Abstract
Purpose:
To compare two different tasks and kinematic measures in terms of their ability to detect ALS and differences in ALS severity in order to establish potential candidate markers of bulbar decline.
Method:
We tracked jaw kinematics during speech and chewing to determine which is more affected by bulbar motor deterioration, based on measures of maximum speed and articulatory working space. Data were collected from 31 individuals diagnosed with ALS and 17 neurologically intact controls.
Result:
1) Both sentence and chewing tasks were effective in distinguishing between the groups of individuals with ALS and controls, 2) jaw maximum speed for both chewing and speech was a more sensitive marker for bulbar dysfunction than articulatory working space, 3) the sentence task distinguished between ALS subgroups stratified by severity and 4) distinct jaw kinematic differences existed between chewing and sentence tasks. More specifically, movement speed for speech decreased with severity while movement speed for chewing increased with disease severity.
Conclusion:
The findings from the current investigation suggest that measures of jaw movement speed during chewing and sentence tasks are affected by bulbar deterioration, and jaw speed during a sentence task may serve as a candidate marker of bulbar disease onset and severity.
Keywords: Amyotrophic lateral sclerosis, biomechanics, speech, mastication, jaw, kinematics
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease affecting motor neurons in the brain, brainstem, and spinal cord. With disease progression, individuals lose control over skeletal muscles including those needed for speech, chewing, and swallowing. Symptoms are typically first reported in either spinal (e.g. limbs) or bulbar (e.g. head and neck) regions. The bulbar-onset variant accounts for approximately 30% of all cases (Brown & Al-Chalabi, 2017; van Es, Hardiman, Chio, Al-Chalabi, Pasterkamp, Veldink, et al., 2017), although most individuals with spinal-onset eventually experience bulbar dysfunction (Tomik & Guiloff, 2010; Wijesekera & Leigh, 2009). The resulting impairments to speech and swallowing have significant negative impact on length of survival and quality of life (Hecht, Hillemacher, Grӓsel, Tigges, Winterholler, Heuss, et al., 2002).
Clinically, sensitive and specific markers of bulbar motor involvement are urgently needed to improve: early detection, prediction of the rate of bulbar motor decline, and intervention planning. Early sensitive measures of dysfunction would identify individuals in need of nutritional management and augmentative and alternative communication systems as the bulbar disease may progress rapidly (Beukelman, Fager, & Nordness, 2011; Beukelman, Fager, Ball, & Dietz, 2007). They would also provide a method for more accurate staging of ALS progression (Shellikeri, Green, Kulkarni, Rong, Martino, Zinman, et al., 2016) and provide much needed endpoints for clinical trials (Green, Yunusova, Kuruvilla, Wang, Pattee, Synhorst, et al., 2013a).
Most common clinical practices for assessing bulbar motor involvement include the measurement of speech intelligibility (% words understood/ transcribed correctly by an unfamiliar listener) and speaking rate (number of words produced per minute; WPM) (Green, et al., 2013a; Yorkston, Strand, Miller, Hillel, & Smith, 1993). However, converging sources suggest that these clinical measures are not as sensitive to early stages of bulbar disease as instrumental physiological subsystem measures, including articulatory kinematics (Allison, Yunusova, Campbell, Wang, Berry, & Green, 2017; Mefferd, Green, & Pattee, 2012; Rong, Yunusova, Wang & Green, 2015b; Yunusova, Green, Lindstrom, Ball, Pattee, & Zinman, 2010). Kinematic measures are of great interest because a number of studies demonstrated disease-related changes in speech and speech-like movements in ALS, including reduced displacement and speed of the tongue, lip, and jaw (Green et al., 2013a; Hirose, Kiritani, & Sawashima, 1982; Mefferd et al., 2012; Rong, et al., 2015b; Shellikeri et al., 2016; Yunusova et al., 2010; Yunusova, Weismer, Westbury, & Lindstrom, 2008) Although the existing research supports the efficacy of kinematic approaches in the assessment of bulbar motor dysfunction for diagnostic and prognostic purposes, additional research is needed to determine exactly which tasks (e.g. speech or non-speech) and which measures (e.g. movement volume, movement speed) are most responsive to bulbar motor dysfunction.
