Abstract
Eisosomes are furrows in the yeast plasma membrane that form a membrane domain with distinct lipid and protein composition. Recent studies highlighted the importance of this domain for the regulation of proton-nutrient symporters. The amino acids and other nutrients which these transporters deliver to the cytoplasm not only feed into metabolic pathways but also activate the metabolic regulator TORC1. Eisosomes have also been shown to harbor the membrane stress sensors Slm1 and Slm2. Membrane tension caused by hypoosmotic shock results in the redistribution of Slm1/2 from eisosomes to TORC2 which in turn regulates lipid synthesis. Therefore, eisosomes function upstream of both TORC1 and TORC2 regulation.
Keywords: Traffic, Intracellular Transport, Eisosomes, nutrient transporter, APC transporter, TORC1, TORC2, metabolism, lipid synthesis
Graphical Abstract
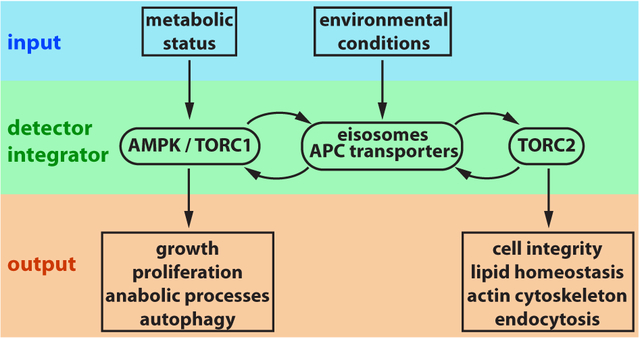
Yeast cells are exposed to dramatically changing environmental conditions and accordingly have evolved stress response pathways to efficiently adapt to these changes. Because of the high metabolic rate, fast growing and dividing yeast cells are particularly affected by environmental changes. These cells require both efficient nutrient uptake and conditions favorable to metabolize these nutrients. Furthermore, dividing cells are not able to abort the cell cycle program during stress conditions but have to finish the cycle before entering a more resilient stationary phase (a G0 state). Therefore, stress response pathways are important to allow dividing cells to safely exit the cell cycle.
During acute stress, yeast cells terminate non-essential activities to preserve energy that is redirected towards the stress response pathways. One group of proteins that responds very strongly to environmental changes is the protein superfamily of the Amino Acid Polyamine Organocation (APC) type nutrient transporters. These transporters are evolutionary conserved from archaea to humans and function in the import of small molecules such as amino acids and nucleosides (reviewed in1. However, yeast is able to synthesize all these molecules from glucose and salts (yeast is a prototroph) and therefore APC transporters are not essential, affording cells the capacity to degrade them when the need arises.
The yeast genome encodes 26 APC transporters, most of which are involved in the uptake of amino acids from the extracellular medium. Although APC transporters function as permeases (facilitating movement of molecules across the membrane in both directions), their activity is usually linked to an ion gradient that drives the import of the nutrient. In yeast, the strong proton gradient across the plasma membrane serves as the driving force for APC transporters. A yeast culture acidifies a standard synthetic defined growth medium to pH ~4, resulting in a 1000-fold concentration gradient of protons across the plasma membrane. This proton gradient is maintained by the proton pump Pma1, the most abundant plasma membrane protein in yeast (estimated copy number of >200,000 molecules per cell;2) and one of the largest energy consumers (reviewed in3. The proton pumping activity of Pma1 requires ATP and thus nutrient import by APC transporters is indirectly an ATP driven process. Because the APC transporters are among the largest consumers of the proton gradient, it is not surprising that these transporters are rapidly removed from the cell surface under most stress conditions4–9. Endocytosis of APC transporters dramatically reduces the proton flux across the plasma membrane which in turn lowers the energy consumption by Pma1. Therefore, environmental changes that impact cellular ATP levels are among the conditions that cause rapid APC transporter endocytosis and degradation (e.g. starvation conditions). An imbalance between proton import and the proton pumping activity of Pma1 could rapidly lead to acidification of the cytoplasm and a collapse of the proton gradient, both of which are detrimental for the cell (Pma1 function is essential;10). This problem is exemplified by studies that analyzed the link between cytoplasmic pH and glucose availability. Acute glucose starvation leads to a rapid drop in cytoplasmic pH, which is likely caused by the drop in ATP levels and the resulting low Pma1 activity11. The same study also indicated that loss of the vacuolar ATPase, which pumps protons into the endosomal/vacuolar compartments (reviewed in12), did not affect the glucose dependent changes in cytoplasmic pH, suggesting that this proton pump does not significantly contribute to the regulation of the cytoplasmic pH during these stress conditions.
It should be noted that glucose import is independent of the proton gradient. Hexose permeases, although similar to the APC transporters in structure and mechanism, facilitate the diffusion of sugars into the cell where phosphorylation by hexokinase traps the nutrient. Hexose permeases are mainly regulated by carbon source availability and thus their regulation is distinct from that of APC transporters.
APC transporters and metabolism
APC transporters and the cells’ metabolism are tightly interconnected. These transporters import amino acids, nutrients that can be rate limiting for fast growing yeast (proteins constitute for ~40% of yeast dry weight). Loss of amino acid import causes the cell to reroute the metabolism and use a portion of glucose as carbon source for the amino acid synthesis (glycolytic metabolism) (reviewed in13. On the other hand, if the conditions allow for amino acid import, the resulting increase in proton flux has to be accommodated by an increase in ATP production (to allow for higher Pma1 activity), which results in a higher demand for respiration and/or fermentation. Therefore, APC transporter activity can drive metabolic control of the cell. However, the reverse is also true: the metabolic state of the cell as well as nutrient availability also regulate the expression of APC transporters.
