Abstract
Background:
Low adherence to statin (HMG-CoA reductase inhibitors) medication is common. Here, we report on the design and implementation of the Habit Formation Trial. This clinical trial assessed whether the interventions, based on principles from behavioral economics, might improve statin adherence and lipid control in at-risk populations. We describe the rationale and methods for the trial, recruitment, conduct and follow-up. We also report on several barriers we encountered with recruitment and conduct of the trial, solutions we devised and efforts we will make to assess their impact on our study.
Methods:
Habit Formation is a four-arm randomized controlled trial. Recruitment of 805 participants at elevated risk for atherosclerotic cardiovascular disease with evidence of sub-optimal statin adherence and low density lipoprotein (LDL) control is complete. Initially, we recruited from large employers (Employers) and a national health insurance company (Insurers) using mailed letters; individuals with a statin Medication Possession Ratio less than 80% were invited to participate. Respondents were enrolled if a laboratory measurement of LDL was > 130 mg/dl. Subsequently, we recruited participants from the Penn Medicine Health System; individuals with usual-care LDL of >100 mg/dl in the electronic medical record were recruited using phone, text, email, and regular mail. Eligible participants self-reported incomplete medication adherence.
During a 6-month intervention period, all participants received a wireless-enabled pill bottle for their statins and daily reminder messages to take their medication. Principles of behavioral economics were used to design three financial incentives, specifically a Simple Daily Sweepstakes rewarding daily medication adherence, a Deadline Sweepstakes where participants received either a full or reduced incentive depending on whether they took their medication before or after a daily reminder, or Sweepstakes Plus Deposit Contract with incentives divided between daily sweepstakes and a monthly deposit. Six months post-incentives, we compared the primary outcome, mean change from baseline LDL, across arms.
Results & lessons learned:
Health system recruitment yielded substantially better enrollment and was cost-efficient. Despite unexpected systematic failure and/or poor availability of two wireless pill bottles we achieved enrollment targets and implemented the interventions. For new trials, we will routinely monitor device function, and have contingency plans in the event of systemic failure.
Conclusions:
Interventions used in the Habit Formation trial could be translated into clinical practice. Within a large health system, successful recruitment depended on identification of eligible individuals through their electronic medical record, along with flexible ways of contacting these individuals. Challenges with device failure were manageable. The study will add to our understanding of optimally structuring and implementing incentives to motivate durable behavior change.
Keywords: Behavioral economics, financial incentive, coronary vascular disease, diabetes, statin medication, pragmatic trial, randomized trial, electronic pill bottle
Introduction
Atherosclerotic cardiovascular disease is the leading cause of death in the United States. Reducing low-density lipoproteins (LDL) with statins successfully lowers risk of atherosclerotic cardiovascular disease, but medication adherence is often poor.1–6 For example, around 40% to 50% of patients with prior myocardial infarction have low adherence or stop taking statins within one to two years of initial prescription.7–12 The Habit Formation trial examined the effectiveness of three behavioral economic interventions in achieving sustained reductions in LDL-cholesterol.
Concepts from behavioral economics offers promise in advancing health. Interventions based on financial incentives can successfully change behavior during the intervention, but post-intervention, the behavior may regress.13–15 For the Habit Formation trial, we had particular interest in determining which interventions would foster sustained change following cessation of the active intervention.
All participants in the four-arm randomized study received daily reminders for statin adherence. Participants in the intervention arms received daily financial incentives in the form of (1) simple daily sweepstakes, (2) deadline sweepstakes whereby a participant who missed a reminder was eligible for only half of the financial incentive, or (3) sweepstake plus deposit contract, whereby a non-adherent participant was both ineligible for the daily sweepstakes and received a deduction from the balance of their experimenter-provided virtual account. Compared to control, we asked whether each arm improved LDL levels during the intervention, and if so, whether the behavior change persisted post-intervention. Cost-effectiveness will be determined for interventions showing efficacy.
The Habit Formation study utilized the University of Pennsylvania ‘Way To Health’ system, a web-based platform to support research into patient engagement strategies16 The platform implemented the behavioral intervention without on-site study visits, facilitating remote participant recruitment, consent, enrollment, randomization, communication, reimbursement and data collection. We describe the design of Habit Formation with perspectives on recruiting and enrolling participants through to study completion.
Methods
The study protocol, including recruitment procedures, was approved by the institutional review board of the University of Pennsylvania.
