Abstract
Chronic pain states have resulted in an over-reliance on opioid pain relievers, which can carry significant risks when used long-term. As such, alternative pain treatments are increasingly desired. Although emerging research suggests that cannabinoids have therapeutic potential regarding pain, results from studies across pain populations have been inconsistent. To provide meta-analytic clarification regarding cannabis’s impact on subjective pain, we identified studies that assessed drug-induced pain modulations under cannabinoid and corresponding placebo conditions. A literature search yielded 25 peer-reviewed records that underwent data extraction. Baseline and end-point data were used to compute standardized effect size estimates (Cohen’s d) across cannabinoid administrations (k = 39) and placebo administrations (k = 26). Standardized effects were inverse-variance weighted and pooled across studies for meta-analytic comparison. Results revealed that cannabinoid administration produced a medium-to-large effect across included studies, Cohen’s d = −0.58, 95% CI (−0.74, −0.43), while placebo administration produced a small-to-medium effect, Cohen’s d = −0.39, 95% CI (−0.52, −0.26). Meta-regression revealed that cannabinoids, β = −0.43, 95% CI (−0.62, −0.24), p < 0.05, synthetic cannabinoids, β = −0.39, 95% CI (−0.65, −0.14), p < 0.05, and sample size, β = 0.01, 95% CI (0.00, 0.01), p < 0.05, were associated with marked pain reduction. These outcomes suggest that cannabinoid-based pharmacotherapies may serve as effective replacement/adjunctive options regarding pain, however, additional research is warranted. Additionally, given demonstrated neurocognitive side-effects associated with some constituent cannabinoids (i.e., THC), subsequent work may consider developing novel therapeutic agents that capitalize on cannabis’s analgesic properties without producing adverse effects.
Keywords: Cannabis, Cannabinoid, Marijuana, Pain, Meta-Analysis
Chronic pain is an ever-growing concern in the United States. There is a rising economic burden – currently estimated to be between $560 billion and $635 billion annually – that stems from pain-related costs to patients, patient-care providers, healthcare systems, and poor treatment outcomes among clinical pain populations (e.g., chronic lower back pain, neuropathic pain, and fibromyalgia) (Henschke, Kamper, & Maher, 2015). These, and other, conditions have resulted in an over-reliance on opioid-based pharmacotherapies. Although some patients are appropriate for focused treatments involving opioids (e.g., acute pain), patients with more chronic conditions (e.g., cancer) can achieve better outcomes by managing pain through more comprehensive approaches (Chou et al., 2009). Thus, it has become increasingly important to explore additional therapeutic opportunities. In recent decades, cannabinoids – such as molecular compounds found in cannabis, including delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) – have been considered viable treatment options regarding pain (Savage et al., 2016). As recently as 2018, 30 states had enacted policies that permit cannabis use to treat various medical conditions, with 27 states citing pain-related conditions as inclusionary criteria. Despite growing access to medicinal cannabis, mixed (and on occasion, null) effects have been reported, underscoring the need to expand research efforts regarding cannabinoid-induced pain mitigation.
Recently, several reports have examined cannabinoid administration effects on subjective reports of pain (Hill, Palastro, Johnson, & Ditre, 2017). However, these accounts have produced variant, and sometimes contradictory, conclusions. In one example, Johnson and colleagues (2010) examined the impact of nabiximols (Sativex®), a standardized whole-plant cannabis extract oromucosal spray, on cancer-related pain. In that double-blind, randomized controled trial (RCT), patients with intractable cancer pain entered a two-week administration regimen and received THC:CBD extract (2.7 mg THC and 2.5 mg CBD), THC extract (2.7 mg THC), or placebo. Patients were free to titrate their dosage as needed. Following the drug administration regimen, Johnson et al. observed significant reductions in subjective pain ratings among patients receiving THC:CBD extract compared to patients receiving placebo. THC alone was less effective. In a similar example, Portenoy and colleagues (2012) evaluated nabiximols as an add-on therapy for advanced cancer patients with opioid-refractory (unresponsive) pain. Patients were placed into low-, medium-, or high-administration conditions and pain was measured following a five-week intervention interval. At the end of treatment, Portenoy et al. found that THC:CBD extract was associated with greater pain reduction in the low-administration condition (1–4 sprays/day), but not in the medium-administration (6–10 sprays/day) or high-administration conditions (11–16 sprays/day). Taken together, these outcomes suggest that cannabinoids may represent potential pharmacological tools for pain reduction. On the other hand, several studies have shown no difference between cannabinoids and corresponding placebo administrations. For example, Lichtman and colleagues (2018) leveraged a double-blind RCT to examine pain outcomes among cancer patients with uncontrolled pain following a two-week nabiximols administration period. Following the intervention, Lichtman et al. compared pain modulations from baseline between cannabinoid and placebo conditions, revealing no superior effects associated with THC:CBD extract. Moving forward, an important challenge facing biomedical research involves coalescing results from studies involving various pain populations receiving cannabinoid administrations to determine overall therapeutic potential.
Towards this goal, several systematic reviews have endeavored to summarize cannabis’s putative pain-related therapeutic effects (Abrams, 2018; Campbell et al., 2001; Colombo, Annovazzi, & Comi, 2006; Deshpande, Mailis-Gagnon, Zoheiry, & Lakha, 2015; Lynch & Campbell, 2011; Lynch & Ware, 2015; Sznitman & Zolotov, 2015; Wright, 2007). These reviews have provided competing conclusions. In one review, Campbell and colleagues (2001) considered outcomes from nine randomized active- and placebo-controlled trials involving cannabinoids (five trials involved cancer-related pain, two involved chronic pain, and two acute post-operative pain), with a focus on pain intenseness scores, pain relief scores, and adverse effects. Those authors concluded that the cannabinoids considered were no more effective than active control conditions, including the opioid analgesic codeine, stressing that cannabinoid administration to treat post-operative pain would be “undesirable,” given unwanted central nervous system depressant effects. However, opioids have also been linked with depressant/sedative effects (Chou et al., 2009). Moreover, other – perhaps more severe – opioid-related adverse effects include respiratory depression, especially when paired with other substances, such as benzodiazepines and alcohol (Chou et al., 2009). Given the abuse potential associated with opioids, these (and other) side-effects underscore the need to consider replacement and/or adjunctive pain management approaches. Additionally, Campbell et al. noted that, among RCTs considered in the systematic review, none had examined active cannabis. That is, the trials examined pain reduction associated with THC, nitrogen-containing benzopyran derivative, benzopyranoperidine, or levonantradol. Importantly, cannabinoid-induced analgesia may stem from compound or synergistic effects associated with several cannabinoids. For example, preclinical evidence suggests that high-dose CBD modulates antinociceptive effects associated with low-dose THC, indicating that both cannabinoids may be involved in pain reduction (Varvel et al., 2006). Furthermore, work from Comelli and colleagues (Comelli, Giagnoni, Bettoni, Colleoni, & Costa, 2008) demonstrates that whole-plant cannabis extract provides improved nociceptive efficacy compared to corresponding doses of constituent cannabinoids. As such, as the corpus of cannabis-related pain investigations continues growing, it is possible that more comprehensive assessments could reach alternative conclusions regarding cannabinoid analgesia. In a more recent review, The National Academies of Sciences, Engineering and Medicine considered more than 10,000 peer-reviewed abstracts to characterize cannabis’s potential therapeutic utility across several domains, including pain. That committee concluded that “there was conclusive or substantial evidence that Cannabis or cannabinoids are effective for the treatment of pain in adults,” (Abrams, 2018). However, narrative and systematic reviews often omit representative estimates of effect magnitude and therefore cannot provide quantitative conclusions about outcomes of interest. As such, objective techniques that determine statistical convergence across published studies involving cannabinoid-induced pain reduction are needed to more accurately characterize potential therapeutic effects.
