Abstract
PrEP’s potential benefit for men who have sex with men (MSM) who use stimulants may be limited by adherence or prescriber willingness to recommend PrEP due to concerns of non-compliance. Using data from PATH-PrEP, a 48-week study evaluating PrEP for MSM in Los Angeles, we modeled an interaction between stimulant use and condomless sex with multiple partners (CAS-MP) on prevention-effective dried blood spot tenofovir-diphosphate concentrations. At week 4, participants reporting stimulant use and CAS-MP had a decreased odds of prevention-effective adherence compared to non-stimulant use and non-CAS-MP (AOR 0.15, 95% CI 0.04-0.57). From week 4 to 48, participants reporting stimulant use and CAS-MP had increased odds of prevention-effective adherence (AOR 1.06 per week, 95%CI 1.01-1.12). Participants reporting CAS-MP without stimulant use had no significant change in prevention-effective adherence (AOR 0.99 per week, 95%CI 0.96-1.02). Stimulant use moderated the association of CAS-MP on prevention-effective PrEP adherence over time.
Keywords: PrEP, adherence, MSM, stimulant, condomless anal sex
Introduction
Pre-exposure prophylaxis (PrEP) with daily oral tenofovir disoproxil fumarate and emtricitabine (TDF/FTC, Truvada ®, Gilead Sciences) has been proven to be highly effective for preventing HIV acquisition in diverse populations including men who have sex with men (MSM) (Baeten et al., 2012; Grant et al., 2010; Molina et al., 2015; Thigpen et al., 2012). Effectiveness is contingent on adherence, with efficacy highly correlated with biomarkers of TDF/FTC use in clinical trials (Marrazzo et al., 2015; Van Damme et al., 2012). Data suggest that individuals may alter PrEP taking behavior in response to temporal changes in sexual risk (Grant et al., 2014; Haberer et al., 2015; 2017; Liu et al., 2016). In some studies, increased PrEP adherence was associated with condomless anal sex (CAS) (Hoagland et al., 2017; Hosek et al., 2017) or CAS with multiple partners (CAS-MP) (Grant et al., 2014; Liu et al., 2016).
Stimulant use (cocaine, methamphetamine, or ecstasy) (Lim et al., 2012) is an important risk factor for HIV acquisition (Drumright, Patterson, & Strathdee, 2009; Koblin et al., 2006) and may challenge an individual’s ability to maintain prevention-effective adherence to PrEP. Qualitative studies have reported MSM’s concerns regarding potential interactions between recreational stimulants and antiretroviral medications (Oldenburg et al., 2016), and difficulties remembering to take PrEP while under the influence of stimulants (Closson, Mitty, Malone, Mayer, & Mimiaga, 2017; Storholm, Volk, Marcus, Silverberg, & Satre, 2017). Additionally, providers reported that they would be less likely to prescribe PrEP to users of illicit drugs (Castel et al., 2015). Data are limited and conflicting with regard to PrEP adherence among stimulant users. One study reported increased adherence in frequent substance users (Hoenigl et al., 2018); while another study reported that MSM who used stimulants were five times less likely to have prevention-effective adherence early after initiation of PrEP (Hojilla et al., 2018). The objective of this analysis was to test the hypothesis that stimulant use may moderate the association of CAS-MP with PrEP adherence, decreasing prevention-effective adherence over time.
Methods
The PrEP and Testing/linkage to care for HIV Prevention (PATH-PrEP) study was a single-arm open label demonstration project in Los Angeles, California conducted between April 2014 and July 2016. Detailed methods and primary results have been reported (Landovitz et al., 2017). Screening and enrollment occurred at two clinics in Los Angeles. Eligible participants were HIV uninfected MSM or transgender women age 18 years or older who reported receptive anal sex within the last 12 months. Participants were offered PrEP if they met criteria suggesting high-risk for HIV (see Landovitz et al., 2017 for full inclusion/exclusion criteria). Those not offered PrEP were provided with as-needed post-exposure prophylaxis (PEP) and could be escalated to the PrEP cohort if high-risk criteria were met.
