Abstract
Objective:
To evaluate the properties of a frailty index (FI), constructed using data from the Systemic Lupus International Collaborating Clinics (SLICC) inception cohort, as a novel health measure in SLE.
Methods:
For this secondary analysis, the baseline visit was defined as the first study visit at which both organ damage (SLICC/ACR Damage Index [SDI]) and health-related quality of life (Short-Form 36 [SF-36]) were assessed. The SLICC-FI was constructed using baseline data. The SLICC-FI comprises 48 health deficits, including items related to organ damage, disease activity, comorbidities, and functional status. Content, construct, and criterion validity of the SLICC-FI were assessed. Multivariable Cox regression was used to estimate the association between baseline SLICC-FI values and mortality risk, adjusting for demographic and clinical factors.
Results:
The 1683 SLE patients in the baseline dataset were 89% female with mean (SD) age 35.7 (13.4) years and mean (SD) disease duration 18.8 (15.7) months. At baseline, the mean (SD) SLICC-FI score was 0.17 (0.08) with a range from 0 to 0.51. Baseline SLICC-FI values exhibited the expected measurement properties and were weakly correlated with baseline SDI scores (r=0.262; p<0.0001). Higher baseline SLICC-FI values (per 0.05 increment) were associated with increased mortality risk (Hazard Ratio 1.59; 95%CI 1.35–1.87), after adjusting for age, sex, steroid use, ethnicity/region, and baseline SDI scores.
Conclusion:
The SLICC-FI demonstrates internal validity as a health measure in SLE and predicts future mortality risk. The SLICC-FI is potentially valuable for quantifying vulnerability among patients with SLE, and adds to existing prognostic scores.
The clinical course of SLE is highly variable(1) and difficult to predict. Evaluation of SLE patients encompasses three core dimensions(1) – disease activity, organ damage, and health-related quality of life (HRQoL), with each domain providing valuable prognostic information. In particular, the Systemic Lupus International Collaborating Clinics (SLICC) / ACR Damage Index (SDI)(2) consistently predicts adverse outcomes, including future organ damage(3–5) and mortality(3–7). However, the associations between disease activity, organ damage, and HRQoL in SLE are complex(3,8,9) and the optimal approach for aggregating data across these domains is unclear. A comprehensive instrument is required to more accurately predict the risk of adverse health outcomes among SLE patients.
In geriatric medicine(10), and increasingly in other disciplines(11–13), differences in susceptibility to adverse outcomes are quantified using the construct of frailty, which represents a state of increased vulnerability resulting in diminished ability to respond to physiologic stressors(14). One approach to operationalizing frailty is the construction of a frailty index (FI)(15), which conceptualizes frailty as a loss of physiologic reserve due to the accumulation of health deficits across multiple systems(16). Individuals with few deficits are considered relatively fit, while those with a greater number of health problems are considered increasingly frail and thus more vulnerable to adverse outcomes(17). Prior work in non-lupus populations has identified properties of the FI that remain remarkably consistent across settings(15,18–21), demonstrating the robustness and generalizability of this approach. Patients with higher FI values have increased risk of adverse outcomes, including mortality(18–20,22,23). Although utilized in many different clinical contexts(18,19,24,25), this approach has not previously been applied in SLE.
We hypothesized that evaluating frailty through deficit accumulation could help explain the heterogeneous health outcomes in SLE. Using data from the SLICC inception cohort, we constructed an FI for SLE patients, known as the SLICC-FI. Our primary aim was to evaluate the properties of the SLICC-FI, including its ability to predict mortality within the SLICC inception cohort. Secondarily, we assessed whether the SLICC-FI provides additional prognostic information compared to existing SLE measures. To this end, we compared the predictive validity of the SLICC-FI and the SDI for mortality risk.
PATIENTS & METHODS
Data source:
This was a secondary analysis of longitudinal data from the Systemic Lupus International Collaborating Clinics (SLICC) inception cohort. SLICC comprises 52 investigators at 43 academic centers in 16 countries. From 1999 to 2011, 1826 SLE patients were recruited from 31 SLICC sites in Europe, Asia, and North America. Patients were enrolled within 15 months of SLE diagnosis, based on ≥4 revised ACR classification criteria for SLE(26). Data were collected per a standardized protocol and submitted to the coordinating centers at the University of Toronto (Toronto, ON, Canada) and Dalhousie University (Halifax, NS, Canada). The study was approved by the Institutional Research Ethics Boards of participating centers and patients provided written informed consent.
Clinical and laboratory assessments:
Assessments were performed at enrolment and annually thereafter. Demographic features included age, sex, race/ethnicity, geographic location, and post-secondary education. Use of corticosteroids, antimalarials, and immunosuppressives was noted. We documented ACR classification criteria for SLE(26), neuropsychiatric events(27), and medical comorbidities at the enrolment visit, and between follow-up visits. SLE disease activity [SLE Disease Activity Index 2000 (SLEDAI-2K)(28)], cumulative organ damage [SLICC/ACR Damage Index (SDI)(2)], and health-related quality of life [Medical Outcomes Survey Short-Form 36 (SF-36)(29)] were documented at each visit. Blood pressure (in mmHg), height (in metres), and weight (in kilograms) were also recorded. Laboratory investigations to assess SLE disease activity and organ damage were performed locally at each visit(3).
Construction of the SLICC Frailty Index (SLICC-FI):
A standard procedure for FI construction, described in detail elsewhere(15), was used to generate the SLICC-FI. Briefly, we established a baseline dataset, consisting of the first visit for each patient at which both the SDI and the SF-36 were completed. Variables were selected for the SLICC-FI if they met the criteria for a health deficit, defined as any symptom, disease process, functional impairment, or laboratory abnormality that is: (i) acquired, (ii) associated with chronological age, (iii) associated with adverse health outcomes, (iv) present in ≥1% and ≤80% of the sample, and (v) missing values for <5% of the sample(15). Of 222 candidate variables, 48 items met the inclusion criteria. SLICC-FI health deficits spanned a range of organ systems and included variables related to organ damage, disease activity, comorbidities, and functional status. Each health deficit was assigned a score from 0 (complete absence) to 1 (fully present) using established cut points(2,26–29). More detailed information regarding the SLICC-FI health deficits and their scoring can be found in the supplementary file.