Meta-analyses present powerful opportunities to coalesce conventional effect size estimates (e.g., Cohen’s d) across published studies, providing clarification regarding results and permitting assessments not possible within the original, single report. Within this framework, several study-level effect size estimates derived under comparable experimental conditions are averaged, producing one pooled (representative) effect size estimate (Hedges & Olkin, 1985). Regarding pharmacologic manipulations, pooled effect sizes are used to characterize cross-study drug administration effects on specific end-points (Wilkinson et al., 2018), or to make comparisons between two (or more) drug administration conditions (Bushe et al., 2016). Towards this goal, several meta-analyses have provided some insight into cannabinoid-related pain reduction (Andreae et al., 2015; Aviram & Samuelly-Leichtag, 2017; De Vita, Moskal, Maisto, & Ansell, 2018; Goldenberg, Reid, IsHak, & Danovitch, 2017; Iskedjian, Bereza, Gordon, Piwko, & Einarson, 2007; Martin-Sanchez, Furukawa, Taylor, & Martin, 2009; Phillips, Cherry, Cox, Marshall, & Rice, 2010; Whiting et al., 2015). For example, Iskedjian and colleagues (2007) synthesized results from six studies examining cannabinoid administration within the limited context of multiple sclerosis (MS). When considering baseline versus end-point pain ratings among 298 patients, Iskedjian et al. observed that cannabinoids were associated with greater pain reduction relative to placebo. However, whether these effects extend beyond MS-related pain (e.g., neuropathic pain) remained unclear. In a more comprehensive meta-analysis, Aviram and Samuelly-Leichtag (2017) examined pain reduction associated with cannabinoid-based medicines across 24 RCTs. Those researchers considered several pain populations, including neuropathic pain, cancer-related pain, non-cancer pain, and post-operative pain, as well as active-control and placebo-control designs. Overall, Aviram and Samuelly-Leichtag reported “limited” support for cannabinoid-based medicines across considered RCTs. However, a more focused assessment that excluded active-control designs – which were believed to have increased analgesic efficacy compared to placebo – demonstrated improved analgesic outcomes associated with cannabinoid-based medicines. Surprisingly, the extent to which specific study-level characteristics, such as sample size, age, and sex composition (sex ratio), may modulate observed pain outcomes remains to be meta-analytically explored. Indeed, these active research areas have received considerable attention in recent years (Lauer, 2016). Here, we address this open-ended question using meta-regression to examine cannabinoid- and placebo-related pain reduction with respect to several study-level characteristics (Baker et al., 2009).
To determine cross-study cannabinoid-related standardized effect sizes regarding self-reported pain reduction, and to examine potential associations with important study-level characteristics, we leveraged a combined meta-analysis and meta-regression approach. In a primary assessment, we used meta-analysis techniques to coalesce drug-induced pain reduction standardized effect sizes associated with cannabinoid and placebo administrations to produce pooled effects and enable statistical comparison. In a second assessment (Glass, Smith, & McGaw, 1981), we used meta-regression to examine relationships between various continuous and categorical explanatory variables and drug-induced pain reduction effect sizes. Specifically, we used multiple linear regression to examine relationships between several study-level characteristics (sample size, age, sex composition, experimental design, and pain population) and drug administration conditions. Overall, we posited that cannabinoid administration would be associated with pain reduction across included studies, and that placebo administration would be less effective. Furthermore, we expected that study-level characteristics would be associated with pain reduction standardized effect sizes. Providing clarification about potential pain-mitigating effects associated with cannabinoids should enable enhanced scientific understanding about possible therapeutic applications.
Methods
Search
We conducted a literature search to identify pharmacological manipulation studies that assessed cannabinoid-induced alterations in subjective pain ratings. Primary searches were carried out using PudMed (www.ncbi.nlm.nih.gov/pumed/) and Web of Science (http://webofknowledge.com) with the search terms: cannabis OR cannabinoids OR delta-9-tetrahydrocannabinol OR THC OR cannabidiol OR CBD OR marijuana OR nabilone OR dronabinol OR nabiximols AND pain OR noxious OR analgesia OR visual analog scale OR VAS OR numeric rating scale OR NRS. We further reviewed the reference sections of each record identified during the exhaustive search, in particular, systematic and narrative review papers (Campbell et al., 2001; Colombo et al., 2006; Deshpande et al., 2015; Lynch & Campbell, 2011; Lynch & Ware, 2015; Sznitman & Zolotov, 2015; Wright, 2007) and existing meta-analyses (Andreae et al., 2015; Aviram & Samuelly-Leichtag, 2017; De Vita et al., 2018; Goldenberg et al., 2017; Iskedjian et al., 2007; Martin-Sanchez et al., 2009; Phillips et al., 2010)
Screen
During screening, record abstracts were inspected to determine appropriateness. Specifically, records that did not represent peer-reviewed original research studies were removed from the meta-analysis review pipeline (e.g., letters to editors, reviews, conference proceedings). Records involving non-human models were also not considered. This meta-analysis was restricted to RCTs that: (A) assessed drug-induced pain reductions following cannabinoid administration across studies, including whole-plant cannabis, whole-plant cannabis extracts, and synthetic cannabinoids (i.e., Dronabinol, Nabilone, CT3), and corresponding active or placebo administrations, (B) described pain reductions as differences between baseline (pre-administration) and end-point (post-administration) measurements, and (C) used a parallel-groups (i.e., independent samples) or crossover (i.e., repeated measures) design to examine pain reductions. Importantly, although active control studies were considered in the current meta-analysis (Frank, Serpell, Hughes, Matthews, & Kapur, 2008; Pini et al., 2012), drug-induced pain reductions associated with active control administration (e.g., ibuprofen) were not included in placebo sub-group analyses. The current meta-analysis reflects papers published through August 2018.
Data Extraction and Primary Meta-Analysis
Remaining records were obtained as complete published articles and assessed by two reviewers (J.A.Y and Z.E.M). Reviewers cross-checked extracted data points and resolved disagreement before commencing meta-analyses. Extracted data points included: author, publication year, sample size(s), pharmacological manipulation(s) (whole-plant cannabis, whole-plant cannabis extract, synthetic cannabinoid, and placebo), pain population (pain linked with various medical conditions), baseline mean pain score, end-point mean pain score, and associated variance estimates. Studies that involved more than two (ki) administration conditions (e.g., THC:CBD extract, THC extract, and placebo) contributed ki (k = 3) mean gain standardized effect sizes to quantitative assessment, where k describes total standardized effect sizes considered in the current meta-analysis. Because we sought to pool cannabinoid-related standardized effect sizes across included studies, and because we sought to pool placebo-related standardized effect sizes across included studies, baseline and end-point pain severity scores were extracted from cannabinoid and placebo conditions separately. Studies that omitted baseline and/or end-point pain severity scores were excluded. When required, pain severity scores were computed using available summary data (e.g., mean pain percent reduction (Abrams et al., 2007; Johnson et al., 2010; Langford et al., 2013; Narang et al., 2008; Skrabek, Galimova, Ethans, & Perry, 2008). Data points collected from one record required reverse scoring (Wade, Robson, House, Makela, & Aram, 2003). Although baseline and end-point pain severity scores were necessary for inclusion, several records omitted associated variance estimates (Abrams et al., 2007; Blake, Robson, Ho, Jubb, & McCabe, 2006; Buggy et al., 2003; Chou et al., 2009; Corey-Bloom et al., 2012; Johnson et al., 2010; Karst et al., 2003; Langford et al., 2013; Narang et al., 2008; Nurmikko et al., 2007; Portenoy et al., 2012; Rog, Nurmikko, Friede, & Young, 2005; Skrabek et al., 2008; Svendsen, Jensen, & Bach, 2004; Wilsey et al., 2008). In such cases, we employed several strategies to secure missing variance data. First, we contacted the lead and/or corresponding authors with data requests. Second, to supplement remaining records, we leveraged the freely available service WebPlotDigitizer (https://automeris.io/WebPlotDigitizer) to compute variance estimates using manuscript figures – an accepted technique to extract numeral data from data visualizations (Rohatgi, 2018). Third, when data requests and data extraction from visualizations were not possible, missing variance estimates were reconciled via mean imputation using assembled variance estimates (Cooper, Hedges, & Valentine, 2009). Notably, imputed variance estimates represented approximately 35% (46/130) of total variance data. Outcome measures included quantitative pain-rating scales, such as numeric rating scales (NRS) (Hartrick, Kovan, & Shapiro, 2003) and visual analog scales (VAS) (Ferraz et al., 1990). Quantitative pain-rating scales involve asking participants to describe pain severity, routinely anchored by zero, indicating “no pain,” and 10, indicating “worst pain.” Results from studies using 100-point ranges were scaled to enable pooling and comparison.
Following data extraction, baseline pain severity scores, end-point pain severity scores, and associated variance estimates, were used to compute study-level standardized mean gain effect size (i.e., Cohen’s d) (Becker, 1988). Standardized effect sizes were used to calculate associated standard errors (Lipsey & Wilson, 2001) and confidence intervals (Nakagawa & Cuthill, 2007). To facilitate meta-analytic comparison, study-level standardized effect sizes were then inverse-variance weighted and pooled to produce an average cannabinoid-induced effect and an average placebo-induced effect (Hedges & Olkin, 1985). Monte Carlo simulations suggest that inverse-variance weighting produces optimal pooled effect sizes in meta-analysis assessments (Sánchez-Meca & Marin-Martinez, 1998). Forest plots were created to visualize standardized effect sizes. We assessed the degree to which variation among cannabinoid and placebo administrations was attributed to chance via the I2 statistic and associated confidence intervals (Higgins & Thompson, 2002; Higgins, Thompson, Deeks, & Altman, 2003). Pooled effects were compared with an independent-samples mean difference test (Hedges & Olkin, 1985; Hedges & Pigott, 2001).
