomal formulation of BPD followed by irradiation. (Reprinted with permission from Ref. [85]).
Another study showed compete eradication of tumors when long-circulating liposomes comprising of Ce6:DLPC (dilauroylphosphatidylcholine):DOPE:DSPE-N-PEG-2000 in a molar ratio 23:46:46:10 were used for PDT in human gastric cancer cell lines [86]. Further, when 2,3-dihydro-5,15-di(3,5-dihydroxyphenyl)porphyrin (SIM01), a new diphenylchlorin PS encapsulated in DMPC based liposomes, were studied in vivo in HT29 human adenocarcinoma mice xenografts, it showed significant tissue uptake and phototoxicity of SIM01 leading to significant cellular destruction [87].
Yuan et al. reported reduction of phototoxicity of Ce6 by loading it in indocyanine green (ICG) loaded liposomes. The liposomes (soya lecithin: CHOL in mass ratio 7:3) loaded with Ce6 and ICG were tested for photothermal effects by irradiating the liposomes with 808 nm at 1W/cm2. The increase in temperature measured with temperature probe showed photothermal efficacy of the liposomes. In vitro studies conducted by incubating MCF-7 cells with liposomes and irradiation showed effective recovery of phototoxicity of Ce6 when released by phototriggerable ICG-liposomes [88].
3.4.2. Phthalocyanines
Phthalocyanines (Pc) are second generation PS with high absorption of red light at 650–680 nm. Their application in PDT has attracted considerable interest [89]. The behavior of Pc depends on the identity of the central metal ion. Zinc (II) phthalocyanine (ZnPC) and Aluminum (III) phthalocyanine chloride (AlPC) are the most prominent ones among the metal-PC that have been studied for PDT [90]. ZnPC is a popular second generation sensitizer and has favorable spectral properties relative to Photofrin® (longer absorption wavelength and higher extinction coefficient) for deeper light activation in tissue [80]. ZnPc is hydrophobic and insoluble in water, so it can be administered in formulations including liposomes [84, 85]. Substantial research has focused on the development of liposomal formulations of this PS, including development of the CGP55847 liposomal formulation as described above. In another study, interstitially targeted PEG-coated liposomes loaded with ZnPC were used to treat human extrahepatic cholangiocarcinoma (Sk-Cha1) cells. This liposomal formulation consists of DPPC, CHOL and DSPE-PEG (66: 30: 4 mass ratio). It was observed that maximum ROS generation and oxidation potential was obtained when ZnPC was loaded as ZnPC:lipid in molar ratio of 0.003. This study also showed that PDT-induced apoptosis and necrosis depends on size, zeta potential and uptake of the liposomal PS [89]. In a recent study, Machacek et al. reported preparation of PC-liposomes by mixing PC with cargo-loaded pre-formed liposomes. The formulations egg PC:CHOL (45:55 in molar ratio) and DOPC:CHOL (60:40 in molar ratio) were loaded with Basic Orange or Doxorubicin such that the final concentration of the cargo was 1.2 mg/mL. Then, the cargo-loaded liposomes were mixed with stock solutions of a newly synthesized amphiphilic cationic PC in loading ratio of 1:1 to 500:1. This study showed that the rate of light triggered release from the liposomes changed with change in the amount of PS added to the liposomes [91].
3.4.3. Bodipy
Bodipy or 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene possesses several interesting photophysical properties such as high extinction coefficients, photobleaching resistance and environmental insensitivity. Although it has been widely used as a fluorescence imaging probe, it also has potential as a PDT agent [92]. Chen et al. reported a liposomal formulation of a dimeric Bodipy (di-BDP) conjugated with anti-HIF antibodies for treatment and imaging of hypoxic tumors. In this study, an orthogonal BDP dimer (Ab-DiBDP NPs) was designed by the substitution of a nitro group at the meso position. In vitro and in vivo studies showed that this dimer with higher production of singlet oxygen encapsulated in liposomes (DOPE, CHEMS, DSPE-mPEG2000 and DiBDP with a molar ratio of 50:50:0.5:0.5) were capable of selective destruction of cancer cells using NTR controllable phototoxicity and fluorescence and dual hypoxia marker imaging. The main advantage of this nanosystem system was the Cy 7-marked anti-HIF-1a antibody and the NTR-activatable DiBDP that allowed better hypoxia marker imaging by dual detection of NTR and HIF-1α [93]. Another study showed PDT using liposomes loaded with newly synthesized Bodipy sensitizers. They reported the photophysical properties of the four new Bodipy sensitizers like photobleaching, singlet oxygen generation and triplet quantum yield. They also showed that PDT using Egg PC and DOPE liposomes with Bodipy compounds resulted in significant PDT cell death in vitro in studies done on human ovarian cancer cells [56].
3.4.4. Rose Bengal
Rose Bengal is a hydrophilic xanthene stain used widely in the diagnosis of eye-disorders. It is also a potent PS owing to its ability to absorb energy from visible light and generate long-lived radicals. However, its low lipid soluble nature restricts it from crossing biological membranes and accumulation in tumors. This limits its usage as a potent PDT agent. To improve this, liposomes encapsulated with the PS have been studied. A study showed that the photophysical properties of the PS showed improvement when the PS was encapsulated in DMPC-based liposomes. It is assumed that the enhanced spectral properties could be due to the ability of phospholipids in liposomes to establish an equilibrium between the monomeric and dimeric forms of the PS [94].
3.5. Vessel targeted liposomes
Since solid tumors are highly dependent on angiogenesis for growth and metastasis, destruction of tumor neovasculature by vascular targeted PDT is a compelling targeted approach for cancer therapy [95]. Vascular targeted PDT has been the subject of significant research as the efficacy of PDT can be partly driven by the destruction of blood vessels as is the case with Visudyne for AMD [96, 97] and more recently Tookad for prostate cancer [98]. While these approaches work by conducting light treatment while the PS is circulating in the blood stream, approaches with vascular targeting liposomes have the potential to produce more effective treatments by increasing the PS concentration at the target vascular site. Doddapaneni et. al. demonstrated the ability of gambogic acid loaded PEGylated liposomes containing DOTAP to target and suppress tumor growth [99]. BPD-MA in cationic liposomes can target vessels for effective PDT. The ability to apply this approach to PS was demonstrated by Luo et. al. who prepared porphyrin phospholipid (PoP) liposomes containing DOTAP loaded with and without doxorubicin encapsulation. The results demonstrated the ability of DOTAP to enhance the efficacy of the liposomes by increasing the binding of the liposomes to the tumor vasculature [100].
Oku’s group has carried out pioneering work on this topic and has put forth multiple ways to improve liposomal delivery of BPD-MA for enhanced anti-angiogenic effect. [101]. The basic formulation included DPPC, POPC, CHOL, DPPG and BPD-MA in molar ratio 10:10:10:2.5:0.3 [97]. The first method involved incorporating PEG-distearoylphosphatidylethanolamine to the formulation to prepare stealth liposomes of verteporfin. The second method involved conjugating a peptide specific for angiogenic endothelial cells to the liposomes. The peptide used was Ala-Pro-Arg-Pro-Gly (APRPG). These liposomes comprised of the same basic formulation with or without PEG-DSPE/APRPG-PEG-DSPE such that lipids:PEG-DSPE/APRPG-PEG-DSPE 20:1 [102]. The third method involved incorporating cetylated polyethyleneamine to the formulation to prepare polycationic liposomes. These liposomes were composed of DPPC, CHOL, DPPG, cetyl-PEI and BPD-MA (20:10:5:1.75:0.3 in molar ratio) [103]. These liposomal formulations were tested at different PS doses, light doses and drug-light intervals. Liposomes prepared with the second [102] and third [57, 104] approaches showed lower uptake in tumor tissue but greater tumor shrinkage due to targeted accumulation and eradication of angiogenic vasculature [102, 103].
4. Liposomal PS Treatment Parameters and Performance
4.1. Biodistribution
PS biodistribution is an important parameter for both enhanced PDT efficacy and diminished side-effects, including sunlight toxicity. Higher propensity for a PS to accumulate avidly in target tissue results in superior PDT outcomes. Several mouse studies have shown an increase in the accumulation in tumors and major organs of PS was delivered by photoactivatable liposomes compared to the free PS. Yan et al. showed that internalized RGD modified liposomes loaded with ICG (iRGD-ICG-LP) allowed maximum accumulation of ICG in tumors as compared to ICG loaded in liposomes without iRGD and free ICG [105]. Oku’s group showed the amount of BPD-MA accumulated in tumor and major organs in mice xenografts when injected with BPD-MA liposomes and BPD-MA polycationic liposomes [103]. Reshetov et al. reported the biodistribution of mTHPC in tumors and major organs of HT29 cells bearing mice injected with Foslip and Fospeg at different time points post injection [53].
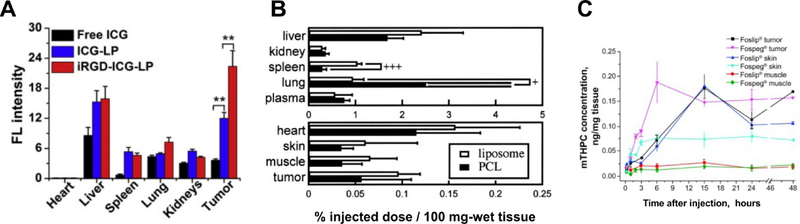
Figure 6 shows representative biodistribution of PS in tumors and major organs in mice xenografts after PDT with liposomal PS. For hydrophilic dyes such as ICG, liposomal formulations and targeted liposomal formulations can enhance uptake in neoplastic tissues (Figure 6A). Generally, most PS target tumors with limited accumulation compared to other organs such as the liver and spleen, and this is still generally the case with liposomal formulations (Figure 6B).
Figure 6: Representative biodistribution of liposomal PS in tumors and organs in mice:
A) Biodistribution of ICG in tumors and different organs of 4T1 mice injected with free ICG, ICG loaded liposomes (ICG-LP) or internalized RGD modified liposomes loaded with ICG (iRGD-ICG-LP), measured quantitatively with fluorescence intensity. It shows iRGD-ICG-LP accumulated the highest in tumors. B) Biodistribution of BPD-MA in tumors and major organs in Meth-A sarcoma mice xenografts injected with BPD-MA liposomes and BPD-MA ploycationic liposomes. C) & D) Biodistribution of mTHPC in tumors and major organs of HT29 cells bearing mice injected with Foslip and Fospeg. (Reprinted with permission from Ref. [105]; Ref. [103]; Ref. [53]).
Pharmacokinetics, which represent the kinetic aspect of biodistribution, are also important for PDT consideration. For example, since PDT is typically applied initially to cutaneous tumors where laser light is easily reached, biodistribution studies have assessed the ratio of PS in the skin to the tumor (Figure 6C). It has been shown that increasing PEGylation of Fospeg-like liposomes can increase blood circulation times while avoiding the skin toxicity compared to the photosensitizers without liposomal formulation [106].
4.2. Anti-tumor parameters
At the preclinical stage, strong anti-tumor efficacy should be demonstrated to warrant further investigation. Numerous studies have reported increase in therapeutic efficiency after PDT with liposomal PS. These studies were conducted with variations in PS dosage, light dosage and time intervals between drug injected and light irradiated or drug-light-interval (DLI). Table 2 shows several advanced formulations of liposomal PS with light doses that can be used for reference. The in vivo survival studies conducted on mice xenografts showed suppression of tumor growth after PDT with liposomal PS.