After providing informed consent, participants were evaluated at baseline, week 4, 8, 12, 24, 36, and 48. At each visit, behavioral data were collected by computer assisted self-interview. Those offered PrEP had laboratory testing for safety monitoring, and both plasma and dried blood spot (DBS) samples to assess adherence. Escalating adherence support based on plasma tenofovir levels was provided. All participants received a comprehensive HIV prevention package that included counseling, condoms, and testing for HIV and STIs (gonorrhea, chlamydia, and syphilis) at baseline and 12-week intervals.
Measures.
Substance use was assessed through a 15-item questionnaire based on the NIDA-ASSIST assessment (NIDA, 2012). Stimulant use was defined as any reported use of methamphetamine, ecstasy, or cocaine. Condomless anal sex with multiple partners (CAS-MP) was defined as self-report of at least two partners with who the participant had condomless receptive anal intercourse. Questions for both substance use and CAS-MP were asked within a time frame of 30-days prior to baseline or since the last study visit (30 or 90 days).
Primary outcome.
The primary outcome was tenofovir-diphosphate (TFV-DP) concentrations in DBS as a measure of PrEP adherence. TFV-DP concentrations were available for weeks 4, 12, 24, 36, and 48. TFV-DP concentrations were categorized as a dichotomous variable: Prevention-effective adherence (≥700 fmol/punch) or suboptimal adherence (<700 fmol/punch). A prevention-effective adherence assignment is consistent with taking 4 or more doses of TDF/FTC per week on average (Anderson et al., 2018).
Statistical analysis.
Participants who completed at least one follow-up visit at which a TFV-DP concentration was obtained from a DBS sample were included in the analysis (1 transgender participant was excluded from the analysis as results would not be generalizable to the transgender population). Generalized linear mixed models with random intercepts and slopes were used to estimate the relationship of predictors of interest with PrEP adherence as measured by TFV-DP concentrations in DBS. An interaction term of stimulant use and CAS-MP was included in the model, which also controlled for age, race and ethnicity, education, income, enrollment site, and sex work. All statistical analyses were performed using SPSS 24.
Results
The PATH-PrEP study offered PrEP to 296 MSM. These analyses included 283 participants who returned for at least one TFV-DP measurement. Table 1 shows baseline demographics by stimulant use and CAS-MP.
Table 1.
Baseline demographic variables by stimulant use and condomless anal sex with multiple partners (CAS-MP) among a cohort of men who have sex with men using PrEP (n=283)
| Stimulant use1 | CAS-MP | |||||
|---|---|---|---|---|---|---|
| No (n=187) n (%) |
Yes (n=96) n (%) |
P value2 | No (n=111) n (%) |
Yes (n=172) n (%) |
P value2 | |
| Age | 0.15 | 0.01 | ||||
| Less than 26 | 20 (10.7) | 17 (17.7) | 21 (18.9) | 16 (9.3) | ||