Overview of design and study objectives
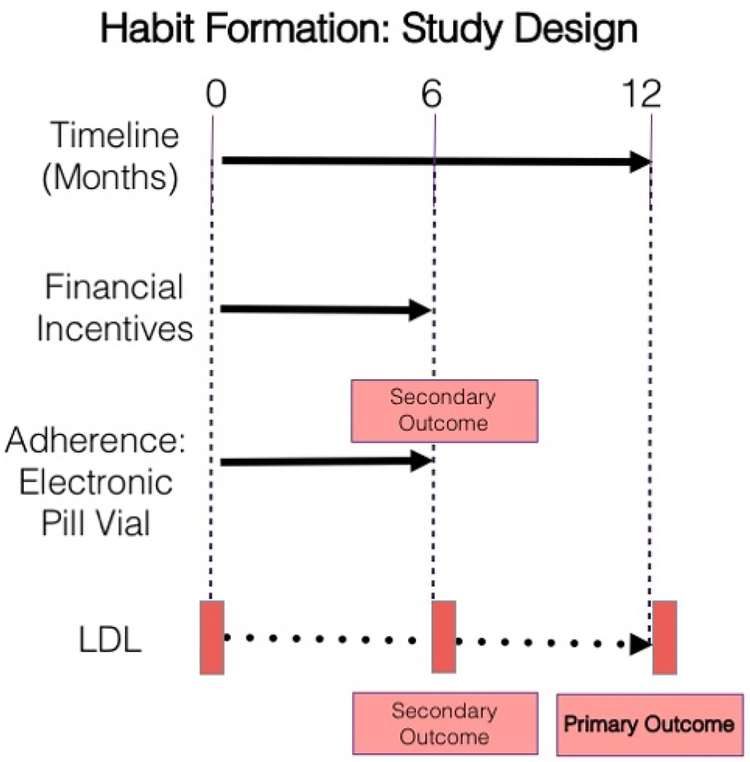
Habit Formation is a four-arm randomized controlled trial (RCT) with sequential 6-month intervention and observation phases (Figure 1).
Figure 1.
Using the Way to Health platform, the Habit Formation trial randomized participants to Control or one of three financial incentive arms. Financial incentives ended at 6 months post-randomization and participants were followed for an additional 6 months. The primary outcome was the change in LDL at 12 months versus baseline. Secondary outcomes included the change in LDL at 6 months versus baseline and adherence, measured using an electronic pill bottle during the first 6 months of financial incentives.
During the intervention, participants received either electronic daily reminders alone (Control) or daily reminders plus a financial incentive. The total possible payment was equivalent across the financial incentive arms. LDL-cholesterol was assessed at baseline, 6 and 12 months. The primary endpoint was change in LDL from baseline to 12 months, 6 months post-intervention. Each participant received a wireless-enabled pill bottle for their statin medication; during the 6-month financial incentive period, the bottle transmitted daily information about bottle-opening, our measure of adherence. During the 6–12 month period we ceased all monitoring to approximate a period of ‘usual care’.
All study personnel were initially blinded to treatment assignment. Research coordinators occasionally became unblinded during communication with participants, but did not relay this information to the rest of the research team.
We asked three questions:
At six months post-intervention, how did LDL change from baseline differ among the intervention arms and compared to control?
During the six-month intervention, how did financial incentives affect statin adherence and LDL control?
For arms with improved LDL levels compared to the control arm, how cost-effective were the interventions?
Study populations
Initially, potential participants were employees of several companies with benefits from a national pharmacy benefits manager (Employers) and beneficiaries of a large health plan (Insurers). Due to slow recruitment, we subsequently enrolled exclusively from the University of Pennsylvania Health System (Penn Medicine).
Eligibility
Table 1 shows eligibility criteria. Initially, we identified eligible participants from the Employer or Insurer populations based on an annual statin Medication Possession Ratio (Medication Possession Ratio) of less than 80%. Medication Possession Ratio is based on pharmacy claims. The numerator is the number of statin pills supplied during a time period; the denominator is the number of days in the period. In this case, we calculated Medication Possession Ratio for statins over 12 months. Individuals with diabetes as well as an LDL > 130 mg/dl, measured by the study, were eligible. Enrollment was low, so we reduced our LDL criterion to >100 mg/dl and started enrollment within Penn Medicine. We broadened diagnostic criteria to include individuals with a history of atherosclerotic cardiovascular disease, an American Heart Association/American Cardiologist Association cardiovascular disease risk score of at least 7.5% over 10 years, or LDL > 190 mg/dl.17 Eligible participants self-reported imperfect medication adherence based on a positive response to one or more validated queries about whether the individual sometimes missed taking their medicine or experienced difficulty adhering to the medication regime (see Supplementary Material).
Table 1.