Table 2:
Representative effective preclinical anti-tumor PDT parameters for liposomal PS
| PS | Liposomal formulation | Tumor model | PS dose (mg/kg) | Light dose (J/cm2) | DLI (hr) | Ref |
|---|---|---|---|---|---|---|
| mTHPC (Foslip) | DPPC, DPPG | HT29 | 0.15 | 10 | 24 | [53] |
| mTHPC (Fospeg) | DPPC, DPPG and DSPE-PEG | HT29 | 0.15 | 10 | 24 | [53] |
| Zn-Pc (CGP55847) | POPC:DOPC | Meth A | 0.125 | 180 | 48 | [78] |
| BPD-MA | DPPC, POPC, CHOL, DPPG, PEG | Meth A | 0.5 | 150 | 3 | [102] |
| BPD-MA | DPPC, CHOL, DPPG | Meth A | 0.25 | 150 | 0.25 | [103] |
DLI: drug-light-interval
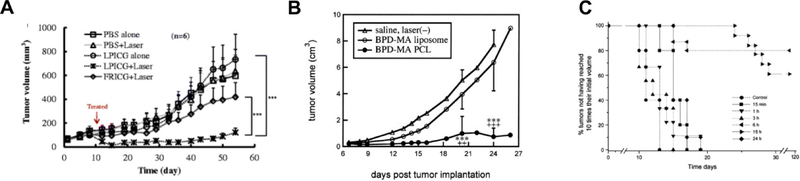
Formulating a PS in liposomes may or may not enhance anti-tumor efficacy for PDT compared to other formulation methods. But there is a likelihood that liposomal formulations will modulate behavior and interaction with cellular targets [107]. More hydrophilic dyes can have altered pharmacokinetic benefit from encapsulation owing to altered behavior in vivo. For example, liposomal ICG has been used for PDT and shown greater anti-tumor efficacy, compared to the free dye, in suppression of tumor growth in a breast cancer model (Figure 7A). [60]. In addition, the surface charge of liposomes is an attribute that can be varied readily by, for example, incorporating cationic lipids. This has been shown to result in superior anti-tumor efficacy in a METH-A model using liposomal ICG (Figure 7B) [103].
Figure 7: Suppression of tumor growth after PDT with different liposomal PS:
A) Tumor growth delay in mice with Meth-A sarcoma cells xenograft after PDT treatment with BPD-MA liposomes or BPD-MA polycationic liposomes (“PCL”) (0.25 mg/kg BPD-MA) and laser treatment at 689 nm at 150 J/cm2 15 mins post injection. Treatment with BPD-MA polycationic liposomes showed least tumor growth over time. B) MDA-MB-468 xenograft tumor growth delay after PDT with PBS alone, PBS plus laser, LPICG (liposomes loaded with ICG) alone, LPICG plus laser, and FRICG (Free ICG) plus laser and laser dose at 808 nm and 2 W/cm2 fluence level for desired groups after 6 h from injection. LPICG plus laser showed best therapeutic results. C) EMT6 tumor growth delay after PDT treatment with Foslip (0.3 mTHPC g/kg) and light dose of 10 J/cm2 at fluence rate of 30 mW/cm2 at different drug-light intervals. (Reprinted with permission from Ref. [103]; Ref. [60]; Ref. [108]).
Another parameter that can be considered for effective PDT is the drug-light-interval. After intravenous administration, the PS levels in blood decrease over time, and the PS levels in the tumor increase. A study using liposomal BDP-MA demonstrated that optimal anti-tumor efficacy came when the liposomal PS had moderate concentration in both the tumor and in plasma (Figure 7C) [108].
5. Liposomal formulations for photothermal therapy
Photothermal therapy (PTT), is a therapeutic approach in which a light-absorbing dye, PS or nanoparticle is used to convert light into heat for local ablation. This method has been previously studied using inorganic nanoparticles such as graphene, gold nanoparticles. Lovell et al. demonstrated the use of organic nanoparticles for photothermal therapy [109]. They reported that when porphysomes (42 mg/kg porphyrin phospholipid), made up of porphyrin phospholipids and CHOL (70:30 in molar ratio), were intravenously injected into mice xenografts bearing KB tumor cells, followed by laser treatment after 24 hours with 658 nm laser providing 750 mW at a power density of 1.9 W/cm2 for 1 min, the tumor temperature reached 60 °C whereas tumor temperature in mice injected with PBS only reached 40 °C. After two weeks, the mice that received both porphysomes and laser treatment demonstrated appreciable tumor growth suppression while tumors in other groups kept growing. Wu et al. reported a drug loaded liposomal formulation for photothermal therapy in which they fabricated liposome/SiO2/gold nanoshell nanocomposites loaded with anticancer drug doxorubicin. The liposomes were made with soybean lecithin and CHOL (30:2 mass ratio). In vitro studies with these liposomes followed by irradiation showed significant cancer cell destruction [110]. Luo et al. demonstrated the fabrication of photosensitive and pH-responsive nanocarriers with gold nanoshells coated oleanolic acid (OA) liposomes with chitosan for combined photothermal therapy and chemotherapy. The liposomal formulation comprised of soya lecithin, CHOL and oleanolic acid (50:60:5 in mass ratio). When these liposomes were administered followed by irradiation, the pH sensitive liposomes releasing OA at low pH tumor environment (pH 5.5) and gold nanoshells generating hyperthermia resulted in significant antitumor effects both in vitro and in vivo [111, 112]. Oh et al. demonstrated plasmonic liposomes loaded with ZnPc and coated with gold nanofilm for combinatorial photothermal therapy and PDT. ZnPc loaded liposomes (HSPC: ZnPc:PE-NH2 at a molar ratio of 69:2:6) were coated with gold ion solution to prepare plasmonic liposomes. In vitro studies conducted by incubating cells from mouse brain endothelial cell line, a human breast cancer cell line (MDA-MB-231) and a mouse colon cancer cell line (CT-26) with ZnPc-PL for 4 hours and then irradiating the cells at 660 nm for 5 min. showed enhanced anti-cancer efficacy as compared to PDT or PTT alone [65].
6. PS and drug co-delivery systems
Efficiency of chemotherapy treatments is restricted by limited tumoral uptake of anticancer drugs in bioavailable form [113–116]. To resolve this, drug delivery systems are used for selective delivery of bioavailable drugs at target sites [20, 116–118]. PDT has the capability to enhance drug delivery through vascular damage and in the case of drugs and PS co-delivery systems, release of entrapped cargo [119].
Liposomes can integrate PS and drugs simultaneously. Figure 8 shows various cryo TEM images of liposomes with PS encapsulated with drugs. Figure 8A and 8B show liposomal irinotecan and doxorubicin, respectively loaded inside the liposomes containing porphyrin-phospholipid (PoP) within the bilayer. Because PoP behaves like a lipid, formulating liposomes for chemotherapeutic drug loading can be simplified and several variants of PoP have recently been examined [120–123]. Conventional photosensitizers can also be used in liposomal drug formulations. Figure 8C shows a photoactivatable multi-inhibitor nanoliposome that induces light-induced cytotoxicity with photoinitiated release of inhibitors that suppress tumor regrowth and treatment escape signaling pathways [124]. This design consists of a liposome doped with a PS (BPD-MA) in the lipid bilayer, and a polymeric particle containing cabozantinib (XL184), a multikinase inhibitor, encapsulated inside.
Figure 8: Morphology of various PS and drug co-delivery systems:
A) Cryo TEM image of Porphyrin-phospholipid liposomes loaded with anticancer drug Irinotecan (Scale bars, 50 nm) B) Cryo TEM image of Porphyrin-phospholipid liposomes loaded with anticancer drug Doxorubicin (Scale bars, 50 nm) C) Photoactivatable multi-inhibitor nanoliposome (PMIL) containing BPD in bilayer and a multikinase inhibitor cabozantinib (XL184) loaded inside it. (Scale bars, 50 nm). D) Representative cryo transmission electron microscopy images of Dox-PoP liposomes before and after laser treatment. Arrows in the pre-laser treatment images indicate the presence of Doxorubicin-sulphate crystals inside the liposomes. The absence of the crystals in the post-laser treatment images indicate that the crystals dissolved upon irradiation and simultaneously the drug was released with minimal effect on the structure of the liposomes. (Scale bars, 100 nm). (Reprinted with permission from Ref. [125]; Ref. [124]; Ref. [126]).
Many liposomal formulations have been prepared for co-delivery of PS and drugs. Table 3 shows representative formulations of liposomal PS and drug co-delivery systems. In 2013, it was shown that spatially controlled light-triggered release of encapsulated gentamicin and temporal controlled light-triggered release of encapsulated fluorophores could be induced by the inclusion of PoP in liposomes. The formulation developed for that study was DSPC:CHOL:HPPH PoP:DSPE-PEG-2K (50:35:10:5 in molar ratio). This study showed effective single treatment anticancer treatment by light-triggered release of entrapped doxorubicin from liposomes [126].
Table 3:
Representative cargo-loaded liposomal PS
| PS | Cargo | Liposomal formulation | Lipid ratio (mol.) | PS ratio | Ref. |
|---|---|---|---|---|---|
| PoP | Doxorubicin | DSPC: DSPE-PEG-2K: CHOL | 53:5:40 | 2 mol.% | [127] |
| PoP | Doxorubicin | DSPC: DSPE-PEG-2K: DOPC: CHOL | 53:5:5:40 | 0.3 mol.% | [128] |
| PoP | Irinotecan | SPM:CHOL | 53:45 | 2 mol.% | [125] |
| PoP | Doxorubicin | DSPC: DSPE-PEG-2K: CHOL | 53:5:40 | 2 mol.% | [129] |
| PoP | Doxorubicin | DSPC: DSPE-PEG-2K:CHOL | 53:5:4 | 2 mol.% | [130] |
| HPPH lipid | Doxorubicin | DSPC:CHOL:HPPH:DSPE-PEG-2K | 50:35:10:5 | 10 mol.% | [126] |
| PoP | Doxorubicin | DSPC:DOTAP:CHOL | 38:20:40 | 2 mol.% | [100] |
| PdPC(OBu)8 | Tetrodotoxin | DSPC:DLPC:DSPG:CHOL | 3:3:2:3 | Post-loading 0.45 mol.% | [131] |
| ICG | Calcein [132], Calcein and FITC dextran [133] | DPPC:DSPC:Lyso PC: DSPE-PEG | 75:15:10:4 | Post-loading 0.2 umol. | [132, 133] |
| hCe6 | AQ4N | DPPC: CHOL: DSPE-mPEG5k | 6:4:0.5 | 0.5 mol.% | [134] |
| hCe6 | Metformin | DPPC: CHOL: DSPE-mPEG5k: | 6:4:0.5 | 0.5 mol.% | [135] |
Luo et al. built upon this work and reported the development of stealth liposomes known as LC-Dox-PoP liposomes for long circulation in blood. This study showed that drug-loading, light triggered drug release and serum stability is affected by the type of PoP used. It showed that though incorporation of PEG and CHOL was essential for Doxorubicin entrapment in liposomes, it reduced the speed of light-triggered release from liposomes. The stealth Dox-PoP liposomes not only showed 21.9 h long circulation half-life of stealth liposomes but also showed long storage stability. This liposomal formulation (DSPC:DSPE-PEG-2K:PoP:Chol in molar ratio 53:5:2:40) encapsulated with Doxorubicin when intravenously injected into mice subcutaneous pancreatic xenografts at dose of 5–7 mg/kg Dox followed by NIR irradiation, showed complete eradication of tumors whereas other photodynamic and chemotherapies were ineffective. This was made possible by high Dox accumulation (~7 fold) in tumor post irradiation [127].