| Between 26-35 | 81 (43.3) | 44 (45.8) | 53 (47.7) | 72 (41.9) | ||
| Between 36-45 | 52 (27.8) | 18 (18.8) | 18 (16.2) | 52 (30.2) | ||
| 46 or Older | 34 (18.2) | 17 (17.7) | 19 (17.1) | 32 (18.6) | ||
| Race/ethnicity | 0.13 | 0.21 | ||||
| White | 93 (49.7) | 52 (54.2) | 49 (44.1) | 96 (55.8) | ||
| Hispanic or Latino | 58 (31.) | 20 (20.8) | 35 (31.5) | 43 (25.0) | ||
| Black or African-American | 17 (9.1) | 12 (12.5) | 14 (12.6) | 15 (8.7) | ||
| Asian/Pacific Islander | 8 (4.3) | 9 (9.4) | 9 (8.1) | 8 (4.7) | ||
| Other | 11 (5.9) | 3 (3.1) | 4 (3.6) | 10 (5.8) | ||
| Education | 0.45 | 0.94 | ||||
| High School or Less | 22 (11.8) | 10 (10.4) | 14 (12.6) | 18 (10.5) | ||
| Some College | 63 (33.7) | 33 (34.4) | 38 (34.2) | 58 (33.7) | ||
| Bachelors | 62 (33.2) | 39 (40.6) | 38 (34.2) | 63 (36.6) | ||
| Graduate | 40 (21.4) | 14 (14.6) | 21 (18.9) | 33 (19.2) | ||
| Family income | 0.10 | 0.34 | ||||
| $20,000 or Less | 48 (25.7) | 36 (37.5) | 37 (33.3) | 47 (27.3) | ||
| Between $20,000 and $50,000 | 74 (39.6) | 35 (36.5) | 44 (39.6) | 65 (37.8) | ||
| More than $50,000 | 65 (34.8) | 25 (26.0) | 30 (27.0) | 60 (34.9) | ||
| Enrollment site | 0.29 | <0.01 | ||||
| Los Angeles LGBT Center | 161 (86.1) | 78 (81.3) | 102 (91.9) | 137 (79.7) | ||
| Oasis | 26 (13.9) | 18 (18.8) | 9 (8.1) | 35 (20.3) | ||
Stimulant use defined as any use of methamphetamine, ecstasy, or cocaine in the preceding 30 days.
Significance level determined at a p value of less than 0.05. Chi-square was used for categorical variables.
At baseline, methamphetamine use was reported by 35 (13%) participants in the past 30 days, ecstasy by 58 (21%), and cocaine by 58 (21%). Use of one or more stimulant types was reported by 96 (34%). CAS was reported by 241 (85%) participants with a median of 3 (IQR 1–5) condomless sex partners in the previous 30-days. CAS-MP was reported by 172 (61%) participants. Both CAS-MP and stimulant use were reported by 70 participants (25%), CAS-MP alone by 102 (36%), stimulant use alone by 26 (9%) and neither by 85 (30%).
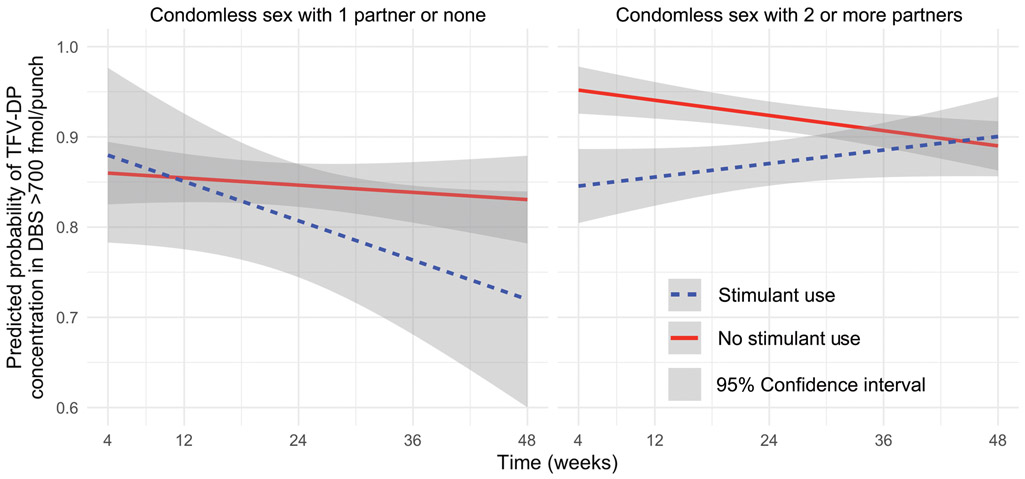
Prevention-effective TFV-DP concentrations were found in 80% of samples over the course of the study (see Landovitz et al., 2017 for full adherence results). In the multivariable model (Table 2), when considering the interaction of stimulant use and CAS-MP at week 4 and over time, participants engaging in CAS-MP had a statistically significant difference in prevention-effective adherence compared to non-CAS-MP: At week 4, those that reported using stimulants had decreased odds of prevention-effective adherence (adjusted odds ratio [AOR] 0.15, 95% CI 0.04–0.57) whereas those not using stimulants had increased odds of prevention-effective adherence (AOR 2.69, 95% CI 1.36–5.31) compared to non-stimulant non-CAS-MP participants. Only those with both stimulant use and CAS-MP had increased odds of prevention-effective adherence over time (Figure 1) (AOR 1.06 per week, 95% CI 1.01–1.12).