Calculation of SLICC-FI scores:
The SLICC-FI score is the sum of an individual’s health deficit scores divided by the total number of deficits. For example, if 12 of 48 deficits are fully present, the SLICC-FI score is 12/48=0.25. Changes in FI values of ≥0.03 are clinically relevant. SLICC-FI scores were calculated for each patient using baseline data.
Evaluating the properties of the SLICC-FI:
We considered content, construct, and criterion validity(30). Content validity was inherent in the derivation of health deficits from existing, well-validated SLE instruments(2,26–29). The use of a standard procedure(15) for the identification of health deficits further enhanced face validity. For construct validity, we compared the properties of the SLICC-FI to existing FI measures in non-SLE populations. We estimated the relationship between patient age and SLICC-FI values (FI values typically increase by 1% per year on a log scale(19,20)), the distribution of SLICC-FI values (typically Gaussian(19)) and its 99th percentile value (typically <0.7(15,18–20)). We also estimated the correlation between baseline SLICC-FI scores and baseline values of the SLEDAI-2K, SDI, and SF-36. For criterion validity, we estimated the predictive validity of baseline SLICC-FI scores for mortality.
Statistical analysis:
Descriptive statistics were calculated for demographic and clinical characteristics, and for SLICC-FI values at baseline and last visit. The distributions of SLICC-FI scores were compared to theoretical distributions using goodness-of-fit tests. The association between age and baseline SLICC-FI scores was estimated using correlation coefficients and simple linear regression. Spearman rank correlation coefficients estimated the association of SLICC-FI values with SDI, SLEDAI-2K, and SF-36 scores at baseline. Correlation coefficients estimated the association of baseline SLICC-FI scores with SLICC-FI values at last follow-up. All linear regression models met the required assumptions of linearity, homoscedasticity, and normality of errors.
Kaplan-Meier survival curves illustrated the risk of mortality following the baseline visit. The event date was the date of death, with survivors censored at their last visit. To evaluate the association between baseline frailty and mortality risk, an FI cut point validated in non-SLE populations(22,31,32) was used to dichotomize patients into those who were frail (SLICC-FI >0.21) and not frail (SLICC-FI ≤0.21) at baseline. We then compared mortality risk between these two groups using a log-rank test.
The predictive validity of baseline SLICC-FI scores for mortality risk was further evaluated using Cox proportional hazards regression. First, a univariable model was constructed with the baseline SLICC-FI (per 0.05 increase) as the independent variable. Demographic and clinical variables were identified as potential confounders and univariable models for mortality risk were constructed for each of these variables. The full multivariable model included baseline SLICC-FI scores and potential confounders associated with p-values <0.1 in univariable analysis. A backwards stepwise procedure was used to remove potential confounders that were no longer statistically significant in multivariable analysis. The final multivariable model included the baseline SLICC-FI and any potential confounders for which removal from the model resulted in a statistically significant likelihood ratio (LR) test (p<0.05). Age and sex were retained in the final model regardless of statistical significance. A similar procedure was followed to construct unadjusted and adjusted models for mortality risk with 1) baseline SDI scores as the independent variable of interest; and 2) both baseline SLICC-FI and baseline SDI scores as independent variables in the same model. We then used LR tests to compare the goodness-of-fit of the models containing both baseline SLICC-FI and baseline SDI scores to the models containing 1) the baseline SLICC-FI alone and 2) the baseline SDI alone. We also compared the relative performance of these alternative models using the Akaike information criterion (AIC), with smaller AIC values indicating better predictive quality. For all models, the proportional hazards assumption was tested using log-log plots, time-varying covariates, and Schoenfeld residuals. Data analysis was conducted using STATA-IC Version 14 (StataCorp, TX, USA).
Sensitivity analyses:
The SLICC-FI contains several health deficits related to organ damage that could overlap with items captured by the SDI. To assess for a relationship between baseline SLICC-FI scores and mortality risk independent of organ damage, we repeated the above analyses omitting all damage-related items from the SLICC-FI and recalculating SLICC-FI scores using the remaining 33 health deficits.
As many SLE patients have SDI scores of zero, particularly early in disease(3), we investigated whether the SLICC-FI could predict mortality risk in the subgroup of patients with no organ damage (SDI=0) at baseline. Finally, to evaluate the influence of disease duration, we repeated these analyses in patients whose baseline visits occurred within two years of SLE diagnosis.
RESULTS
Baseline dataset characteristics:
There were 1683 patients (92.2% of cohort) with ≥1 visit where both the SDI and SF-36 were recorded. Each patient’s first such visit was included in the baseline dataset. This occurred within two years of SLE diagnosis for 1390 [82.6%] patients. The demographic and clinical characteristics are shown in Table 1. At baseline, 70.1% of patients were receiving corticosteroids, 68.3% antimalarials, and 40.5% immunosuppressives.
Table 1 -.
Demographic and clinical characteristics of SLE patients in the SLICC inception cohort at the time of their baseline visit and last follow-up visit.