Eligibility criteria for participants recruited initially from the Employer or Insurer populations and subsequently from Penn Medicine. With the exception of participants with a diagnosis of diabetes, all participants were 18 years of age or older. Participants enrolled through Employer and Insurer Populations from July 2013 until October 2014, when we switched to recruitment from Penn Medicine.
| Eligibility Criteria | Diagnosis or Risk Criterion | Population | |
|---|---|---|---|
| Employers or Insurers | Penn Medicine | ||
| Statin Prescription | Any | Pharmacy Record | Self-verified |
| Statin Adherence | Any | Medication Possession Ratio <80%a | Self-reported Imperfect Adherence |
| LDLi cholesterol | Any | >190 mg/dl | >190 mg/dld |
| Diabetesb | >130 mg/dlc,e >100 mg/dlc,f |
>100 mg/dld | |
| Diagnosis of clinical ASCVDh | >130 mg/dlc,e >100 mg/dlc,f |
>100 mg/dld | |
| ASCVDh 10-year Risk >7.5% |
NAg | >100 mg/dld | |
Based on pharmacy records
And age of 40 to 75 years
Measured by the study
Recorded as part of usual care
July 2013-April 2014
May 2014-October 2014
No participants were recruited from Employers or Insurers based on Atherosclerotic Cardiovascular Disease Risk
ASCVD: Atherosclerotic Cardiovascular Disease
LDL low density lipoprotein cholesterol
Exclusions.
We excluded individuals under 18 years of age, those with contraindications to further statin use or side effects from statins such as active or progressive liver disease, and those who did not or could not give consent. For Penn Medicine, we excluded individuals enrolled in another clinical trial or on PCSK9 inhibitors.
Recruitment through randomization
Table 2 summarizes recruitment through randomization. For Employers and Insurers, individuals with diabetes and a statin Medication Possession Ratio < 80% were invited by mail to create a profile through the webpage or enroll over the phone with assistance from a study coordinator. Upon creating the profile, participants completed a screening questionnaire, and qualified participants were offered a laboratory measurement to determine LDL-based eligibility. Once enrolled, participants received an electronic pill-bottle.
Table 2.
Steps in the process of recruitment through randomization
| Step | Population | |
|---|---|---|
| Employer or Insurer | Penn Medicine | |
| Prescreen | Medication Possession Ratio, Statin Prescription & Eligibility criteria other than LDL level | LDL from usual care; Statin Prescription & Eligibility criteria other than LDL level |
| Invitation to Enroll | Mail only | Mail with follow-up by phone, text and email, pending available contact information |
| Creation of online profile (demographics, confirm non-LDL related eligibility criteria) | Way To Health Website Completed online or by phone with staff | |
| Assessment of LDL criterion | Lab visit required for everyone who satisfied eligibility criteria other than LDL level | Based on usual care data from the electronic medical record. Confirmed by lab visit for those with usual care LDL measured > 4 weeks before randomization |
| Consent | Online or by phone with follow up by paper | Online or by phone with follow up by paper |
| Randomization | Upon receipt and activation of electronic pill-bottle | |
Enrollment among employers and insurers was low: under 1% of those contacted. We thus altered our protocol and recruited exclusively from Penn Medicine, a large health system serving an urban and suburban population around Philadelphia, PA. We limited recruitment to participants with LDL eligibility based on cholesterol measured during usual care. These individuals received letters using “opt-out” language indicating they were “pre-enrolled” and inviting them to complete their study profile on the webpage. Because we had health and contact information we could target recruitment, using multiple methods. After one week, participants who did not complete enrollment received a phone call and, if possible, an email. When reached by phone, the coordinator verbally administered the screening questionnaire, obtained consent and recorded study information on the webpage. During the second week, if not already enrolled, potential participants received a Google voice text message that was Health Insurance Portability and Accountability Act-compliant and a phone call. Participants who agreed to consent received a copy of the completed consent form and an electronic pill bottle by mail.
The Way To Health platform block-randomized participants upon activation of their device using variable block sizes (4, 8 or 12 participants). (see Supplementary Material) Participants could opt out of study participation at any time.
Conceptual basis for financial incentive interventions
Elevated LDL is asymptomatic. Statins do not improve quality of life in the short term, but reduce future risk. Adherence may challenge patients because they discount future benefits or simply forget to take their statin. Financial incentives can motivate individuals, in part by bringing benefits of adherence into the present. Our financial incentives built on established behavioral economics research, highlighting the benefits of: daily positive reinforcement of behavior; probabilistic delivery of rewards; regret lotteries; and reminder messaging.18–21 We disbursed all accumulated incentive payments on the last Friday of each month. This avoided the ‘peanuts’ effect, where individuals are poorly motivated by small incentives.22
The Control received a daily reminder to take their study medication if they had not already done so. The reminder time was either 10 PM (the default) or a time specified by the subject at baseline. The other participants were enrolled in sweepstakes as described below (see also Supplementary Material):
Simple Sweepstakes.
Before randomization, each participant received a two-digit number, ranging from 00 to 99. Daily, a two-digit randomly generated number was compared to this number. Participants who adhered the previous day and had a two-digit match won $100; if only one digit matched, the participant won $10. The chance of a two-digit match was one in 100, and the chance of a one-digit match was 18 in 100. On average, a fully-adherent participant could expect $2.80/day and $504.00 during the whole trial. A fully adherent participant had an 83.6% chance of winning the $100 sweepstake at least once over the six-month period, and a 99.7% chance of winning the $10 sweepstake at least once each 30-day ‘month’. Adherent participants received feedback the following day indicating whether they won; non-adherent participants received comparable feedback reminding them to take their medication. In the case of a one or two-digit match, we informed non-adherent participants of the lost incentive.