Table 2.
Multivariable generalized linear mixed model of interaction of stimulant use and condomless anal sex with multiple partners and their interaction with prevention-effective tenofovir diphosphate concentration (≥700 fmol/punch) in dried blood spots among a cohort of MSM using PrEP (n=283).
| 95% CI | ||||||
|---|---|---|---|---|---|---|
| B | SE | AOR | Lower | Upper | P value | |
| Interaction | ||||||
| Week 4 | ||||||
| No stimulant use or CAS-MP (reference) | ||||||
| Stimulant use1 only | 0.67 | 0.56 | 1.96 | 0.65 | 5.87 | 0.23 |
| CAS-MP only | 0.99 | 0.35 | 2.69 | 1.36 | 5.31 | <0.01 |
| Stimulant use and CAS-MP | −1.89 | 0.67 | 0.15 | 0.04 | 0.57 | 0.01 |
| Over time2 (per week increase) | ||||||
| No stimulant use or CAS-MP | 0.01 | 0.01 | 1.01 | 0.99 | 1.04 | 0.36 |
| Stimulant use only | −0.03 | 0.02 | 0.97 | 0.93 | 1.02 | 0.24 |
| CAS-MP only | −0.02 | 0.02 | 0.99 | 0.96 | 1.02 | 0.33 |
| Stimulant use and CAS-MP | 0.06 | 0.03 | 1.06 | 1.01 | 1.12 | 0.02 |
| Age | ||||||
| Less than 26 (reference) | ||||||
| Between 26-35 | 0.00 | 0.33 | 1.00 | 0.53 | 1.89 | 0.99 |
| Between 36-45 | 1.22 | 0.45 | 3.39 | 1.40 | 8.16 | 0.01 |
| 46 or older | 0.58 | 0.41 | 1.78 | 0.80 | 3.95 | 0.16 |
| Race/ethnicity | ||||||
| White (reference) | ||||||
| Hispanic | −0.04 | 0.29 | 0.96 | 0.55 | 1.70 | 0.90 |
| Black or African-American | −1.61 | 0.33 | 0.20 | 0.11 | 0.38 | <0.001 |
| Asian/Pacific Islander | 0.73 | 0.55 | 2.08 | 0.70 | 6.18 | 0.19 |
| Other | −0.22 | 0.57 | 0.80 | 0.26 | 2.47 | 0.70 |
| Enrollment site | ||||||
| Los Angeles LGBT Center (reference) | ||||||
| OASIS Clinic | −0.66 | 0.29 | 0.52 | 0.29 | 0.92 | 0.03 |
| Education | ||||||
| High School or Less (reference) | ||||||
| Some College | −0.08 | 0.37 | 0.93 | 0.45 | 1.93 | 0.84 |
| Bachelors | 0.39 | 0.40 | 1.48 | 0.68 | 3.22 | 0.33 |
| Graduate | 0.22 | 0.45 | 1.25 | 0.52 | 2.99 | 0.62 |
| Family income | ||||||
| Less than $20,000 (reference) | ||||||
| Between $20,000 and $50,000 | 0.51 | 0.28 | 1.66 | 0.95 | 2.90 | 0.08 |
| More than $50,000 | −0.06 | 0.32 | 0.95 | 0.50 | 1.78 | 0.87 |
| Sex work | ||||||
| No sex work (reference) | ||||||
| Sex work | −0.05 | 0.40 | 0.95 | 0.43 | 2.09 | 0.89 |
Abbreviations: AOR, adjusted odds ratio; CAS-MP, condomless anal sex with multiple partners; CI, confidence interval; MSM, men who have sex with men; PrEP, pre-exposure prophylaxis; SE, standard error.