Multiple Linear Regression (Meta-Regression)
Meta-regression examines the relationships between continuous and/or categorical explanatory variables (e.g., sample size, sample age, sample sex composition) and a continuous outcome variable (e.g., study-level standardized effect sizes) (Green & Higgins, 2005). Specifically, we used an exploratory fixed-effects multiple linear regression (meta-regression) approach (Greenland, 1987; Luebke & Brunkwall, 2015), to explore relationships between pain reduction effects and: drug administration condition [placebo, cannabinoid (whole-plant, whole-plant extract), synthetic cannabinoid (Dronabinol, Nabilone, CT3)], sample size (reported sample size), sample age (mean sample age), sample sex composition (sample sex ratio), experimental design (parallel versus crossover), and pain population (abdominal pain, arthritis, cancer, chronic pain, diabetes, fibromyalgia, headache, human immunodeficiency virus (HIV), multiple sclerosis, neuropathic pain, post-operative pain, and “various,” or mixed pain populations within one effect). Data were examined using statistical assumptions associated with regression, including normality, residual normality, and equal variances. Outliers among standardized effect sizes [i.e., median effect +/− interquartile range (IQR) × 1.5] were adjusted using upper/lower quartile replacement (Tukey, 1977). Categorical variables (e.g., placebo, cannabinoid, synthetic cannabinoid) were dummy coded to facilitate meta-regression assessment (Wolf & Cartwright, 1974).
Ethics and Open Science Practices
As is common with meta-analytic assessments, the current report did not involve human subjects and therefore did not require institutional review board approval (Sullivan, 2011). In line with current recommendations and open science best practices (Open Science, 2015), we have made meta-data and corresponding code associated with this work freely available on GitHub (https://doi.org/10.5281/zenodo.1463262).
Results
Primary Meta-Analysis
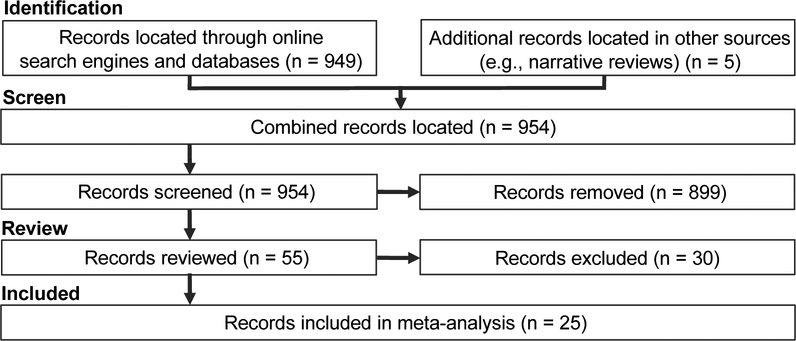
Literature search and review results are depicted in Table 1 and Figure 1. The search produced 954 records which underwent screening. Using exclusion criteria described above, 899 records were removed during abstract review, and another 30 were removed during full-text review. The current meta-analysis included data from 25 records that met inclusion criteria, providing data from k = 65 individual pharmacologic manipulations (39 cannabinoid manipulations versus 26 placebo manipulations), involving 2,248 participants. On average, studies reported that participants’ mean age ranged from 43.50 to 62.80 years (mean = 52.09). Included studies assessed drug-induced pain reductions associated with several cannabinoid administration conditions, including whole-plant cannabis (n = 5), whole-plant cannabis extract (n = 11), and synthetic cannabinoids (n = 9). Pain-related clinical samples (pain populations) considered were neuropathic pain (n = 7), cancer (n = 4) diabetes (n = 3), MS (n = 3), abdominal pain (n = 1), arthritis (n = 1), chronic pain (n = 1), fibromyalgia (n = 1), headache (n = 1), HIV (n = 1), post-operative pain (n = 1), and “various” (n = 1). Standardized effect sizes are organized according to pain population in Supplemental Figure 1. On average, studies reported that 51.57% of participants were women. Fifteen studies provided data from parallel-group designs and 10 provided data from crossover designs.
Table 1. Studies Meeting Inclusion Criteria.
Numbering corresponds to studies meeting inclusion criteria. Extracted variables were administration condition (administration), including cannabis whole plant, cannabis extract, synthetic cannabinoid, and placebo, administration dose (dose), administration route (route), population with pain-related clinical condition (pain population), subjective pain outcome measure (pain measure), and associated scale (scale). THC, delta-9-tetrahydrocannabinol; CBD, cannabidiol. CT3, dimethylheptyl-delta-8-tetrahydrocannabinol-11-oic acid; HIV, human immunodeficiency virus;
| No. | Author | Year | Details Regarding Sampled Studies | |||||
|---|---|---|---|---|---|---|---|---|
| Administration | Dose | Route | Pain Population | Pain Measure | Scale | |||
| 1 | Abrams et al. | 2007 | Whole Plant | 3.56 % THC | Smoke | HIV | Visual Analog Scale | 0 – 100 |
| 2 | Blake et al. | 2006 | Extract (Sativex) | 2.7 mg THC / 2.5 mg CBD | Oromucosal Spray | Arthritis | Numeric Rating Scale | 0 – 10 |
| 3 | Buggy et al. | 2003 | Extract | 5.0 mg THC | Capsule | Post-Operation | Visual Analog Scale | 0 – 10 |
| 4 | Corey-Bloom et al. | 2012 | Whole Plant | 4.0% THC | Cigarette | Multiple Sclerosis | Visual Analog Scale | 0 – 100 |
| 5 | De Vries et al. | 2017 | Synthetic (Dronabinol; | 8 mg | Tablet | Abdominal Pain | Visual Analog Scale | 0 – 10 |
| De Vries et al. | 2017 | Synthetic (Dronabinol) | 8 mg | Tablet | Abdominal Pain | Visual Analog Scale | 0 – 10 | |
| 6 | Fallon et al. | 2017 | Extract (Sativex) | 2.7 mg THC / 2.5 mg CBD | Capsule | Cancer | Numeric Rating Scale | 0 – 10 |
| 7 | Frank et al. | 2008 | Synthetic (Nabilone) | 0.25 mg | Capsule | Neuropathic Pain | Visual Analog Scale | 0 – 100 |
| 8 | Johnson et al. | 2010 | Extract (Sativex) | 2.7 mg THC / 2.5 mg CBD | Capsule | Cancer | Numeric Rating Scale | 0 – 10 |
| Extract | 2.7 mg THC | Capsule | Cancer | Numeric Rating Scale | 0 – 10 | |||
| 9 | Karst et al. | 2003 | Synthetic (CT-3) | 10.0 mg | Capsule | Neuropathic Pain | Visual Analog Scale | 0 – 100 |
| 10 | Langford et al. | 2012 | Extract (Sativex) | 2.7 mg THC / 2.5 mg CBD | Oromucosal Spray | Neuropathic Pain | Numeric Rating Scale | 0 – 10 |
| 11 | Lichtman et al. | 2017 | Extract (Sativex) | 2.7 mg THC / 2.5 mg CBD | Cancer | Numeric Rating Scale | 0 – 10 | |
| 12 | Narang et al. | 2008 | Synthetic (Dronabinol) | 20.0 mg | Capsule | Chronic Pain | Numeric Rating Scale | 0 – 10 |
| 2008 | Synthetic (Dronabinol) | 10.0 mg | Capsule | Chronic Pain | Numeric Rating Scale | 0 – 10 | ||
| 13 | Nurmikko et al. | 2007 | Extract (Sativex) | 2.7 mg THC / 2.5 mg CBD | Oromucosal Spray | Neuropathic Pain | Numeric Rating Scale | 0 – 10 |
| 14 | Pini et al. | 2012 | Synthetic (Nabilone) | 0.5 mg | Capsule | Headache | Visual Analog Scale | 0 – 10 |
| 15 | Portenoy et al. | 2012 | Extract (Sativex) | 2.7 mg THC / 2.5 mg CBD | Oromucosal Spray | Cancer | Visual Analog Scale | 0 – 10 |
| Extract (Sativex) | 2.7 mg THC / 2.5 mg CBD | Oromucosal Spray | Cancer | Visual Analog Scale | 0 – 10 | |||
| Extract (Sativex) | 2.7 mg THC / 2.5 mg CBD | Oromucosal Spray | Cancer | Visual Analog Scale | 0 – 10 | |||
| 16 | Rog et al. | 2007 | Extract (Sativex) | 2.7 mg THC / 2.5 mg CBD | Oromucosal Spray | Neuropathic Pain | Numeric Rating Scale | 0 – 10 |
| 17 | Schimrigk et al. | 2017 | Synthtic (Nabilone) | 7.5 mg – 15.0 mg | Capsule | Multiple Sclerosis | Numeric Rating Scale | 0 – 10 |
| 18 | Selvarajah et al. | 2010 | Extract (Sativex) | 2.7 mg THC / 2.5 mg CBD | Oromucosal Spray | Diabetes | Visual Analog Scale | 0 – 100 |
| 29 | Skrabek et al. | 2007 | Synthetic (Nabilone) | 0.5 mg | Capsule | Fibromyalgia | Visual Analog Scale | 0 – 10 |
| 20 | Svedson et al. | 2004 | Synthetic (Dronabinol) | 2.5 mg – 10.0 mg | Capsule | Multiple Sclerosis | Numeric Rating Scale | 0 – 10 |
| 2004 | Synthetic (Dronabinol) | 2.5 mg – 10.0 mg | Capsule | Multiple Sclerosis | Numeric Rating Scale | |||
| 21 | Toth et al. | 2012 | Synthetic (Nabilone) | 2.0 mg – 4.0 mg | Capsule | Diabetes | Numeric Rating Scale | 0 – 10 |
| 22 | Wade et al. | 2003 | Extract | 2.5 mg THC / 2.5 mg CBD | Oromucosal Spray | Various | Visual Analog Scale | 0 – 100 |
| Extract | 2.5 mg THC | Oromucosal Spray | Various | Visual Analog Scale | 0 – 100 | |||
| Extract | 2.5 mg CBD | Oromucosal Spray | Various | Visual Analog Scale | 0 – 100 | |||
| 23 | Wallace et al. | 2015 | Whole Plant | 7% THC | Humidified | Diabetes | Visual Analog Scale | 0 – 10 |
| Whole Plant | 4% THC | Humidified | Diabetes | Visual Analog Scale | 0 – 10 | |||
| Whole Plant | 1 % THC | Humidified | Diabetes | Visual Analog Scale | 0 – 10 | |||
| 24 | Ware et al. | 2010 | Whole Plant | 9.4 % THC | Smoke | Neuropathic Pain | Numeric Rating Scale | 0 – 10 |
| Whole Plant | 6.0 % THC | Smoke | Neuropathic Pain | Numeric Rating Scale | 0 – 10 | |||
| Whole Plant | 2.5 % THC | Smoke | Neuropathic Pain | Numeric Rating Scale | 0 – 10 | |||
| 25 | Wisley et al. | 2008 | Whole Plant | 7.0 % THC | Smoke | Neuropathic Pain | Visual Analog Scale | 0 – 100 |
| Whole Plant | 3.5 % THC | Smoke | Neuropathic Pain | Visual Analog Scale | 0 – 100 | |||
Figure 1. Literature Search and Review Pipeline.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) pipeline diagram showing search and review results. A preliminary search produced 949 records, with an additional 5 assembled from additional resources (e.g., narrative reviews), totaling 954 records overall. During abstract review, 899 records were removed from the meta-analysis pipeline. During complete manuscript review, an additional 30 records were discarded based on study exclusion criteria. Finally, the 25 remaining records underwent data extraction and subsequent meta-analytic assessment.