Deadline Sweepstakes.
We sent the reminder prior to the time of the day when the participant indicated they usually took their statin. The financial incentive when adherent was identical to Simple Sweepstakes unless the participant took their statin after receiving the reminder. In this case the payment was halved.
Sweepstakes Plus Deposit Contract.
Participants were eligible for daily sweepstake rewards, but winnings were halved compared to those in Simple Sweepstakes. Thus, a participant received $50 for a two-digit match and $5 for a one-digit match. The study placed $45 in a virtual account at the start of each 30-day month. Every day that a participant was non-adherent, $1.50 was deducted from the account. The amount remaining at the conclusion of the 30-day month was included in the monthly payment. During the intervention phase, the deposit amount reset to the initial value of $45 on the first day of each 30-day month.
Electronic pill bottles
As summarized in Supplementary Table 1, we deployed three different electronic pill bottles. During the first 33 months, we distributed 541 Model 1 devices. Model 1 used a 2G cellphone network; by 27 months these devices began failing as the network provider eliminated 2G towers. We identified another viable device (Model 2), switched 34 participants (6.3% of Model 1 devices) to Model 2 and distributed 80 Model 2 devices to new enrollees. Unfortunately, Model 2 had high failure rates, and was supplied intermittently, forcing suspension of trial recruitment for several months while we identified a new device (Model 3). Model 3 runs on the 3G/4G network, and has proven reliable and available. A total of 184 subjects were started on Model 3.
A total of 17 patients of those who initially used Model 2 (21.2%) and who had not completed the 6-month intervention phase were switched to Model 3. Additionally, three participants randomized using Model 1 switched to Model 2 and then switched again to Model 3. Improper pill bottle use was a minor anticipated problem across devices.
Outcome measures
The primary outcome was change in LDL-cholesterol from baseline to 12 months, i.e., six months after completion of financial incentives intervention (Figure 1). Baseline LDL-cholesterol was either calculated or measured LDL,23 determined within 30 days of randomization and completed prior to study enrollment. Secondary endpoints included LDL change from baseline to six months and medication adherence during the 6-month and final 30 days of the intervention.
Following enrollment, we measured LDL-cholesterol at 6 and 12 months at a lab chosen by the participant. We offered the option of having labs drawn at home for the 12-month follow-up.
Each day the pill bottle was opened, it electronically transmitted a signal to Way To Health. A participant could also become eligible for the sweepstakes if they reported pill bottle malfunction, and indicated adherence data were not properly collected. In this case, staff promptly diagnosed and corrected the problem.
Adherence was pre-defined as the number/rate of daily pill bottle openings during the six-month intervention. Adherence during the final 30 days of the intervention will also be reported.
In addition to the primary outcomes, a series of survey instruments at 6 and 12 months were designed to answer questions about self-perceived characteristic of their health, risk perception, motivation to engage in behavioral change related to health and perceived value of a financial reward. The costs of the pill bottle, staff time and computer platform and the financial incentives were measured as inputs for the cost effectiveness analysis (Further details appear in Supplementary Methods).
Financial compensation outside of financial incentives
Initially, all participants who completed eligibility screening received $25, irrespective of whether they obtained an LDL measurement through a lab visit. Later, we added a participant pool with existing baseline LDL data and compensated participants who enrolled $25 for their baseline LDL result, irrespective of the source of an eligible result. Finally, we moved recruitment to Penn Medicine where we contacted eligible participants with current LDL values and compensated participants $25 if they were eligible, activated their device and were randomized. Participants received $75 for each LDL-cholesterol measurement at 6 and 12 months. If the LDL at 12 months was measured by a technician sent to the participant’s home, the compensation was reduced to $25.
Study advising and monitoring
We had an independent Advisory Board. A Data Safety Monitoring Board met at 6 month intervals to assess study conduct and adverse events (Supplementary Material). Participants reported adverse events on the Way To Health portal and by phone to coordinators. We specifically surveyed participants for adverse events at 6 and 12 months.
Statistical considerations
Determination of sample size.
We used a 10 mg/dl change as our detectable effect, based on a meta-analysis by the Cholesterol Treatment Trialists Collaboration on 90,000 participants from 14 trials; over a year, a 10 mg/dl LDL reduction would predict about a 5% reduction in atherosclerotic cardiovascular disease events.24 LDL-cholesterol is also a useful surrogate measure to study an intervention aimed at improving the behavior of improved statin adherence.
In a two-step approach, the analysis will compare each financial incentive arm with control (3 comparisons), and then compare any financial incentive arms differing from the control to each other (up to 3 additional comparisons). Results from a prior trial suggested a standard deviation of the change from baseline in LDL-cholesterol of 24.5 mg/dl. Step I (comparison of each incentive arm with control) will use a Holm-Bonferroni correction to maintain the experiment-wise Type I error at the nominal level of 0.05.25
Step II (comparison of intervention arms) will use a Tukey Honest Significant Difference approach. We further assumed 20% loss to follow-up for the LDL-cholesterol measurement at 12 months. There were no formal stopping rules.