| Variables | Baseline visit (n = 1683) | Last follow-up visit (n=1507) |
|---|---|---|
| Patient age (years) | ||
| Mean (S.D.) | 35.7 (13.4) | 42.8 (13.6) |
| Sex | ||
| Female, n (%) | 1493 (88.7) | 1337 (88.7) |
| Male, n (%) | 190 (11.3) | 170 (11.3) |
| Race/Ethnicity | ||
| Caucasian, n (%) | 834 (49.6) | 742 (49.2) |
| African ancestry, n (%) | 280 (16.6) | 246 (16.3) |
| Asian, n (%) | 260 (15.5) | 245 (16.3) |
| Hispanic, n (%) | 248 (14.7) | 222 (14.7) |
| Other, n (%) | 61 (3.6) | 52 (3.5) |
| Geographic location | ||
| United States, n (%) | 467 (27.7) | 377 (25.0) |
| Canada, n (%) | 395 (23.5) | 376 (25.0) |
| Mexico, n (%) | 197 (11.7) | 181 (12.0) |
| Europe, n (%) | 461 (27.4) | 419 (27.8) |
| Asia, n (%) | 163 (9.7) | 154 (10.2) |
| Education | ||
| Post-secondary education, n (%) | 847 (50.3) | 767 (50.9) |
| Missing, n (%) | 22 (1.3) | 20 (1.3) |
| SLE disease duration (years) | ||
| Median (I.Q.R.) | 1.2 (0.9–1.5) | 8.5 (5.6 – 11.3) |
| SLEDAI-2K | ||
| Median (I.Q.R.) | 2 (0–6) | 2 (0–4) |
| SLICC/ACR Damage Index (SDI) | ||
| SDI = 0, n (%) | 1270 (75.5) | 721 (47.8) |
| SF-36 Physical Component Score (PCS) | ||
| Median (I.Q.R.) | 41.7 (32.5–50.8) | 43.7 (32.6–52.6) |
| SF-36 Mental Component Score (MCS) | ||
| Median (I.Q.R.) | 48.8 (37.4–55.8) | 49.8 (39.3–56.1) |
Notes: S.D. = standard deviation; I.Q.R. = interquartile range; SLICC = Systemic Lupus International Collaborating Clinics; SLEDAI-2K = SLE disease activity index 2000; SF-36 = Short-Form 36
Baseline SLICC-FI properties:
SLICC-FI scores were calculated for 1682/1683 patients in the baseline dataset. A baseline SLICC-FI score could not be calculated for one patient due to missing data for >20% of health deficits(33)). Baseline SLICC-FI scores ranged from 0 to 0.51, with a median (I.Q.R.) of 0.16 (0.11–0.22) and a slightly higher mean (S.D.) of 0.17 (0.08).
The distribution of baseline SLICC-FI scores closely approximated a beta distribution with shape parameters α=3.51 and β=17.49 (Figure 1). This was confirmed using goodness-of-fit tests. There was a positive, linear relationship between patient age and baseline SLICC-FI values (Pearson correlation coefficient [r]=0.20; p<0.0001), although age accounted for only 4% of the total variation in baseline SLICC-FI scores. The submaximal limit (99th percentile value) of baseline SLICC-FI values was 0.39. A significant relationship between age and SLICC-FI scores was absent in this 99th percentile sample.
Figure 1:
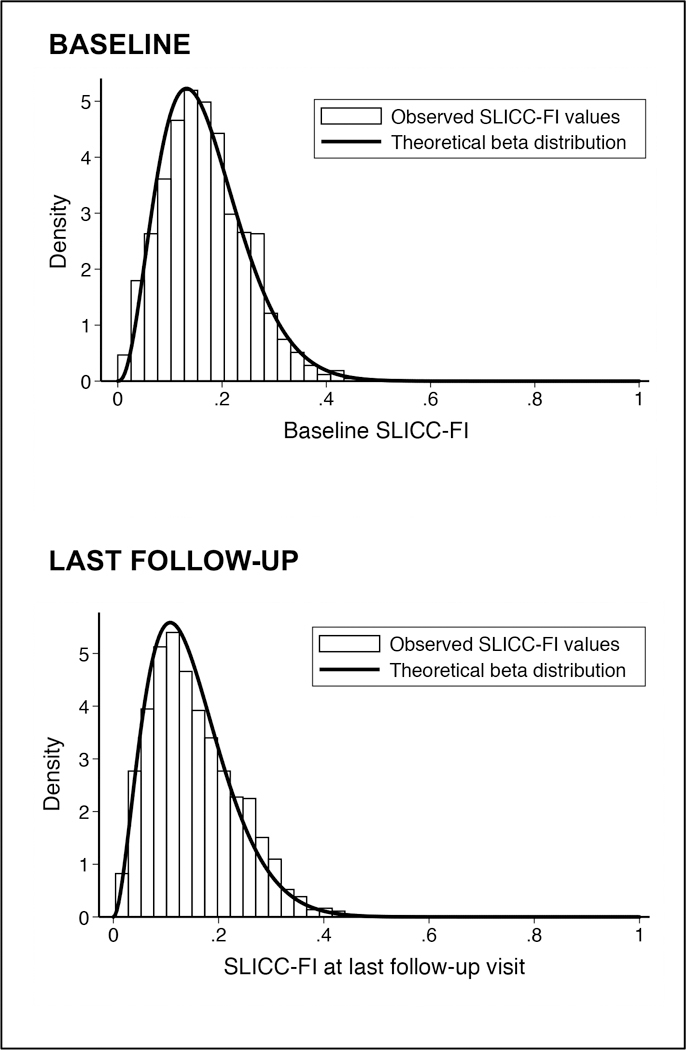
Observed distribution of SLICC-FI values at baseline (n=1682) and at last follow-up visit (n=1507) among SLE patients in the SLICC inception cohort.
Baseline SLICC-FI scores were not significantly different (t-test p-value=0.12) between males (mean [S.D.] 0.16 [0.08]) and females (mean [S.D.] 0.17 [0.08]). However, males were significantly older (mean [S.D.] age 40.0 [16.4] years) than females (mean [S.D.] age 35.1 [12.8] years) at baseline (t-test p-value <0.0001). After adjusting for age, male sex was associated with lower baseline SLICC-FI scores (β=−0.02; p=0.01).
At baseline, higher SLICC-FI values were associated with higher SDI (Spearman rank correlation coefficient [rs]=0.26; p<0.0001) and SLEDAI-2K (rs=0.23; p<0.0001) scores. These associations were weak, despite the presence of overlapping SDI and SLEDAI-2K variables that were also captured as health deficits in the SLICC-FI. These correlations remained statistically significant after removing overlapping items from the SLICC-FI (rs=0.15, p<0.0001 for the SDI; rs=0.11, p <0.0001 for the SLEDAI-2K).
At baseline, there was a moderately strong, negative association between SLICC-FI values and SF-36 Physical Component Summary (PCS) scores (rs=−0.62; p<0.0001). Higher SLICC-FI scores were also associated with lower SF-36 Mental Component Summary (MCS) scores (rs= −0.33; p<0.0001) at baseline. These negative correlations remained statistically significant after removing SF-36 variables from the SLICC-FI (rs=−0.35, p<0.0001 for the PCS; rs=−0.12, p<0.0001 for the MCS).