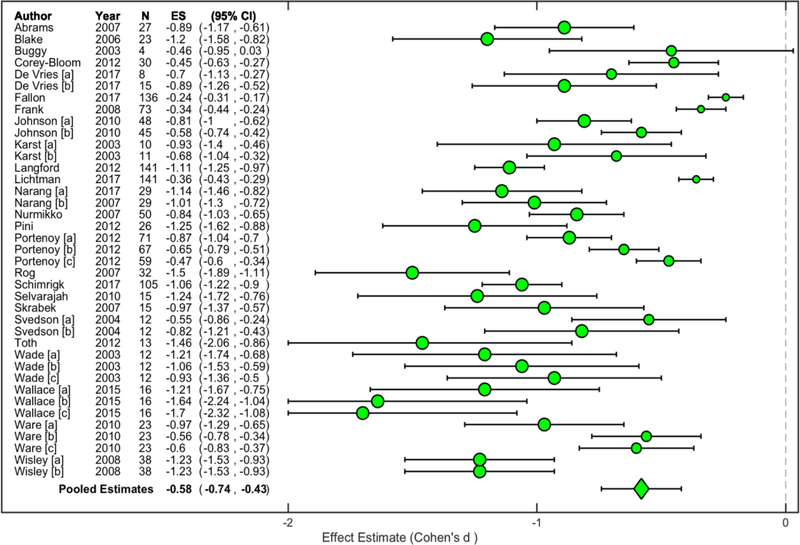
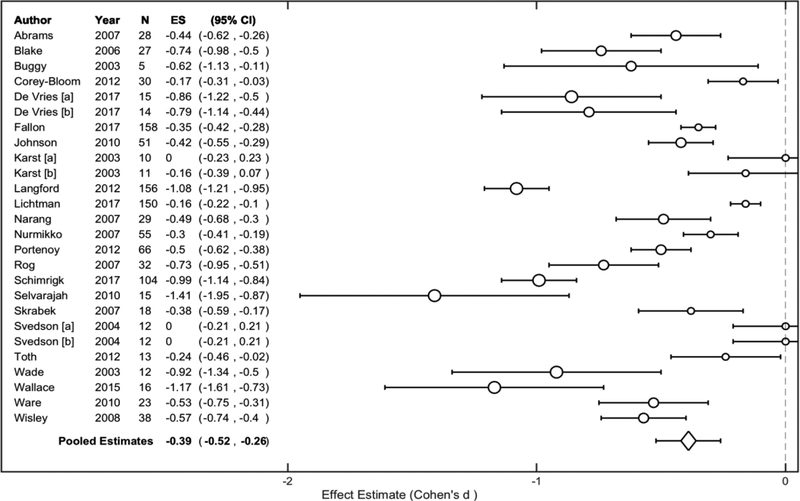
Inverse-variance weighting and pooling across cannabinoid standardized effect sizes revealed that cannabinoid administration was associated with a ‘medium-to-large’ effect, Cohen’s d = −0.58, 95% CI (−0.74, −0.43) (Figure 2). An assessment of variation revealed considerable heterogeneity among cannabinoid effect sizes, I2 = 91.47%, 95% CI (87.93%, 92.37%). On the other hand, inverse-variance weighting and pooling across placebo standardized effect sizes revealed that placebo administration was associated with a ‘small-to-medium’ effect, Cohen’s d = −0.39, 95% CI (−0.52, −0.26) (Figure 3). An assessment of variation revealed considerable heterogeneity among placebo effect sizes, I2 = 92.66%, 95% CI (89.18%, 93.70%). Overall, cannabinoid administration was associated with greater pain reduction compared to placebo administration, t (64) = −4.06, p < 0.05. Visual inspection revealed some overlap between drug administration condition confidence intervals.
Figure 2. Pooled Cannabinoid Administration Effect.
Study-level standardized effect size estimates (Cohen’s d) were computed for each cannabinoid administration across included studies. Circle sizes are proportional to small, medium, and large effect size estimate interpretations (Cohen, 1988). Study-level estimates were inverse-variance weighted and pooled to determine a representative estimate. When considering overall pain reduction effects, cannabinoid administration was associated with a medium-to-large effect across studies, Cohen’s d = −0.58, 95% CI (−0.74, −0.43).
N, sample size; ES, standardized effect size estimate; CI, confidence interval.
Figure 3. Pooled Placebo Administration Effect.
Study-level standardized effect size estimates (Cohen’s d) were computed for each placebo administration across included studies. Circle sizes are proportional to small, medium, and large effect size estimate interpretations (Cohen, 1988). Study-level estimates were inverse-variance weighted and pooled to determine a representative estimate. When considering overall pain reduction effects, placebo administration was associated with a small-to-medium effect across studies, Cohen’s d = −0.39, 95% CI (−0.52, −0.26).
N, sample size; ES, standardized effect size estimate; CI, confidence interval.