Transcriptional regulation of APC transporter expression
Transcriptional regulation of genes encoding APC transporters ensures the expression of these proteins is upregulated if their substrate is present in the environment. Therefore, transcriptional regulation varies between the different transporters. Amino acid transporter expression has been studied in great detail and I refer to several comprehensive reviews for an in-depth read on this subject13–15. In brief, the main system that regulates the transcription of amino acid APC transporters is the SPS sensor (named after the key components Ssy1, Ptr3 and Ssy5;16) that is able to detect the presence of micromolar concentrations of amino acids in the medium and induces expression of the transporter genes with the help of the transcription factors Stp1 and Stp2. An interesting aspect of the SPS sensor is the mechanism by which the presence of amino acids is recognized. The amino acid detector Ssy1 is homologous to APC transporters and thus its function might have evolved from transporting to sensing amino acids. Since amino acids also serve as a nitrogen source, the transcriptional control of the corresponding transporters is also affected by the nitrogen regulatory system of the cell17.
Substrate-dependent downregulation of APC transporters
Numerous studies have shown that post-translational regulation of transporters is the main mechanism by which yeast controls the number of active transporters and thus the influx of nutrients. This post-translational regulation includes changes in the transporter trafficking as well as regulation of transporter turnover. These regulatory mechanisms are particularly well suited to respond rapidly to changing environmental conditions, with the goal to maintain a steady influx of nutrients, high enough to promote fast growth of the cell, but not too high to avoid the toxic effects of nutrients. Several studies have shown that overexpression of APC transporters leads to growth inhibition, suggesting that excess nutrient influx is toxic for the cell18–20. Although the reason for this toxicity is in most cases not known, it is likely that the high cytoplasmic nutrient concentrations are able to disrupt metabolic pathways. This potential toxic effect of nutrients highlights the need for an efficient regulatory system that rapidly adjusts the import activity of APC transporters. Transcriptional regulation is too slow for a rapid response to nutrient fluctuations. However, endocytosis is able to remove transporters from the cell surface within seconds/minutes, thereby preventing toxic accumulation of nutrients in the cytoplasm.
Substrate-dependent downregulation of APC transporters refers to the regulatory mechanism that increases transporter degradation in response to high substrate concentrations. Interestingly, the sensing of high nutrient concentrations is built into the APC transporters itself and does not require auxiliary proteins. The key to understanding this sensing mechanism is in the large structural changes APC transporters undergo during the substrate import cycle. In the ground state the central substrate-binding site of the transporter is accessible to the extracellular medium (outward-open conformation). Protonation and nutrient binding cause the transporter to change structure into the inward-open conformation that allows the nutrient and the proton to diffuse into the cytoplasm. This type of nutrient import is referred to as the “alternate-access model of transport”21,22. Detailed studies of 2 APC transporters suggested that the cytoplasmic, N-terminal region of these proteins function as conformation sensors that are able to detect the structural changes involved in the import cycle of the protein23,24. In the ground state, the N-terminal domain (called LID for Loop Interaction Domain) is in close contact with the cytoplasmic loops of the transporter and thus is in a protected conformation. In contrast, nutrient import causes changes in the structure of the transporter which disrupts the interactions between the cytoplasmic loops and the N-terminal LID, exposing amino acid sequences that recruit the ubiquitination machinery and ultimately cause the degradation of the protein (reviewed in25. This activity-based degradation system of APC transporters is highly sensitive to the cytoplasmic substrate concentration. Because these transporters can work in both directions (the proton gradient drives directionality), increasing cytoplasmic substrate concentrations stabilize the inward-facing conformation, a structure that promotes ubiquitination of the protein. This results in a negative feedback loop that increases the chance of transporter degradation with increasing cytoplasmic substrate concentration. The result is an elegant regulatory system that rapidly adjusts the number of APC transporters at the cell surface depending on the supply and demand of the nutrient (Figure 1).
Figure 1.
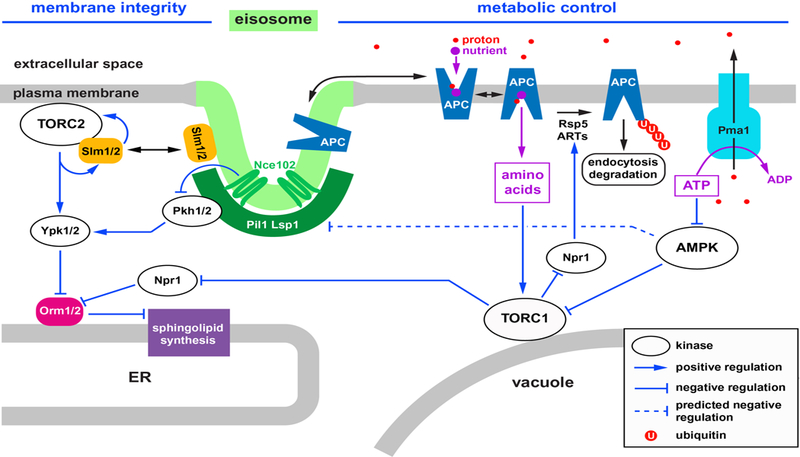
Model of the regulatory connections between eisosomes, TORC1, TORC2 and AMPK. The green-marked membrane of the eisosome indicates a special lipid composition (sphingolipid-ergosterol rich). Short descriptions of the proteins shown in this model are listed in Table 1.