Another study showed that when these liposomes were intravenously injected (2 mg/kg Dox) into mice bearing subcutaneous human pancreatic xenografts, shorter drug-light intervals (0.5–3h) enabled higher Dox deposition in tumor and better treatment results [130]. When these liposomes were used to study pharmacokinetics and pharmacodynamics modeling of chemophototherapy of liposomal PS administered and irradiated with short drug-light intervals in patient-derived pancreatic cancer xenografts, there was about 7.4-fold increase in Dox accumulation which matched the amount of Dox (7 fold) obtained through modeling [136].
It has been shown that inclusion of an unsaturated phospholipid (DOPC) in small amount (5 mol.%) can significantly accelerate light-triggered release of Dox from Dox-PoP liposomes. When intravenously injected (6 mg/kg Dox) into mice with subcutaneous human pancreatic xenografts followed by NIR irradiation showed considerably delayed growth in tumors [128]. Another anticancer drug Irinotecan (IRT) have also been encapsulated in photoactivatable liposomes. Three formulations of IRT-PoP liposomes that were studied were SPM:pyro-lipid:chol (53:45:2 in molar ratio), SPM:DSPE-PEG2000:pyro-lipid:chol (48:5:2:45 in molar ratio) and DSPC:DSPE- PEG2000:pyrolipid:chol (48:5:2:45 in molar ratio). This demonstrated that incorporation of Sphingomyelin into IRT-PoP liposomes enabled rapid light-triggered IRT release and enhanced serum stability as compared to the other formulations [125]. Rwei et al. reported a liposomal formulation of a PS for sciatic nerve block. The liposomal formulation was DSPC: DLPC: DSPG: CHOL (3:3:2:3 in molar ratio). PS 1,4,8,11,15,18,22,25-octabutoxyphthalocyaninato-palladium(II), PdPC(OBu)8 was encapsulated in the bilayer of the liposomes (0.45 mol.% to total lipid in liposomes). Later, this liposomal PS formulation was loaded with local anesthesia drug Tetrodotoxin When Adult male Sprague-Dawley rats were administered with the liposomes and irradiated at 730 nm, it showed a nerve block of up to 13.5 +− 3.1 hours [131]. Lajunen et al. suggested indocyanine green (ICG) loaded liposomes for controlled ocular drug delivery. The liposomal preparation was DPPC:DSPC:LysoPC:DSPE-PEG (75:15:10:4 in molar ratio). Later, the liposomes were mixed with calcein solution (60 mM, 280 mOsm, pH 7.4) and 0.2 umol ICG was loaded in the liposomes. In vitro studies conducted by incubating ARPE-19 cells with 1.5 mM of ICG-liposomes for 3 hours and then irradiating the cells with light (9.7 W / cm2) for 2 minutes at 37 °C showed controlled release of liposomal contents in the cells. Release of calcein from liposomes in cells was determined by increase in fluorescence of the cells measured by flow cytometry [132]. In another study, Lajunen et al. reported that the same liposomal formulation of ICG (DPPC:DSPC:LysoPC:DSPE-PEG in molar ratio of 75:15:10:4) loaded with calcein and FITC dextran was stable at temperatures 4 – 22 °C whereas laser irradiation at body temperature led to complete release of the cargo [133].
In another approach to induce light-triggered drug release from liposomes, Sine et al. developed the concept of pocket liposomes [137]. Enhanced fluidity in certain regions of liposomal membranes allow triggered release, using HPPH as the PDT PS and calcein as a marker for drug release. They entrapped calcein and/ HPPH in the liposomal formulation- DPPC :DC8,9PC:DiR in different molar ratios (86:10:0 or 86:10:0.5). Laser irradiation showed release of calcein only from the liposomes with HPPH. When these liposomes were used for in vitro PDT with MDA-MB-231-LM2 breast cancer cell line, significant cell damage was induced and likewise, tumor shrinkage was observed in in vivo studies with the same cell line in mice xenografts
7. Emerging applications of liposomal PS
7.1. Image-guided interventions
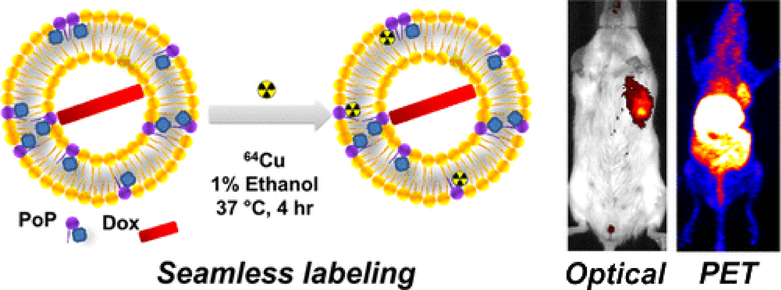
One interesting capability of liposomal formulations of PDT agents is the imaging of the formulation in tissues following injection. Several studies have shown the success of this application. One such study showed that when long circulating liposomes (DSPC:DSPE-PEG-2K:PoP:Chol in molar ratio 53:5:2:40) encapsulated with anticancer drug doxorubicin, labeled with 64Cu in the bilayer were intravenously injected into mice xenografts, it not only enabled fluorescence and positron emission tomography (PET) imaging of orthotopic mammary tumors, but also showed significant tumor growth shrinkage with single treatment with light treatment of the tumors [129].This is shown in Figure 9.
Figure 9:
Seamless radiolabeling of 64Cu on Dox-PoP liposomes with 1% Ethanol at 37 °C for 4 hours. The second figure shows images of mice tumor xenografts treated with 64Cu radiolabeled Dox-PoP liposomes and irradiation. The images were obtained using near-infrared fluorescence imaging and positron emission tomography. (Reprinted with permission from Ref. [129]).
Other demonstrations of liposomes for enabling imaging and PS delivery have been presented. The aqueous core of liposomes were loaded with an X-ray contrast agent, iodixanol along with a hydrophilic photosensitizer, meso-tetrakis (4-sulphonatophenyl) porphine (TPPS4). These liposomes were used for computed tomography, fluorescence imaging and PDT [138]. Notably, co-encapsulation of the iodinated contrast agent resulted in an increase in singlet oxygen generation by PS via the intraparticle heavy-atom (iodine) effect. Other liposomal PS formulations have involved co-loading of gadolinium-based contrast agents for magnetic resonance contrast for PDT [139] and PTT [140] applications.
7.2. Overcoming Hypoxia
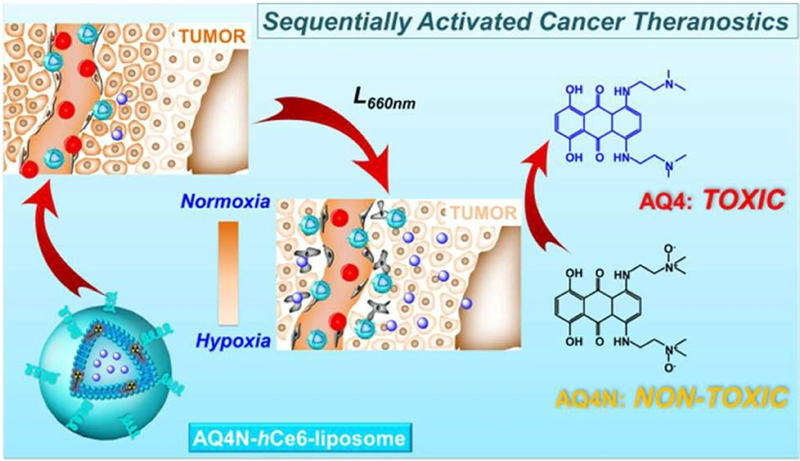
The therapeutic efficacy of PDT is restricted in hypoxic areas of tumors owing to PDT dependency on oxygen availability. To resolve this, new strategies have been explored. Song et al. reported a study on using metformin loaded liposomes to overcome hypoxia in PDT. Metformin (Met), an oral hypoglycemic agent used in the treatment of type II diabetes, is known to enhance tumor oxygenation by decreasing oxygen consumption in tumors. PDT was conducted with liposomes (DPPC: CHOL: DSPE-PEG5K: Ce6 at a molar ratio of 6:4:0.5:0.5) entrapped with hydrophilic metformin in the inner core which would be continuously released from the liposomes increasing the tumor oxygen levels. In vivo photoacoustic imaging and ex vivo immunofluorescence staining showed enhanced tumor oxygenation leading to significant tumor cell killing [135]. Another study demonstrated improved anti-tumor efficacy by PDT with liposomes encapsulated with a prodrug AQ4N that contributes to cell killing only in hypoxic environment. PDT with liposomes (DPPC: CHOL: DSPE-mPEG5k: hCe6 at a molar ratio of 6:4:0.5:0.5) loaded with AQ4N upon irradiation at 660 nm showed enhanced cancer therapy owing to dual effect of PDT along with activation of AQ4N due to PDT induced hypoxia. Figure 10 shows this. Further, in vivo positron emission tomography, in vivo fluorescence and photoacoustic imaging of liposomal uptake post intravenous administration was made possible by radiolabeling AQ4N-64Cu-hCe6-liposomes (prepared by conjugating 64Cu with Ce6 before liposome preparation) [134].
Figure 10:
Schematic illustration of enhanced cancer therapy owing to dual effect of PDT along with activation of prodrug AQ4N, selectively delivered to site by AQ4N-hCe6-liposomes, due to PDT induced hypoxia. Reprinted with permission from Ref. [134].
In another study, Song et al. demonstrated a tumor oxygenation strategy using perfluorocarbon nanodroplets, which have capability for oxygen dissolution [141]. When a nanoperflurocarbon emulsion, stabilized using albumin, was intravenously injected into mice bearing tumor xenografts under hyperoxic breathing, ultrasound transducers applied on tumor stimulated nanoperflurocarbon droplets to absorb oxygen in lungs, release it in tumor and circulate back into lungs for reoxygenation. These repeated cycles provided enough oxygen in tumor for enhanced radiotherapy and PDT. When mice bearing 4T1 tumors were treated with photosensitive liposomes made of DPPC, CHOL, DSPE-PEG (5k) and hydrophobic Ce6 (6:4:0.5:0.5 in molar ratio) followed by intravenous injection of nanoperflurocarbon emulsion along with hyperoxic breathing conditions and tumor focused irradiation, remarkably improved tumor inhibition was observed.