Exploratory Multiple Linear Regression (Meta-Regression)
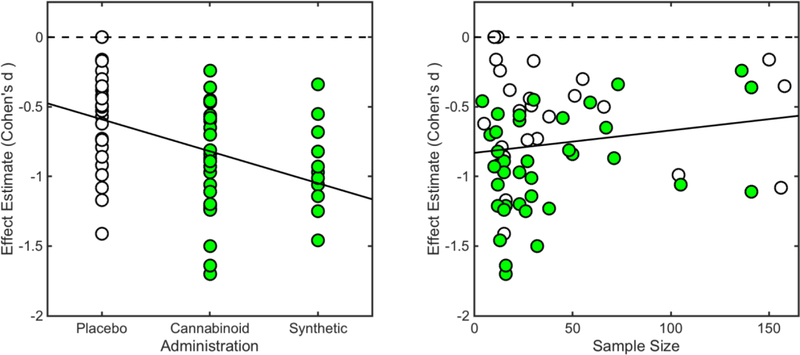
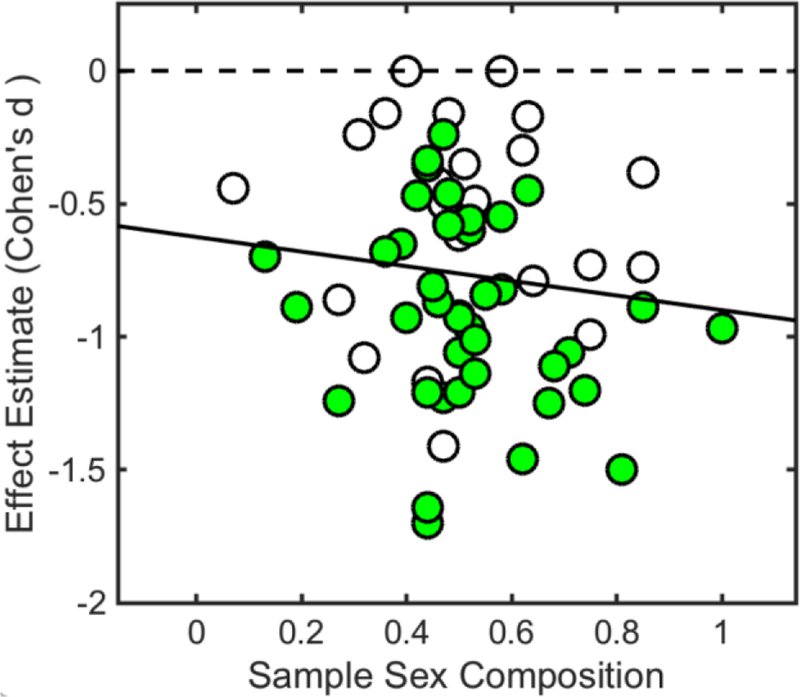
Overall, the meta-regression model explained a moderate proportion of variance among individual studies, R2 = 0.37 (adjusted R2 = 0.30), F (6,48) = 4.62, p < 0.05. Reported p-values are associated with corresponding coefficient hypotheses tests. Meta-regression results revealed that, when controlling for other explanatory variables, drug administration conditions were linked with pain reduction among included studies, such that cannabinoids (whole-plant cannabis and whole-cannabis extracts) β = −0.43, 95% CI (−0.62, −0.24), p < 0.05 (Figure 4), and synthetic cannabinoids (Dronabinol, Nabilone, and CT3) β = −0.39, 95% CI (−0.65, −0.14), p < 0.05 (Figure 4), performed better than placebo. Furthermore, meta-regression results showed that, when controlling for other explanatory variables, sample size was linked with pain reduction, β = 0.01, 95% CI (0.00, 0.01), p < 0.05, such that studies involving smaller samples tended to report greater pain reduction effects (Figure 4). There were no observed interactions between drug administration conditions and sample size. Finally, meta-regression results showed that, when controlling for other explanatory variables, sample sex composition was linked with a modest, however non-significant, effect, β = −0.64, 95% CI (−1.37, 0.09), p = 0.09, such that studies including more female participants tended to report greater pain reductions (Figure 5).
Figure 4. Bivariate Relationship Between Effect Size Estimates and Significant Predictors.
Meta-regression results revealed that, when controlling for other explanatory variables, drug administration conditions were linked with pain reduction among included studies, such that cannabinoids (whole-plant cannabis and whole-cannabis extracts) β = −0.43, 95% CI (−0.62, −0.24), p < 0.05, and synthetic cannabinoids (Dronabinol, Nabilone, and CT3) β = −0.39, 95% CI (−0.62, −0.24), p < 0.05, performed better than placebo. Furthermore, meta-regression results showed that, when controlling for other explanatory variables, sample size was linked with pain reduction, β = 0.01, 95% CI (0.00, 0.01), p < 0.05, such that studies involving smaller samples tended to report greater pain reduction.
Cannabinoids = shaded (green) circles; placebo = unshaded circles.
Figure 5. Bivariate Relationship Between Effect Size Estimates and Sample Sex Composition (Sex Ratio).
Meta-regression results showed that, when controlling for other explanatory variables, sample sex composition was linked with a modest, albeit non-significant, effect, β = −0.64, 95% CI (−1.37, 0.09), p = 0.09, such that studies including more female participants tended to report greater pain reductions.
Cannabinoids = shaded (green) circles; placebo = unshaded circles.
Discussion
In this meta-analytic study, we coalesced results from peer-reviewed primary research articles that characterized cannabinoid- and placebo-induced reductions of subjective pain ratings across medical conditions. Our findings extend current understanding about cannabinoids and pain, taking a meta-regression approach to examine relationships between various study-level characteristics and drug-induced pain reductions. When considering reductions in self-reported pain, we observed that cannabinoid administration was associated with a ‘medium-to-large’ (Cohen, 1988) pooled effect size across included studies. Importantly, cannabinoid administration was associated with statistically significant greater pain reduction than placebo administration, which yielded a ‘small-to-medium’ (Cohen, 1988) pooled effect size. Indeed, placebo administration has been shown to enhance expectations about pain reduction (Bushe et al., 2016), potentially assuaging negative emotional/motivational aspects about pain experiences. Finally, results from our meta-regression analysis suggested that, when controlling for other explanatory variables, drug administration conditions and sample size predicted observed pain reduction. Taken together, these meta-analytic outcomes provide some evidence that cannabinoids, relative to placebo, might mitigate subjective pain reporting among those experiencing chronic pain tied to various medical conditions. However, more research is needed to understand nuances in cannabinoid-induced pain reduction, including outcome differences between single-dose versus long-term cannabinoid treatments, complex interactions with concurrent analgesic pharmacotherapies, and changes in cannabis conditional dependence rates as a function of increased access.
Neuropsychological Impact of Cannabinoid-Based Administrations
When considering cannabis’s effect on pain, our primary meta-analysis outcomes suggest that cannabinoids may represent a viable option regarding pain management and treatment- outperforming corresponding placebo conditions across included studies. That cannabinoids were associated with pain reduction is not surprising, given that the most common medicinal cannabis applications throughout documented human history involve administration for pain (Parker, 2017). Indeed, early evidence suggests that medicinal cannabis may have been used to relieve pain around 400 CE (Zlas et al., 1993). However, it was just in the 1990s that several reports described an endogenous cannabinoid framework embedded within the central nervous system (Devane et al., 1992) and peripheral nervous system (Munro, Thomas, & Abu-Shaar, 1993), which interacts with exogenous cannabinoids to modulate pain.
Processing pain signals starts with nociceptive sensation signal transduction throughout the peripheral nervous system and terminates with subjective pain perception within the central nervous system (for an exteded review, see Millan, 1999). First, peripheral sensory neurons detect noxious stimulation, which is then communicated to neuronal bodies around the spinal column. Next, sensory neurons synapse onto central dorsal horn neurons within the spinal cord, where pain signals are integrated across pathways. Finally, central dorsal horn neurons forward pain signals via ascending pathways to the brainstem, thalamus, and cortical brain regions, which process higher-order pain behavior. Notably, cannabinoid receptors are densely concentrated in the frontal and limbic cortices- brain regions also associated with processing pain, including the anterior cingulate cortex (ACC) (Glass, Faull, & Dragunow, 1997). As such, cannabinoid receptor agonists may work to mitigate subjective pain experiences via interactions with brain regions responsible for processing more complex mental operations, such as pain-related affective and motivational dimensions. Consistent with such an interpretation, recent reports have examined the relationship between cannabis and pain-related brain function. For example, Lee and colleagues (2013) used functional magnetic resonance imaging (fMRI) to investigate cannabis’s impact on blood-oxygen-level dependent (BOLD) signal fluctuations in response to experimental chemical pain (i.e., capsaicin) among normal participants. Those researchers observed that, when compared to placebo, cannabinoid administration (i.e., 15 mg THC) reduced pain unpleasantness, but not pain intenseness. That is, cannabinoid administration may modulate pain perception (unpleasantness) without affecting pain sensation (intenseness), a position supported by a recent meta-analysis of cannabinoid-induced modulations in experimental pain (De Vita et al., 2018). Moreover, cannabinoid-induced reductions in pain unpleasantness correlated with less ACC activation. Indeed, ACC functioning has been implicated in various affective-motivational components in higher-order pain processing, such as conditioned place avoidance (Johansen, Fields, & Manning, 2001; LaGraize, Labuda, Rutledge, Jackson, & Fuchs, 2004), perceived threat from noxious stimulation (Foltz & White Jr, 1962), and monitoring survival-relevant goals (Lieberman & Eisenberger, 2015). Although acute cannabinoid receptor agonism dampens ACC responding to pain, effectively reducing pain-related negative affect, whether these effects endure beyond acute administration remains unclear. In a recent neuroimaging meta-analysis, Yanes and colleagues (2018) examined neurofunctional alterations associated with chronic cannabis use. When considering cannabis’s impact across various mental tasks, those researchers observed that chronic cannabis was linked with, among other changes, decreased ACC activation. Furthermore, ancillary assessments revealed that activity within the ACC has been consistently linked with pain-related taxonomic descriptors (i.e., Pain, Pain Monitor/Discrimination) across the functional neuroimaging literature. To summarize, the neurobiological outcomes discussed here may represent potential higher-order, brain-level mechanisms that support demonstrated cannabis-induced pain reduction.
Outcomes from Meta-Regression
Meta-regression results showed that sample size was associated with pain reduction standardized effect sizes across studies, such that studies involving smaller samples reported greater pain reduction. Moreover, there was no interaction between reported sample size and drug administration conditions (i.e., cannabinoid, synthetic cannabinoid, and placebo), suggesting that this was the case across pharmacologic manipulations considered. Sample size represents an important determinant regarding how generalizable research results are to target populations (Wiedermann & Wiedermann, 2015). Often, studies with smaller samples have reported better therapeutic outcomes (Sterne & Egger, 2001). This phenomenon has been linked to outcome reporting biases (Chan & Altman, 2005), such as data omission when results lack statistical significance, poorer methodological parameters (Kjaergard, Villumsen, & Gluud, 2001), and increased between-study heterogeneity among studies with small samples (IntHout, Ioannidis, Borm, & Goeman, 2015). Moving forward, it is important that researchers, healthcare providers, and law makers consider outcomes from studies on cannabinoid-induced pain reductions within the context of the sample sizes that derived them.