In addition to controlling nutrient import, APC transporter regulation is also essential to limit proton influx into the cell. The APC transporters are among the largest consumers of the proton gradient and an imbalance between proton import and the proton pumping activity of Pma1 could rapidly lead to acidification of the cytoplasm and a collapse of the proton gradient. Therefore, regulatory systems have to be in place that maintain the balance of proton import/export.
ARTs
An additional level of APC transporter regulation is provided by a group of proteins referred to as alpha-arrestins or ART (Arrestin Related Trafficking) adaptors. As the name indicates, these proteins function as adaptors that aid in the recruitment of the ubiquitin ligase Rsp5 to the transporters at the plasma membrane26,27. Each ART adaptor (at least 14 different proteins in yeast) interacts with a subset of transporters, thereby promoting their ubiquitination and turnover. ARTs have been shown to be regulated by phosphorylation and ubiquitination, which in turn regulates the ubiquitination and degradation of the transporters interacting with the ARTs28–33. Several APC transporters have been shown to be downregulated in response to TORC1 inactivation, either by rapamycin treatment or starvation6,34–38. Furthermore, Npr1, a kinase that functions downstream of TORC1, has been found to be required for the starvation induced degradation of APC transporters6,36. Together, the data suggests that inactivation of TORC1 (caused by starvation) releases inhibition of Npr1, which in turn phosphorylates ART adaptors, thereby increasing APC transporter turnover. In addition, a proteome study indicated that Npr1 phosphorylates Rsp539, possibly enhancing the activity of this ubiquitin ligase towards the transporters (Figure 1).
It should be noted that there is a well-studied exception to the rule. Gap1 (General amino acid permease 1) is an APC transporter and a broad specificity amino acid importer that seems to be regulated in the opposite manner to most other APC transporters. Gap1 is expressed during prototrophic growth (in absence of amino acids in the medium) when other amino acid transporters are not present40–42. Gap1 does not localize to eisosomes (see below;41,43) and Gap1 is stabilized during starvation when other transporters are degraded31. Together, it seems that Gap1 is the transporter that replaces all the specialized, high-affinity amino acid transporters during stress conditions and prototrophic growth. This reversed regulation of Gap1 has led to confusion in the field with regard to the effects of TORC1 regulation on APC transporter stability. The problem has been further exacerbated by the publication of MacGurn et al. that proposed a model in which, under starvation conditions, the TORC1-Npr1-ART system stabilizes high-affinity amino acid transporters such as Can1 at the plasma membrane44. As mentioned above, several publications have shown that high-affinity transporters in fact are rapidly degraded during starvation (e.g. leucine starvation)6,34–38, which indicates a regulation that is opposite to the model presented by MacGurn et al. (Figure 1).
Eisosomes are storage compartments for APC transporters
In contrast to ABC-type transporters that require ATP for the pump activity, the conformational changes that APC transporters undergo during the import cycle are mainly driven by thermal energy. As a consequence, even in absence of the proton gradient, APC transporters allow nutrients to cross the plasma membrane according to their concentration gradient. In addition, in vitro studies have determined a proton-to-nutrient ratio for APC transporters of ~3/1, suggesting that these transporters exhibit nutrient independent proton import45. Together, these findings suggest that APC transporters are highly flexible proteins with low energy barriers between the different conformational states, a property with important consequences for the function of these transporters. For one, the high protein flexibility allows the transporter to switch even in absence of the substrate to a non-ground state (mimicking the import cycle), exposing these transporters to ubiquitination and degradation. Therefore, amino acid transporters are among the shortest-lived proteins in yeast2. In addition, APC transporters are leaky in case of both protons (flux protons without nutrients) as well as nutrients (in absence of a strong proton gradient cells leak nutrients;41). Yeast addresses these problems in part by the presence of eisosomes, membrane domains at the cell surface that harbor the APC transporters.
Eisosomes are ~50 nm deep and ~300 nm long membrane furrows that are enriched in sphingolipids and ergosterol46,47. In this regard, eisosomes share similarity to other membrane domains referred to as “membrane rafts” (reviewed in48. Similar to rafts, the eisosome membrane is thicker than the surrounding membrane (~1.6 times thicker;49) and transmembrane proteins present in the eisosome exhibit reduced diffusion50. Eisosomes are formed by membrane-associated proteins including Pil1 and Lsp1; BAR domain containing proteins that form a polymer at the curved bottom of the membrane furrow. Furthermore, transmembrane proteins such as the tetraspan proteins Nce102 and Sur7 are important to maintain the eisosome structure (Figure 1). Many additional eisosome-localized proteins have been identified, most of which are poorly characterized (for a review see51).