The role of task
The neural organization of a specific behaviour is task-dependent (Bernstein, 1967; Bunton, 2008; Turvey, 1990; Weismer, 2006) and task-specific strategies (e.g. specified movement parameters) are imposed based on the goal of a given motor behaviour. The progressive muscle dysfunction of ALS is expected to impact motor tasks differently over the course of the disease depending on the demands on muscle strength, endurance, range of motion, speed, spatiotemporal precision, and coordination. For example, Rong, Yunusova, and Green (2015a) showed that during the early stages of bulbar involvement, impaired oromotor performance was only evident when the participants with ALS performed a motorically challenging task (i.e. producing the maximum number of syllables per breath or alternating motion rate, AMR task). At the same time, bulbar deficit was not detected by the measures of speaking rate and speech intelligibility, or by a symptom report by means of the ALS Functional Rating Scale – Revised (ALSFRS-R) (Cedarbaum, Stambler, Malta, Fuller, Hilt, Thurmond et al., 1999). Similarly, Yunusova and colleagues (2008) demonstrated that speech tasks requiring “larger, longer, and faster movements” (p. 610) may be more effective for detecting bulbar dysfunction.
In the search for the most sensitive measure of bulbar decline in individuals with ALS, we compare jaw motion in a non-speech task (i.e. chewing) to that of a speech task. To the best of our knowledge, kinematics of non-speech movements such as those in chewing have not been examined in relation to disease severity in ALS or compared to speech movements. Our rationale for investigating chewing is that specified tasks parameters (e.g. jaw speed and distance) for chewing are different than those required for speech (see Bunton, 2008 and Weismer, 2006 for review). For example, the primary kinematic parameters of the jaw during chewing are specified for the generation of adequate occlusal force for bolus breakdown. Articulatory parameters specified during speech are related to the modification of the vocal tract for production of a precise acoustic target. Further, the muscular effort used to generate speech movements is estimated to be a fraction of the force generated for maximum force tasks (see Kent, 2015 for review). The greater force requirement for chewing may provide additional rationale for positing that chewing may be more affected by bulbar deterioration than speech in ALS.
Research question
In search of candidate markers of bulbar dysfunction, we assessed jaw motor performance during two distinct oromotor tasks – speech and chewing – based on kinematic measures of maximum movement speed and articulatory working space (i.e. range of motion) to determine their ability to detect ALS and differences in ALS severity. We focused on movement of the jaw because the jaw is fundamental to both tasks and, unlike the tongue, jaw movements have the potential to be more easily quantified in clinical settings.
Methods
Participants
Data were collected from 31 individuals diagnosed with ALS. Seven individuals presented with bulbar-onset ALS, and 24 showed spinal onset. Data from 17 neurologically intact control participants were also collected. All participants were fluent speakers of English, able to read at grade-four level or higher, and had no history of other neurologic conditions (e.g. stroke). Participants were also screened for hearing and cognitive dysfunction at the onset of the study and had to score within normal limits (MoCA scores = 27.76 ±2.44) to participate. Summary demographic and clinical information at session 1 is shown in Table I. Fifteen participants were assessed only once due to factors associated with disease progression (e.g. significant respiratory or bulbar changes, fatigue). Sixteen participants with ALS were assessed more than once (Mean N of repeated sessions = 2.94 SD = 2.80; average time in study = 12.14 (±11.29) months). All available sessions were used in the analyses (Rong, et al., 2015a).
Table I: Summary Demographic Information.
Means and ± standard deviations for demographic and clinical measures of the patient sample (at session 1) and healthy controls
| ALS (n = 31) | Controls (n = 17) | |
|---|---|---|
| Age | 57.81 (10.51) | 62.58 (7.62) |
| Sex (M:F) | 22:9 | 5:11 |
| Disease duration (months) | 19.63 (11.45) | - |
| ALSFRS-R total score | 36.86 (7.12) | - |
| ALSFRS-R bulbar subscore | 11.15 (0.93) | - |
| Speech intelligibility (%) | 95.22 (11.90) |
Procedure
Clinical Measures
Measures of speech intelligibility and speaking rate were obtained at every session using the Speech Intelligibility Test (SIT) for Windows (Beukelman, Yorkston, Hakel, & Dorsey, 2007). The SIT program generated an 11-sentence test with each sentence displayed individually on a screen. The speakers were asked to read each sentence aloud at their normal comfortable speed and loudness level. Per the SIT protocol, each production was recorded and later orthographically transcribed by a naïve listener, unfamiliar with dysarthria and individuals’ speaking patterns. An intelligibility score was calculated by the SIT program based on the transcription. The SIT program also calculated a measure of speaking rate (WPM) based on the duration of recorded samples. These testing procedures have been used to index severity of bulbar ALS in multiple studies (Mefferd et al., 2012; Yunusova et al., 2010). Each participant also completed the ALS Functional Rating Scale – Revised (ALSFRS-R), a questionnaire to provide a metric of disease effects on activities of daily living (Cedarbaum et al., 1999). The ALSFRS-R provided a bulbar subscore based on three questions devoted to speech, swallowing and salivation, with a value of 4 indicating a lack of bulbar symptoms and 0 indicating a complete loss of function.