7.3. Activatable liposomal PS
To decrease non-specific phototoxicity, activatable PS have been developed which can be “turned on” by factors found in the tumor microenvironment, such as proteases or lower pH [142]. This approach has also been adopted by nanoparticle PS formulations. Feng et al. developed a nano drug delivery system by encapsulating liposomes with a PS, Ce6 conjugated with hexylamine (hCe6) along with a hydrophobic near-infrared (NIR) dye 1,1’-dioctadecyl-3,3,30,3’-tetramethylindotricarbocyanine iodide (DiR) that can quench the photosensitizing effect of the PS by fluorescence resonance energy transfer (FRET). When liposomes (DPPC:CHOL:DSPE-mPEG5k:hCe6:DiR at a molar ratio of 6:4:0.5:0.5:0.5) were intravenously injected into mice followed by NIR irradiation at 785 nm, it activates the photodynamic and fluorescence effect of hCe6 that results in increasing the temperature of the tissue causing photothermal effect which increases the blood flow and prevents tumor hypoxia. Further, this procedure reduces the phototoxicity of skin while the PS remained quenched by the dye, thus ensuring lesser photo-damage while causing enhanced therapeutic results [143].
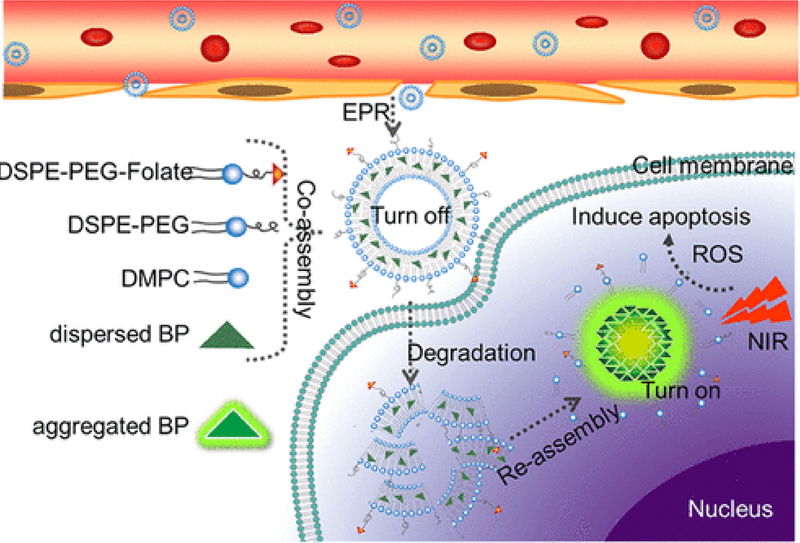
Yang et al. demonstrated PDT using liposomes loaded with aggregation induced emission PS or (AIE) PS. [144]. In this approach, when AIE-PS entrapped in lipid bilayer of liposomes are injected, phototoxicity to healthy tissue due to photosensitive AIE-PS is reduced to the photosensitizer being inactivated within the liposomes. When these AIE-PS-liposomes accumulate in tumor, the liposomes disintegrate releasing the AIE-PS which assemble into aggregated allowing them to produce ROS upon NIR irradiation leading to cell death [145]. Figure 11 demonstrates this approach.
Figure 11:
Schematic diagram showing co-assembly of aggregation induced emission PS (AIE-PS) entrapped in liposomes for antitumor effect using PDT. Reprinted with permission from Ref. [145].
Liposomal approaches for activatable PS can gain insights from other strategies. This includes approaches that activate the nanoparticle upon targeting to molecular targets [146]. Also, tumor microenvironment factors such as pH can be exploited. For instance, pH sensitive CaCO3-polydopamine hollow nanoparticles loaded with Ce6 were used for multifunctional theranostic applications with pH dependent activation [147]. Likewise, PEG modified CaCO3 nanoparticles loaded with doxorubicin and a Mn-chelated Ce6 (Ce6(Mn)) was also used for activation in the reduced pH of tumor environments with real time monitoring of drug release by MR and fluorescence imaging imaging [148].
7.4. Upconversion Liposomal PS
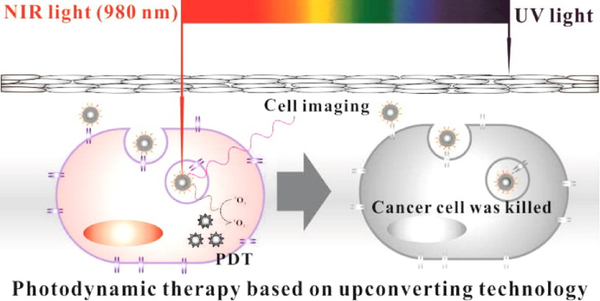
Poor tissue penetration of light used to activate PS for the treatment is key limitations to the application of PDT. As a result, PDT remains almost ineffective in treating large tumors. To improve this, new strategies are being explored for better therapeutic results. One such strategy is using upconversion nanoparticles (UCNPs) which absorbs NIR light and emits high energy visible light. These high energy photons, in turn, activate the PS molecules in the adjoining areas resulting in enhanced tumor tissue killing via PDT [149]. Figure 12 illustrates this approach. A study showed encapsulation of UCNPs and a PS MC540 into liposomes made with octadecyl-quaternized lysine-modified chitosan or OQLCS, TAT-OQLCS or transmembrane peptide TAT-grafted OQLCS, and FAOQLCS or targeting molecule folate acid grafted OQLCS (weight ratio 1:1:1) to produce a functionalized liposomal nanocarrier (FPL) [150]. This combination of liposomal nanocarrier showed several advantages over UCNPs and MC540 used without it. In vitro studies showed significant improvement in tumor cell killing owing to better targeting with folate liposomes as well as the combined effect of UCNPs and MC540.
Figure 12:
Schematic illustration of cancer therapy by PDT using upconversion technology. Reprinted with permission from Ref. [150].
Another study showed usage of liposomes encapsulated with triplet-triplet annihilation upconversion (TTA-UC) molecules with ruthenium(II) polypyridyl compounds as PS. TTA-UC is a phenomenon that involves absorption of low energy photons by a sensitizer to reach a triplet state and transfers the energy by triplet-triplet energy transfer(TTET) to an annihilator molecule, which in turn, combines with another annihilator by TTA or triplet-triplet annihilation, thus promoting one molecule to a singlet excited state and the other one to ground state. This singlet excited annihilator emits high energy photon to come back to ground state thus causing upconversion. The TTA-UC couples used contained sensitizers: platinum octaethylporphyrin and palladium tetraphenyltetrabenzoporphyrin and annihilators: 9,10-diphenylanthracene; and perylene. When upconversion liposomes (made with DMPC, DSPE-MPEG-2000, one of the sensitizers and/or one of the annihilators) was mixed with ruthenium functionalized liposomes and irradiated, the energy transfer in TTA-UC was capable of triggering hydrolysis of Ru-S bond in the Ruthenium functionalized liposomes [151].
7.5. Antibacterial liposomal PS
Antibacterial therapy of infections caused by bacteria resistant to antibiotics has been a challenge with increasing incidence of antibiotic resistant bacteria. To overcome this, approaches have been developed for antibacterial PDT [152]. Some have used liposomal PS. Park et al. reported PDT of acne by treating antibiotic resistant Propionibacterium acnes (P. acnes) using photoactivatable transfersomes containing a DSPE-PEG-Pheo A conjugate (DPP). DPP transfersomes were prepared from DPP conjugates, CHOL and TWEEN-80. Both in vitro and in vivo studies using DPP transfersomes with irradiation showed enhanced treatment efficiency. In vitro studies showed loss in P. acnes viability and in vivo studies showed reduced swelling and thickness of nude mice skin infected by P. acnes [153]. In another study, Zhao et al. demonstrated an antibiotic therapeutic method for the treatment of bacterial biofilm using photoactivatable thermosensitive liposomes. When liposomal formulations containing DSPC and cypate to Betainylated Cholesterol (BC) in three different molar ratios 4:0.5:1, 4:0.5:2, and 4:0.5:3, loaded with antibiotic Tobramycin were used for in vitro and in vivo studies, it showed very effective treatment of Pseudomonas aeruginosa biofilm, 7 to 8 fold biofilm dispersion in in vitro studies and rapid healing in mice infection in in vivo studies as compared to treatment with antibiotic alone [154]. Also, Jeong et al. reported lipase-sensitive singlet oxygen-producible and erythromycin-loaded liposomes (DPPC: CHOL: Erythromycin in mass ratio 10:1.5:1 coated with pullulanpheophorbide or PU-Pheo A conjugates) for combinatorial antibacterial and PDT for skin disorder caused by Propionibacterium acnes (P. acnes). The extracellular lipases secreted by P. acnes was reported to disrupt lipase sensitive ester linkages in the liposomes releasing the antibiotic and the irradiation onto Pheo-A further enabled combinatorial treatment method of nude mice skin disorders caused by P. acnes [155]. Another study showed using photodynamic antibacterial therapy with perfluorohexane (PFH; known for oxygen dissolving capacity) - IR780 liposomes for the treatment of bacterial infections on the surface of Ti implants. In this study when PFH - IR780 liposomes (lecithin: CHOL: DSPE-PEG-2000:IR780 in mass ratio 24.7:4.3:3.8:0.2 and PFH later added to it) prepared on Ti plates with irradiation were used for in vitro and in vivo studies, it showed significant inhibition of bacterial growth as compared to the groups without PFH. In vitro studies showed over 99% killing of bacterial growth on implants caused by Escherichia coli and Staphylococcus aureus with PFA and in vivo studies showed much improved treatment results in rats with PFH - IR780 liposomes on Ti implants with bacterial infection [156].
7.6. Immunotherapy
PDT holds promise for immunotherapy [157]. Cancer immunotherapy using liposomal PS has been considered. Kim et al demonstrated a treatment for cholangiocarcinoma using immunotherapy enabled by photosensitive liposomes loaded with Gemcitabine. The liposomal formulation was DPPC:CHOL:DOPE (1,2-dioleoyl-sn-glycero- 3-phosphoethanolamine): CHEMS (cholesteryl hemisuccinate): DPP (5:1.3:5:1.6:5.5 in mass ratio) where DPP was synthesized before such that the molar ratio of DSPE-PEG-amine: PheoA: NHS(N-hydroxysuccinimide): DCC (1,3-Dicyclohexyl carbodiimide) was 1: 1.2: 1.8: 1.8. Both in vitro and in vivo studies using these liposomes loaded with Gemcitabine (GDPPL) followed by irradiation showed improved treatment results. In vitro studies using human liver bile duct carcinoma cell line (HuCCT-1) treated with GDPPL followed by irradiation showed better cell killing behavior and in vivo studies using HuCCT-1 tumor-bearing xenograft mice model showed 3-fold antitumoral efficiency compared to untreated group. Further, immunohistochemical analysis on Balb/c mice treated with GDPPL with irradiation showed rapid production of immune cells for enhanced antitumoral immunotherapy [158]. In another study, Yoon et al. reported a cancer therapeutic method combining effects of chemotherapy and hyperthermia using photothermally amplified liposomes. The liposomal ICG formulation was prepared from DPPC, DSPC-mPEG (2000), and ICG at a molar ratio of 950:50:16. These liposomes loaded with chemotherapy drug cisplatin with laser irradiation showed enhanced therapeutic results over chemotherapy and photothermal therapy alone in both in vitro studies conducted on HeLa (human cervical cancer cell line) and 4T1 (murine breast cancer cell line) cancer cells and in vivo studies on 4T1 cells Balb/c mice xenografts [159].