At least 5 APC transporters have been localized to eisosomes41,47,50,52,53, and therefore it is tempting to speculate that most of the 26 transporters are eisosome components (exception is Gap1, as noted before). Localization to eisosomes reduces the turnover of APC transporters41,54–56, suggesting that either the ubiquitination system (Rsp5 and ARTs) does not have access to this membrane domain or that the lipid environment stabilizes the ground state of the transporter (or both). The latter of these two models is based on the fact that during the import reaction the transporters undergo large conformational changes that require movements of the surrounding lipids. Since the lipid environment in eisosomes is more rigid it can be assumed that transporters are likely stabilized in the ground state and thus are less likely to expose the ubiquitination site. Support for this model has been also provided by an in vitro study of a bacterial APC transporter, which showed evidence that thicker membranes, similar to those observed in eisosomes, inhibit the import activity of the transporter57. Consistent with this finding, studies have shown that transporters preferentially localize to eisosomes in absence of substrate, but leave the eisosomes when importing nutrients41,54,56. The simplest explanation for this observation is that the presence or absence of substrate shifts the equilibrium between the pools of eisosomal and non-eisosomal transporters. In the absence of substrate the transporters are mainly in the ground state that thus prefer the lipid environment of the eisosomes. However, the addition of substrate drives the import reaction of the transporters which requires large conformational changes that are accommodated by the fluid lipid environment outside of the eisosomes. Therefore, in the presence of substrate the equilibrium is shifted towards the transporter pool outside of eisosomes. A prediction of this model is that the stabilization effect of eisosomes on the APC transporters comes with a price, a reduced import activity. This prediction has been confirmed by a recent study showing increased APC transporter activity in strains mutated for key eisosome components41. However, it should be noted that two other studies came to different conclusions with regard to transporter activity in eisosomes. Whereas the data of Spira et al. indicated increased Can1 (arginine transporter) activity when localized to eisosomes, the experiments by Gournas et al. showed no effect of the presence of eisosomes on Can1 activity55,56. This controversy is most likely the result of the different methodologies used to determine import activity of the transporters.
Together, the published data suggest that eisosomes function in reducing transporter turnover by protecting the transporters from ubiquitination. In addition, the data by Moharir et al. would argue that eisosomes stabilize the non-active ground state of the transporter41, thereby preventing the leaking of protons in absence of substrate (Figure 1).
Eisosomes regulate APC transporters
Eisosomes have a limited capacity to store APC transporters41,50. If the number of transporters exceeds the eisosome capacity, the pool of transporters outside of eisosomes increases and thus the turnover of these proteins is elevated. This negative feed-back regulation allows the cell to control the total number of APC transporters by changing eisosome number and/or size. Because APC transporters are among the largest importers of protons, limiting the number of APC transporters is essential to maintain both the cytoplasmic pH as well as the proton gradient across the plasma membrane. In addition, the proposed stabilizing effect of eisosomes on the APC transporters is expected to limit proton flux of inactive transporters (as discussed above), further minimizing acidification of the cytoplasm. The ability of Pma1 to export protons is tightly linked to ATP production and thus the energy metabolism of the cell. It is therefore not surprising that under energy starvation conditions, such as glucose depletion, APC transporters are rapidly endocytosed and degraded8,41. This response reduces proton import and thus allows the cell to conserve energy by reducing the need for ATP consumption by Pma1.
In addition to starvation conditions, other stresses such as high extracellular pH also effect the cellular ATP levels41,58. Yeast acidifies the growth medium to a low pH of 3–5 (depending on composition of the growth medium), which is the basis for the strong proton gradient across the plasma membrane. Rapidly adjusting the pH to >7 experimentally causes the collapse of the proton gradient which increases Pma1 activity in the effort to reestablish the proton gradient. As result, ATP levels drop, triggering a stress response that tunes down Pma1 and causes rapid endocytosis of APC transporters. One part of the rapid endocytic response is a remodeling of the eisosomes which causes not only APC transporters but also other eisosome components such as Nce102 to leave this membrane compartment41. The movement out of eisosomes is predicted to increase the access of transporters to the ubiquitination machinery, thereby facilitating the rapid removal of the transporters from the cell surface. The stress-induced eisosome remodeling requires AMPK (AMP-dependent kinase)41, a key sensor of cellular ATP levels59,60. A drop in the ratio of ATP-to-ADP/AMP or low glucose levels induce the kinase activity of this highly conserved hetero-trimeric protein complex, which phosphorylates and regulates many downstream effectors. The goal of this regulation is to reestablish energy homeostasis by upregulating/adapting ATP production and downregulate nonessential energy consumers. Interestingly, a study indicated that the AMPK response differs between alkaline stress and glucose starvation58. Yeast expresses three isoforms of AMPK that exhibit unique cellular localization, which might explain why different insults result in distinct responses.
AMPK mutants exposed to alkaline-stress exhibit reduced eisosome remodeling and the endocytic response is impaired41. Therefore, it is likely that AMPK senses the alkaline-stress induced drop in ATP levels and aids in the rapid shutdown of one of the largest energy consumers in yeast, the Pma1-APC transporter system (Figure 1). It is expected that many different environmental stressors either cause a rapid increase in energy consumption due to activation of stress response pathways or affect energy production, which might explain why rapid APC transporter endocytosis is a common phenomenon during most stress conditions4–8.
Eisosomes are sensors of membrane stress
Eisosomes have been implicated in sensing plasma membrane stress. Under normal growth conditions the two homologous sensor proteins Slm1 and Slm2 localize to eisosomes61,62. However, osmotic stress or drugs that block sphingolipid synthesis cause the redistribution of Slm1/2 from the eisosomes to the plasma membrane localized TORC2 complex63. Both of these stress conditions are predicted to cause increased plasma membrane tension (either by stretching or by blocking new membrane delivery), which is somehow sensed by the eisosome localized pools of Slm1/2. Slm1/2 contain a BAR domain that might sense the curvature of the associated eisosome. It is plausible that increased membrane tension might cause the deformation of eisosomes and the subsequent dissociation of the Slm1/2 proteins. The binding of Slm1/2 to TORC2 activates this protein kinase complex, which in turn activates the downstream kinases Ypk1/263,64. Two of the downstream effectors of Ypk1/2 are the ER-localized Orm1 and Orm2 proteins, which function as suppressors of sphingolipid synthesis65. Phosphorylation of Orm1/2 by Ypk1/2 releases the inhibitory effect of these proteins on lipid synthesis and the resulting increase in membrane delivery to the cell surface help alleviate membrane tension (Figure 1). In addition, Ypk1 has been shown to directly activate ceramide synthase, thereby further activating lipid synthesis at the ER66.
