Abstract
Psychosocial Assessment of Candidates for Transplant (PACT) is a tool originally developed to address psychosocial risks in solid organ transplant recipients and has the potential for application to hematopoietic cell transplantation (HCT) recipients. In a retrospective cohort study, we reviewed 404 adult allogeneic HCT cases from 2003 to 2014 to identify predictors of adverse psychosocial status as determined by PACT. Final PACT rating was poor/borderline (score 0–1) in 5%, acceptable (score 2) in 22%, good (score 3) in 44%, and excellent (score 4) in 29% recipients. In multivariable regression, higher PACT score was associated with White race (odds ratio [OR] 2.95, P < 0.001), having a related donor (OR 1.61, P = 0.015), and a higher quality of life score (OR 1.22/ 10-point increase in FACT-BMT total score, P < 0.001). PACT score correlated with all quality of life subscales. The final PACT score was associated with non-relapse mortality (HR 0.82/ 1-point increase, p = 0.03) in multivariable analysis that considered patient and disease factors, but not in models that also included transplant-related factors and performance status. PACT score was not associated with overall survival. PACT can be considered as part of a comprehensive psychosocial assessment for identifying patients who may require additional resources around allogeneic HCT.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a complex treatment with a prolonged recovery course. Variables beyond disease and transplant-related risk factors have been evaluated as predictors for recovery and transplantation outcomes. The association of socioeconomic status and race/ethnicity with access to and outcomes after HCT has been previously studied and is well-recognized [1–6]. In addition, psychiatric comorbidities such as depression have been reported to impair pre- and post-HCT quality of life (QoL) and are associated with non-relapse mortality (NRM) and survival after HCT [7–11]. Other psychosocial factors such as lack of a consistent care giver, mental health needs, psychological issues such as depression and anxiety, substance abuse, and poor compliance may also increase risks of toxicity and adverse outcomes after transplantation but have been less well studied. Many patients report high levels of anxiety and distress prior to HCT, which can be further exacerbated during the post-transplant recovery phase and interfere with their work, family, and social life [12–15]. In a relatively small study, pre-transplant emotional support and presence of a committed care giver have been shown to be associated with better HCT outcomes [16]. An important aspect of patient selection for HCT frequently involves a subjective assessment of psychosocial status and support, and patients who are determined to be at high risk for adverse outcomes from this perspective may not be offered transplantation. However, validated instruments to specifically assess psychosocial status among HCT recipients are not widely utilized since data on their use specifically in HCT recipient population are lacking.
Historically, psychosocial evaluations for solid organ transplantations have been conducted as a standard of practice for candidacy selection and patient care because there is a limited supply of donor organs [17–19]. While there is a lack of a standard instrument, the Psychosocial Assessment of Candidates for Transplantation (PACT) scale is a tool that is validated and routinely used to stratify psychosocial risk among solid organ transplant recipients [19, 20]. It consists of an 8-item rating scale with four sections: social support, psychological health, lifestyle factors, and understanding of transplant and follow-up. In addition, the instrument can capture the clinician’s assessment of a patient’s substance abuse, compliance, and coping strategies. The overall impression of the patient’s suitability for transplantation is assigned a final PACT score. While a possible association between survival and PACT score has been reported in solid organ transplant recipients [21], there are conflicting data on the influence of psychosocial comorbidities on HCT outcomes [22–26]. In a previous prospective study, we evaluated the utility of the PACT assessment and association of outcomes in HCT recipients [27]. However, this was a relatively small study with short follow-up and it did not consider sociodemographic and patient-reported factors. We conducted a follow-up study to evaluate the association of pre-transplant PACT assessment with HCT outcomes in a larger cohort of allogeneic HCT recipients. In addition, we also explored whether baseline demographic, clinical factors, and pre-HCT QoL are associated with the PACT score.
Methods
Patients
From our institutional Blood and Marrow Transplant (BMT) database that prospectively collects data on patient demographic, clinical and transplant-related characteristics, we identified 610 consecutive adult patients who received their first allogeneic HCT between November 2003 and December 2014 and had consented for using their data for research. Among these patients, 404 had PACT assessments performed pre-transplantation. Baseline characteristics of patients who received and did not receive PACT assessments were comparable, although patients who were not assessed were older (median age HCT 52 years vs. 50 years, P = 0.01), transplanted in an earlier time period (P < 0.01), were more likely to have received a reduced intensity conditioning (RIC) regimen (40 vs. 22%, P < 0.01) and peripheral blood as the graft source (47 vs. 34%, P < 0.01). There were also relatively small but significant differences in diagnosis for patients who were and were not assessed. Of note, there was no significant difference in sex or race/ethnicity between the two groups. Finally, 1-year overall survival between the two groups was comparable (P = 0.29). The study was conducted under the guidance of the Cleveland Clinic’s Institutional Review Board.