8. Conclusion
The formulation and delivery of PS is a major consideration for PDT and PTT compounds. Liposomes represent a clinically-validated and versatile platform that should be considered for such purposes. Additionally, more complex and advanced therapeutic PDT designs using photoactivatable PS, chemophototherapy, or integrated imaging guidance are suited for liposomal formulations. The ability of liposomes to encapsulate PS in their hydrophobic bilayer or aqueous core is a relatively simple approach. Liposomal formulations of PS can be modulated to prolong circulation half-life and decrease the PS accumulation in skin. Early works on liposomal formulations used phospholipids from egg and soy, whereas more recently, synthetic lipids are being used more frequently. The use of CHOL can improve liposome stability, and PEGylated liposomes enable longer blood circulation.
Visudyne®, a formulation of BPD-MA, has been widely used for PDT treatment of AMD and was the first clinically-approved liposomal PS formulation. Considering the translation of this successful formulation, it is likely that liposomal formulations will be considered for new PS that enter clinical testing. However, since the approval of Photofrin® in the United States, few if any new PS have gained regulatory approval for phototreatment of solid tumors. Therefore, PDT in general must overcome challenges through innovation and by showing advantages to alternative and more established ablative modalities treatments such as radiofrequency and microwave ablation. Demonstration of preclinical efficacy and safety, as well as demonstration of enabling new therapeutic paradigms will drive future PS research, and liposomal formulations are likely to be considered for these applications.
9. Acknowledgement
This work was supported by the National Institutes of Health (R01EB017270, DP5OD017898) and the National Science Foundation (1555220),
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
10. References
- [1].Brown SB, Brown EA, Walker I, The present and future role of photodynamic therapy in cancer treatment, Lancet Oncol. 5(8) (2004) 497–508. [DOI] [PubMed] [Google Scholar]
- [2].Henderson BW, Dougherty TJJP, photobiology, How does photodynamic therapy work?, Photochem. Photobiol. 55(1) (1992) 145–157. [DOI] [PubMed] [Google Scholar]
- [3].Gomer CJ, Razum NJJP, photobiology, Acute skin response in albino mice following porphyrin photosensitization under oxic and anoxic conditions, Photochem. Photobiol 40(4) (1984) 435–439. [DOI] [PubMed] [Google Scholar]
- [4].Kessel D, Oleinick NL, Cell Death Pathways Associated with Photodynamic Therapy: An Update, Photochem. Photobiol 94(2) (2018) 213–218. [DOI] [PubMed] [Google Scholar]
- [5].Bechet D, Couleaud P, Frochot C, Viriot M-L, Guillemin F, Barberi-Heyob M, Nanoparticles as vehicles for delivery of photodynamic therapy agents, Trends Biotechnol. 26(11) (2008) 612–621. [DOI] [PubMed] [Google Scholar]
- [6].Skupin-Mrugalska P, Piskorz J, Goslinski T, Mielcarek J, Konopka K, Düzgüneş N, Current status of liposomal porphyrinoid photosensitizers, Drug Discov. Today 18(15–16) (2013) 776–784. [DOI] [PubMed] [Google Scholar]
- [7].Vemuri S, Rhodes C, Preparation and characterization of liposomes as therapeutic delivery systems: a review, Pharm. Acta Helv 70(2) (1995) 95–111. [DOI] [PubMed] [Google Scholar]
- [8].Derycke AS, de Witte PA, Liposomes for photodynamic therapy, Adv. Drug Del. Rev 56(1) (2004) 17–30. [DOI] [PubMed] [Google Scholar]
- [9].Drummond DC, Meyer O, Hong K, Kirpotin DB, Papahadjopoulos D, Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors, Pharmacol. Rev 51(4) (1999) 691–744. [PubMed] [Google Scholar]
- [10].Samad A, Sultana Y, Aqil M, Liposomal drug delivery systems: an update review, Curr. Drug Del 4(4) (2007) 297–305. [DOI] [PubMed] [Google Scholar]
- [11].Berlanda J, Kiesslich T, Engelhardt V, Krammer B, Plaetzer K, Comparative in vitro study on the characteristics of different photosensitizers employed in PDT, J. Photochem. Photobiol. B: Biol 100(3) (2010) 173–180. [DOI] [PubMed] [Google Scholar]
- [12].Bellnier DA, Greco WR, Loewen GM, Nava H, Oseroff AR, Pandey RK, Tsuchida T, Dougherty TJ, Population pharmacokinetics of the photodynamic therapy agent 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a in cancer patients, Cancer Res. 63(8) (2003) 1806–13. [PubMed] [Google Scholar]
- [13].Obaid G, Broekgaarden M, Bulin A-L, Huang H-C, Kuriakose J, Liu J, Hasan T, Photonanomedicine: a convergence of photodynamic therapy and nanotechnology, Nanoscale 8(25) (2016) 12471–12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jin CS, Zheng G, Liposomal nanostructures for photosensitizer delivery, Lasers Surg. Med 43(7) (2011) 734–48. [DOI] [PubMed] [Google Scholar]
- [15].Chen B, Pogue BW, Hasan T, Liposomal delivery of photosensitising agents, Expert Opin. Drug Deliv 2(3) (2005) 477–487. [DOI] [PubMed] [Google Scholar]
- [16].Jori G, Tomio L, Reddi E, Rossi E, Corti L, Zorat PL, Calzavara F, Preferential delivery of liposome-incorporated porphyrins to neoplastic cells in tumour-bearing rats, Br. J. Cancer 48(2) (1983) 307–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jiang F, Lilge L, Grenier J, Li Y, Wilson MD, Chopp M, Photodynamic therapy of U87 human glioma in nude rat using liposome-delivered photofrin, Lasers Surg. Med 22(2) (1998) 74–80. [DOI] [PubMed] [Google Scholar]
- [18].Keene J, Kessel D, Land EJ, Redmond R, Truscott T, Direct detection of singlet oxygen sensitized by haematoporphyrin and related compounds, Photochem. Photobiol 43(2) (1986) 117–120. [DOI] [PubMed] [Google Scholar]
- [19].Wang ZJ, He YY, Huang CG, Huang JS, Huang YC, An JY, Gu Y, Jiang LJ, Pharmacokinetics, tissue distribution and photodynamic therapy efficacy of liposomal-delivered hypocrellin A, a potential photosensitizer for tumor therapy, Photochem. Photobiol 70(5) (1999) 773–780. [PubMed] [Google Scholar]
- [20].Allen TM, Cullis PR, Drug delivery systems: entering the mainstream, Science 303(5665) (2004) 1818–1822. [DOI] [PubMed] [Google Scholar]
- [21].Torchilin VP, Recent advances with liposomes as pharmaceutical carriers, Nat. Rev. Drug Discov 4(2) (2005)145. [DOI] [PubMed] [Google Scholar]
- [22].Allen TM, Long-circulating (sterically stabilized) liposomes for targeted drug delivery, Trends Pharmacol. Sci 15(7) (1994) 215–220. [DOI] [PubMed] [Google Scholar]
- [23].Gomez-Hens A, Fernandez-Romero J, Analytical methods for the control of liposomal delivery systems, TrAC, Trends Anal. Chem 25(2) (2006) 167–178. [Google Scholar]
- [24].Dua J, Rana A, Bhandari A, Liposome: methods of preparation and applications, Int J Pharm Stud Res 3(2) (2012)14–20. [Google Scholar]
- [25].Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K, Liposome: classification, preparation, and applications, Nanoscale Res. Lett 8(1) (2013) 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Patil YP, Jadhav S, Novel methods for liposome preparation, Chem. Phys. Lipids 177 (2014) 8–18. [DOI] [PubMed] [Google Scholar]
- [27].Maeda H, Wu J, Sawa T, Matsumura Y, Hori K, Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review, J. Controlled Release 65(1) (2000) 271–284. [DOI] [PubMed] [Google Scholar]
- [28].Jain RK, Stylianopoulos T, Delivering nanomedicine to solid tumors, Nature Reviews Clinical Oncology 7 (2010) 653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Allen TM, Hansen CB, Stuart DD, Targeted sterically stabilized liposomal drug delivery, Medical applications of liposomes (1998) 297–323. [Google Scholar]
- [30].Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, Marks JD, Benz CC, Park JW, Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models, Cancer Res. 66(13) (2006) 6732–40. [DOI] [PubMed] [Google Scholar]
- [31].Schiffelers RM, Koning GA, ten Hagen TLM, Fens MHAM, Schraa AJ, Janssen APCA, Kok RJ, Molema G, Storm G, Anti-tumor efficacy of tumor vasculature-targeted liposomal doxorubicin, J. Controlled Release 91(1) (2003) 115–122. [DOI] [PubMed] [Google Scholar]
- [32].Allison RR, Mota HC, Sibata CH, Clinical PD/PDT in North America: An historical review, Photodiagnosis Photodyn. Ther 1(4) (2004) 263–277. [DOI] [PubMed] [Google Scholar]
- [33].Allison RR, Sibata CH, Oncologic photodynamic therapy photosensitizers: a clinical review, Photodiagnosis Photodyn. Ther 7(2) (2010) 61–75. [DOI] [PubMed] [Google Scholar]
- [34].Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Photodynamic therapy of cancer: an update, CA Cancer J. Clin 61(4) (2011) 250–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Maiya BG, Photodynamic therapy (PDT), Resonance 5(6) (2000) 15–29. [Google Scholar]
- [36].Sadasivam M, Avci P, Gupta Gaurav K, Lakshmanan S, Chandran R, Huang Y-Y, Kumar R, Hamblin Michael R, Self-assembled liposomal nanoparticles in photodynamic therapy, Eur. J. Nanomedicine, 2013, p. 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rechtman E, Ciulla TA, Criswell MH, Pollack A, Harris A, An update on photodynamic therapy in age-related macular degeneration, Expert Opin. Pharmacother 3(7) (2002) 931–938. [DOI] [PubMed] [Google Scholar]
- [38].Detty MR, Gibson SL, Wagner SJ, Current clinical and preclinical photosensitizers for use in photodynamic therapy, J. Med. Chem 47(16) (2004) 3897–3915. [DOI] [PubMed] [Google Scholar]
- [39].Leman J, Morton C, Photodynamic therapy: applications in dermatology, Expert Opin. Biol. Ther 2(1) (2002) 45–53. [DOI] [PubMed] [Google Scholar]
- [40].Hasan T, Ortel B, Solban N, Pogue B, Photodynamic therapy of cancer, Cancer medicine 7 (2003) 537–48. [Google Scholar]