Using simulations, we estimated that 200 participants recruited into each of the four arms would provide over 90% power to detect a 10 mg/dl difference between interventions and control, and at least 80% power to detect an 8.5 mg/dl mean difference between at least one pair of intervention arms.
Data analysis plans.
The primary intent-to-treat analysis will fit a linear model and test whether (1) the change from baseline in mean LDL-cholesterol at 12 months differs between each intervention arm and control, and (2) mean LDL-cholesterol differs between pairs of intervention arms showing significance in Step I. For efficiency, the primary analysis will include baseline LDL-cholesterol as a covariate.26 Using multiple imputation, we will adjust for missing outcomes and compare with the results from the complete case analysis.27 Secondarily, we will adjust for study group (Employers, Insurers or Penn Medicine), patient sex, income, educational attainment and race. Sensitivity analyses will assess the impact of pill bottle model and malfunction. The analysis of adherence will use summary measures of individual participants’ frequency of pill bottle openings in the first six months or the final 30 days as the outcome. Further analyses will explore self-assessed barriers to medication adherence and how financial incentives might impact those with specific barriers to medication adherence. Details appear in Supplementary Materials.
The sample size calculation was conducted in R (v2.5.1). Statistical methods are described further in Supplementary Materials.
Cost effectiveness
For each intervention deemed effective, we will conduct cost-effectiveness analyses from the healthcare sector perspective for the short term, the 12-month study period, and for the long term, the lifetimes of participants (See Supplementary Methods).28
Results
Recruitment and enrollment
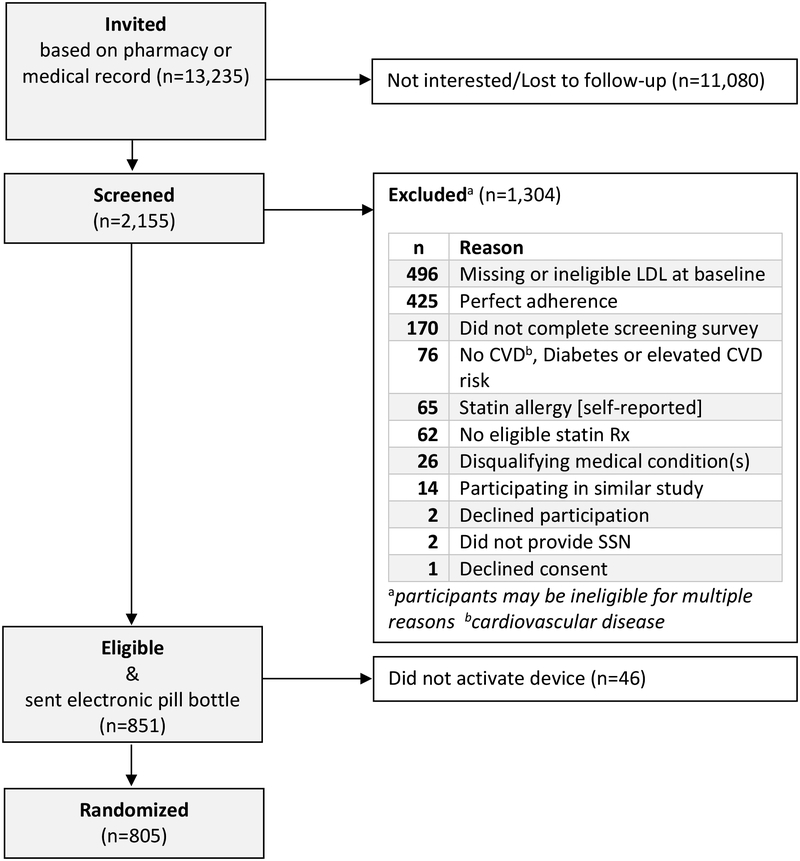
Figure 2 summarizes participant flow. We invited over 10,000 individuals and randomized 805. Of 2146 participants who were screened, common reasons for ineligibility included: LDL-cholesterol missing or ineligible at baseline (n=496), self-reported perfect statin adherence (n=425) and failure to complete screening survey (n=161), Among 851 screened and eligible participants, 5.4% (n=46) never activated their device and were never randomized.
Figure 2.
Consort Diagram: The Habit Formation Trial
Table 3 provides details on recruitment through Employer or Insurers. By mail, we invited over 8500 participants from Employer and Insurer groups, but eventually randomized only 67 participants; the yield (proportion of invited) was only 0.8%. Notably, only 7.2% of invitees were screened using a Way To Health account. Of these, only 10.7% were eligible and consented. For those screened, an LDL that was too low (n=123) or failure to get a baseline LDL (n=222) were common reasons for ineligibility. This strategy was expensive, because the research study covered the cost of labs for both eligible and ineligible participants.