Stimulant use was defined as any use of methamphetamine, ecstasy, or cocaine in the 30-day horizon at baseline, weeks 4, 8, and 12, and in the 90-day horizon at weeks 24, 36, and 48.
TFV-DP concentrations in DBS were tested at weeks 4, 12, 24, 36 and 48.
Figure 1.
Predicted probabilities of prevention-effective adherence (TFV-DP ≥700 fmol/punch) by condomless anal sex with multiple partners and stimulant use in a cohort of MSM on PrEP (n=283). Abbreviations: DBS, dried blood spot; PrEP, pre-exposure prophylaxis; TFV-DP, tenofovir diphosphate.
Discussion
This study evaluated the interaction of CAS-MP and stimulant use on prevention-effective PrEP adherence in a population of MSM prescribed PrEP in Los Angeles. The analyses found that stimulant users engaging in CAS-MP had decreased prevention-effective adherence compared to non-CAS-MP and non-stimulant using participants early after initiation of PrEP. The early finding of decreased adherence in people who used stimulants early after initiation of PrEP concurs with those reported by Hojilla et al. (2018). Yet, when analyses were extended over a 48-week period, MSM who reported stimulant use and CAS-MP were able to achieve the same or better levels of adherence over time as their non-stimulant using counterparts. The findings in the PATH-PrEP cohort suggest that MSM who used stimulants and were at high-risk for HIV infection due to CAS-MP may have subsequently assessed their risk adequately and adjusted their PrEP adherence to sustain prevention-effective concentrations over time similar.
In the PrEP continuum of care (Kelley et al., 2015; Nunn et al., 2017), substance use has been identified as a potential contributor to the ‘step off’ at each stage of the prevention continuum. A critical step in the continuum is for at-risk individuals to be prescribed PrEP by their providers (Kelley et al., 2015). In a study in Miami and Washington, DC, only 13% of providers reported that they would prescribe PrEP to non-injection drug users (Castel et al., 2015). In the current analysis, the results contradict prevailing beliefs that stimulant users are unable to achieve high levels of HIV protection in the setting of stimulant use – particularly for those reporting CAS-MP.
These analyses have several limitations. Stimulant use was documented by self-report that may be complicated by recall and social desirability bias, yet, use of CASI may minimize these biases. Combining all three stimulants may have obscured associations of smaller sub-groups (i.e., individual stimulants or high frequency use) with achieving or not achieving prevention-effective adherence. The inclusion in this study of a brief intervention based on PrEP drug concentrations with a rapid turnaround time may preclude generalizing the intervention to many real-world settings where such biomarkers and/or interventions are not routinely available or feasible. Strengths of our study include an ethnically diverse population, using biologic markers to characterize adherence, and statistical methods that take into account participants’ risk behavior changing over time in longitudinal analyses.
These analyses suggest that MSM who use stimulants can adequately adhere to PrEP over time. Stimulant use should therefore not be viewed as a contraindication to PrEP prescription; rather the increased risk of HIV acquisition associated with stimulant use in MSM increases the potential benefits of PrEP use in this population. Future studies should continue to evaluate the barriers and motivators stimulant users encounter in initiating, adhering to, and persisting on PrEP.
Acknowledgements:
We would like to acknowledge the participants in the study for their time and effort and the staff that recruited, screened, enrolled the participants, and collected the data for the parent study.
Funding: The original data collection was funded by the California HIV Research Program via Award EI11-LA-002. Gilead Sciences provided drug supply and additional support for some drug-assay testing. DGM and MRB were supported by the US National Institute on Mental Health (NIMH T32 Grant in Global HIV Prevention DA-2T32MH080634–11). This work was also supported by the UCLA Center for HIV Identification, Prevention, and Treatment Services (CHIPTS) NIMH grant P30MH58107; the UCLA Center for AIDS Research (CFAR) grant 5P30AI028697; and the UCLA Clinical Translational Science Institute (CTSI) Grant UL1TR001881. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Footnotes
Conflicts of interest: RJL has served as a consultant to and received honoraria and travel support from Gilead Sciences. KRA received an educational grant through the University of Michigan from Gilead Sciences. PLA reports grant and contract work with Gilead Sciences, paid to his institution. JFR is employed by Gilead Sciences. All other authors declare that they have no conflicts of interest.