An additional input into the sphingolipid regulation is provided by two homologues eisosome-localized kinases, Pkh1 and Pkh2. These kinases are negatively regulated by the presence of Nce102 in eisosomes67,68. However, stress conditions that affect the plasma membrane cause Nce102 to leave the eisosome (e.g. loss of proton gradient, as discussed above, or block in lipid synthesis) which is predicted to activate Pkh1/2. Important targets of these kinases are the Ypk1/2 kinases that require the phosphorylation by Pkh1/2 for activity69,70. Therefore, the Nce102-Pkh1/2 system also feeds into the regulation of sphingolipid synthesis at the ER (Figure 1).
Link between starvation and osmotic stress?
With regard to eisosomes an obvious question remains: is the regulation of APC transporters and sensing membrane tension functionally linked or are these two separate processes that coincidentally are localized to the same membrane domain? To our knowledge, no study has yet tested if membrane stress (e.g. hypoosmotic shock) affects APC transporter localization to eisosomes. One study identified Slm1/2 as important factors for heat-shock dependent downregulation of the APC transporter Fur4, suggesting that the Slm1/2 proteins are not only acting upstream of TORC2 but also affect the stress induced degradation of transporters5. One potential link between osmolarity and nutrient transporters can be found in the lifestyle of yeast. S. cerevisiae has specialized to grow in the sugar-rich environment of fruits, which are rather high in osmolarity. However, a rain storm can rapidly change the yeast environment from fruit juice to distilled water, resulting not only in starvation conditions but also in a hypoosmotic shock. Therefore, changes in nutrient availability and osmolarity are often linked, which might explain why eisosomes are the site for nutrient transporter regulation as well as membrane tension sensing.
Eisosomes are connected to the two TORC regulatory systems
The two TOR (Target Of Rapamycin) complexes in yeast, TORC1 and TORC2, are kinases that have discrete but overlapping functions in regulating cell growth and stress response pathways (reviewed in71. TORC1 localizes to the vacuole and responses mainly to metabolic inputs. In contrast, plasma membrane localized TORC2 seems to sense environmental stresses by monitoring the condition of the plasma membrane (see discussion of Slm1/2 above). Eisosomes collaborate with TORC1 in regulating the turnover of amino acid transporters and in doing so regulate nutrient import. Stress conditions that cause a drop in ATP levels activate AMPK, which causes the disassembly of eisosomes and the inactivation of TORC141,72. Both of these regulatory events aid in the rapid ubiquitination and subsequent degradation of APC transporters (as discussed above; Figure 1). As a consequence, nutrients such as amino acids are no longer imported and thus have be synthesized using glucose as the carbon source. Similarly, eisosomes also collaborate with TORC2 in maintaining plasma membrane integrity. Eisosomes function as sensors for membrane stress that relay this information via Slm1/2 and Pkh1/2 to TORC2 and its downstream kinases Ypk1/2. Interestingly, the two eisosome-mediated stress responses (metabolic regulation and membrane integrity) are often linked. Loss of the plasma membrane proton gradient results in the rapid degradation of APC transporters as well as the redistribution of Slm141. Similarly, the switch from growth medium to water (a common stress in yeast’s natural environment) causes a combination of hypoosmotic shock and starvation, conditions that are predicted to activate both eisosomal/TORC stress pathways.
In summary, eisosomes are on many levels interconnected with the major regulatory systems of the cell’s metabolism and stress response pathways (Figure 1). Eisosomes respond to environmental changes and aid in the necessary downregulation of APC transporters as well as in the adaptation of the plasma membrane lipid composition. Although eisosomes are only found in fungi and possibly plants, caveolae seem to fulfill analogous function in higher eukaryotes. Caveolae are sphingolipid-rich membrane domains that form small, cup-shaped structures, present either in single units or as larger assemblies (reviewed in73. Caveolae play an important role in plasma membrane lipid homeostasis and in sensing mechanical stress on the membrane, which is particularly important in muscle cells. Therefore, similar to eisosomes, caveolae act as sensors for extracellular insults affecting the plasma membrane.
Table 1.
Proteins discussed in this review (see also Figure 1)
| Eisosome components (subset discussed here): | |
| APC Transporters: |
|
| Pil1/Lsp1: |
|
| Nce102: |
|
| Slm1/2: |
|
| Proton pump | |
| Pma1: |
|
| Ubiquitination system | |
| Rsp5: |
|
| ARTs: |
|
| Kinases | |
| TORC1: |
|
| TORC2: |
|
| AMPK: |
|
| Npr1: |
|
| Ypk1/2: |
|
| Pkh1/2: |
|
Synopsis.
Yeast eisosomes and the associated APC-type nutrient transporters are affected by environmental conditions and function both upstream and downstream of TORC1 and TORC2 signaling.
Acknowdgements
I thank Lincoln Gay for critical reading of the review. The research in my laboratory on eisosomes is supported by the National Institute of Health (NIH R01 GM123147).