When considering sex-dependent effects in cannabinoid-induced pain reduction, meta-regression results suggested that among included studies, those studies that recruited more female participants reported greater, although non-significant, standardized effect sizes across drug administration conditions. It is worth noting that meta-regression outcomes derived using summary statistics (e.g., sample sex composition) may exhibit ecological confounding (Morgenstern, 1982) compared to using patient-level data (Thompson & Higgins, 2002). As such, the relationship between biological sex and cannabinoid analgesia should become clearer as new studies emerge that provide within-sample comparisons. Accumulating preclinical evidence suggests that females may be more sensitive to cannabis’s pain-reducing effects. Indeed, greater pain reduction among females following cannabinoid-receptor agonism has been shown in acute pain and non-acute pain animal models (Craft, Marusich, & Wiley, 2013; Craft, Wakley, Tsutsui, & Laggart, 2012; Tseng & Craft, 2001). However, whether these sex-dependent effects extend to humans remains unclear. One recent report from Cooper and Haney (2016) examined pain reduction among male and female cannabis users following active cannabis consumption (3.65–5.60% THC) and placebo consumption (0.00% THC). Among male cannabis users, those researchers found that cannabis consumption increased pain-onset latency compared to placebo- presumably by reducing pain sensitivity. Among female cannabis users, however, no differences were observed between active cannabis and placebo conditions. These discordant outcomes may highlight important nuances about cannabinoid-related reductions in reported pain. Specifically, findings from the current meta-analysis represent data from participants with various clinical conditions. Growing evidence suggests that women experience greater clinical pain (Rosseland & Stubhaug, 2004; Unruh, 1996), often endorsing increased pain-related distress (Paller, Campbell, Edwards, & Dobs, 2009). It is then possible that reported sex-differences in cannabinoid-induced pain reduction stem from differences in pain reporting- not pain sensation and/or perception. With this in mind, one important question facing subsequent research involves our current understanding of sex-dependent effects in cannabinoid-induced pain reduction. Moreover, subsequent research may consider sex differences across complimentary pain outcomes, such as pain tolerance, pain ratings, and pain questionnaires/scales.
Limitations
Findings presented here should be considered in the context of several methodological limitations. First, as is common with meta-analyses, our outcomes and associated interpretations are constrained by the state of the current literature. Accordingly, results obtained here should be considered preliminary given the modest sample size (i.e., 25 papers). Moreover, recommendations regarding sub-group analyses and meta-analytic modeling prevented more refined assessments, such as estimating standardized effect sizes as a function of cannabinoid sub-classifications (e.g., whole-plant cannabis, whole-plant cannabis extract, synthetic cannabinoid, THC, CBD, THC/CBD), dose (e.g., 2.5mg THC, 5mg THC), administration route (e.g., smoke, oromucosal spray, capsule), and pain population (e.g., central/peripheral neuropathic pain, cancer pain, multiple sclerosis pain) (Green & Higgins, 2005). The inclusion of studies that involved several drug conditions and clinical samples into the same meta-analysis presumably contributed to observed between-study heterogeneity. More granular meta-analytic approaches should become possible as additional relevant studies are made available. Second, even though included studies involved comparable end-point measures (i.e., numeric rating scale, visual analog scale), these studies may contain confounds and/or biases that have not been addressed, such as temporal variation in societal attitudes towards cannabis, regional policies that promote medicinal cannabis, and inter-individual differences regarding cannabis’s expected effectiveness. With this in mind, we used fixed-effects multiple linear regression (meta-regression) to control confounding effects where possible (e.g., experimental design) (Stanley & Jarrell, 1989). Also, moving forward, researchers may consider systematically collecting/reporting concomitant end-point measures [e.g., McGill Pain Questionnaire (Melzack, 1975)], to provide more complete characterizations of cannabis-related analgesic effects. Third, despite rigorous review methods, several records were excluded from the current meta-analysis due to missing data. According to the Open Science Collaboration (2015), problematic practices within psychological science include selective reporting, omitting analyses, and insufficient specification regarding experimental parameters. Moreover, the current meta-analysis cannot consider studies that were conducted but never reported (i.e., “the file drawer problem”) (Rosenthal, 1979). Thus, improved reporting practices should enable enhanced meta-analysis assessments in general, and regarding cannabinoids in particular. Finally, despite showing that cannabinoid administration was associated with pain reduction, many studies included in this meta-analysis did not give full consideration to neurocognitive side-effects linked with cannabis (for an extended review, see Crane, Schuster, Fusar-Poli, & Gonzalez, 2013). Future investigations should systematically examine cannabis’s therapeutic properties in the context of co-occurring undesired neurocognitive effects.
Conclusions
Our meta-analysis outcomes show that cannabinoid administration was associated with reductions in subjective pain across included studies, making them viable candidates for pain management and treatment. Moreover, meta-regression results suggested that drug administration condition and sample size predicted pain reduction effects. Finally, we observed that sample sex composition was associated (although, not statistically significant) with observed pain reduction, suggesting that this may be an important biological variable when considering cannabis-induced pain reduction. As social, societal, and political attitudes towards cannabis evolve, it is becoming increasingly important to provide enhanced scientific understanding regarding risks and potential therapeutic applications. Such understanding should lead to more informed decision-making regarding cannabis among patients, care providers, and law makers.
Supplementary Material
Public Significance Statement.
Chronic pain states are an ever-growing concern in the United States, costing an estimated $600 billion annually in lost labor and healthcare expenses. These, and other, conditions have resulted in an over-reliance on opioid-based pharmacotherapies. Results from the current meta-analysis provide some support that cannabinoids might mitigate subjective pain among patients with pain-related clinical conditions.
References:
- Abrams DI (2018). The therapeutic effects of Cannabis and cannabinoids: An update from the National Academies of Sciences, Engineering and Medicine report. European Journal of Internal Medicine, 49, 7–11. doi: 10.1016/j.ejim.2018.01.003 [DOI] [PubMed] [Google Scholar]
- Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, … Petersen KL (2007). Cannabis in Painful HIV-associated Neuropathy: a randomized placebo-controlled clinical trial. Neurology, 68(7), 515–521. [DOI] [PubMed] [Google Scholar]
- Andreae MH, Carter GM, Shaparin N, Suslov K, Ellis RJ, Ware MA, … Sacks HS (2015). Inhaled Cannabis for Chronic Neuropathic Pain: A Meta-analysis of Individual Patient Data. Journal of Pain, 16(12), 1221–1232. doi: 10.1016/j.jpain.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviram J, & Samuelly-Leichtag G (2017). Efficacy of Cannabis-Based Medicines for Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pain Physician, 20(6), E755–E796. [PubMed] [Google Scholar]
- Baker WL, White CM, Cappelleri JC, Kluger J, Coleman CI, Health Outcomes, P., & Economics Collaborative, G. (2009). Understanding heterogeneity in meta-analysis: the role of meta-regression. International Journal of Clinical Practice, 63(10), 1426–1434. doi: 10.1111/j.1742-1241.2009.02168.x [DOI] [PubMed] [Google Scholar]
- Becker BJ (1988). Synthesizing standardized mean‐change measures. British Journal of Mathematical and Statistical Psychology, 41(2), 257–278. [Google Scholar]
- Blake DR, Robson P, Ho M, Jubb RW, & McCabe CS (2006). Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Journal of Rheumatology, 45(1), 50–52. doi: 10.1093/rheumatology/kei183 [DOI] [PubMed] [Google Scholar]
- Buggy DJ, Toogood L, Maric S, Sharpe P, Lambert DG, & Rowbotham D (2003). Lack of analgesic efficacy of oral Δ−9-tetrahydrocannabinol in postoperative pain. Pain, 106(1–2), 169–172. [DOI] [PubMed] [Google Scholar]