Properties of the SLICC-FI at last follow-up visit:
There were 1507 patients with final study visits after a mean (S.D.) follow-up of 7.2 (3.7) years from baseline. Demographic characteristics including race/ethnicity, post-secondary education status, and sex distribution were similar to baseline (Table 1). Compared to baseline, these patients had less active disease (mean [S.D.] SLEDAI-2K 2.82 [3.37] vs. 3.98 (4.28) at baseline) and more organ damage (mean [S.D.] SDI 1.19 [1.61] vs. 0.40 (0.84)) at last follow-up, although 721 patients (47.8%) still had no organ damage (SDI=0) at their last visit.
Last SLICC-FI values ranged from 0.004 to 0.49, with a mean (S.D.) value of 0.15 (0.08) and a median (I.Q.R.) of 0.14 (0.09–0.21). Compared to baseline SLICC-FI values, the properties of final SLICC-FI scores were similar (Figure 1), including their distribution, 99th percentile value (0.38), and weakly positive, linear relationship with age (r=0.26; p<0.0001). Similar to baseline, SLICC-FI values at last follow-up were positively associated with both SDI (rs=0.44; p<0.0001) and SLEDAI-2K (rs=0.26; p<0.0001) scores from the same visit. SLICC-FI values also remained negatively associated with SF-36 PCS (rs=−0.68; p<0.0001) and MCS (rs=−0.36; p<0.0001) scores at last follow-up. Final SLICC-FI values were moderately correlated with baseline SLICC-FI scores (r=0.57; p<0.0001).
During follow-up (n=1506), 67.8% of patients had a clinically meaningful change (±0.03) in their SLICC-FI scores (Figure 2). There were 395 patients (26.2%) with a clinically meaningful increase in SLICC-FI scores, while 626 patients (41.6%) had a clinically meaningful decrease in SLICC-FI scores over time. Longer follow-up was weakly associated with more positive changes (i.e. increases) in SLICC-FI scores (r=0.10; p=0.0001).
Figure 2:
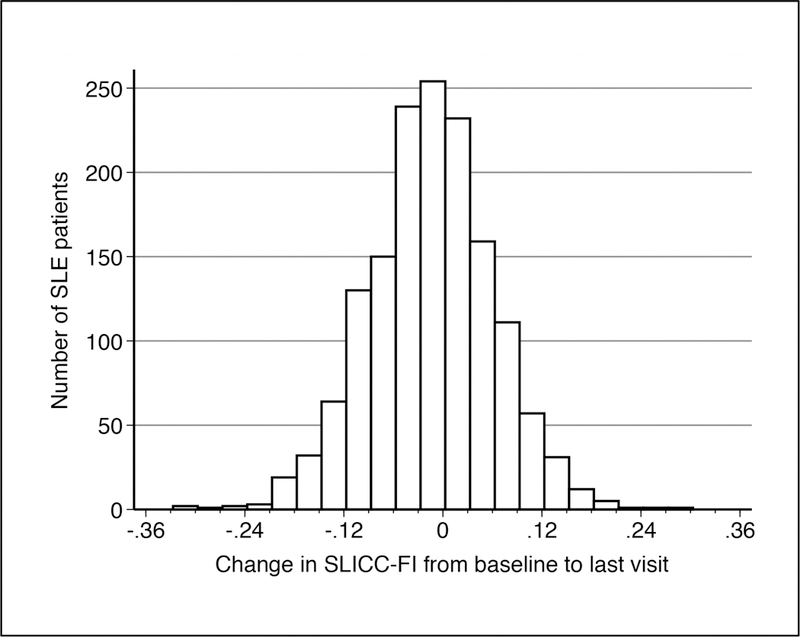
Distribution of the change in SLICC-FI values from the baseline assessment to the last follow-up visit among SLE patients in the SLICC inception cohort (n=1506).
The association of the baseline SLICC-FI with mortality risk:
There were 66 deaths after a mean (S.D.) follow-up of 5.4 (3.7) years (Figure 3). As 117 patients had no available follow-up data after their baseline visit, 1566 patients were included in the survival analysis for mortality, with mean (S.D.) follow-up time among censored individuals of 6.7 (4.0) years.
Figure 3:
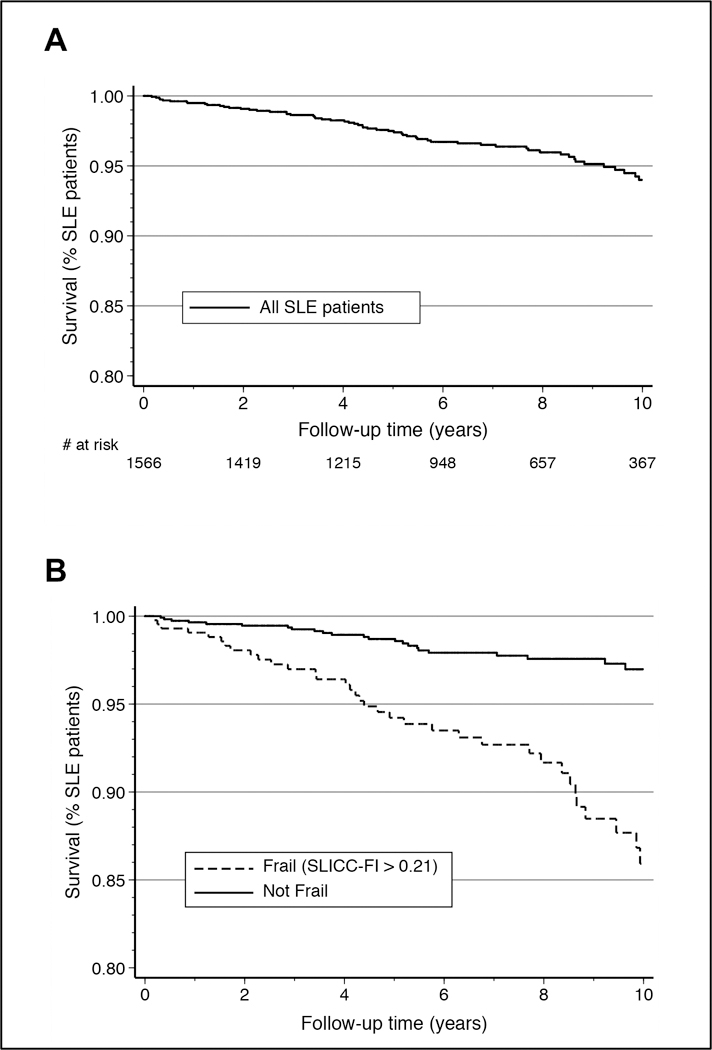
Kaplan-Meier survival curves for the risk of mortality during follow-up among SLE patients in the SLICC inception cohort, overall (A) and stratified by baseline frailty status (B).