In the current study, speaking rate was used to stratify the ALS group into subgroups of “affected” (speaking rate < 145 WPM) and “unaffected” (speaking rate ≥ 145 WPM) individual sessions. Recent work conducted on a large sample of individuals with ALS, tracked longitudinally, indicated a 143 WPM as a critical point in intelligibility decline (Rong, et al., 2015a). The cut-off of 145 WPM, therefore, was empirically determined to be a conservative estimate of the point in disease progression after which bulbar disease begins to progress rapidly.
Physiological Measures
Along with the clinical measures, physiological measures were also obtained at every session. Jaw motion was also recorded at every session using 3-dimensional motion capture technology (with Eagle Cameras, Motion Analysis Corporation and Optotrak Certus Motion Capture System, NDI, Northern Digital Inc). Markers were placed on the participants’ face as seen in Figure 1. The analyses only included data from the jaw right (JR) marker (see Green, Wilson, Wang, & Moore, 2007). The head markers were used to subtract rotational and translational components of head movement so that the JR data reflected motion of the jaw only, exclusive of head movement (Green & Wilson, 2006).
Figure 1:
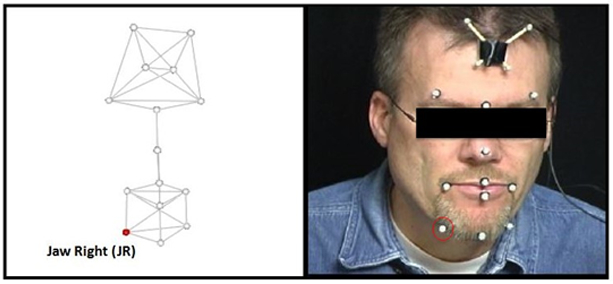
Marker Array. The marker array (left) and marker placement on the participant (right). The head markers were used to subtract rotational and translational components of head movement. For each participant and task, movement from the jaw right (JR) marker (circled) was selected for analysis.
At each session, participants: (1) read the sentence (“Buy Bobby a puppy”; BBP) five times at their normal rate and loudness level (speech task) and (2) chewed a bolus of 5 Cheerios® across 3 trials (non-speech task). Cheerios® were selected after careful consideration of the risk/benefit ratio of a variety of consistency options including puree, mechanical soft, and solid. Cheerios® are a solid consistency, yet they are not a particularly challenging solid, which provided a means to maximize participant safety when working with individuals with known oromotor dysfunction. Further, they are recognizable and familiar, thereby decreasing participant anxiety related to trialing a new foodstuff. Cheerios® are also free of many common allergens and are a standardized size which allowed for ease of controlling bolus size across trials, sessions, and even various research assistants. Finally, an obvious and defined chewing pattern was clearly observed for all participants, leaving no question two distinct tasks were being compared, (i.e. chewing and speech).
Signal Processing
All speech and chewing movement signals were digitally low-pass filtered (flp = 10 Hz) using a zero-phase shift forward and reverse digital filter (Butterworth, 8 pole) prior to measurement (Wilson & Green, 2009). The associated onset and offset of the jaw movements were determined using procedures that were different for each task (Figure 2). For the speech task (i.e. BBP), the movement onset was denoted at the point of maximum lip closure for the initial /b/ and the offset was denoted as the point of maximum lip closure for the 2nd /p/ in “puppy”. For the chewing task, data were parsed in two stages. During the first stage, the onset was denoted at the point of maximum jaw closure following bolus placement in the mouth. The offset was denoted at the point of the swallow as defined by visual observation of laryngeal elevation (Nip, Wilson, & Kearney, 2018; Wilson, Green, & Weismer, 2012). The secondary stage of editing involved algorithmically parsing the mid 80% of the chewing sequence to avoid the inclusion of non-chewing movement related to initial bolus manipulation and adjustment for swallowing preparation (e.g. clearing of the teeth) (Wilson et al., 2009). The mid 80% of the chewing sequences were then analysed. Sentence productions were analysed in their entirety.