- [41].Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q, Photodynamic therapy, J. Nat. Cancer Institute 90(12) (1998) 889–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kotagiri N, Sudlow GP, Akers WJ, Achilefu S.J.N.n., Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers, Nat. Nanotechnol 10(4) (2015) 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ni D, Ferreira CA, Barnhart TE, Quach V, Yu B, Jiang D, Wei W, Liu H, Engle JW, Hu P, Magnetic Targeting of Nanotheranostics Enhances Cerenkov Radiation-Induced Photodynamic Therapy, J. Am. Chem. Soc. 140(44) (2018) 14971–14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Abrahamse H, Hamblin MR, New photosensitizers for photodynamic therapy, Biochem. J. 473(4) (2016) 347–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Reshetov V, Kachatkou D, Shmigol T, Zorin V, D’Hallewin MA, Guillemin F, Bezdetnaya L, Redistribution of meta-tetra(hydroxyphenyl)chlorin (m-THPC) from conventional and PEGylated liposomes to biological substrates, Photochem. Photobiol. Sci 10(6) (2011) 911–9. [DOI] [PubMed] [Google Scholar]
- [46].Kuntsche J, Freisleben I, Steiniger F, Fahr A, Temoporfin-loaded liposomes: physicochemical characterization, Eur. J. Pharm. Sci 40(4) (2010) 305–315. [DOI] [PubMed] [Google Scholar]
- [47].Chen M, Liu X, Fahr A, Skin penetration and deposition of carboxyfluorescein and temoporfin from different lipid vesicular systems: in vitro study with finite and infinite dosage application, Int. J. Pharm 408(1–2) (2011) 223–234. [DOI] [PubMed] [Google Scholar]
- [48].Dragicevic-Curic N, Winter S, Stupar M, Milic J, Krajisnik D, Gitter B, Fahr A, Temoporfin-loaded liposomal gels: viscoelastic properties and in vitro skin penetration, Int. J. Pharm 373(1–2) (2009) 77–84. [DOI] [PubMed] [Google Scholar]
- [49].Chang H-I, Yeh M-K, Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy, Int. J. Nanomed 7 (2012) 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chowdhary RK, Shariff I, Dolphin D, Drug release characteristics of lipid based benzoporphyrin derivative, J. Pharm. Pharm. Sci 6(1) (2003) 13–9. [PubMed] [Google Scholar]
- [51].Fahr A, van Hoogevest P, May S, Bergstrand N, Leigh ML, Transfer of lipophilic drugs between liposomal membranes and biological interfaces: consequences for drug delivery, Eur. J. Pharm. Sci 26(3–4) (2005) 251–265. [DOI] [PubMed] [Google Scholar]
- [52].Reshetov V, Zorin V, Siupa A, D’Hallewin MA, Guillemin F, Bezdetnaya L, Interaction of Liposomal Formulations of Meta-tetra (hydroxyphenyl) chlorin (Temoporfin) with Serum Proteins: Protein Binding and Liposome Destruction, Photochem. Photobiol 88(5) (2012) 1256–1264. [DOI] [PubMed] [Google Scholar]
- [53].Reshetov V, Lassalle H-P, François A, Dumas D, Hupont S, Gräfe S, Filipe V, Jiskoot W, Guillemin F, Zorin V, Photodynamic therapy with conventional and PEGylated liposomal formulations of mTHPC (temoporfin): comparison of treatment efficacy and distribution characteristics in vivo, Int. J. Nanomed 8 (2013) 3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Isele U, Hoogevest P.v., Leuenberger H, Capraro H-G, Schieweck K, Pharmaceutical development of CGP 55847: a liposomal Zn-phthalocyanine formulation using a controlled organic solvent dilution method, SPIE1994. [Google Scholar]
- [55].Pierre MBR, Tedesco AC, Marchetti JM, Bentley MVL, Stratum corneum lipids liposomes for the topical delivery of 5-aminolevulinic acid in photodynamic therapy of skin cancer: preparation and in vitro permeation study, BMC Dermatol. 1(1) (2001) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Gayathri T, Vijayalakshmi A, Mangalath S, Joseph J, Rao NM, Singh SP, Study on Liposomal Encapsulation of New Bodipy Sensitizers for Photodynamic Therapy, ACS Med. Chem. Lett 9(4) (2018) 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Takeuchi Y, Ichikawa K, Yonezawa S, Kurohane K, Koishi T, Nango M, Namba Y, Oku N, Intracellular target for photosensitization in cancer antiangiogenic photodynamic therapy mediated by polycation liposome, J. Controlled Release 97(2) (2004) 231–240. [DOI] [PubMed] [Google Scholar]
- [58].Shim G, Lee S, Kim YB, Kim C-W, Oh Y-K, Enhanced tumor localization and retention of chlorin e6 in cationic nanolipoplexes potentiate the tumor ablation effects of photodynamic therapy, Nanotechnology 22(36) (2011) 365101. [DOI] [PubMed] [Google Scholar]
- [59].Segalla A, Milanesi C, Jori G, Capraro H, Isele U, Schieweck K, CGP 55398, a liposomal Ge (IV) phthalocyanine bearing two axially ligated cholesterol moieties: a new potential agent for photodynamic therapy of tumours, Br. J. Cancer 69(5) (1994) 817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Shemesh CS, Moshkelani D, Zhang H, Thermosensitive liposome formulated indocyanine green for near-infrared triggered photodynamic therapy: in vivo evaluation for triple-negative breast cancer, Pharm. Res 32(5) (2015)1604–1614. [DOI] [PubMed] [Google Scholar]
- [61].Jiang F, Lilge L, Logie B, Li Y, Chopp M, Photodynamic therapy of 9L gliosarcoma with liposome-delivered Photofrin, Photochem. Photobiol 65(4) (1997) 701–706. [DOI] [PubMed] [Google Scholar]
- [62].Leunig M, Richert C, Gamarra F, Lumper W, Vogel E, Jocham D, Goetz A, Tumour localisation kinetics of photofrin and three synthetic porphyrinoids in an amelanotic melanoma of the hamster, Br. J. Cancer 68(2) (1993)225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Temizel E, Sagir T, Ayan E, Isik S, Ozturk R , Delivery of lipophilic porphyrin by liposome vehicles: Preparation and photodynamic therapy activity against cancer cell lines, Photodiagnosis Photodyn. Ther 11(4) (2014) 537–545. [DOI] [PubMed] [Google Scholar]
- [64].Ibrahim H, Kasselouri A, You C, Maillard P, Rosilio V, Pansu R, Prognon P, Meso-tetraphenyl porphyrin derivatives: The effect of structural modifications on binding to DMPC liposomes and albumin, J. Photochem. Photobiol. A: Chem 217(1) (2011) 10–21. [Google Scholar]
- [65].Oh J, Yoon H-J, Park J-H, Plasmonic liposomes for synergistic photodynamic and photothermal therapy, J. Mater. Chem. B 2(17) (2014) 2592–2597. [DOI] [PubMed] [Google Scholar]
- [66].Ichikawa K, Hikita T, Maeda N, Takeuchi Y, Namba Y, Oku N, PEGylation of liposome decreases the susceptibility of liposomal drug in cancer photodynamic therapy, Biol. Pharm. Bull 27(3) (2004) 443–444. [DOI] [PubMed] [Google Scholar]
- [67].Chan WM, Lim T-H, Pece A, Silva R, Yoshimura N, Verteporfin PDT for non-standard indications—a review of current literature, Graefe’s Archive Clin. Exp. Ophthalmol 248(5) (2010) 613–626. [DOI] [PubMed] [Google Scholar]
- [68].Therapy VIP, Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-year results of a randomized clinical trial—VIP Report No. 3, Ophthalmology 110 (2003) 667–673. [DOI] [PubMed] [Google Scholar]
- [69].Richter AM, Kelly B, Chow J, Liu DJ, Towers G, Dolphin D, Levy JG, Preliminary studies on a more effective phototoxic agent than hematoporphyrin, JNCI J. Nat. Cancer Institute 79(6) (1987) 1327–1332. [PubMed] [Google Scholar]
- [70].Yano S, Hirohara S, Obata M, Hagiya Y, S.-i. Ogura, A. Ikeda, H. Kataoka, M. Tanaka, T. Joh, Current states and future views in photodynamic therapy, J. Photochem. Photobiol. C 12(1) (2011) 46–67. [Google Scholar]
- [71].Aveline BM, Hasan T, Redmond RW, The effects of aggregation, protein binding and cellular incorporation on the photophysical properties of benzoporphyrin derivative monoacid ring A (BPDMA), J. Photochem. Photobiol. B 30(2–3) (1995) 161–169. [DOI] [PubMed] [Google Scholar]
- [72].Richter AM, Waterfield E, Jain AK, Canaan AJ, Allison BA, Levy JG, Liposomal delivery of a photosensitizer, benzoporphyrin derivative monoacid ring A (BPD), to tumor tissue in a mouse tumor model, Photochem. Photobiol. 57 (1993) 1000–1006. [DOI] [PubMed] [Google Scholar]
- [73].Cramers P, Ruevekamp M, Oppelaar H, Dalesio O, Baas P, Stewart F, Foscan® uptake and tissue distribution in relation to photodynamic efficacy, Br. J. Cancer 88(2) (2003) 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Copper MP, Tan IB, Oppelaar H, Ruevekamp MC, Stewart FA, Meta-tetra (hydroxyphenyl) chlorin photodynamic therapy in early-stage squamous cell carcinoma of the head and neck, Archives of Otolaryngology-Head & Neck Surgery 129(7) (2003) 709–711. [DOI] [PubMed] [Google Scholar]
- [75].Hopper C, Kübler A, Lewis H, Tan IB, Putnam G, Group FS, mTHPC-mediated photodynamic therapy for early oral squamous cell carcinoma, Int. J. Cancer 111(1) (2004) 138–146. [DOI] [PubMed] [Google Scholar]
- [76].Kiesslich T, Berlanda J, Plaetzer K, Krammer B, Berr F, Comparative characterization of the efficiency and cellular pharmacokinetics of Foscan®-and Foslip®-based photodynamic treatment in human biliary tract cancer cell lines, Photochemical & Photobiological Sciences 6(6) (2007) 619–627. [DOI] [PubMed] [Google Scholar]
- [77].Compagnin C, Moret F, Celotti L, Miotto G, Woodhams JH, MacRobert AJ, Scheglmann D, Iratni S, Reddi E, Meta-tetra (hydroxyphenyl) chlorin-loaded liposomes sterically stabilised with poly (ethylene glycol) of different length and density: characterisation, in vitro cellular uptake and phototoxicity, Photochem. Photobiol. Sci 10(11) (2011) 1751–1759. [DOI] [PubMed] [Google Scholar]
- [78].Schieweck K, Capraro H-G, Isele U, Hoogevest P.v., Ochsner M, Maurer T, Batt E, CGP 55 847, liposome-delivered zinc(II)-phthalocyanine as a phototherapeutic agent for tumors, Proc. SPIE 2078 (1994) 107–118. [Google Scholar]
- [79].Love WG, Duk S, Biolo R, Jori G, Taylor PW, Liposome-mediated delivery of photosensitizers: localization of zinc (II)-phthalocyanine within implanted tumors after intravenous administration, Photochem. Photobiol 63(5) (1996) 656–61. [DOI] [PubMed] [Google Scholar]
- [80].Ochsner M, Light scattering of human skin: A comparison between zinc (II)-phthalocyanine and photofrin II®, J. Photochem. Photobiol. B: Biol 32(1–2) (1996) 3–9. [DOI] [PubMed] [Google Scholar]