Ethical Approval: The Institutional Review Boards at the University of California Los Angeles (approval number 12–001265) and Charles R. Drew University of Medicine and Science (approval number 13–01-2395–01) approved the parent study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All participants provided written informed consent before screening.
References
- Anderson PL, Liu AY, Castillo-Mancilla JR, Gardner EM, Seifert SM, McHugh C, et al. (2018). Intracellular Tenofovir-Diphosphate and Emtricitabine-Triphosphate in Dried Blood Spots following Directly Observed Therapy. Antimicrobial Agents and Chemotherapy, 62(1), 2587 10.1128/AAC.01710-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. (2012). Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. The New England Journal of Medicine, 367(5), 399–410. 10.1056/NEJMoa1108524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel AD, Feaster DJ, Tang W, Willis S, Jordan H, Villamizar K, et al. (2015). Understanding HIV Care Provider Attitudes Regarding Intentions to Prescribe PrEP. Journal of Acquired Immune Deficiency Syndromes, 70(5), 520–528. 10.1097/QAI.0000000000000780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closson EF, Mitty JA, Malone J, Mayer KH, & Mimiaga MJ (2017). Exploring strategies for PrEP adherence and dosing preferences in the context of sexualized recreational drug use among MSM: a qualitative study. AIDS Care, 1(4), 1–8. 10.1080/09540121.2017.1360992 [DOI] [PubMed] [Google Scholar]
- Drumright LN, Patterson TL, & Strathdee SA (2009). Club Drugs as Causal Risk Factors for HIV Acquisition Among Men Who Have Sex with Men: A Review. Substance Use & Misuse, 41(10–12), 1551–1601. 10.1080/10826080600847894 [DOI] [PubMed] [Google Scholar]
- Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, et al. (2014). Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. The Lancet Infectious Diseases, 14(9), 820–829. 10.1016/S1473-3099(14)70847-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. (2010). Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men. The New England Journal of Medicine, 363(27), 2587–2599. 10.1056/NEJMoa1011205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberer JE, Bangsberg DR, Baeten JM, Curran K, Koechlin F, Amico KR, et al. (2015). Defining success with HIV pre-exposure prophylaxis: a prevention-effective adherence paradigm. Aids, 29(11), 1277–1285. 10.1097/QAD.0000000000000647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberer JE, Kidoguchi L, Heffron R, Mugo N, Bukusi E, Katabira E, et al. (2017). Alignment of adherence and risk for HIV acquisition in a demonstration project of pre-exposure prophylaxis among HIV serodiscordant couples in Kenya and Uganda: a prospective analysis of prevention-effective adherence. Journal of the International AIDS Society, 20(1), 21842 10.7448/IAS.20.1.21842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland B, Moreira RI, De Boni RB, Kallas EG, Madruga JV, Vasconcelos R, et al. (2017). High pre-exposure prophylaxis uptake and early adherence among men who have sex with men and transgender women at risk for HIV Infection: the PrEP Brasil demonstration project. Journal of the International AIDS Society, 20(1). 10.7448/IAS.20.1.21472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenigl M, Jain S, Moore D, Collins D, Sun X, Anderson PL, et al. (2018). Substance Use and Adherence to HIV Preexposure Prophylaxis for Men Who Have Sex with Men. Emerging Infectious Diseases, 24(12). 10.3201/eid2412.180400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojilla JC, Vlahov D, Glidden DV, Amico KR, Mehrotra M, Hance R, et al. (2018). Skating on thin ice: stimulant use and sub-optimal adherence to HIV pre-exposure prophylaxis. Journal of the International AIDS Society, 21(3), e25103 10.1002/jia2.25103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosek SG, Rudy B, Landovitz R, Kapogiannis B, Siberry G, Rutledge B, et al. (2017). An HIV Preexposure Prophylaxis Demonstration Project and Safety Study for Young MSM. Journal of Acquired Immune Deficiency Syndromes, 74(1), 21–29. 