Table 3.
Recruitment based on Medication Possession Ratio from either the Employer or Insurer populations. Furthest left column describes each step of the recruitment process. Values shown are numbers of participants, or percentage of either the total number invited, or the number who remained eligible and available at the prior step. For example, only 10.7% (68 participants) of the 638 participants who agreed to be screened were eligible & consented for the study.
| Employer or Insurer Population | |||
|---|---|---|---|
| Step of Recruitment Process | N | % of Invited | % of Prior Step |
| Invited based on Medication Possession Ratio | 8831 | 100 | NAa |
| Screened | 638 | 7.2 | 7.2 |
| Eligible & Consented & Sent Device | 68 | 0.8 | 10.7 |
| Randomized | 67 | 0.8 | 98.5 |
No prior step
Recruitment through Penn Medicine was more successful, both in terms of yield and expense (Table 4). A total of 4404 participants were ‘pre-enrolled’ based on a usual-care LDL measurement and invited to complete enrollment by regular mail. We followed up by phone, text or email. Of these, 1508 completed a Way To Health profile and 738 completed enrollment and were randomized, for an overall yield of 16.8%. Notably, 34.2% of potentially-eligible invited participants were screened using a Way To Health account and a screening questionnaire expressing interest in participating. Of these, over half were eligible and consented. The most frequent reason for ineligibility in this group of invited participants was self-reported perfect adherence to their statin (n=425 screened participants). Using usual-care LDL to prescreen lipid levels meant that we paid for an LDL measurement only for those participants with usual-care LDL more than 30 days prior to their randomization date. Participants who were ineligible based on self-reports of perfect adherence were not costly because the research study did not pay for LDL assessment. A small number of participants required a study-based LDL measurement at baseline, and a few of these (n=13) were ineligible based on their second measurement. For Penn Medicine, exclusions based on ineligible lipids were small compared to Employer and Insurers, where over nine-fold more subjects (n=123) were excluded for ineligible lipid values.
Table 4.
Recruitment within the Penn Medicine Health System and relying on usual care LDL. Furthest left column describes each step of the recruitment process. Values shown are numbers of participants, or percentage of either the total number invited, or the number who remained eligible and available at the prior step. For example, 52.4% (783 participants) of the 1495 participants from Penn Medicine who were eligible based on diagnosis, medication adherence and LDL-cholesterol were eligible based on the remaining criteria and were sent a pill bottle.
| Step | Penn Medicine | ||
|---|---|---|---|
| N | % of Invited | % of Prior Step | |
| Pre-enrolled in Way To Health based on usual care LDL-cholesterol | 4404 | 100 | NAb |
| Screened and eligible based on diagnosis and medication adherence | 1508 | 34.2 | 34.2 |
| Eligible usual-care LDL-cholesterol within 30 days of randomization OR eligible by follow-up LDLa | 1495 | 33.9 | 99.1 |
| Eligible and sent electronic pill bottle | 783 | 17.8 | 52.4 |
| Randomized | 738 | 16.8 | 94.3 |
Participants with usual-care LDL-cholesterol outside of 30 days were required to obtain another measurement of LDL at the lab of their choice. They remained eligible if they satisfied the LDL criteria in Table 1.
No prior step
Challenges and lessons learned
Recruitment of eligible patients was challenging. Using Penn Medicine to identify potential participants with LDL-cholesterol measured in routine clinical practice, following up by phone to encourage participation, and enrolling with minimal further participant effort proved effective. In contrast, recruiting through Employer or Insurer populations, where potential participants required an initial LDL measurement paid for by the study, was inefficient and costly. Few participants enrolled and a preponderance had well-controlled LDL-cholesterol, rendering them ineligible. Engaging multiple avenues of reaching potential participants, and minimizing the participant’s burden, were key. A pilot or feasibility study might have allowed us to recognize these issues earlier.
Incomplete follow-up is generally a concern for longitudinal clinical trials, and is of special concern here, given that our primary endpoint (LDL-cholesterol) was measured at 12 months (within a window of 60 days). Thus, at 12 months, we offered participants the option of an LDL measurement at a lab, with payment of $75, or at home, with a $25 payment. At study completion, our 12-month follow-up rate was 79% (range of 77.6 to 79.9% by arm) and only one participant chose the in-home option. In-home visits, at least with this level of reduced compensation, were not an attractive option. It is possible that offering the home-visit option at a reduced payment increased uptake of the full-payment lab-visit option.
Over the study period of 36 months, we tracked electronic bill bottle reliability and availability. Our research coordinators monitored device function and investigated signal failures or participant concerns. Our coordinators were experts in pill bottle function and worked with participants to troubleshoot common problems. We had contingency plans in case the pill bottle could not be used as intended. Specifically, we maintained a supply of ‘spare’ bottles and were in regular contact with vendors both to troubleshoot problems and maintain a sense of the company’s commitment to their product and the study. This process continues in preparation for future studies. An important lesson: staff should be trained and proactive in supporting device issues.