PACT Assessment
The PACT assessment was performed pre-transplantation by three BMT Program social workers as a part of psychosocial assessment during transplant workup. The scale includes 8 individual questions which focus on four domains: social support (two questions about “support stability” and “support availability”), psychological health (two questions about presence of “psychopathology” and “risk for psychopathology”), lifestyle factors (three questions about “healthy lifestyle”, “drug and alcohol use”, and “compliance”), and understanding of transplant and follow-up (one question about “relevant knowledge”). Each question is rated on an ordinal scale from 1 to 5, with a higher score reflective of a more positive status. The social worker completes each item and assigns an overall or final PACT score, which represents his or her overall impression of the patient’s suitability for transplantation [21, 26, 28]. Final PACT rating scores range from 0 (poor candidate) to 4 (excellent candidate), and is scored independent of the scores assigned to subscales. Although the PACT score by itself was not a factor that determined patient candidacy for transplantation, it was part of the comprehensive assessment conducted by social workers which was considered along with other pre-BMT assessments. We did not track PACT scores of patients who did not proceed to HCT.
Definitions
Conditioning regimens and supportive care practices have been reported previously [2, 29–31]. Disease status at transplant was classified by standard criteria [32]. HCT Comorbidity Index (HCT-CI) was used to stratify comorbidity risks [7]. Residence setting at the time of transplant (urban vs. rural) was based on Rural Urban Commuting Area (RUCA) classification that is based on ZIP Code of residence [33]. Urban areas were defined as metropolitan (>50,000 population) or micropolitan (>10,000 population) areas, or small town (9999–25,000 population) with 30–49% work commuting flow to an urbanized area [34]. Other areas were classified as rural. The median annual household income was derived from the 2010 United States Census data based on ZIP Code of primary residence at the time of HCT [35]. Data on actual income or educational status were not available. Measurement of QoL at baseline was based on Functional Assessment of Cancer Therapy-Bone Marrow Transplantation (FACT-BMT), that is composed of 50 items in domains of physical wellbeing, social/family wellbeing, emotional wellbeing, functional wellbeing, and additional concerns [36].
Statistical Analysis
Patient and disease characteristics were compared between patients with and without PACT scores using Chi-square or Wilcoxon rank-sum test, and survival with log-rank test.
Correlation of final PACT score with individual PACT scores and quality of life at baseline and day 100 was assessed with Spearman correlation. Given the small number of patients in PACT score categories of 0 and 1 (total n = 21 [n0 = 3, n1 = 18]), we combined them into one group for our analysis. We were interested in addressing two questions. First, whether the PACT provided any additional information beyond other baseline characteristics that may describe or be a surrogate for a given patient’s psychosocial status. Hence, we evaluated the association between final PACT score and its subscales with performance status, other clinical factors, place of residence, household income, and total FACT-BMT QoL scores. Univariable associations were assessed between PACT score and other characteristics with Cochran-Armitage trend tests (binary variables), Cochran-Mantel-Haenszel (CMH) score test (categorical variables with 3 or more groups), CMH correlation test (ordinal categorical variables), or Jonckheere-Terpstra test (continuous variables); all of these methods look for trends as PACT score increases. Ordinal logistic regression analysis was used also to identify univariable and multivariable prognostic factors for the final PACT score. Results are presented as odds ratio (OR) and 95% confidence interval (CI) for OR. Multivariable prognostic factors were identified with stepwise logistic regression with a variable entry criterion of P < 0.10 and a variable retention criterion of P < 0.05.
Second, we evaluated the association of PACT total scores and scores of individual subscales with HCT outcomes, including overall survival (OS), NRM, and days alive and out of hospital in the first 100 days post-transplant. For univariable analysis, OS was estimated with the Kaplan–Meier method but compared with Cox proportional hazards analysis to account for PACT as an ordinal variable. NRM was estimated with cumulative incidence and compared with Fine and Gray regression. Days alive and out of hospital was estimated with median and inter-quartile range and compared with Jonckheere-Terpstra test. Multivariable analyses were done with Cox regression, Fine and Gray regression, or linear regression respectively, to account for the following demographic and clinical factors: age, sex, ethnicity/race, median household income, place of residence, HCT-Comorbidity Index, disease status, diagnosis, time from diagnosis to transplant, and pre-HCT QoL. We performed two separate multivariable analyses without and with transplant characteristics (conditioning intensity, donor source and graft source) as a patient’s psychosocial assessment may have determined the type of transplant received. We performed analyses with the PACT score assigned ordinally (0–1 vs. 2 vs. 3 vs. 4) and categorically (0–3 vs. 4); results were comparable, and results of the former are presented. Analyses were performed using the SAS software (version 9.4, Cary, NC, USA) and all P-values were two sided. P < 0.05 was defined as statistically significant.
Results
Patient Characteristics
The final PACT score was 0–1 in 5% (n = 21), 2 in 22% (n = 87), 3 in 44% (n = 177), and 4 in 29% (n = 119) patients. Table 1 shows patient characteristics by PACT score. There was a significant trend towards higher PACT scores in Whites, patients receiving HCT from a related donor, patients with higher median household income, patients with better Karnofsky performance status (KPS), and in patients with lower HCT-CI score at transplantation.
Table 1.