- [81].Paszko E, Ehrhardt C, Senge MO, Kelleher DP, Reynolds JV, Nanodrug applications in photodynamic therapy, Photodiagnosis Photodyn. Ther 8(1) (2011) 14–29. [DOI] [PubMed] [Google Scholar]
- [82].Zhou Y, Liang X, Dai Z, Porphyrin-loaded nanoparticles for cancer theranostics, Nanoscale 8(25) (2016) 12394–12405. [DOI] [PubMed] [Google Scholar]
- [83].Igarashi A, Konno H, Tanaka T, Nakamura S, Sadzuka Y, Hirano T, Fujise Y, Liposomal photofrin enhances therapeutic efficacy of photodynamic therapy against the human gastric cancer, Toxicol. Lett 145(2) (2003) 133–141. [DOI] [PubMed] [Google Scholar]
- [84].Jia H-R, Zhu Y-X, Xu K-F, Liu X, Wu F-G, Plasma membrane-anchorable photosensitizing nanomicelles for lipid raft-responsive and light-controllable intracellular drug delivery, J. Controlled Release 286 (2018) 103–113. [DOI] [PubMed] [Google Scholar]
- [85].Wang S, Hüttmann G, Zhang Z, Vogel A, Birngruber R, Tangutoori S, Hasan T, Rahmanzadeh R, Light-controlled delivery of monoclonal antibodies for targeted photoinactivation of Ki-67, Mol. Pharm 12(9) (2015) 3272–3281. [DOI] [PubMed] [Google Scholar]
- [86].Namiki Y, Namiki T, Date M, Yanagihara K, Yashiro M, Takahashi H, Enhanced photodynamic antitumor effect on gastric cancer by a novel photosensitive stealth liposome, Pharmacol. Res 50(1) (2004) 65–76. [DOI] [PubMed] [Google Scholar]
- [87].Bourré L, Thibaut S, Fimiani M, Ferrand Y, Simonneaux G, Patrice T, In vivo photosensitizing efficiency of a diphenylchlorin sensitizer: interest of a DMPC liposome formulation, Pharmacol. Res 47(3) (2003) 253–261. [DOI] [PubMed] [Google Scholar]
- [88].Yuan A, Tang X, Qiu X, Jiang K, Wu J, Hu Y, Activatable photodynamic destruction of cancer cells by NIR dye/photosensitizer loaded liposomes, Chem. Commun 51(16) (2015) 3340–3342. [DOI] [PubMed] [Google Scholar]
- [89].Allen CM, Sharman WM, Van Lier JE, Current status of phthalocyanines in the photodynamic therapy of cancer, J. Porphyrins Phthalocyanines 5(02) (2001) 161–169. [Google Scholar]
- [90].Urizzi P, Allen CM, Langlois R, Ouellet R, La Madeleine C, Van Lier JE, Low-density lipoprotein-bound aluminum sulfophthalocyanine: targeting tumor cells for photodynamic therapy, J. Porphyrins Phthalocyanines 5(02) (2001) 154–160. [Google Scholar]
- [91].Macháček M, Carter KA, Kostelanský F, Miranda D, Seffouh A, Ortega J, Šimůnek T, Zimčík P, Lovell JF, Binding of an amphiphilic phthalocyanine to pre-formed liposomes confers light-triggered cargo release, J. Mater. Chem. B 6(44) (2018) 7298–7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Kamkaew A, Lim SH, Lee HB, Kiew LV, Chung LY, Burgess K, BODIPY dyes in photodynamic therapy, Chem. Soc. Rev 42(1) (2013) 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Chen H, Bi Q, Yao Y, Tan N, Dimeric BODIPY-loaded liposomes for dual hypoxia marker imaging and activatable photodynamic therapy against tumors, J. Mater. Chem. B 6(26) (2018) 4351–4359. [DOI] [PubMed] [Google Scholar]
- [94].Chang C-C, Yang Y-T, Yang J-C, Wu H-D, Tsai T, Absorption and emission spectral shifts of rose bengal associated with DMPC liposomes, Dyes and Pigments 79(2) (2008) 170–175. [Google Scholar]
- [95].Pastorino F, Brignole C, Marimpietri D, Cilli M, Gambini C, Ribatti D, Longhi R, Allen TM, Corti A, Ponzoni M, Vascular damage and anti-angiogenic effects of tumor vessel-targeted liposomal chemotherapy, Cancer Res. 63(21) (2003) 7400–7409. [PubMed] [Google Scholar]
- [96].Chen B, Pogue BW, Hoopes PJ, Hasan T, Combining vascular and cellular targeting regimens enhances the efficacy of photodynamic therapy, International Journal of Radiation Oncology* Biology* Physics 61(4) (2005)1216–1226. [DOI] [PubMed] [Google Scholar]
- [97].Ichikawa K, Takeuchi Y, Yonezawa S, Hikita T, Kurohane K, Namba Y, Oku N, Antiangiogenic photodynamic therapy (PDT) using Visudyne causes effective suppression of tumor growth, Cancer Lett. 205(1) (2004) 39–48. [DOI] [PubMed] [Google Scholar]
- [98].Trachtenberg J, Bogaards A, Weersink R, Haider M, Evans A, McCluskey S, Scherz A, Gertner M, Yue C, Appu S, Vascular targeted photodynamic therapy with palladium-bacteriopheophorbide photosensitizer for recurrent prostate cancer following definitive radiation therapy: assessment of safety and treatment response, J. Urol 178(5) (2007) 1974–1979. [DOI] [PubMed] [Google Scholar]
- [99].Doddapaneni R, Patel K, Owaid IH, Singh M, Tumor neovasculature-targeted cationic PEGylated liposomes of gambogic acid for the treatment of triple-negative breast cancer, Drug Deliv. 23(4) (2016) 1232–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Luo D, Geng J, Li N, Carter KA, Shao S, Atilla-Gokcumen GE, Lovell JF, Vessel-Targeted Chemophototherapy with Cationic Porphyrin-Phospholipid Liposomes, Mol. Cancer Ther (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Kurohane K, Tominaga A, Sato K, North JR, Namba Y, Oku N, Photodynamic therapy targeted to tumor-induced angiogenic vessels, Cancer Lett. 167(1) (2001) 49–56. [DOI] [PubMed] [Google Scholar]
- [102].Ichikawa K, Hikita T, Maeda N, Yonezawa S, Takeuchi Y, Asai T, Namba Y, Oku N, Antiangiogenic photodynamic therapy (PDT) by using long-circulating liposomes modified with peptide specific to angiogenic vessels, Biochimica et Biophysica Acta (BBA)-Biomembranes 1669(1) (2005) 69–74. [DOI] [PubMed] [Google Scholar]
- [103].Takeuchi Y, Kurohane K, Ichikawa K, Yonezawa S, Nango M, Oku N, Induction of intensive tumor suppression by antiangiogenic photodynamic therapy using polycation-modified liposomal photosensitizer, Cancer: Interdisciplinary Int. J. Am. Cancer Soc 97(8) (2003) 2027–2034. [DOI] [PubMed] [Google Scholar]
- [104].Takeuchi Y, Kurohane K, Ichikawa K, Yonezawa S, Ori H, Koishi T, Nango M, Oku N, Polycation liposome enhances the endocytic uptake of photosensitizer into cells in the presence of serum, Bioconj. Chem 14(4) (2003) 790–796. [DOI] [PubMed] [Google Scholar]
- [105].Yan F, Wu H, Liu H, Deng Z, Liu H, Duan W, Liu X, Zheng H, Molecular imaging-guided photothermal/photodynamic therapy against tumor by iRGD-modified indocyanine green nanoparticles, J. Controlled Release 224 (2016) 217–228. [DOI] [PubMed] [Google Scholar]
- [106].Bovis MJ, Woodhams JH, Loizidou M, Scheglmann D, Bown SG, MacRobert AJ, Improved in vivo delivery of m-THPC via pegylated liposomes for use in photodynamic therapy, J. Controlled Release 157(2) (2012) 196–205. [DOI] [PubMed] [Google Scholar]
- [107].Rizvi I, Obaid G, Bano S, Hasan T, Kessel D, Photodynamic therapy: Promoting in vitro efficacy of photodynamic therapy by liposomal formulations of a photosensitizing agent, Lasers Surg. Med 50(5) (2018) 499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Lassalle H-P, Dumas D, Gräfe S, D’Hallewin M-A, Guillemin F, Bezdetnaya L, Correlation between in vivo pharmacokinetics, intratumoral distribution and photodynamic efficiency of liposomal mTHPC, J. Controlled Release 134(2) (2009) 118–124. [DOI] [PubMed] [Google Scholar]
- [109].Lovell JF, Jin CS, Huynh E, Jin H, Kim C, Rubinstein JL, Chan WC, Cao W, Wang LV, Zheng G, Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents, Nature materials 10(4) (2011) 324. [DOI] [PubMed] [Google Scholar]
- [110].Wu C, Yu C, Chu M, A gold nanoshell with a silica inner shell synthesized using liposome templates for doxorubicin loading and near-infrared photothermal therapy, Int. J. Nanomed 6 (2011) 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Luo L, Bian Y, Liu Y, Zhang X, Wang M, Xing S, Li L, Gao D, Combined near infrared photothermal therapy and chemotherapy using gold nanoshells coated liposomes to enhance antitumor effect, Small 12(30) (2016) 4103–4112. [DOI] [PubMed] [Google Scholar]
- [112].Bian Y, Gao D, Liu Y, Li N, Zhang X, Zheng RY, Wang Q, Luo L, Dai K, Preparation and study on antitumor effect of chitosan-coated oleanolic acid liposomes, RSC Advances 5(24) (2015) 18725–18732. [Google Scholar]
- [113].Minchinton AI, Tannock IF, Drug penetration in solid tumours, Nature Reviews Cancer 6(8) (2006) 583. [DOI] [PubMed] [Google Scholar]
- [114].Hambley TW, Is anticancer drug development heading in the right direction?, Cancer Res. 69(4) (2009) 1259–1262. [DOI] [PubMed] [Google Scholar]
- [115].Tannock IF, Lee CM, Tunggal JK, Cowan DS, Egorin MJ, Limited penetration of anticancer drugs through tumor tissue: a potential cause of resistance of solid tumors to chemotherapy, Clin. Cancer. Res 8(3) (2002) 878–884. [PubMed] [Google Scholar]
- [116].Luo D, Carter KA, Lovell JF, Nanomedical engineering: shaping future nanomedicines, Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 7(2) (2015) 169–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Needham D, Dewhirst MW, The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors, Adv. Drug Del. Rev 53(3) (2001) 285–305. [DOI] [PubMed] [Google Scholar]
- [118].Shum P, Kim J-M, Thompson DH, Phototriggering of liposomal drug delivery systems, Adv. Drug Del. Rev 53(3) (2001) 273–284. [DOI] [PubMed] [Google Scholar]
- [119].Luo D, Carter KA, Miranda D, Lovell JF, Chemophototherapy: An Emerging Treatment Option for Solid Tumors, Advanced science (Weinheim, Baden-Wurttemberg, Germany) 4(1) (2017) 1600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Chitgupi U, Shao S, Carter KA, Huang WC, Lovell JF, Multicolor Liposome Mixtures for Selective and Selectable Cargo Release, Nano Lett. 18(2) (2018) 1331–1336. [DOI] [PubMed] [Google Scholar]