10.1097/QAI.0000000000001179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley CF, Kahle E, Siegler A, Sanchez T, del Rio C, Sullivan PS, & Rosenberg ES (2015). Applying a PrEP Continuum of Care for Men Who Have Sex With Men in Atlanta, Georgia. Clinical Infectious Diseases, 61(10), 1590–1597. 10.1093/cid/civ664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koblin BA, Husnik MJ, Colfax G, Huang Y, Madison M, Mayer K, et al. (2006). Risk factors for HIV infection among men who have sex with men. Aids, 20(5), 731–739. 10.1097/01.aids.0000216374.61442.55 [DOI] [PubMed] [Google Scholar]
- Landovitz RJ, Beymer M, Kofron R, Amico KR, Psaros C, Bushman L, et al. (2017). Plasma Tenofovir Levels to Support Adherence to TDF/FTC Preexposure Prophylaxis for HIV Prevention in MSM in Los Angeles, California. Journal of Acquired Immune Deficiency Syndromes, 76(5), 501–511. 10.1097/QAI.0000000000001538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SH, Ostrow D, Stall R, Chmiel J, Herrick A, Shoptaw S, et al. (2012). Changes in stimulant drug use over time in the MACS: evidence for resilience against stimulant drug use among men who have sex with men. AIDS and Behavior, 16(1), 151–158. 10.1007/s10461-010-9866-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AY, Cohen SE, Vittinghoff E, Anderson PL, Doblecki-Lewis S, Bacon O, et al. (2016). Preexposure Prophylaxis for HIV Infection Integrated With Municipal- and Community-Based Sexual Health Services. JAMA Internal Medicine, 176(1), 75 10.1001/jamainternmed.2015.4683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. (2015). Tenofovir-Based Preexposure Prophylaxis for HIV Infection among African Women. The New England Journal of Medicine, 372(6), 509–518. 10.1056/NEJMoa1402269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina J-M, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. (2015). On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. The New England Journal of Medicine, 373(23), 2237–2246. 10.1056/NEJMoa1506273 [DOI] [PubMed] [Google Scholar]
- NIDA. (2012, March 1). Resource Guide: Screening for Drug Use in General Medical Settings. Retrieved February 1, 2019, from https://www.who.int/substance_abuse/activities/assist_v3_english.pdf
- Nunn AS, Brinkley-rubinstein L, Oldenburg CE, Mayer KH, Mimiaga M, Patel R, & Chan PA (2017). Defining the Hiv pre-exposure prophylaxis care continuum. Aids, 31(5), 731–734. 10.1097/QAD.0000000000001385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenburg CE, Mitty JA, Biello KB, Closson EF, Safren SA, Mayer KH, & Mimiaga MJ (2016). Differences in Attitudes About HIV Pre-Exposure Prophylaxis Use Among Stimulant Versus Alcohol Using Men Who Have Sex with Men. AIDS and Behavior, 20(7), 1451–1460. 10.1007/s10461-015-1226-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storholm ED, Volk JE, Marcus JL, Silverberg MJ, & Satre DD (2017). Risk Perception, Sexual Behaviors, and PrEP Adherence Among Substance-Using Men Who Have Sex with Men: a Qualitative Study. Prevention Science, 18(6), 737–747. 10.1007/s11121-017-0799-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. (2012). Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. The New England Journal of Medicine, 367(5), 423–434. 10.1056/NEJMoa1110711 [DOI] [PubMed] [Google Scholar]
- Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. (2012). Preexposure prophylaxis for HIV infection among African women. The New England Journal of Medicine, 367(5), 411–422. 10.1056/NEJMoa1202614 [DOI] [PMC free article] [PubMed] [Google Scholar]