Patient characteristics by final PACT score
| Characteristic | Final PACT score |
P-value as score increase | |||
|---|---|---|---|---|---|
| Poor (score 0–1, N = 21) | Acceptable (score 2, N = 87) | Good (score 3, N = 177) | Excellent (score 4, N = 119) | ||
| Age in years, median (range) | 46 (22–61) | 50 (21–73) | 50 (18–73) | 49 (19–72) | 0.43 |
| Sex, N (%) | 0.85 | ||||
| Male | 15 (71%) | 40 (46%) | 99 (56%) | 63 (53%) | |
| Female | 6 (29%) | 47 (54%) | 78 (44%) | 56 (47%) | |
| Race, N (%) | 0.004 | ||||
| White | 17 (81%) | 73 (84%) | 158 (89%) | 113 (95%) | |
| Othera | 4 (19%) | 14 (16%) | 19 (11%) | 6 (5%) | |
| Annual household income, median (range) | $46,404 (22,820–95,740) | $49,152 (20,097–112,530) | $48,753 (14,028–100,309) | $53,576 (22,820–100,309) | 0.004 |
| Place of residence, N (%) | 0.38 | ||||
| Urban | 16 (76%) | 68 (78%) | 140 (79%) | 98 (82%) | |
| Rural | 5 (24%) | 19 (22%) | 37 (21%) | 21 (18%) | |
| Karnofsky performance status, median (range)b | 90 (70–100) | 90 (70–100) | 90 (60–100) | 90 (60–100) | 0.010 |
| HCT-CI score, N (%) | 0.007 | ||||
| Low | 4 (19%) | 15 (17%) | 46 (26%) | 42 (35%) | |
| Intermediate | 9 (43%) | 30 (35%) | 61 (34%) | 37 (31%) | |
| High | 8 (38%) | 42 (48%) | 70 (40%) | 40 (34%) | |
| Diagnosis, N (%) | 0.12 | ||||
| AML/MDS | 13 (62%) | 60 (69%) | 98 (55%) | 67 (56%) | |
| Other | 8 (38%) | 27 (31%) | 79 (45%) | 52 (44%) | |
| Disease risk, N (%)b | 0.85 | ||||
| Low | 7 (33%) | 33 (39%) | 82 (49%) | 44 (38%) | |
| Intermediate | 7 (33%) | 18 (21%) | 35 (21%) | 31 (27%) | |
| High | 7 (33%) | 33 (39%) | 49 (30%) | 40 (35%) | |
| Time from diagnosis in months, median (range) | 11 (4–165) | 7 (<1–106) | 7 (1–218) | 7 (1–174) | 0.19 |
| Donor relationship, N (%) | 0.039 | ||||
| Unrelated | 15 (71%) | 48 (55%) | 105 (59%) | 55 (46%) | |
| Related | 6 (29%) | 39 (45%) | 72 (41%) | 64 (54%) | |
| Donor source, N (%) | 0.19 | ||||
| Matched related donor | 6 (29%) | 35 (40%) | 67 (38%) | 61 (51%) | |
| Matched unrelated donor | 12 (57%) | 42 (48%) | 81 (46%) | 46 (39%) | |
| Umbilical cord blood | 3 (14%) | 6 (7%) | 24 (14%) | 9 (8%) | |
| Haploidentical donor | 0 | 4 (5%) | 5 (3%) | 3 (3%) | |
| Graft source, N (%) | 0.84 | ||||
| Bone marrow | 13 (62%) | 52 (60%) | 90 (51%) | 69 (58%) | |
| Peripheral blood | 5 (24%) | 29 (33%) | 63 (36%) | 41 (34%) | |
| Umbilical cord blood | 3 (14%) | 6 (7%) | 24 (14%) | 9 (8%) | |
| CMV serostatus, N (%)b | 0.14 | ||||
| Donor + / recipient + | 7 (33%) | 27 (31%) | 51 (29%) | 33 (28%) | |
| Donor +/ recipient − | 2 (10%) | 11 (13%) | 26 (15%) | 5 (4%) | |
| Donor −/ recipient + | 8 (38%) | 32 (37%) | 59 (34%) | 47 (40%) | |
| Donor −/ recipient − | 4 (19%) | 16 (19%) | 39 (22%) | 32 (27%) | |
| Conditioning regimen, N (%) | 0.46 | ||||
| Myeloablative | 18 (86%) | 73 (84%) | 127 (72%) | 96 (81%) | |
| Reduced intensity | 3 (14%) | 14 (16%) | 50 (28%) | 23 (19%) | |
| FACT-BMT total score, median (range)b | 147 (93–175) | 138 (77–185) | 148 (87–187) | 153 (99–193) | <0.001 |
HCT CI hematopoietic cell transplantation comorbidity index, CMV cytomegalovirus, FACT-BMT functional assessment of cancer therapy-bone marrow transplantation instrument
Other ethnic groups included African Americans, Asians, Hispanics, etc
Karnofsky performance status was missing in 49 patients; disease risk was not classified in 18 patients; CMV serostatus was missing in 5 patients; FACT BMT quality of life assessments were missing in 34 patients
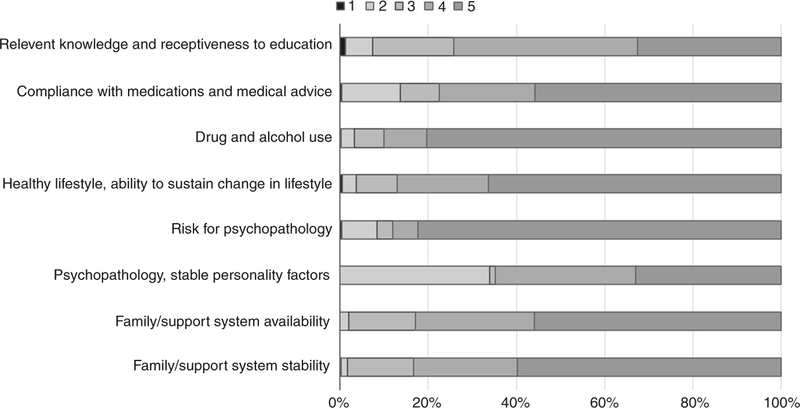