Abbreviations
- AMPK
AMP-activated protein kinase
- APC
amino acid polyamine organocation
- TORC1
target of rapamycin complex 1
- TORC2
target of rapamycin complex 2
- SPS
Ssy1, Ptr3, Ssy5
- ARTs
arrestin related trafficking adaptors
- ER
endoplasmic reticulum
References
- 1.Gournas C, Athanasopoulos A, Sophianopoulou V. On the Evolution of Specificity in Members of the Yeast Amino Acid Transporter Family as Parts of Specific Metabolic Pathways. Int J Mol Sci. 2018;19(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin-Perez M, Villen J. Determinants and Regulation of Protein Turnover in Yeast. Cell Syst. 2017;5(3):283–294 e285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morsomme P, Slayman CW, Goffeau A. Mutagenic study of the structure, function and biogenesis of the yeast plasma membrane H(+)-ATPase. Biochim Biophys Acta. 2000;1469(3):133–157. [DOI] [PubMed] [Google Scholar]
- 4.Volland C, Urban-Grimal D, Geraud G, Haguenauer-Tsapis R. Endocytosis and degradation of the yeast uracil permease under adverse conditions. J Biol Chem. 1994;269(13):9833–9841. [PubMed] [Google Scholar]
- 5.Bultynck G, Heath VL, Majeed AP, Galan JM, Haguenauer-Tsapis R, Cyert MS. Slm1 and slm2 are novel substrates of the calcineurin phosphatase required for heat stress-induced endocytosis of the yeast uracil permease. Mol Cell Biol. 2006;26(12):4729–4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones CB, Ott EM, Keener JM, Curtiss M, Sandrin V, Babst M. Regulation of membrane protein degradation by starvation-response pathways. Traffic. 2012;13(3):468–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crapeau M, Merhi A, Andre B. Stress conditions promote yeast Gap1 permease ubiquitylation and down-regulation via the arrestin-like Bul and Aly proteins. J Biol Chem. 2014;289(32):22103–22116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang MJ, Martinez-Marquez JY, Prosser DC, et al. Glucose starvation inhibits autophagy via vacuolar hydrolysis and induces plasma membrane internalization by down-regulating recycling. J Biol Chem. 2014;289(24):16736–16747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikko E, Pelham HR. Arrestin-mediated endocytosis of yeast plasma membrane transporters. Traffic. 2009;10(12):1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serrano R, Kielland-Brandt MC, Fink GR. Yeast plasma membrane ATPase is essential for growth and has homology with (Na+ + K+), K+- and Ca2+-ATPases. Nature. 1986;319(6055):689–693. [DOI] [PubMed] [Google Scholar]
- 11.Dechant R, Binda M, Lee SS, Pelet S, Winderickx J, Peter M. Cytosolic pH is a second messenger for glucose and regulates the PKA pathway through V-ATPase. EMBO J. 2010;29(15):2515–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kane PM. Proton Transport and pH Control in Fungi. Advances in experimental medicine and biology. 2016;892:33–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ljungdahl PO, Daignan-Fornier B. Regulation of amino acid, nucleotide, and phosphate metabolism in Saccharomyces cerevisiae. Genetics. 2012;190(3):885–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ljungdahl PO. Amino-acid-induced signalling via the SPS-sensing pathway in yeast. Biochem Soc Trans. 2009;37(Pt 1):242–247. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Du G, Zhou J, Chen J. Regulation of Sensing, Transportation, and Catabolism of Nitrogen Sources in Saccharomyces cerevisiae. Microbiology and molecular biology reviews: MMBR. 2018;82(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forsberg H, Ljungdahl PO. Genetic and biochemical analysis of the yeast plasma membrane Ssy1p-Ptr3p-Ssy5p sensor of extracellular amino acids. Mol Cell Biol. 2001;21(3):814–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boban M, Ljungdahl PO. Dal81 enhances Stp1- and Stp2-dependent transcription necessitating negative modulation by inner nuclear membrane protein Asi1 in Saccharomyces cerevisiae. Genetics. 2007;176(4):2087–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaur J, Bachhawat AK. Yct1p, a novel, high-affinity, cysteine-specific transporter from the yeast Saccharomyces cerevisiae. Genetics. 2007;176(2):877–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe D, Kikushima R, Aitoku M, et al. Exogenous addition of histidine reduces copper availability in the yeast Saccharomyces cerevisiae. Microb Cell. 2014;1(7):241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshikawa K, Tanaka T, Ida Y, Furusawa C, Hirasawa T, Shimizu H. Comprehensive phenotypic analysis of single-gene deletion and overexpression strains of Saccharomyces cerevisiae. Yeast. 2011;28(5):349–361. [DOI] [PubMed] [Google Scholar]
- 21.Boudker O, Verdon G. Structural perspectives on secondary active transporters. Trends in pharmacological sciences. 2010;31(9):418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forrest LR, Kramer R, Ziegler C. The structural basis of secondary active transport mechanisms. Biochim Biophys Acta. 2011;1807(2):167–188. [DOI] [PubMed] [Google Scholar]