At baseline, 431/1566 patients (27.5%) were considered frail (SLICC-FI >0.21) and frailty was associated with a significant increase in the risk of mortality (log rank test p<0.0001) (Figure 3). Specifically, mortality risk was over four times higher among frail individuals (SLICC-FI >0.21) when compared to patients classified as non-frail (SLICC-FI ≤0.21) at baseline (hazard ratio [HR] 4.37; 95% CI 2.67–7.17).
In unadjusted Cox regression, higher baseline SLICC-FI values (per 0.05 increment) were associated with increased risk of mortality (HR 1.62; 95% CI 1.41–1.85). Baseline SDI scores (per one-unit increase) demonstrated a similar association with mortality risk in unadjusted analysis (HR 1.65; 95% CI 1.38, 1.97). Looking at potential confounders, we found that older age, male sex, steroid use, immunosuppressive use, and higher disease activity (SLEDAI-2K) at baseline were associated with increased risk of mortality (Table 2). Antimalarial use and post-secondary education were associated with lower mortality risk. There were also differences in mortality risk based on race/ethnicity and geographic location (Table 2). However, the effects of race/ethnicity and geographic location were not independent of one another. Therefore, for the purposes of multivariable analysis, a combined ethnicity/region variable was created.
Table 2 -.
Univariable Cox regression models for the association of baseline demographic and clinical variables with mortality risk during follow-up among SLE patients in the SLICC inception cohort (n=1566).
| Independent variable | Log-rank test p valuea |
Hazard ratio (95% CI) |
p value |
|---|---|---|---|
| Baseline age (years)b | 1.055 (1.040 – 1.072) | <0.0001 | |
| Sex: Female | 0.061 | Referent | |
| Male | 1.80 (0.96 – 3.37) | 0.065 | |
| Race/ethnicity: Caucasian | 0.025 | Referent | |
| Hispanic | 1.54 (0.86 – 2.77) | 0.146 | |
| African ancestry | 1.11 (0.56 – 2.19) | 0.765 | |
| Asian | 0.25 (0.08 – 0.82) | 0.023 | |
| Other | 0.41 (0.06 – 3.03) | 0.386 | |
| Geographic location: USA | 0.052 | Referent | |
| Canada | 1.07 (0.52 – 2.21) | 0.860 | |
| Mexico | 1.71 (0.81 – 3.64) | 0.162 | |
| Europe | 0.86 (0.41 – 1.81) | 0.692 | |
| Asia | 0.26 (0.06 – 1.18) | 0.080 | |
| Post-secondary educationc: No | 0.009 | Referent | |
| Yes | 0.46 (0.27 – 0.77) | 0.003 | |
| Corticosteroid use: No | 0.002 | Referent | |
| Yes | 3.12 (1.49 – 6.55) | 0.003 | |
| Immunosuppressive use: No | 0.002 | Referent | |
| Yes | 2.19 (1.33 – 3.59) | 0.002 | |
| Antimalarial use: No | 0.007 | Referent | |
| Yes | 0.52 (0.32 – 0.84) | 0.008 | |
| SLEDAI-2K (per 1.0) | 1.05 (1.00 – 1.09) | 0.039 | |
| SLE disease duration (years) | 1.00 (0.98 – 1.02) | 0.649 | |
For categorical variables only
Time-varying covariate (proportional hazards assumption not met)
A “missing” indicator was included for the 1.3% of patients for whom this data was lacking.
SLEDAI-2K = SLE disease activity index 2000
In multivariable analysis, higher baseline SLICC-FI values remained significantly associated with increased risk of mortality after accounting for potentially confounding variables (Table 3–Model 1). Similarly, there was a persistent association between higher baseline SDI scores and increased mortality risk following multivariable adjustment (Table 3–Model 2). Comparing these models using AIC values (Table 3), the multivariable models containing the SLICC-FI (Model 1) demonstrated relatively smaller AIC values than the models containing the SDI (Model 2). When baseline SLICC-FI and SDI scores were included in the same models for mortality risk, both measures maintained independent associations with the risk of death during follow-up (Table 3–Model 3). Compared to the models containing either the baseline SLICC-FI or the baseline SDI alone, the models containing both baseline SLICC-FI and SDI scores demonstrated superiority for predicting mortality risk (Table 3). In particular, the addition of the baseline SLICC-FI to the model containing the baseline SDI alone was associated with significant improvement in model fit (Model 2 vs. Model 3: LR test statistic 30.07 [p<0.0001] for the final model) and relative predictive quality (Model 2 AIC=796.3 vs. Model 3 AIC=768.3 for the final model).
Table 3 -.
Multivariable Cox regression models for the association of baseline SLICC-FI and SDI scores with mortality risk during follow-up among SLE patients in the SLICC inception cohort.
| Full multivariable model a (n = 1556) |
Final multivariable model b (n = 1565) |
|||
|---|---|---|---|---|
| Hazard Ratio (95% CI) |
p value | Hazard Ratio (95% CI) |
p value | |
| Model 1: SLICC-FI | ||||
| SLICC-FI (per 0.05) | 1.62 (1.36 – 1.92) | <0.001 | 1.66 (1.42 – 1.94) | <0.001 |
| Model 2: SDI | ||||
| SDI (per 1.0) | 1.45 (1.18 – 1.78) | <0.001 | 1.50 (1.23 – 1.83) | <0.001 |
| Model 3: SLICC-FI & SDI | ||||
| SLICC-FI (per 0.05) | 1.55 (1.30 – 1.84) | <0.001 | 1.59 (1.35 – 1.87) | <0.001 |
| SDI (per 1.0) | 1.27 (1.03 – 1.57) | 0.025 | 1.27 (1.03 – 1.57) | 0.023 |
| Overall model comparisons | LR test statistic | p value | LR test statistic | p value |
| Model 1 vs. Model 3 | 4.34 | 0.037 | 4.71 | 0.030 |
| Model 2 vs. Model 3 | 27.79 | <0.001 | 30.07 | <0.001 |
| Akaike information criteria (AIC) | Full model | Final model | ||
| Model 1: SLICC-FI | AIC = 770.2 | AIC = 771.0 | ||
| Model 2: SDI | AIC = 789.1 | AIC = 796.3 | ||
| Model 3: SLICC-FI & SDI | AIC = 767.7 | AIC = 768.3 | ||
Models adjusted for the following baseline characteristics: age, sex, steroid use, antimalarial use, immunosuppressive use, ethnicity/location, post-secondary education, and SLEDAI-2K.