A final lesson learned concerns staffing and organization. By using shared staff from the Way To Health platform, we required only a small study staff of two full-time coordinators, several students, a half-time project manager and part-time statistical analyst. Weekly one-hour meetings between staff and the multidisciplinary team of principal and co-investigators, were central to the successful conduct and completion of this trial.
Discussion
The Habit Formation study examined the efficacy of three interventions to promote sustained adherence to statins. We hypothesized that the structure of a financial incentive may be fundamental to its success. This study will provide information about how to optimally structure financial incentives to achieve a durable reduction in LDL-cholesterol. Our design will assess the effects of sweepstakes-based interventions over and above any effect due to a daily reminder, which, on their own, appear to exert limited effects on medication adherence.29
Our study confirms the importance of adapting recruitment procedures to changing circumstances. Based on discussions with colleagues in industry, we anticipated the Employer and Insurer populations would have an abundance of eligible participants who would accrue over a reasonable timeframe. Once recruitment began, we experienced challenges attracting potential participants, and the pool of eligible participants was smaller than anticipated. In retrospect, an initial feasibility study might have shed light on the problem earlier. In light of poor accrual, the team explored recruitment options within our local health system, where we accessed baseline LDL, ‘pre-enrolled’ individuals, and had flexibility to directly contact potential participants.30 Notably, phone calls appeared particularly effective. The switch from Employer and Insurer populations to Penn Medicine enabled completion of the study in a timely manner.
Medication adherence can be monitored using multiple technologies, which vary in terms of patient burden and technological aptitude required for successful use. In the Habit Formation Trial, participants transferred their statin medication from pharmacy bottles into the wireless pill bottles. However, there was no need for a smartphone application, and little further usage burden on the patient. Inaccurate adherence reporting was possible if participants opened the bottle without taking their medication, or took more than one dose at an opening. To date, we have observed little evidence of such behavior. Other technologies include video-assisted, directly-observed therapy, monitored via a mobile smartphone application.31,32 For monitoring adherence to multiple medications, the direct-observation approach may be more effective, and less burdensome than wireless pill bottles, but requires a smartphone and dexterity with an application, undoubtedly a barrier for some individuals. Finally, sensors can be embedded into pills.33 These sensors transmit information when swallowed, and may pose the lowest patient burden while providing the most accurate reports of adherence. But, some individuals may consider the technology intrusive and either refuse to use it or to participate in a study involving the technology.
In behavioral studies, device problems have the potential to contaminate behavioral treatment interventions. With maturing technologies, these problems should diminish. In our experience, despite the potential for contamination, close monitoring of technology is critical to a successful trial; to mitigate study risk, it is essential to have an alternative device available at all times. Participants were encouraged to report problems to a coordinator. Our research coordinators were key to identifying device problems. At weekly meetings, our research coordinators brought the problems to the team’s attention, and we brainstormed solutions.
Our study has the potential to impact clinical practice. While many financial incentive studies focus on adherence alone, we measured changes in LDL-cholesterol, an outcome that is undoubtedly more relevant to risk of atherosclerotic cardiovascular disease. The recruitment process leveraged our health system’s electronic medical records to efficiently identify patients at high risk of atherosclerotic cardiovascular disease, who could benefit by adhering well to statin medications. The interventions focused on optimally structuring financial incentives. Lastly, commercially-available, wireless pill bottles could be integrated into financial incentives programs offered by employers or insurers in partnership with healthcare providers.
Supplementary Material
Acknowledgements
We gratefully acknowledge the study participants, members of our Advisory Board and Data Safety and Monitoring Boards. Drs. Andrea Troxel and Scarlett Bellamy contributed to the design/accrual portion of the study. We thank three anonymous reviewers for their thoughtful comments.
Funding
The National Institute on Aging of the National Institutes of Health sponsored the study (R01AG043844: Volpp, Barankay, Reese).