Figure 2:
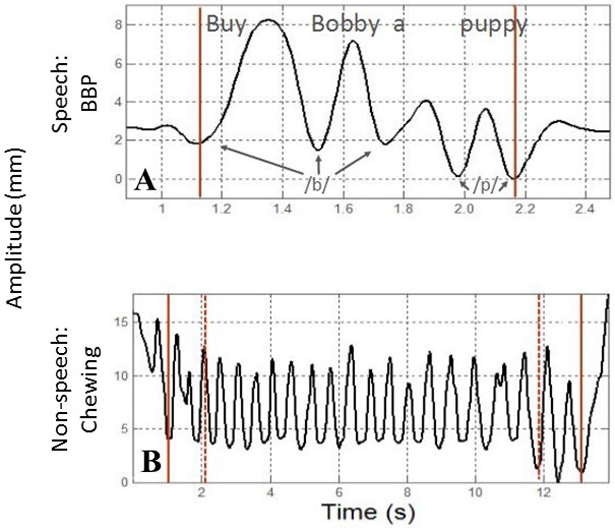
An exemplar of the parsing strategy for the sentence task (panel A) and chewing task (panel B).
Measurements
The following two kinematic measurements were obtained from each task using a custom Matlab-based software, Speech Movement Analysis for Speech and Hearing research (SMASH, Green, Wang, & Wilson, 2013b).
(1) Jaw articulatory working space (mm3) represented changes in the total movement size over the duration of the task. The three-dimensional volume was calculated for each task specific movement (BBP and chewing) by fitting a 2.0 SD ellipsoid around each movement trajectory (see Figure 3). Data were then averaged across repetitions for each task. This measure has been shown to be affected in ALS during speech (Weismer, Yunusova, & Bunton, 2012).
Figure 3:
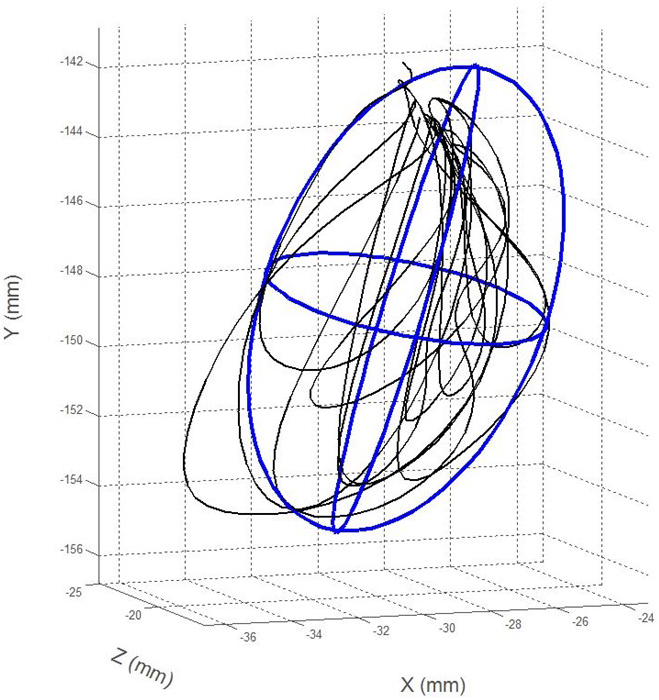
An exemplar of a jaw movement trajectory for a single chewing sequence in 3-dimensions fit with a 2.0 SD ellipse representing articulatory working space.
(2) Maximum jaw movement speed (mm/sec) was also algorithmically determined in the time history as the peak of the first derivative of the 3-dimensional path distance. Within session trials were then averaged. This measure has been consistently important in the assessment of the disease effect on speech production (Kent, 2015).