Models adjusted for the following baseline characteristics: age, sex, steroid use, and ethnicity/location.
Notes: SLICC = Systemic Lupus International Collaborating Clinics; FI = Frailty Index
SDI = SLICC/ACR Damage Index; LR = Likelihood Ratio
Sensitivity analyses:
In a subgroup analysis including only patients without organ damage (SDI=0) at baseline (n=1187), frailty was still associated with increased mortality risk. In our final multivariable model for this subgroup, an increase in baseline SLICC-FI by 0.05 was associated with an increase in mortality risk by approximately 50% (HR 1.47; 95% CI 1.18–1.83), after adjusting for age, sex, steroid use, and ethnicity/region (Table S1).
Similar results were also obtained when we repeated the survival analyses for mortality after removing all health deficits from the SLICC-FI that relate to organ damage (Table S2). Finally, we repeated the analyses for mortality in the subgroup of patients whose baseline visits occurred within two years of SLE diagnosis (n=1390), and found a similar relationship between baseline SLICC-FI values and mortality risk (Table S3).
DISCUSSION
In a well-characterized, international cohort of recently-diagnosed SLE patients, we have demonstrated the feasibility of using an FI to quantify vulnerability to adverse outcomes in SLE. The SLICC-FI was correlated with existing measures of SLE disease activity, organ damage, and HRQoL. Higher SLICC-FI values were associated with increased risk of mortality, independent of other demographic and clinical factors known to predict mortality in SLE.
The SLICC-FI exhibited measurement properties similar to those consistently demonstrated by other frailty indices in non-lupus populations(15,18–21). For example, at all ages, women demonstrated higher mean SLICC-FI values than men(15,20). We found a positive, linear association between chronological age and SLICC-FI values that was very weak. As expected, the relationship between age and SLICC-FI values attenuated to zero at the highest levels of frailty, as severely frail individuals die rather than accumulate further deficits(34). Finally, although the mean SLICC-FI value (0.17) was high compared to estimates for similarly-aged individuals in the general population(22,35,36), the upper limit of SLICC-FI scores was not higher than expected (maximum value 0.51), suggesting that the SLICC-FI was not overestimating the prevalence of frailty among SLE patients.
After a mean follow-up interval of 7.2 years, mean SLICC-FI values, as well as the overall distribution of SLICC-FI scores, remained largely unchanged compared to baseline. This is uncommon in FI studies with such prolonged follow-up and may reflect the impact of treatment, as demonstrated by the large number of patients in whom SLICC-FI scores improved during follow-up. This finding may help to provide insight into the relationship between frailty and chronological age in disease-specific cohorts.
The lack of change in mean SLICC-FI scores during follow-up could also reflect a tradeoff between deficits related to SLE disease activity and those related to organ damage. Early in disease, frailty may be driven by disease activity, with minimal organ damage. With treatment, disease activity recedes and damage accumulates consequent to the disease, its treatment, and other comorbidities(4,37,38). With longer follow-up, we expect that mean SLICC-FI scores will increase, as deficits continue to accumulate with increasing age(36) and increasing disease duration. This hypothesis is supported by the finding that longer follow-up time was weakly associated with worsening SLICC-FI scores over time.
While the overall distribution of SLICC-FI values remained largely unchanged between baseline and last follow-up, approximately 2/3 of patients had clinically meaningful changes in their SLICC-FI values between the two time points. The potential for SLICC-FI scores to decrease, in contrast to SDI scores, supports the view that frailty itself can be reversed(10). To this end, the SLICC-FI warrants investigation as a possible outcome measure for future intervention studies.
Similar to the findings of FI studies in non-lupus populations(18,19,23), we identified a significant association between baseline SLICC-FI scores and mortality risk. Given that prior work has emphasized the importance of the SDI for predicting mortality in SLE(3,6,7), some may question whether the ability of the SLICC-FI to predict mortality is heavily reliant upon the inclusion of deficits related to organ damage. However, sensitivity analysis demonstrated persistence of the relationship between baseline SLICC-FI values and mortality risk, despite removal of all damage-related deficits from the index. This finding highlights a key strength of the deficit accumulation approach to frailty – it is the cumulative impact of multiple small effects, rather than specific individual deficits, that is important(17,39). As long as a sufficient number of variables are included in an FI (generally more than 30), its predictive ability for adverse outcomes remains robust, even when a subset of the included deficits are removed(15,20,21,33,40).
Traditionally, the core dimensions of SLE – disease activity, organ damage, and HRQoL - have been evaluated separately(1). However, this approach does not capture interactions between these domains, thereby potentially missing their impact on prognosis. Conversely, the SLICC-FI combines aspects of all three dimensions into a single measure. The relationships that exist between deficits from different domains within the SLICC-FI are critical to its performance as a prognostic tool. For example, the scoring of the “Cerebrovascular Disease” health deficit weighs transient ischemic attacks and debilitating strokes equally, despite clear differences in the likely impact of these events on prognosis. However, an individual with a disabling stroke is likely to have additional deficits related to their functional performance that will be reflected in their SLICC-FI score. As shown in this example, including deficits from different domains ensures that the overall impact of complex health events is accurately represented in the SLICC-FI.
The baseline SLICC-FI and SDI were both significant predictors of mortality risk. Despite some overlap in the items captured, these two instruments are likely measuring separate constructs and each provides valuable prognostic information. The SDI can be viewed as a measure of SLE disease severity in one of three core dimensions(1). In contrast, the SLICC-FI provides a more holistic approach, incorporating both patient and healthcare provider perspectives of the impact of the disease, its treatment, and other comorbidities, on the health of SLE patients. Prior FI studies in other disease-specific cohorts have yielded similar findings, namely that both the FI and existing measures of disease severity maintain independent associations with the risk of future adverse health outcomes(18,19).