- Bushe C, Day K, Reed V, Karlsdotter K, Berggren L, Pitcher A, … Haynes V (2016). A network meta-analysis of atomoxetine and osmotic release oral system methylphenidate in the treatment of attention-deficit/hyperactivity disorder in adult patients. Journal of Psychopharmacology, 30(5), 444–458. doi: 10.1177/0269881116636105 [DOI] [PubMed] [Google Scholar]
- Campbell FA, Trameèr MA, Carroll D, Reynolds DJM, Moore A, & McQuay HJ (2001). Are cannabinoids an effective and safe treatment option in the management of pain? A qualitative systematic review. BMJ, 323(7303), 13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A, & Altman DG (2005). Identifying outcome reporting bias in randomised trials on PubMed: review of publications and survey of authors. BMJ, 330(7494), 753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, … Miaskowski C (2009). Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. Journal of Pain, 10(2), 113–130. doi: 10.1016/j.jpain.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988). (Statistical power analysis for the behavioral sciences Vol. 2). Hillsdale, NJ: Lawrence Earlbaum Associates. [Google Scholar]
- Colombo B, Annovazzi PO, & Comi G (2006). Medications for neuropathic pain: current trends. Neurological Sciences, 27 Suppl 2, S183–189. doi: 10.1007/s10072-006-0598-7 [DOI] [PubMed] [Google Scholar]
- Comelli F, Giagnoni G, Bettoni I, Colleoni M, & Costa B (2008). Antihyperalgesic effect of a Cannabis sativa extract in a rat model of neuropathic pain: mechanisms involved. Phytotherapy Research, 22(8), 1017–1024. doi: 10.1002/ptr.2401 [DOI] [PubMed] [Google Scholar]
- Cooper H, Hedges LV, & Valentine JC (2009). The handbook of research synthesis and meta-analysis. New York, NY: Russell Sage Foundation. [Google Scholar]
- Cooper ZD, & Haney M (2016). Sex-dependent effects of cannabis-induced analgesia. Drug and Alcohol Dependence, 167, 112–120. doi: 10.1016/j.drugalcdep.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey-Bloom J, Wolfson T, Gamst A, Jin S, Marcotte TD, Bentley H, & Gouaux B (2012). Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. Canadian Medical Association Journal, 184(10), 1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Marusich JA, & Wiley JL (2013). Sex differences in cannabinoid pharmacology: a reflection of differences in the endocannabinoid system? Life Sciences, 92(8–9), 476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Wakley AA, Tsutsui KT, & Laggart JD (2012). Sex differences in cannabinoid 1 vs. cannabinoid 2 receptor-selective antagonism of antinociception produced by Δ9-tetrahydrocannabinol and CP55, 940 in the rat. Journal of Pharmacology and Experimental Therapeutics, 340(3), 787–800. [DOI] [PubMed] [Google Scholar]
- Crane NA, Schuster RM, Fusar-Poli P, & Gonzalez R (2013). Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Neuropsychology Review, 23(2), 117–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vita MJ, Moskal D, Maisto SA, & Ansell EB (2018). Association of Cannabinoid Administration With Experimental Pain in Healthy Adults: A Systematic Review and Meta-analysis. JAMA Psychiatry, 75(11), 1118–1127. doi: 10.1001/jamapsychiatry.2018.2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande A, Mailis-Gagnon A, Zoheiry N, & Lakha SF (2015). Efficacy and adverse effects of medical marijuana for chronic noncancer pain. Canadian Family Physician, 61, 10. [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, … Mechoulam R (1992). Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science, 258(5090), 1946–1949. [DOI] [PubMed] [Google Scholar]
- Ferraz MB, Quaresma M, Aquino L, Atra E, Tugwell P, & Goldsmith C (1990). Reliability of pain scales in the assessment of literate and illiterate patients with rheumatoid arthritis. Journal of Rheumatology, 17(8), 1022–1024. [PubMed] [Google Scholar]
- Foltz EL, & White LE Jr (1962). Pain “relief” by frontal cingulumotomy. Journal of Neurosurgery, 19(2), 89–100. [DOI] [PubMed] [Google Scholar]
- Frank B, Serpell MG, Hughes J, Matthews JN, & Kapur D (2008). Comparison of analgesic effects and patient tolerability of nabilone and dihydrocodeine for chronic neuropathic pain: randomised, crossover, double blind study. BMJ, 336(7637), 199–201. doi: 10.1136/bmj.39429.619653.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass GV, Smith ML, & McGaw B (1981). Meta-analysis in social research. Beverly Hills, CA: Sage Publications, Incorporated. [Google Scholar]
- Glass M, Faull R, & Dragunow M (1997). Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Journal of Neuroscience, 77(2), 299–318. [DOI] [PubMed] [Google Scholar]
- Goldenberg M, Reid MW, IsHak WW, & Danovitch I (2017). The impact of cannabis and cannabinoids for medical conditions on health-related quality of life: A systematic review and meta-analysis. Drug and Alcohol Dependence, 174, 80–90. doi: 10.1016/j.drugalcdep.2016.12.030 [DOI] [PubMed] [Google Scholar]
- Green S, & Higgins J (2005). Cochrane handbook for systematic reviews of interventions. Available from www.handbook.cochrane.org: The Cochrane Collaboration. [Google Scholar]
- Greenland S (1987). Quantitative methods in the review of epidemiologic literature. Epidemiologic Reviews, 9(1), 1–30. [DOI] [PubMed] [Google Scholar]
- Hartrick CT, Kovan JP, & Shapiro S (2003). The numeric rating scale for clinical pain measurement: a ratio measure? Pain Practice, 3(4), 310–316. [DOI] [PubMed] [Google Scholar]
- Hedges LV, & Olkin I (1985). Statistical Methods for Meta-Analysis. Cambridge, MA: Academic Press, Inc. [Google Scholar]
- Hedges LV, & Pigott TD (2001). The power of statistical tests in meta-analysis. Psychological Methods, 6(3), 203–217. [PubMed] [Google Scholar]
- Henschke N, Kamper SJ, & Maher CG (2015). The epidemiology and economic consequences of pain. Mayo Clinic Proceedings, 90(1), 139–147. doi: 10.1016/j.mayocp.2014.09.010 [DOI] [PubMed] [Google Scholar]
- Higgins JP, & Thompson SG (2002). Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine, 21(11), 1539–1558. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, & Altman DG (2003). Measuring inconsistency in meta-analyses. BMJ, 327(7414), 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KP, Palastro MD, Johnson B, & Ditre JW (2017). Cannabis and Pain: A Clinical Review. Cannabis and Cannabinoid Research, 2(1), 96–104. doi: 10.1089/can.2017.0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IntHout J, Ioannidis JP, Borm GF, & Goeman J (2015). Small studies are more heterogeneous than large ones: a meta-meta-analysis. Journal of Clinical Epidemiology, 68(8), 860–869. [DOI] [PubMed] [Google Scholar]
- Iskedjian M, Bereza B, Gordon A, Piwko C, & Einarson TR (2007). Meta-analysis of cannabis based treatments for neuropathic and multiple sclerosis-related pain. Current Medical Research and Opinion, 23(1), 17–24. doi: 10.1185/030079906X158066 [DOI] [PubMed] [Google Scholar]
- Johansen JP, Fields HL, & Manning BH (2001). The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proceedings of the National Academy of Sciences, 98(14), 8077–8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, & Fallon MT (2010). Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. Journal of Pain and Symptom Management, 39(2), 167–179. doi: 10.1016/j.jpainsymman.2009.06.008 [DOI] [PubMed] [Google Scholar]
- Karst M, Salim K, Burstein S, Conrad I, Hoy L, & Schneider U (2003). Analgesic effect of the synthetic cannabinoid CT-3 on chronic neuropathic pain: a randomized controlled trial. JAMA, 290(13), 1757–1762. [DOI] [PubMed] [Google Scholar]
- Kjaergard LL, Villumsen J, & Gluud C (2001). Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Annals of Internal Medicine, 135(11), 982–989. [DOI] [PubMed] [Google Scholar]
- LaGraize SC, Labuda CJ, Rutledge MA, Jackson RL, & Fuchs PN (2004). Differential effect of anterior cingulate cortex lesion on mechanical hypersensitivity and escape/avoidance behavior in an animal model of neuropathic pain. Experimental Neurology, 188(1), 139–148. [DOI] [PubMed] [Google Scholar]
- Langford RM, Mares J, Novotna A, Vachova M, Novakova I, Notcutt W, & Ratcliffe S (2013). A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. Journal of Neurology, 260(4), 984–997. doi: 10.1007/s00415-012-6739-4 [DOI] [PubMed] [Google Scholar]
- Lauer M (2016). Consideration of Relevant Biological Variables in NIH Grant Applications. Open Mike. Retrieved from https://nexus.od.nih.gov/all/2016/01/29/consideration-of-relevant-biological-variables-in-nih-grant-applications/
- Lee MC, Ploner M, Wiech K, Bingel U, Wanigasekera V, Brooks J, … Tracey I (2013). Amygdala activity contributes to the dissociative effect of cannabis on pain perception. Pain, 154(1), 124–134. doi: 10.1016/j.pain.2012.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Lux EA, McQuade R, Rossetti S, Sanchez R, Sun W, … Fallon MT (2018). Results of a Double-Blind, Randomized, Placebo-Controlled Study of Nabiximols Oromucosal Spray as an Adjunctive Therapy in Advanced Cancer Patients with Chronic Uncontrolled Pain. Journal of Pain and Symptom Management, 55(2), 179–188 e171. doi: 10.1016/j.jpainsymman.2017.09.001 [DOI] [PubMed] [Google Scholar]