- 23.Keener JM, Babst M. Quality control and substrate-dependent downregulation of the nutrient transporter fur4. Traffic. 2013;14(4):412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gournas C, Saliba E, Krammer EM, Barthelemy C, Prevost M, Andre B. Transition of yeast Can1 transporter to the inward-facing state unveils an alpha-arrestin target sequence promoting its ubiquitylation and endocytosis. Mol Biol Cell. 2017;28(21):2819–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Babst M Quality control: quality control at the plasma membrane: one mechanism does not fit all. J Cell Biol. 2014;205(1):11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin CH, MacGurn JA, Chu T, Stefan CJ, Emr SD. Arrestin-related ubiquitin-ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell. 2008;135(4):714–725. [DOI] [PubMed] [Google Scholar]
- 27.Nikko E, Sullivan JA, Pelham HR. Arrestin-like proteins mediate ubiquitination and endocytosis of the yeast metal transporter Smf1. EMBO Rep. 2008;9(12):1216–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho HC, MacGurn JA, Emr SD. Deubiquitinating enzymes Ubp2 and Ubp15 regulate endocytosis by limiting ubiquitination and degradation of ARTs. Mol Biol Cell. 2017;28(9):1271–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Donnell AF, Schmidt MC. AMPK-Mediated Regulation of Alpha-Arrestins and Protein Trafficking. Int J Mol Sci. 2019;20(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Becuwe M, Vieira N, Lara D, et al. A molecular switch on an arrestin-like protein relays glucose signaling to transporter endocytosis. J Cell Biol. 2012;196(2):247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merhi A, Andre B. Internal amino acids promote Gap1 permease ubiquitylation via TORC1/Npr1/14-3-3-dependent control of the Bul arrestin-like adaptors. Mol Cell Biol. 2012;32(22):4510–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hovsepian J, Defenouillere Q, Albanese V, et al. Multilevel regulation of an alpha-arrestin by glucose depletion controls hexose transporter endocytosis. J Cell Biol. 2017;216(6):1811–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Donnell AF, Huang L, Thorner J, Cyert MS. A calcineurin-dependent switch controls the trafficking function of alpha-arrestin Aly1/Art6. J Biol Chem. 2013;288(33):24063–24080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galan JM, Volland C, Urban-Grimal D, Haguenauer-Tsapis R. The yeast plasma membrane uracil permease is stabilized against stress induced degradation by a point mutation in a cyclin-like “destruction box”. Biochem Biophys Res Commun. 1994;201(2):769–775. [DOI] [PubMed] [Google Scholar]
- 35.Penalver E, Lucero P, Moreno E, Lagunas R. Catabolite inactivation of the maltose transporter in nitrogen-starved yeast could be due to the stimulation of general protein turnover. FEMS Microbiol Lett. 1998;166(2):317–324. [DOI] [PubMed] [Google Scholar]
- 36.Beck T, Schmidt A, Hall MN. Starvation induces vacuolar targeting and degradation of the tryptophan permease in yeast. J Cell Biol. 1999;146(6):1227–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Omura F, Kodama Y, Ashikari T. The N-terminal domain of the yeast permease Bap2p plays a role in its degradation. Biochem Biophys Res Commun. 2001;287(5):1045–1050. [DOI] [PubMed] [Google Scholar]
- 38.Krampe S, Boles E. Starvation-induced degradation of yeast hexose transporter Hxt7p is dependent on endocytosis, autophagy and the terminal sequences of the permease. FEBS Lett. 2002;513(2–3):193–196. [DOI] [PubMed] [Google Scholar]
- 39.Breitkreutz A, Choi H, Sharom JR, et al. A global protein kinase and phosphatase interaction network in yeast. Science. 2010;328(5981):1043–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen EJ, Kaiser CA. Amino acids regulate the intracellular trafficking of the general amino acid permease of Saccharomycescerevisiae. Proc Natl Acad Sci U S A. 2002;99(23):14837–14842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moharir A, Gay L, Appadurai D, Keener J, Babst M. Eisosomes are metabolically regulated storage compartments for APC-type nutrient transporters. Mol Biol Cell. 2018;29(17):2113–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Springael JY, Andre B. Nitrogen-regulated ubiquitination of the Gap1 permease of Saccharomyces cerevisiae. Mol Biol Cell. 1998;9(6):1253–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lauwers E, Grossmann G, Andre B. Evidence for coupled biogenesis of yeast Gap1 permease and sphingolipids: essential role in transport activity and normal control by ubiquitination. Mol Biol Cell. 2007;18(8):3068–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacGurn JA, Hsu PC, Smolka MB, Emr SD. TORC1 regulates endocytosis via Npr1-mediated phosphoinhibition of a ubiquitin ligase adaptor. Cell. 2011;147(5):1104–1117. [DOI] [PubMed] [Google Scholar]
- 45.Hopkins NRC P, Jund R and Eddy AA. Use of plasmid vectors to show that the uracil and cytosine permeases of the yeast Saccharomyces cerevisiae are electrogenic proton pumps. FEMS Microbiol Lett. 1988;49:4. [Google Scholar]
- 46.Stradalova V, Stahlschmidt W, Grossmann G, et al. Furrow-like invaginations of the yeast plasma membrane correspond to membrane compartment of Can1. J Cell Sci. 2009;122(Pt 16):2887–2894. [DOI] [PubMed] [Google Scholar]
- 47.Grossmann G, Opekarova M, Malinsky J, Weig-Meckl I, Tanner W. Membrane potential governs lateral segregation of plasma membrane proteins and lipids in yeast. EMBO J. 2007;26(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simons K, Sampaio JL. Membrane organization and lipid rafts. Cold Spring Harbor perspectives in biology. 2011;3(10):a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bharat TAM, Hoffmann PC, Kukulski W. Correlative Microscopy of Vitreous Sections Provides Insights into BAR-Domain Organization In Situ. Structure. 2018;26(6):879–886 e873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bianchi F, Syga L, Moiset G, et al. Steric exclusion and protein conformation determine the localization of plasma membrane transporters. Nat Commun. 2018;9(1):501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Douglas LM, Konopka JB. Fungal membrane organization: the eisosome concept. Annu Rev Microbiol. 2014;68:377–393. [DOI] [PubMed] [Google Scholar]