Another important distinction between the SDI and the SLICC-FI is that the SDI does not capture damage accrued prior to SLE diagnosis, and therefore does not consider the likely effects of preexisting organ damage on mortality risk. Conversely, health deficits accrued prior to SLE diagnosis can be included in the SLICC-FI. Damage captured by the SDI often does not occur until several years after diagnosis of SLE (3,6,7). Thus, the added prognostic value of the SLICC-FI may be highest early in the disease course. This was demonstrated in our subgroup analysis of patients without baseline organ damage (SDI=0), where each 0.05 increase in the baseline SLICC-FI was associated with a 50% increase in mortality risk.
An alternative approach to the measurement of frailty uses rules-based tools(17) such as the Fried frailty phenotype(41), which classifies individuals as frail if any three of five specific criteria (slow gait speed, impaired grip strength, reduced physical activity, weight loss, and exhaustion) are met. The Fried frailty phenotype was recently evaluated in a prevalent cohort of 152 women with SLE(42). Similar to our findings, they reported an association between frailty and increased mortality risk(42). Phenotypic frailty was also associated with significantly worse physical functioning, measured using the Physical Function subscale of the SF-36(42). Interestingly, we observed a similar association between concurrent SLICC-FI and SF-36 PCS scores. Phenotypic frailty at baseline was also associated with significant declines in physical functioning during follow-up(42). Future work will evaluate the association of baseline SLICC-FI values with changes in functional status and quality of life over time.
Our study has some limitations. First, a relatively low number of deaths occurred during follow-up, which limited statistical power in our analysis of mortality. Although this would increase our type II error rate, it would not change the direction of our finding that baseline SLICC-FI values are a significant predictor of mortality risk. The low mortality rate in the SLICC inception cohort reflects improved survival among SLE patients compared to previous eras(43). As such, future work will evaluate the ability of the SLICC-FI to predict other clinically meaningful outcomes. Second, we have only evaluated the change in SLICC-FI values between two time points. Future work will focus on better understanding the trajectories of SLICC-FI values over multiple time points. Third, we were unable to calculate SLICC-FI values for 144 patients (7.9%) due to missing data. However, the characteristics of the included patients were very similar to those reported in previous studies from the SLICC cohort(3), suggesting that our dataset was representative of the overall cohort. Missing data also precluded the use of SLICC enrolment visits as baseline visits for many patients. Despite this, over 80% of patients had their baseline visit within two years of SLE diagnosis and our results were unchanged in a subgroup analysis including only these individuals. Last, it should be acknowledged that we have evaluated the SLICC-FI in the same cohort used for its initial construction. This is a cohort of relatively young, recently diagnosed SLE patients. External validation of the SLICC-FI in other SLE cohorts is required to confirm our findings and to investigate their generalizability to older patients with more longstanding SLE.
In conclusion, evaluating frailty through deficit accumulation provides a holistic approach to prognostication among SLE patients, incorporating aspects of disease activity, organ damage, and HRQoL into a single measure. We have demonstrated the SLICC-FI to be a meaningful health measure in SLE with the ability to vary over time and to predict mortality. Although the practical utility of frailty assessment in routine clinical care of SLE patients remains unexplored, the SLICC-FI holds promise as a clinical and research tool for the identification of vulnerable SLE patients. It may also be a valuable outcome measure for future intervention studies.
Supplementary Material
Acknowledgments
Financial support:
Dr. Sang-Cheol Bae’s work was supported in part by NRF-2017M3A9B4050335, Republic of Korea.
Dr. Caroline Gordon is supported by Lupus UK, Sandwell and West Birmingham Hospitals NHS Trust and the National Institute for Health Research (NIHR)/Wellcome Trust Birmingham Clinical Research Facility. The views expressed are those of the authors(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
The Hopkins Lupus Cohort is supported by the NIH (grant AR43727 and 69572).
The Montreal General Hospital Lupus Clinic is partially supported by the Singer Family Fund for Lupus Research.
Dr. Clarke holds The Arthritis Society Chair in Rheumatic Diseases at the University of Calgary.
Dr. Paul R. Fortin presently holds a tier 1 Canada Research Chair on Systemic Autoimmune Rheumatic Diseases at Université Laval, and part of this work was done while he was still holding a Distinguished Senior Investigator of The Arthritis Society.
Dr. Bruce is a National Institute for Health Research (NIHR) Senior Investigator and is supported by Arthritis Research UK, the NIHR Manchester Biomedical Centre and the NIHR/Wellcome Trust Manchester Clinical Research Facility. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Dr. Soren Jacobsen is supported by the Danish Rheumatism Association (A3865) and the Novo Nordisk Foundation (A05990).
Dr. Ramsey-Goldman’s work was supported by the NIH (grants 5UL1TR001422–02, formerly 8UL1TR000150 and UL-1RR-025741, K24-AR-02318, and P60AR064464 formerly P60-AR-48098).
Dr. Mary Anne Dooley’s work was supported by the NIH grant RR00046.
Dr. Ruiz-Irastorza is supported by the Department of Education, Universities and Research of the Basque Government.
Dr. Isenberg and Dr. Rahman are supported by the National Institute for Health Research, University College London Hospitals Biomedical Research Center.
Dr. John G. Hanly is supported by the Canadian Institutes of Health Research (grant MOP-88526).