- [121].Baglo Y, Liang BJ, Robey RW, Ambudkar SV, Gottesman MM, Huang HC, Porphyrin-lipid assemblies and nanovesicles overcome ABC transporter-mediated photodynamic therapy resistance in cancer cells, Cancer Lett. 457 (2019) 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Rizvi I, Nath S, Obaid G, Ruhi MK, Moore K, Bano S, Kessel D, Hasan T, A Combination of Visudyne and a Lipid-anchored Liposomal Formulation of Benzoporphyrin Derivative Enhances Photodynamic Therapy Efficacy in a 3D Model for Ovarian Cancer, Photochem. Photobiol 95(1) (2019) 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Keca JM, Chen J, Overchuk M, Muhanna N, MacLaughlin CM, Jin CS, Foltz WD, Irish JC, Zheng G, Nanotexaphyrin: One-Pot Synthesis of a Manganese Texaphyrin-Phospholipid Nanoparticle for Magnetic Resonance Imaging, Angew. Chem. Int. Ed. Engl 55(21) (2016) 6187–91. [DOI] [PubMed] [Google Scholar]
- [124].Spring BQ, Sears RB, Zheng LZ, Mai Z, Watanabe R, Sherwood ME, Schoenfeld DA, Pogue BW, Pereira SP, Villa E, A photoactivable multi-inhibitor nanoliposome for tumour control and simultaneous inhibition of treatment escape pathways, Nat. Nanotechnol 11(4) (2016) 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Carter KA, Luo D, Razi A, Geng J, Shao S, Ortega J, Lovell JF, Sphingomyelin liposomes containing porphyrin-phospholipid for irinotecan chemophototherapy, Theranostics 6(13) (2016) 2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Carter KA, Shao S, Hoopes MI, Luo D, Ahsan B, Grigoryants VM, Song W, Huang H, Zhang G, Pandey RK, Porphyrin-phospholipid liposomes permeabilized by near-infrared light, Nat. Commun 5 (2014) 3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Luo D, Carter KA, Razi A, Geng J, Shao S, Giraldo D, Sunar U, Ortega J, Lovell JF, Doxorubicin encapsulated in stealth liposomes conferred with light-triggered drug release, Biomaterials 75 (2016) 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Luo D, Li N, Carter KA, Lin C, Geng J, Shao S, Huang WC, Qin Y, Atilla-Gokcumen GE, Lovell JF, Rapid Light-Triggered Drug Release in Liposomes Containing Small Amounts of Unsaturated and Porphyrin–Phospholipids, Small 12(22) (2016) 3039–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Luo D, Goel S, Liu H-J, Carter KA, Jiang D, Geng J, Kutyreff CJ, Engle JW, Huang W-C, Shao S, Intrabilayer 64Cu Labeling of Photoactivatable, Doxorubicin-Loaded Stealth Liposomes, ACS nano 11(12) (2017) 12482–12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Luo D, Carter KA, Geng J, He X, Lovell JF, Short Drug-Light Intervals Improve Liposomal Chemophototherapy in Mice Bearing MIA PaCa-2 Xenografts, Molecular pharmaceutics 15(9) (2018) 3682–3689. [DOI] [PubMed] [Google Scholar]
- [131].Rwei AY, Lee J-J, Zhan C, Liu Q, Ok MT, Shankarappa SA, Langer R, Kohane DS, Repeatable and adjustable on-demand sciatic nerve block with phototriggerable liposomes, Proc. Nat. Acad. Sci 112(51) (2015) 15719–15724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Lajunen T, Nurmi R, Kontturi L, Viitala L, Yliperttula M, Murtomäki L, Urtti A, Light activated liposomes: functionality and prospects in ocular drug delivery, J. Controlled Release 244 (2016) 157–166. [DOI] [PubMed] [Google Scholar]
- [133].Lajunen T, Kontturi L-S, Viitala L, Manna M, Cramariuc O, Róg T, Bunker A, Laaksonen T, Viitala T, Murtomaki L, Indocyanine green-loaded liposomes for light-triggered drug release, Mol. Pharm 13(6) (2016) 2095–2107. [DOI] [PubMed] [Google Scholar]
- [134].Feng L, Cheng L, Dong Z, Tao D, Barnhart TE, Cai W, Chen M, Liu Z, Theranostic liposomes with hypoxia-activated prodrug to effectively destruct hypoxic tumors post-photodynamic therapy, ACS Nano 11(1) (2016) 927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Song X, Feng L, Liang C, Gao M, Song G, Liu Z, Liposomes co-loaded with metformin and chlorin e6 modulate tumor hypoxia during enhanced photodynamic therapy, Nano Research 10(4) (2017) 1200–1212. [Google Scholar]
- [136].Luo D, Carter KA, Molins EA, Straubinger NL, Geng J, Shao S, Jusko WJ, Straubinger RM, Lovell JF, Pharmacokinetics and pharmacodynamics of liposomal chemophototherapy with short drug-light intervals, J. Controlled Release 297 (2019) 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Sine J, Urban C, Thayer D, Charron H, Valim N, Tata DB, Schiff R, Blumenthal R, Joshi A, Puri A, Photo activation of HPPH encapsulated in “Pocket” liposomes triggers multiple drug release and tumor cell killing in mouse breast cancer xenografts, Int. J. Nanomed 10 (2015) 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Xu H, Ohulchanskyy TY, Yakovliev A, Zinyuk R, Song J, Liu L, Qu J, Yuan Z, Nanoliposomes Co-Encapsulating CT Imaging Contrast Agent and Photosensitizer for Enhanced, Imaging Guided Photodynamic Therapy of Cancer, Theranostics 9(5) (2019) 1323–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Skupin-Mrugalska P, Sobotta L, Warowicka A, Wereszczynska B, Zalewski T, Gierlich P, Jarek M, Nowaczyk G, Kempka M, Gapinski J, Jurga S, Mielcarek J, Theranostic liposomes as a bimodal carrier for magnetic resonance imaging contrast agent and photosensitizer, J. Inorg. Biochem 180 (2018) 1–14. [DOI] [PubMed] [Google Scholar]
- [140].Zhang C, Wu D, Lu L, Duan X, Liu J, Xie X, Shuai X, Shen J, Cao Z, Multifunctional Hybrid Liposome as a Theranostic Platform for Magnetic Resonance Imaging Guided Photothermal Therapy, ACS Biomaterials Sci. Eng 4(7) (2018) 2597–2605. [DOI] [PubMed] [Google Scholar]
- [141].Song X, Feng L, Liang C, Yang K, Liu Z, Ultrasound triggered tumor oxygenation with oxygen-shuttle nanoperfluorocarbon to overcome hypoxia-associated resistance in cancer therapies, Nano Lett. 16(10) (2016) 6145–6153. [DOI] [PubMed] [Google Scholar]
- [142].Lovell JF, Liu TW, Chen J, Zheng G, Activatable photosensitizers for imaging and therapy, Chem. Rev 110(5) (2010) 2839–2857. [DOI] [PubMed] [Google Scholar]
- [143].Feng L, Tao D, Dong Z, Chen Q, Chao Y, Liu Z, Chen M, Near-infrared light activation of quenched liposomal Ce6 for synergistic cancer phototherapy with effective skin protection, Biomaterials 127 (2017) 13–24. [DOI] [PubMed] [Google Scholar]
- [144].Kwok RT, Leung CW, Lam JW, Tang BZ, Biosensing by luminogens with aggregation-induced emission characteristics, Chem. Soc. Rev 44(13) (2015) 4228–4238. [DOI] [PubMed] [Google Scholar]
- [145].Yang Y, Wang L, Cao H, Li Q, Li Y, Han M, Wang H, Li J, Photodynamic therapy with liposomes encapsulating photosensitizers with aggregation-induced emission, Nano Lett. (2019). [DOI] [PubMed] [Google Scholar]
- [146].Feng Y, Wu Y, Zuo J, Tu L, Que I, Chang Y, Cruz LJ, Chan A, Zhang H, Assembly of upconversion nanophotosensitizer in vivo to achieve scatheless real-time imaging and selective photodynamic therapy, Biomaterials 201 (2019) 33–41. [DOI] [PubMed] [Google Scholar]
- [147].Dong Z, Feng L, Hao Y, Chen M, Gao M, Chao Y, Zhao H, Zhu W, Liu J, Liang C, Zhang Q, Liu Z, Synthesis of Hollow Biomineralized CaCO3-Polydopamine Nanoparticles for Multimodal Imaging-Guided Cancer Photodynamic Therapy with Reduced Skin Photosensitivity, J. Am. Chem. Soc. 140(6) (2018) 2165–2178. [DOI] [PubMed] [Google Scholar]
- [148].Dong Z, Feng L, Zhu W, Sun X, Gao M, Zhao H, Chao Y, Liu Z, CaCO3 nanoparticles as an ultra-sensitive tumor-pH-responsive nanoplatform enabling real-time drug release monitoring and cancer combination therapy, Biomaterials 110 (2016) 60–70. [DOI] [PubMed] [Google Scholar]
- [149].Wang C, Cheng L, Liu Z, Upconversion nanoparticles for photodynamic therapy and other cancer therapeutics, Theranostics 3(5) (2013) 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Wang H, Liu Z, Wang S, Dong C, Gong X, Zhao P, Chang J, MC540 and upconverting nanocrystal coloaded polymeric liposome for near-infrared light-triggered photodynamic therapy and cell fluorescent imaging, ACS Applied Materials Interfaces 6(5) (2014) 3219–3225. [DOI] [PubMed] [Google Scholar]
- [151].Askes SH, Bahreman A, Bonnet S, Activation of a photodissociative ruthenium complex by triplet-triplet annihilation upconversion in liposomes, Angew. Chem. Int. Ed 126(4) (2014) 1047–1051. [DOI] [PubMed] [Google Scholar]
- [152].Hamblin MR, Hasan T, Photodynamic therapy: a new antimicrobial approach to infectious disease?, Photochem. Photobiol. Sci 3(5) (2004) 436–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Park H, Lee J, Jeong S, Im BN, Kim MK, Yang SG, Na K, Lipase-Sensitive Transfersomes Based on Photosensitizer/Polymerizable Lipid Conjugate for Selective Antimicrobial Photodynamic Therapy of Acne, Advanced Healthcare Mater. 5(24) (2016) 3139–3147. [DOI] [PubMed] [Google Scholar]
- [154].Zhao Y, Dai X, Wei X, Yu Y, Chen X, Zhang X, Li C, Near-infrared light-activated thermosensitive liposomes as efficient agents for photothermal and antibiotic synergistic therapy of bacterial biofilm, ACS Applied Mater. Interfaces 10(17) (2018) 14426–14437. [DOI] [PubMed] [Google Scholar]
- [155].Jeong S, Lee J, Im BN, Park H, Na K, Combined photodynamic and antibiotic therapy for skin disorder via lipase-sensitive liposomes with enhanced antimicrobial performance, Biomaterials 141 (2017) 243–250. [DOI] [PubMed] [Google Scholar]
- [156].Wang X, Tan L, Liu X, Cui Z, Yang X, Yeung KW, Chu PK, Wu S, Construction of perfluorohexane/IR780@ liposome coating on Ti for rapid bacteria killing under permeable near infrared light, Biomaterials Sci. 6(9) (2018) 2460–2471. [DOI] [PubMed] [Google Scholar]
- [157].Castano AP, Mroz P, Hamblin MR, Photodynamic therapy and anti-tumour immunity, Nat. Rev. Cancer 6(7) (2006) 535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Kim DH, Im BN, Hwang HS, Na K, Gemcitabine-loaded DSPE-PEG-PheoA liposome as a photomediated immune modulator for cholangiocarcinoma treatment, Biomaterials 183 (2018) 139–150. [DOI] [PubMed] [Google Scholar]
- [159].Yoon H-J, Lee H-S, Jung J-H, Kim HK, Park J-H, Photothermally amplified therapeutic liposomes for effective combination treatment of cancer, ACS Applied Mater. Interfaces 10(7) (2018) 6118–6123. [DOI] [PubMed] [Google Scholar]