- 52.Malinska K, Malinsky J, Opekarova M, Tanner W. Visualization of protein compartmentation within the plasma membrane of living yeast cells. Mol Biol Cell. 2003;14(11):4427–4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malinsky J, Opekarova M, Tanner W. The lateral compartmentation of the yeast plasma membrane. Yeast. 2010;27(8):473–478. [DOI] [PubMed] [Google Scholar]
- 54.Grossmann G, Malinsky J, Stahlschmidt W, et al. Plasma membrane microdomains regulate turnover of transport proteins in yeast. J Cell Biol. 2008;183(6):1075–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spira F, Mueller NS, Beck G, von Olshausen P, Beig J, Wedlich-Soldner R. Patchwork organization of the yeast plasma membrane into numerous coexisting domains. Nat Cell Biol. 2012;14(6):640–648. [DOI] [PubMed] [Google Scholar]
- 56.Gournas C, Gkionis S, Carquin M, Twyffels L, Tyteca D, Andre B. Conformation-dependent partitioning of yeast nutrient transporters into starvation-protective membrane domains. Proc Natl Acad Sci U S A. 2018;115(14):E3145–E3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.In 't Veld G, Driessen AJ, Op den Kamp JA, Konings WN. Hydrophobic membrane thickness and lipid-protein interactions of the leucine transport system of Lactococcus lactis. Biochim Biophys Acta. 1991;1065(2):203–212. [DOI] [PubMed] [Google Scholar]
- 58.Chandrashekarappa DG, McCartney RR, O’Donnell AF, Schmidt MC. The beta subunit of yeast AMP-activated protein kinase directs substrate specificity in response to alkaline stress. Cellular signalling. 2016;28(12):1881–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mayer FV, Heath R, Underwood E, et al. ADP regulates SNF1, the Saccharomyces cerevisiae homolog of AMP-activated protein kinase. Cell metabolism. 2011;14(5):707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25(18):1895–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamble C, Jain S, Murphy E, Kim K. Requirements of Slm proteins for proper eisosome organization, endocytic trafficking and recycling in the yeast Saccharomyces cerevisiae. J Biosci. 2011;36(1):79–96. [DOI] [PubMed] [Google Scholar]
- 62.Olivera-Couto A, Grana M, Harispe L, Aguilar PS. The eisosome core is composed of BAR domain proteins. Mol Biol Cell. 2011;22(13):2360–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berchtold D, Piccolis M, Chiaruttini N, et al. Plasma membrane stress induces relocalization of Slm proteins and activation of TORC2 to promote sphingolipid synthesis. Nat Cell Biol. 2012;14(5):542–547. [DOI] [PubMed] [Google Scholar]
- 64.Niles BJ, Mogri H, Hill A, Vlahakis A, Powers T. Plasma membrane recruitment and activation of the AGC kinase Ypk1 is mediated by target of rapamycin complex 2 (TORC2) and its effector proteins Slm1 and Slm2. Proc Natl Acad Sci U S A. 2012;109(5):1536–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roelants FM, Breslow DK, Muir A, Weissman JS, Thorner J. Protein kinase Ypk1 phosphorylates regulatory proteins Orm1 and Orm2 to control sphingolipid homeostasis in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2011;108(48):19222–19227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muir A, Ramachandran S, Roelants FM, Timmons G, Thorner J. TORC2-dependent protein kinase Ypk1 phosphorylates ceramide synthase to stimulate synthesis of complex sphingolipids. eLife. 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frohlich F, Moreira K, Aguilar PS, et al. A genome-wide screen for genes affecting eisosomes reveals Nce102 function in sphingolipid signaling. J Cell Biol. 2009;185(7):1227–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garcia-Marques S, Randez-Gil F, Dupont S, Garre E, Prieto JA. Sng1 associates with Nce102 to regulate the yeast Pkh-Ypk signalling module in response to sphingolipid status. Biochim Biophys Acta. 2016;1863(6 Pt A):1319–1333. [DOI] [PubMed] [Google Scholar]
- 69.Roelants FM, Torrance PD, Bezman N, Thorner J. Pkh1 and Pkh2 differentially phosphorylate and activate Ypk1 and Ykr2 and define protein kinase modules required for maintenance of cell wall integrity. Mol Biol Cell. 2002;13(9):3005–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luo G, Gruhler A, Liu Y, Jensen ON, Dickson RC. The sphingolipid long-chain base-Pkh1/2-Ypk1/2 signaling pathway regulates eisosome assembly and turnover. J Biol Chem. 2008;283(16):10433–10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gonzalez A, Hall MN. Nutrient sensing and TOR signaling in yeast and mammals. EMBO J. 2017;36(4):397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hughes Hallett JE, Luo X, Capaldi AP. Snf1/AMPK promotes the formation of Kog1/Raptor-bodies to increase the activation threshold of TORC1 in budding yeast. eLife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parton RG. Caveolae: Structure, Function, and Relationship to Disease. Annual review of cell and developmental biology. 2018;34:111–136. [DOI] [PubMed] [Google Scholar]