Statistical analysis
All statistical analyses were completed using IBM SPSS Statistics (PC/v. 23). Each ALS participant/session was considered individually (e.g. if the total of 5 sessions were recorded for a patient, all 5 would contribute data as long as both tasks were present) (Rong, Yunusova, Wang, Zinman, Pattee, Berry, et al., 2016). Because the data were non-normally distributed, non-parametric Mann-Whitney U tests were used to determine differences between clinical variables. Statistical significance was accepted at the p < 0.05 level. A linear mixed model analysis was used to compare groups for each measure within a task and to control for within subject variability by including random intercept for subjects, with an identity covariance structure and restricted maximum likelihood estimation. The mixed models have partially accounted for the distinction of within- and between-subject differences. To be exact, the fixed effect tested between-group differences and the random effect accounted for the within-group variations in baseline performance (i.e. intercept). Tukey’s HSD test was used to test for pairwise differences between groups. Tasks were compared indirectly using the standardized difference between means (Cohen’s d) to measure the magnitude of statistical difference between groups by task for each measure.
Result
Differences in clinical measures between groups
A total of 91 subject/sessions were studied across both participant groups. Descriptive statistics for the ALS subgroups stratified by speaking rate are reported in Table II. As expected, there were significant differences in the ALSFRS-R bulbar subscores (U = 100.00, z = −3.155, p < 0.002) as well as speech intelligibility (U = 370.50, z = −3.534, p < 0.001) between the two ALS subgroups, with the affected (i.e. slower) speaking rate subgroup showing more advanced clinical bulbar disease than the unaffected speaking rate subgroup.
Table II:
Medians and interquartile ranges of clinical variables across sessions in the two studied subgroups (≥ and < 145 WPM).
| ALS ≥ 145 (n = 70) |
ALS < 145 (n = 21) |
|
|---|---|---|
| ALSFRS-R bulbar score* | 11.37 (0.84) | 10.08 (1.55) |
| Speech intelligibility* | 97.40 (4.63) | 82.48 (26.24) |
Asterisk indicates statistically significant differences at α = 0.05
Differences in kinematic measures by task between groups
Descriptive statistics for the results of physiological analyses are displayed in Table III.
Table III:
Means, standard deviations, and effect sizes of physiological tasks
| ALS ≥ 145 (n = 70) |
ALS < 145 (n = 21) |
Controls (n = 17) |
|
|---|---|---|---|
| Articulatory Working Space | |||
| Sentence M(SD) | 22.46 (34.41) | 33.79 (31.01) | 25.57 (50.21) |
| Chewing M(SD) | 395.94 (324.95) | 507.06 (323.39) | 479.73 (305.10) |
| Maximum Speed | |||
| Sentence M(SD) | 121.57 (16.79) | 89.39 (57.01) | 144.02 (16.98) |
| Sentence (Cohen’s d) | −1.33a,c 0.77b,c |
− 1.30a,c | - |
| Chewing M(SD) | 152.13 (47.20) | 176.37 (51.88) | 117.74 (39.33) |
| Chewing (Cohen’s d) | 0.79a,c −0.49b |
1.27a,c | - |
Note. Effect sizes were calculated in comparison to Controls;
Effect sizes were calculated in comparison to ALS < 145 group; ≥ 0.2 = small; ≥ 0.5 = medium; ≥ 0.8 = large;
Statistically significant at α = 0.05
Jaw articulatory working space:
No significant differences were observed for jaw articulatory working space during sentence production between the controls or ALS subgroups or between the ALS subgroups. Similarly, no significant differences were detected in jaw articulatory working space during chewing between any of the three participant groups.
Maximum jaw movement speed:
Figure 4 shows the average maximum speed across participant groups for both the chewing and sentence tasks.
Figure 4:
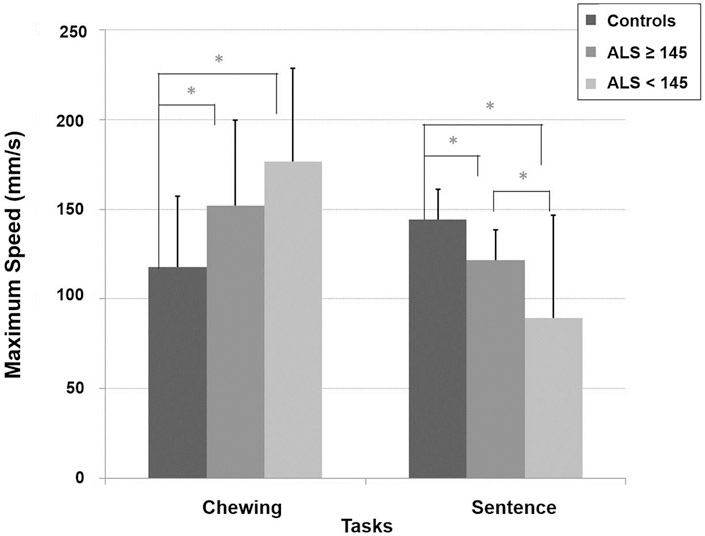
Average maximum jaw movement speed for both the chewing and sentence tasks across participant groups
Sentence:
A significant main effect of group was found (F[2, 60.98] = 8.26, p < 0.001) for the speech task. Healthy controls had a higher sentence maximum speed than ALS participants with unaffected speaking rate (p = 0.006) and those with the affected speaking rate (p < 0.001). ALS participants with a unaffected speaking rate had higher sentence maximum speed as compared to those with affected speaking rate (p = 0.031).