- Lieberman MD, & Eisenberger NI (2015). The dorsal anterior cingulate cortex is selective for pain: Results from large-scale reverse inference. Proceedings of the National Academy of Sciences, 112(49), 15250–15255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsey MW, & Wilson DB (2001). Practical meta-analysis. Beverly Hills, CA: Sage Publications, Inc. [Google Scholar]
- Luebke T, & Brunkwall J (2015). Meta-analysis and meta-regression analysis of the associations between sex and the operative outcomes of carotid endarterectomy. BMC Cardiovascular Disorders, 15(32), 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch ME, & Campbell FA (2011). Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. British Journal of Clinical Pharmacology, 72(5), 735–744. doi: 10.1111/bph.2011.163.issue-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch ME, & Ware MA (2015). Cannabinoids for the Treatment of Chronic Non-Cancer Pain: An Updated Systematic Review of Randomized Controlled Trials. Journal of Neuroimmune Pharmacology, 10(2), 293–301. doi: 10.1007/s11481-015-9600-6 [DOI] [PubMed] [Google Scholar]
- Martin-Sanchez E, Furukawa TA, Taylor J, & Martin JL (2009). Systematic review and meta-analysis of cannabis treatment for chronic pain. Pain Medicine, 10(8), 1353–1368. doi: 10.1111/j.1526-4637.2009.00703.x [DOI] [PubMed] [Google Scholar]
- Melzack R (1975). The McGill Pain Questionnaire: major properties and scoring methods. Pain, 1(3), 277–299. [DOI] [PubMed] [Google Scholar]
- Millan MJ (1999). The induction of pain: an integrative review. Progress in Neurobiology, 57(1), 1–164. [DOI] [PubMed] [Google Scholar]
- Morgenstern H (1982). Uses of ecologic analysis in epidemiologic research. American Journal of Public Health, 72(12), 1336–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, & Abu-Shaar M (1993). Molecular characterization of a peripheral receptor for cannabinoids. Nature, 365(6441), 61–65. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, & Cuthill IC (2007). Effect size, confidence interval and statistical significance: a practical guide for biologists. Biological Reviews, 82(4), 591–605. doi: 10.1111/j.1469-185X.2007.00027.x [DOI] [PubMed] [Google Scholar]
- Narang S, Gibson D, Wasan AD, Ross EL, Michna E, Nedeljkovic SS, & Jamison RN (2008). Efficacy of dronabinol as an adjuvant treatment for chronic pain patients on opioid therapy. Journal of Pain, 9(3), 254–264. doi: 10.1016/j.jpain.2007.10.018 [DOI] [PubMed] [Google Scholar]
- Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, & Haines D (2007). Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial. Pain, 133(1–3), 210–220. doi: 10.1016/j.pain.2007.08.028 [DOI] [PubMed] [Google Scholar]
- Open Science C. (2015). Estimating the reproducibility of psychological science. Science, 349(6251), aac4716. doi: 10.1126/science.aac4716 [DOI] [PubMed] [Google Scholar]
- Paller CJ, Campbell CM, Edwards RR, & Dobs AS (2009). Sex-based differences in pain perception and treatment. Pain Medicine, 10(2), 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA (2017). Cannabinoids and the brain. Cambridge, MA: The MIT Press. [Google Scholar]
- Phillips TJ, Cherry CL, Cox S, Marshall SJ, & Rice AS (2010). Pharmacological treatment of painful HIV-associated sensory neuropathy: a systematic review and meta-analysis of randomised controlled trials. PloS One, 5(12), e14433. doi: 10.1371/journal.pone.0014433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pini LA, Guerzoni S, Cainazzo MM, Ferrari A, Sarchielli P, Tiraferri I, … Zappaterra M (2012). Nabilone for the treatment of medication overuse headache: results of a preliminary double-blind, active-controlled, randomized trial. Journal of Headache and Pain, 13(8), 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portenoy RK, Ganae-Motan ED, Allende S, Yanagihara R, Shaiova L, Weinstein S, … Fallon MT (2012). Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. Journal of Pain, 13(5), 438–449. doi: 10.1016/j.jpain.2012.01.003 [DOI] [PubMed] [Google Scholar]
- Rog DJ, Nurmikko TJ, Friede T, & Young CA (2005). Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology, 65(6), 812–819. [DOI] [PubMed] [Google Scholar]
- Rohatgi A (2018). WebPlotDigitizer. 4.1 Retrieved from https://automeris.io/WebPlotDigitizer
- Rosenthal R (1979). The file drawer problem and tolerance for null results. Psychological Bulletin, 86(3), 638–641. [Google Scholar]
- Rosseland LA, & Stubhaug A (2004). Gender is a confounding factor in pain trials: women report more pain than men after arthroscopic surgery. Pain, 112(3), 248–253. [DOI] [PubMed] [Google Scholar]
- Sánchez-Meca J, & Marin-Martinez F (1998). Weighting by inverse variance or by sample size in meta-analysis: A simulation study. Educational and Psychological Measurement, 58(2), 211–220. [Google Scholar]
- Savage SR, Romero-Sandoval A, Schatman M, Wallace M, Fanciullo G, McCarberg B, & Ware M (2016). Cannabis in Pain Treatment: Clinical and Research Considerations. Journal of Pain, 17(6), 654–668. doi: 10.1016/j.jpain.2016.02.007 [DOI] [PubMed] [Google Scholar]
- Skrabek RQ, Galimova L, Ethans K, & Perry D (2008). Nabilone for the treatment of pain in fibromyalgia. Journal of Pain, 9(2), 164–173. doi: 10.1016/j.jpain.2007.09.002 [DOI] [PubMed] [Google Scholar]
- Stanley TD, & Jarrell SB (1989). Meta‐regression analysis: a quantitative method of literature surveys. Journal of Economic Surveys, 3(2), 161–170. [Google Scholar]
- Sterne JA, & Egger M (2001). Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. Journal of Clinical Epidemiology, 54(10), 1046–1055. [DOI] [PubMed] [Google Scholar]
- Sullivan GM (2011). IRB 101. Journal of Graduate Medical Education, 3(1), 5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen KB, Jensen TS, & Bach FW (2004). Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ, 329(253), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sznitman SR, & Zolotov Y (2015). Cannabis for therapeutic purposes and public health and safety: a systematic and critical review. International Journal on Drug Policy, 26(1), 20–29. doi: 10.1016/j.drugpo.2014.09.005 [DOI] [PubMed] [Google Scholar]
- Thompson SG, & Higgins JP (2002). How should meta‐regression analyses be undertaken and interpreted? Statistics in Medicine, 21(11), 1559–1573. [DOI] [PubMed] [Google Scholar]
- Tseng AH, & Craft RM (2001). Sex differences in antinociceptive and motoric effects of cannabinoids. European Journal of Pharmacology, 430(1), 41–47. [DOI] [PubMed] [Google Scholar]
- Tukey JW (1977). Exploratory data analysis. Boston, MA: Pearson. [Google Scholar]
- Unruh AM (1996). Gender variations in clinical pain experience. Pain, 65(2–3), 123–167. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Wiley JL, Yang R, Bridgen DT, Long K, Lichtman AH, & Martin BR (2006). Interactions between THC and cannabidiol in mouse models of cannabinoid activity. Psychopharmacology, 186(2), 226–234. doi: 10.1007/s00213-006-0356-9 [DOI] [PubMed] [Google Scholar]
- Wade DT, Robson P, House H, Makela P, & Aram J (2003). A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clinical Rehabilitation, 17(1), 21–29. [DOI] [PubMed] [Google Scholar]
- Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, … Kleijnen J (2015). Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA, 313(24), 2456–2473. doi: 10.1001/jama.2015.6358 [DOI] [PubMed] [Google Scholar]
- Wiedermann CJ, & Wiedermann W (2015). Beautiful small: Misleading large randomized controlled trials? The example of colloids for volume resuscitation. Journal of Anaesthesiology, Clinical Pharmacology, 31(3), 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, … Sanacora G (2018). The Effect of a Single Dose of Intravenous Ketamine on Suicidal Ideation: A Systematic Review and Individual Participant Data Meta-Analysis. American Journal of Psychiatry, 175(2), 150–158. doi: 10.1176/appi.ajp.2017.17040472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsey B, Marcotte T, Tsodikov A, Millman J, Bentley H, Gouaux B, & Fishman S (2008). A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. Journal of Pain, 9(6), 506–521. doi: 10.1016/j.jpain.2007.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf G, & Cartwright B (1974). Rules for coding dummy variables in multiple regression. Psychological Bulletin, 81(3), 173–179. [Google Scholar]
- Wright S (2007). Cannabinoid-based medicines for neurological disorders--clinical evidence. Molecular Neurobiology, 36(1), 129–136. doi: 10.1007/s12035-007-0003-4 [DOI] [PubMed] [Google Scholar]
- Yanes JA, Riedel MC, Ray KL, Kirkland AE, Bird RT, Boeving ER, … Sutherland MT (2018). Neuroimaging meta-analysis of cannabis use studies reveals convergent functional alterations in brain regions supporting cognitive control and reward processing. Journal of Psychopharmacology, 32(3), 283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlas J, Stark H, Seligman J, Levy R, Werker E, Breuer A, & Mechoulam R (1993). Early medical use of cannabis. Nature, 363(6426), 215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.