Figure 1 shows distribution of PACT subscales for our patient population, where the scores ranged from 1 (low) to 5 (high). No patients had a score of 1 for the subscale of family and support system availability, since that indicates there is no caregiver support available and such patients would not have met our BMT Program criteria for allogeneic HCT. Since the final PACT rating is scored independent of the individual subscales, we evaluated their correlations to understand whether specific subscales disproportionately contributed to the final score. All subscales were significantly (P < 0.001) but modestly correlated with final PACT score; the lowest correlation was seen for the subscale that assessed drug and alcohol use (R = 0.32) and highest was seen for the psychopathology subscale (R = 0.72).
Fig. 1.
Distribution of PACT subscale scores. Scores range from 1 [low] to 5 [high]
PACT Assessment and QoL
Patients with higher final PACT scores reported better QoL prior to transplantation, as characterized by the total score on the FACT-BMT instrument (Table 1). Table 2 highlights the correlation between final PACT score and QoL at baseline and at day + 100 after HCT. A significant but weak correlation was observed with all FACT-BMT domains at baseline (R ≤ 0.22). A similar weak but significant association was observed at day + 100 FACT-BMT social wellbeing, emotional wellbeing and total scores.
Table 2.
Spearman Correlation between pre-transplant final PACT score and quality of life
| FACT-BMT domain | Baseline pre-transplant (N = 370) |
Day+100 post-transplant (N = 225) |
||
|---|---|---|---|---|
| Correlation coefficient | P-value | Correlation coefficient | P-value | |
| Physical wellbeing | 0.12 | 0.02 | 0.03 | 0.68 |
| Social wellbeing | 0.19 | <0.001 | 0.22 | <0.001 |
| Emotional wellbeing | 0.21 | <0.001 | 0.20 | 0.003 |
| Functional wellbeing | 0.18 | <0.001 | 0.10 | 0.13 |
| BMT subscalea | 0.15 | 0.004 | 0.10 | 0.15 |
| Trial outcome indexa | 0.17 | 0.001 | 0.09 | 0.19 |
| Total score | 0.22 | <0.001 | 0.14 | 0.03 |
FACT-BMT = Functional Assessment of Cancer Therapy-Bone Marrow Transplantation instrument
Trial outcome index is sum of physical wellbeing, functional wellbeing and BMT subscale
Predictors of Pre-transplant Psychosocial Status
We were interested in evaluating whether any patient related factors were associated with the final PACT score, as it would enable identification of a vulnerable population from a psychosocial status perspective. As noted above and in Table 1, patients with better PACT scores were more likely to be of White race, receive HCT from a related donor, have higher median annual household income, and have a higher KPS score, a lower HCT-CI score at transplantation, and a better FACT-BMT total QoL score pre-transplantation. Of note, the correlation between KPS score and final PACT score was weak (R = 0.14, P = 0.011). Multivariable analyses showed that FACT-BMT total QoL score (per 10-point increase, OR 1.22 [95% CI 1.12–1.32], P < 0.001), race (White vs. others, OR 2.95 [1.56–5.58], P < 0.001), and donor relationship (related vs. unrelated, OR 1.61 [1.10–2.37], P = 0.015) remained significantly associated with final PACT score. Among variables tested, it is notable that age, sex, household income, and place of residence were not predictive of final PACT score.
PACT Assessment and Transplant Outcomes
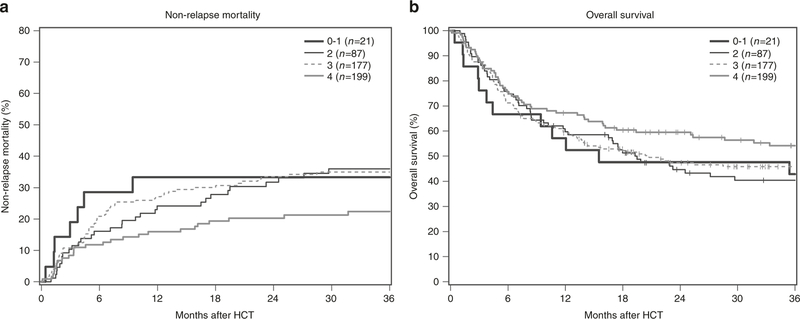
The median follow-up of our cohort was 45 months (range 7–145 months). Table 3 shows HCT outcomes by final PACT score. Patients with higher PACT scores had significantly lower NRM (Fig. 2), and although not statistically significant, a trend towards more days alive and out of the hospital in the first 100 days after transplantation.