Research supported by 5R01AG043844-04 (MultiPI: KGV, IB, PR)
Footnotes
Trial registration at ClinicalTrials.gov Trial Identifier
References
- 1.Armitage J The safety of statins in clinical practice. Lancet 2007; 1781–1790. [DOI] [PubMed] [Google Scholar]
- 2.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278. [DOI] [PubMed] [Google Scholar]
- 3.Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). The Lancet 1994; 344: 1383–1389. [PubMed] [Google Scholar]
- 4.Abramson J, Rosenberg HG, Jewell N, et al. Safety and efficacy of statins. Lancet 2017; 389: 1097. [DOI] [PubMed] [Google Scholar]
- 5.Ho PM, Magid DJ, Shetterly SM, et al. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J 2008; 155: 772–779. [DOI] [PubMed] [Google Scholar]
- 6.Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med 2006; 166: 1836–1841. [DOI] [PubMed] [Google Scholar]
- 7.Booth JN, Colantonio LD, Chen L, et al. Statin discontinuation, reinitiation, and persistence patterns among Medicare beneficiaries after myocardial infarction: a cohort study. Circ Cardiovasc Qual Outcomes 2017; 10: e003626. [DOI] [PubMed] [Google Scholar]
- 8.Shah ND, Dunlay SM, Ting HH, et al. Long-term medication adherence after myocardial infarction: experience of a community. Am J Med 2009; 122: 961.e7–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350: 1495–1504. [DOI] [PubMed] [Google Scholar]
- 10.Jackevicius CA, Mamdani M and Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002; 288: 462–467. [DOI] [PubMed] [Google Scholar]
- 11.Vallejo-Vaz AJ, Robertson M, Catapano AL, et al. LDL-Cholesterol lowering for the primary prevention of cardiovascular disease among men with primary elevations of LDL-cholesterol levels of 190 mg/dL or above: analyses from the WOSCOPS 5- year randomised trial and 20-year observational follow-up. Circulation 2017; 136: 1878–1891. [DOI] [PubMed] [Google Scholar]
- 12.Colantonio LD, Huang L, Monda KL, et al. Adherence to high-Intensity statins following a myocardial infarction hospitalization among medicare beneficiaries. JAMA Cardiol 2017; 2: 890–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loewenstein G, Brennan T and Volpp KG. Asymmetric paternalism to improve health behaviors. JAMA 2007; 298: 2415–2417. [DOI] [PubMed] [Google Scholar]
- 14.Asch DA, Troxel AB, Stewart WF, et al. Effect of financial incentives to physicians, patients, or both on lipid levels. JAMA 2015; 314: 1926–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halpern SD, French B, Small DS, et al. Randomized trial of four financial-incentive programs for smoking cessation. N Engl J Med 2015; 372: 2108–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Way To Health, https://www.waytohealth.org/ (Accessed 20 February 2019).
- 17.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(25 Suppl 2): S1–S45. [DOI] [PubMed] [Google Scholar]
- 18.Connolly T and Butler D. Regret in economic and psychological theories of choice. J Behav Decis Mak 2006; 19: 139–154. [Google Scholar]
- 19.Hoelzl E and Loewenstein G. Wearing out your shoes to prevent someone else from stepping into them: Anticipated regret and social takeover in sequential decisions. Organ Behav Hum Decis Process 2005; 98: 15–27. [Google Scholar]
- 20.Tversky A and Kahneman D. Advances in prospect theory: cumulative representation of uncertainty. J Risk Uncertain 1992; 5: 297–323. [Google Scholar]
- 21.Gonzalez R and Wu G. On the shape of the probability weighting function. Cognit Psychol 1999; 38: 129–166. [DOI] [PubMed] [Google Scholar]
- 22.Weber BJ and Chapman GB. Playing for peanuts: why is risk seeking more common for low-stakes gambles? Organ Behav Hum Decis Process 2005; 97: 31–46. [Google Scholar]
- 23.Nauck M, Warnick GR and Rifai N. Methods for measurement of LDL-cholesterol: a critical assessment of direct measurement by homogeneous assays versus calculation. Clin Chem 2002; 48: 236–254. [PubMed] [Google Scholar]
- 24.Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet 2010; 376: 1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holm S A simple sequentially rejective multiple test procedure. Scand J Stat 1979; 6: 65–70. [Google Scholar]
- 26.Vickers AJ and Altman DG. Analysing controlled trials with baseline and follow up measurements. BMJ 2001; 323: 1123–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carpenter J and Kenward M. Multiple imputation and its application. 1st edition Chichester, West Sussex: Wiley, 2013. [Google Scholar]
- 28.Weinstein MC, Coxson PG, Williams LW, et al. Forecasting coronary heart disease incidence, mortality, and cost: the Coronary Heart Disease Policy Model. Am J Public Health 1987; 77: 1417–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choudhry NK, Krumme AA, Ercole PM, et al. Effect of reminder devices on medication adherence: The REMIND Randomized Clinical Trial. JAMA Intern Med 2017; 177: 624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halpern SD, Ubel PA and Asch DA. Harnessing the power of default options to improve health care. N Engl J Med 2007; 357: 1340–1344. [DOI] [PubMed] [Google Scholar]
- 31.Garfein RS, Liu L, Cuevas-Mota J, et al. Tuberculosis treatment monitoring by video directly observed therapy in 5 health districts, California, USA. Emerg Infect Dis 2018; 24: 1806–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Creary SE, Gladwin MT and Krishnamurti L. Mobile directly observed therapy: Monitoring and improving hydroxyurea adherence in pediatric sickle cell patients. Blood 2012; 120: 2060–2060. [DOI] [PubMed] [Google Scholar]
- 33.Rosenbaum L Swallowing a spy — The potential uses of digital adherence monitoring. N Engl J Med 2018; 378: 101–103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.