REFERENCES
- 1.Strand V, Chu AD. Measuring outcomes in systemic lupus erythematosus clinical trials. Expert Rev Pharmacoecon Outcomes Res 2011;11:455–468. [DOI] [PubMed] [Google Scholar]
- 2.Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996;39:363–369. [DOI] [PubMed] [Google Scholar]
- 3.Bruce IN, O’Keeffe AG, Farewell V, Hanly JG, Manzi S, Su L, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis 2015;74:1706–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alarcón GS, Roseman JM, McGwin G, Uribe A, Bastian HM, Fessler BJ, et al. Systemic lupus erythematosus in three ethnic groups. XX. Damage as a predictor of further damage. Rheumatology (Oxford) 2004;43:202–205. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso CRL, Signorelli FV, Papi JAS, Salles GF. Initial and accrued damage as predictors of mortality in Brazilian patients with systemic lupus erythematosus: a cohort study. Lupus 2008;17:1042–1048. [DOI] [PubMed] [Google Scholar]
- 6.Rahman P, Gladman D, Urowitz MB, Hallett D, Tam LS. Early damage as measured by the SLICCaACR damage index is a predictor of mortality in systemic lupus erythematosus. Lupus 2001;10:93–96. [DOI] [PubMed] [Google Scholar]
- 7.Nivad O, Jonsen A, Bengtsson AA, Bengtsson C, Sturfelt G. High predictive value of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for survival in systemic lupus erythematosus. J Rheumatol 2002;29:1398–1400. [PubMed] [Google Scholar]
- 8.Schmeding A, Schneider M. Fatigue, health-related quality of life and other patient-reported outcomes in systemic lupus erythematosus. Best Pract Res Clin Rheumatol 2013;27:363–375. [DOI] [PubMed] [Google Scholar]
- 9.Mok CC, Ho LY, Cheung MY, Yu KL, To CH. Effect of disease activity and damage on quality of life in patients with systemic lupus erythematosus: a 2‐year prospective study. Scand J Rheumatol 2009;38:121–127. [DOI] [PubMed] [Google Scholar]
- 10.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013;381:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muscedere J, Waters B, Varambally A, Bagshaw SM, Boyd JG, Maslove D, et al. The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive Care Med 2017;43:1105–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abel GA, Klepin HD. Frailty and the management of hematologic malignancies. Blood 2018;131:515–524. [DOI] [PubMed] [Google Scholar]
- 13.Partridge JSL, Harari D, Dhesi JK. Frailty in the older surgical patient: a review. Age Ageing 2012;41:142–147. [DOI] [PubMed] [Google Scholar]
- 14.Fulop T, Larbi A, Witkowski JM, McElhaney J, Loeb M, Mitnitski A, et al. Aging, frailty and age-related diseases. Biogerontology 2010;11:547–563. [DOI] [PubMed] [Google Scholar]
- 15.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitnitski A, Rockwood K. Aging as a process of deficit accumulation: its utility and origin. Interdiscipl Top Gerontol 2015;40:85–98. [DOI] [PubMed] [Google Scholar]
- 17.Theou O, Walston J, Rockwood K. Operationalizing frailty using the frailty phenotype and deficit accumulation approaches. Interdiscipl Top Gerontol 2015;41:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rockwood MR, MacDonald E, Sutton E, Rockwood K, Scleroderma Research Group, Baron M. Frailty index to measure health status in people with systemic sclerosis. J Rheumatol 2014;41:698–705. [DOI] [PubMed] [Google Scholar]
- 19.Guaraldi G, Brothers TD, Zona S, Stentarelli C, Carli F, Malagoli A, et al. A frailty index predicts survival and incident multimorbidity independent of markers of HIV disease severity. AIDS 2015;29:1633–1641. [DOI] [PubMed] [Google Scholar]
- 20.Mitnitski A, Song X, Skoog I, Broe GA, Cox JL, Grunfeld E, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc 2005;53:2184–2189. [DOI] [PubMed] [Google Scholar]
- 21.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med 2011;27:17–26. [DOI] [PubMed] [Google Scholar]
- 22.Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. CMAJ 2011;183:E487–E494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kojima G, Iliffe S, Walters K. Frailty index as a predictor of mortality: a systematic review and meta-analysis. Age Ageing 2018;47:193–200. [DOI] [PubMed] [Google Scholar]
- 24.Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant 2014;14:1870–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hubbard RE, Peel NM, Smith M, Dawson B, Lambat Z, Bak M, et al. Feasibility and construct validity of a Frailty index for patients with chronic kidney disease. Australas J Ageing 2015;34:E9–12. [DOI] [PubMed] [Google Scholar]
- 26.Hochberg MC. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 27.ACR Ad Hoc Committee on Neuropsychiatric Lupus Nomenclature. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum 1999;42:599–608. [DOI] [PubMed] [Google Scholar]
- 28.Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002;29:288–291. [PubMed] [Google Scholar]
- 29.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–483. [PubMed] [Google Scholar]
- 30.Streiner DL, Norman GR. Health measurement scales - A practical guide to their development and use. 4 ed. UK: Oxford University Press; 2008. [Google Scholar]
- 31.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci 2007;62A:738–743. [DOI] [PubMed] [Google Scholar]
- 33.Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc 2013;61:1537–1551. [DOI] [PubMed] [Google Scholar]
- 34.Rockwood K, Rockwood MRH, Mitnitski A. Physiological redundancy in older adults in relation to the change with age in the slope of a frailty index. J Am Geriatr Soc 2010;58:318–323. [DOI] [PubMed] [Google Scholar]
- 35.Rockwood K, Blodgett JM, Theou O, Sun MH, Feridooni HA, Mitnitski A, et al. A frailty index based on deficit accumulation quantifies mortality risk in humans and in mice. Sci Rep 2017;7:43068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitnitski A, Rockwood K. The rate of aging: the rate of deficit accumulation does not change over the adult life span. Biogerontology 2016;17:199–204. [DOI] [PubMed] [Google Scholar]
- 37.Sutton EJ, Davidson JE, Bruce IN. The systemic lupus international collaborating clinics (SLICC) damage index: a systematic literature review. Semin Arthritis Rheum 2013;43:352–361. [DOI] [PubMed] [Google Scholar]
- 38.Gladman DD, Urowitz MB, Rahman P, Ibañez D, Tam L-S. Accrual of organ damage over time in patients with systemic lupus erythematosus. J Rheumatol 2003;30:1955–1959. [PubMed] [Google Scholar]
- 39.Rutenberg AD, Mitnitski AB, Farrell SG, Rockwood K. Unifying aging and frailty through complex dynamical networks. Exp Gerontol 2018;107:126–129. [DOI] [PubMed] [Google Scholar]
- 40.Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc 2006;54:975–979. [DOI] [PubMed] [Google Scholar]
- 41.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56A:M146–M156. [DOI] [PubMed] [Google Scholar]
- 42.Katz PP, Andrews J, Yazdany J, Schmajuk G, Trupin L, Yelin E. Is frailty a relevant concept in SLE? Lupus Sci Med 2017;4:e000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urowitz MB, Gladman DD, Tom B, Ibanez D, Farewell VT. Changing patterns in mortality and disease outcomes for patients with systemic lupus erythematosus. J Rheumatol 2008;35:2152–2158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