Table 3.
Outcome estimates by final PACT score
| Outcomea | Final PACT score |
P-value | |||
|---|---|---|---|---|---|
| Poor (score 0–1, N = 21) | Acceptable (score 2, N = 87) | Good (score 3, N = 177) | Excellent (score 4, N = 119) | ||
| Overall survival at 1-yeara | 57 [34–75] | 60 [49–69] | 59 [52–66] | 67 [58–75] | 0.11 |
| Non-relapse mortality at 1-yearb | 33 [14–54] | 24 [16–34] | 27 [21–34] | 16 [10–23] | 0.03 |
| Relapse at 1-yearb | 24 [8–44] | 30 [21–40] | 24 [18–31] | 25 [18–33] | 0.90 |
| Grade II-IV acute GVHD at 100 daysb | 48 [25–67] | 32 [23–42] | 44 [38–45] | 34 [26–43] | 0.72 |
| Chronic GVHD at 1 yearb | 38 [17–59] | 37 [27–47] | 30 [23–37] | 38 [29–46] | 0.76 |
| Duration of index hospitalizationc | 35 [29–42] | 29 [25–40] | 30 [24–39] | 29 [23–38] | 0.17 |
| Days alive and out of hospital in the first 100-daysc | 58 [45–70] | 65 [44–78] | 66 [45–77] | 70 [54–79] | 0.07 |
[ ] indicates range
GVHD Graft-versus-host disease
Kaplan–Meier estimate with 95% confidence intervals
Cumulative incidence estimate with 95% confidence intervals
Median with inter-quartile range
Fig. 2.
Non-relapse mortality (a) and overall survival (b) by final PACT score
We tested the association of final PACT score with three outcomes in multivariable analyses – days alive and out of hospital in first 100 days after HCT, NRM and OS. There was no significant association between PACT score and days alive and out of hospital in univariable or multivariable analysis (P = 0.09 and 0.32, respectively). For NRM and OS, two sets of multivariable models were analyzed. Since psychosocial status may influence decisions regarding transplant treatment, the first model included patient and disease related characteristics, with the exclusion of KPS score since 12% cases had missing KPS, and it was significantly correlated with final PACT score (R = 0.14, P = 0.011) as well as OS and NRM. The second model added transplant-related factors, so that we could evaluate whether the latter abrogated any association between PACT score and outcomes. In multivariable models that considered patient and disease related factors, final PACT score was associated with NRM (HR 0.82 per 1-point increase [0.69–0.98], P = 0.03). The second model with transplant-related factors led to the similar association although the additional co-variates abrogated the statistical significance (HR 0.84 [0.70–1.01], P = 0.06). Of note, when KPS score was also considered as a covariate in the multivariable analysis, no association was observed between PACT score and NRM in either of the two models. There was no significant association between PACT score and OS (HR for each 1-point increase 0.92 [0.79–1.05], P = 0.18 and 0.90 [0.79–1.04], P = 0.16 in the two models, respectively).
Individual PACT subscale questions were also analyzed for their association with NRM and OS in univariable analysis (Table 4). Multivariable analysis showed that only “relevant knowledge” was significantly associated with NRM (HR 0.81 per 1-point increase [0.69–0.96], P = 0.012). None of the subscales was associated with OS in multivariable analyses.
Table 4.
Associations of Transplant Outcomes with PACT subscales, per 1-point increase
| PACT subscale | Non-relapse mortality |
Overall survival |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Support stability | 0.84 | 0.70–1.01 | 0.07 | 0.93 | 0.80–1.08 | 0.31 |
| Support availability | 0.80 | 0.66–0.97 | 0.03 | 0.88 | 0.76–1.03 | 0.12 |
| Psychopathology | 0.86 | 0.70–1.04 | 0.12 | 0.95 | 0.82–1.10 | 0.51 |
| Risk for psychopathology | 0.92 | 0.76–1.11 | 0.40 | 1.02 | 0.88–1.18 | 0.82 |
| Healthy lifestyle | 0.94 | 0.78–1.13 | 0.50 | 1.05 | 0.90–1.22 | 0.57 |
| Drug and alcohol use | 0.98 | 0.80–1.21 | 0.88 | 0.96 | 0.81–1.13 | 0.60 |
| Compliance | 0.91 | 0.75–1.09 | 0.30 | 0.96 | 0.82–1.13 | 0.64 |
| Relevant knowledge | 0.78 | 0.66–0.92 | 0.003 | 0.91 | 0.80–1.04 | 0.18 |
HR hazard ratio, CI confidence interval
Discussion
The decision-making process for selecting appropriate candidates for allogeneic HCT is complex and considers several patient, disease and transplant-related factors, including an assessment of social and psychological risk factors. We demonstrate that the PACT instrument can provide objective parameters to facilitate psychosocial risk assessment and builds on previous work in a smaller cohort of patients published from our center [27]. Using the PACT scale, we highlight risk factors that may identify a subgroup of vulnerable patients from a psychosocial perspective who may need additional vigilance, support and resources to successfully help them navigate the transplantation procedure. We highlight the association of PACT score with several aspects of QoL impairments, both at baseline and at 100 days after transplantation.
In multivariable analysis, race and donor relationship were significantly associated with psychosocial status. Whites and patients receiving HCT from related donors had a higher final PACT score. Racial minority populations such as Blacks and Hispanics are at risk for healthcare disparities, including disparities in access to and outcomes after HCT [5, 6]. It is likely, that these social and healthcare disparities also contribute to adverse psychosocial status as these patients present for transplantation. It may not be surprising that patients with better psychosocial profile were more likely to have a related donor for transplantation, since many of the factors considered in the PACT scale reflect family structure and dynamics, and siblings, parents, and/or children may be more likely to donate in the setting of a stable family structure. In addition, and not surprisingly, we also found an association between QoL and psychosocial status. It is important to note that impairments in social and emotional wellbeing domains of QoL persisted through at least day 100 among patients with lower PACT scores. This highlights the need for continued support for such patients as they go through the transplantation process. Notably, several variables that can be expected to be associated with psychosocial status were not found to be associated with PACT score, including age, sex, and household income. A larger-scale analysis with more granular information may reveal any associations.
In addition to using PACT as a tool to identify patients who are psychosocially high-risk for allogeneic HCT, the other question is whether this scale is predictive of transplant outcomes. Although there was no association with OS, we did see an association with NRM when only patient and disease related factors were considered in the multivariable models. Not surprisingly, the association was lost when transplant factors such as donor and graft source, conditioning intensity, HLA match, and KPS were included in the analysis. Performance status may have reflected psychosocial status in addition to physical reserve for HCT. Also, strong psychosocial support may improve a patient’s functional or performance status during transplant if patients are recognized to be at risk by the transplant center. One hypothesis is that some decisions made by the treatment team considered psychosocial status and patients at high risk from this perspective may have been offered “less risky” transplants (e.g., preferential use of reduced intensity conditioning), although that was not necessarily the case in our cohort. It is also possible that patients with low PACT scores received interventions during transplantation that abrogated their risk for adverse transplantation outcomes; we did not capture data on follow-up and additional resources that may have been used for such patients and future studies will need to evaluate this further. Finally, patients who were at the highest risk for poor survival based on low PACT score (e.g., no caregiver, serious substance abuse issues) were not offered a transplant and were not included in our analysis and may partly contribute to the lack of an association between psychosocial status and survival.
Among PACT subscales, “relevant knowledge” was the only one associated with NRM. This subscale assesses patients understanding of the transplant process and follow-up, with score 1 indicating “No idea of what is involved, views transplant as cure, no long-range picture” to score 5 “Able to state risks and benefits are realistic”. This finding suggests the importance of patient education in the transplant process, including the need for interventions that may reinforce relevant aspects of transplant care to patients. We did not have health literacy or educational attainment assessments for our patients, and it is possible that some of the effect related to this subscale reflected patients’ ability to comprehend the transplant process. Irrespective, patients who score low on this subscale may offer an opportunity to improve their knowledge of the transplant process which in turn may improve NRM.
Our findings need to be interpreted in the context of some study limitations. First, we did not include patients who received the PACT assessment but did not proceed to allogeneic HCT. Psychosocial reasons may have caused patients to decline transplant or not to be offered HCT. As noted above, we did not capture some variables that may be associated with psychosocial status, such as education, health literacy, more granular metrics of socioeconomic status, and cultural factors. The number of racial/ ethnic minorities was small to conduct a meaningful analysis. Our BMT Program has integrated full-time social workers who actively follow patients through transplantation. We do not have data about inter-rater reliability of the PACT assessment. It is possible that we were not able to identify a strong association between PACT score and NRM and OS since they are able to support and provide resources to psychosocially vulnerable patients. Psychosocial vulnerability may primarily affect the transplant experience and ultimate recovery process rather than survival. Finally, all patients did not undergo PACT assessment and given the retrospective design of our study, we could not ascertain the reason for this. However, OS for patients who did and did not undergo PACT assessment were comparable.
Since most BMT Programs have some mechanism of conducting psychosocial assessment for HCT recipients, the pragmatic question is how a psychosocial assessment can be improved, standardized, and incorporated into routine clinical practice. First, it provides a quantitative measure of psychosocial status which can facilitate team discussions about patient needs and status. Future observational studies on other psychosocial assessment measures such as Transplant Evaluation Rating Scale (TERS) or Stanford Integrated Psychosocial Assessment for Transplant (SIPAT) would be warranted to evaluate the best way to assess psychosocial risks [37, 38]. The PACT scale or its modified version can serve as a tool that facilitates standardization of psychosocial assessment among different clinical providers in the same institution and among transplant centers. The ideal timing of administration of this tool needs further investigation. At our program, it is routinely administered at the time of pre-transplant workup, although an earlier assessment (e.g., at the time of consultation and/or donor search) may be desirable so that adverse psychosocial factors can be identified and addressed before a patient comes in for transplantation. Identifying high psychosocial risk patients based on a standardized psychosocial assessment may improve transplant outcomes by providing additional social support and resources to vulnerable patients.
Acknowledgments
Funding NM is partially supported by a grant from the National Cancer Institute (R01-CA215134).
Footnotes
Publisher's Disclaimer: Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest The authors declare that they have no conflict of interest.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baker KS, Davies SM, Majhail NS, Hassebroek A, Klein JP, Ballen KK, et al. Race and socioeconomic status influence outcomes of unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant 2009;15:1543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fu S, Rybicki L, Abounader D, Andresen S, Bolwell BJ, Dean R, et al. Association of socioeconomic status with long-term outcomes in 1-year survivors of allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2015;50:1326–30. [DOI] [PubMed] [Google Scholar]
- 3.Hari PN, Majhail NS, Zhang MJ, Hassebroek A, Siddiqui F, Ballen K, et al. Race and outcomes of autologous hematopoietic cell transplantation for multiple myeloma. Biol Blood Marrow Transplant 2010;16:395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong S, Rybicki LA, Corrigan D, Schold JD, Majhail NS. Community risk score for evaluating health care disparities in hematopoietic cell transplantation. Biol Blood Marrow Transplant 2018;24:877–9. [DOI] [PubMed] [Google Scholar]
- 5.Majhail NS, Nayyar S, Santibanez ME, Murphy EA, Denzen EM. Racial disparities in hematopoietic cell transplantation in the United States. Bone Marrow Transplant 2012;47:1385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majhail NS, Omondi NA, Denzen E, Murphy EA, Rizzo JD. Access to hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant 2010;16:1070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005;106:2912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pillay B, Lee SJ, Katona L, Burney S, Avery S. Psychosocial factors associated with quality of life in allogeneic stem cell transplant patients prior to transplant. Psychooncology 2014;23:642–9. [DOI] [PubMed] [Google Scholar]
- 9.Pillay B, Lee SJ, Katona L, De Bono S, Burney S, Avery S. A prospective study of the relationship between sense of coherence, depression, anxiety, and quality of life of haematopoietic stem cell transplant patients over time. Psychooncology 2015;24: 220–7. [DOI] [PubMed] [Google Scholar]
- 10.Amonoo HL, Barclay ME, El-Jawahri A, Traeger LN, Lee SJ, Huffman JC. Positive psychological constructs and health outcomes in hematopoietic stem cell transplantation patients: a systematic review. Biol Blood Marrow Transplant 2019;25:e5–16. [DOI] [PubMed] [Google Scholar]
- 11.El-Jawahri A, Chen YB, Brazauskas R, He N, Lee SJ, Knight JM, et al. Impact of pre-transplant depression on outcomes of allogeneic and autologous hematopoietic stem cell transplantation. Cancer 2017;123:1828–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrykowski MA. Psychosocial factors in bone marrow transplantation: a review and recommendations for research. Bone Marrow Transplant 1994;13:357–75. [PubMed] [Google Scholar]
- 13.Hoodin F, Kalbfleisch KR. Factor analysis and validity of the Transplant Evaluation Rating Scale in a large bone marrow transplant sample. J Psychosom Res 2003;54:465–73. [DOI] [PubMed] [Google Scholar]
- 14.McQuellon RP, Russell GB, Rambo TD, Craven BL, Radford J, Perry JJ, et al. Quality of life and psychological distress of bone marrow transplant recipients: the ‘time trajectory’ to recovery over the first year. Bone Marrow Transplant 1998;21:477–86. [DOI] [PubMed] [Google Scholar]
- 15.Keogh F, O’Riordan J, McNamara C, Duggan C, McCann SR. Psychosocial adaptation of patients and families following bone marrow transplantation: a prospective, longitudinal study. Bone Marrow Transplant 1998;22:905–11. [DOI] [PubMed] [Google Scholar]
- 16.Ehrlich KB, Miller GE, Scheide T, Baveja S, Weiland R, Galvin J, et al. Pre-transplant emotional support is associated with longer survival after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2016;51:1594–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.U.S. Department of Health and Human Services. General considerations in assessment for transplant candidacy 2015. https://optn.transplant.hrsa.gov/resources/ethics/general-considerations-in-assessment-for-transplant-candidacy/. Accessed 21 Dec 2018.
- 18.Kuntz K, Weinland SR, Butt Z. Psychosocial challenges in solid organ transplantation. J Clin Psychol Med Settings 2015;22:122–35. [DOI] [PubMed] [Google Scholar]
- 19.Lewandowski AN, Skillings JL. Who gets a lung transplant? Assessing the psychosocial decision-making process for transplant listing. Glob Cardiol Sci Pract 2016;2016:e201626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olbrisch M, Levenson J, Hamer R. The PACT: a rting scale for the study of clinical decision making in psychosocial screening of orang transplant candidates. Clin Transplant 1989;3:164–9. [Google Scholar]
- 21.Hitschfeld MJ, Schneekloth TD, Kennedy CC, Rummans TA, Niazi SK, Vasquez AR, et al. The psychosocial assessment of candidates for transplantation: a cohort study of its association with survival among lung transplant recipients. Psychosomatics 2016;57:489–97. [DOI] [PubMed] [Google Scholar]
- 22.Molassiotis A, Van Den Akker OB, Milligan DW, Goldman JM. Symptom distress, coping style and biological variables as predictors of survival after bone marrow transplantation. J Psychosom Res 1997;42:275–85. [DOI] [PubMed] [Google Scholar]
- 23.Murphy KC, Jenkins PL, Whittaker JA. Psychosocial morbidity and survival in adult bone marrow transplant recipients--a follow-up study. Bone Marrow Transplant 1996;18:199–201. [PubMed] [Google Scholar]
- 24.Hoodin F, Kalbfleisch KR, Thornton J, Ratanatharathorn V. Psychosocial influences on 305 adults’ survival after bone marrow transplantation; depression, smoking, and behavioral self-regulation. J Psychosom Res 2004;57:145–54. [DOI] [PubMed] [Google Scholar]
- 25.Tschuschke V, Hertenstein B, Arnold R, Bunjes D, Denzinger R, Kaechele H. Associations between coping and survival time of adult leukemia patients receiving allogeneic bone marrow transplantation: results of a prospective study. J Psychosom Res 2001;50:277–85. [DOI] [PubMed] [Google Scholar]
- 26.Broers S, Hengeveld MW, Kaptein AA, Le Cessie S, van de Loo F, de Vries T. Are pretransplant psychological variables related to survival after bone marrow transplantation? a prospective study of 123 consecutive patients. J Psychosom Res 1998;45:341–51. [DOI] [PubMed] [Google Scholar]
- 27.Foster LW, McLellan L, Rybicki L, Dabney J, Visnosky M, Bolwell B. Utility of the psychosocial assessment of candidates for transplantation (PACT) scale in allogeneic BMT. Bone Marrow Transplant 2009;44:375–80. [DOI] [PubMed] [Google Scholar]
- 28.Foster LW, McLellan LJ, Rybicki LA, Dabney J, Welsh E, Bolwell BJ. Allogeneic BMT and patient eligibility based on psychosocial criteria: a survey of BMT professionals. Bone Marrow Transplant 2006;37:223–8. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton BK, Rybicki L, Abounader D, Adekola K, Advani A, Aldoss I, et al. Allogeneic hematopoietic cell transplantation for adult T cell acute lymphoblastic leukemia. Biol Blood Marrow Transplant 2017;23:1117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narkhede M, Rybicki L, Abounader D, Bolwell B, Dean R, Gerds AT, et al. The association of histologic grade with acute graft-versus-host disease response and outcomes. Am J Hematol 2017;92:683–8. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton BK, Law AD, Rybicki L, Abounader D, Dabney J, Dean R, et al. Prognostic significance of pre-transplant quality of life in allogeneic hematopoietic cell transplantation recipients. Bone Marrow Transplant 2015;50:1235–40. [DOI] [PubMed] [Google Scholar]
- 32.American Society of Blood and Marrow Transplantation. ASBMT RFI 2015 - disease classifications corresponding to CIBMTR classification 2015. https://www.asbmt.org/resource/resmgr/RFI/RFI_2015_-_CIBMTR_Disease_Cl.pdf. Accessed 15 Oct 2015.
- 33.Hart LG, Larson EH, Lishner DM. Rural definitions for health policy and research. Am J Public Health 2005;95:1149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rural Health Research Center. Rural urban commuting area codes data http://depts.washington.edu/uwruca/ruca-codes.php. Accessed 12 Oct 12, 2016.
- 35.US Census Bureau. American FactFinder 2010. http://factfinder.census.gov/faces/nav/jsf/pages/index.html. Accessed 15 Oct 2015.
- 36.FACIT Measurement System. FACIT measurement system - questionnaires 2016. http://www.facit.org/FACITOrg/Questionnaires. Accessed 12 Oct 2016.
- 37.Maldonado JR, Dubois HC, David EE, Sher Y, Lolak S, Dyal J, et al. The Stanford Integrated Psychosocial Assessment for Transplantation (SIPAT): a new tool for the psychosocial evaluation of pre-transplant candidates. Psychosomatics 2012;53:123–32. [DOI] [PubMed] [Google Scholar]
- 38.Presberg BA, Levenson JL, Olbrisch ME, Best AM. Rating scales for the psychosocial evaluation of organ transplant candidates. Comparison of the PACT and TERS with bone marrow transplant patients. Psychosomatics 1995;36:458–61. [DOI] [PubMed] [Google Scholar]