Chewing:
A significant main effect of group was found (F[2, 86.58] = 7.88, p < 0.001) for the chewing task. Healthy controls had the slowest chewing maximum speed as compared to ALS participants with unaffected speaking rate (p = 0.005) and those with affected speaking rate (p < 0.001). There was no significant difference in chewing movement speed between the two subgroups of participants with ALS.
Determining magnitude of differences in task performance between groups
To compare tasks for their sensitivity to group differences, we computed the Cohen’s d effect sizes. Effect sizes shown in Table III revealed that maximum speeds of both sentence and chewing task were more effective in distinguishing between the subgroups of people with ALS and neurologically intact controls. Sentence maximum speed was more sensitive to ALS subgroup differences.
Discussion
The purpose of this investigation was to determine if jaw function during chewing or speech is more affected by bulbar motor deterioration, based on measures of maximum speed and articulatory working space. The findings suggest both chewing and speech (i.e. sentence reading) were effective in distinguishing between the groups of individuals with ALS and neurologically intact controls. Measures of maximum jaw speed but not articulatory working space were responsive to detecting changes in both ALS severity groups relative to healthy controls, and the sentence task distinguished between the two group of individuals with ALS. These results suggest that the measure of jaw speed during a sentence task may serve as a candidate marker of bulbar disease onset and severity. The speech and chewing findings were divergent with respect to their direction. During speech, movement speed appeared to decrease significantly with severity, while during chewing, movement speed increased with severity, supporting the notion of task specificity in motor control affected by disease.
Physiologic differences in movement speed but not articulatory working space
The measure of jaw maximum speed for both chewing and sentence tasks were effective in distinguishing between the groups of people with ALS and neurologically intact controls, while the articulatory working space measure did not appear to be sensitive to bulbar changes for either speech or chewing. The findings from this investigation corroborate those of prior work showing that articulatory movement speed for speech is affected during the early stages of bulbar motor deterioration when change in speaking rate is either unaffected or minimally affected (Green, et al., 2013a; Mefferd et al., 2012; Yunusova et al., 2010; Yunusova, et al., 2013, Rong et al., 2015b) and are among the first to suggest that measures of maximum speed during chewing are sensitive to differences in the severity of in bulbar dysfunction in ALS.
The lack of significance in articulatory working space is inconsistent with the majority of previous research on the effects of ALS on jaw movements, which have tended to find increased movement extent (i.e. range) during the early stages of the disease and decreased movement extent during the later stages of the disease (Shellikeri et al., 2016; Yunusova et al., 2010). We speculate that the difference between studies is due to differences between measures of the extent of movement and articulatory working space. Specifically, the extent of movement (e.g. displacement) may not be predictive of articulatory working space, the boundaries of which are defined over time by multiple jaw movement extrema that occur across a whole speech or chewing sequence (see Figure 3). In addition, the observed large across-subject variability in articulatory working space suggest this measure may be affected by a range of subject factors including differential impairment among articulators with disease progression, speaking task, and production style (see Shellikeri et al., 2016). Consequently, articulatory working space may be too variable across speakers to yield strong group effects (see Table III) and therefore is likely not a reliable candidate marker of bulbar disease onset and severity.
Sentence task distinguished between ALS subgroups
At the onset, we questioned whether chewing would be more affected by bulbar dysfunction due to the greater force demands required for chewing compared to speech. While to date, the kinematics of chewing have not been thoroughly investigated in the assessment of bulbar dysfunction in ALS, our speed and articulatory working space findings did not suggest that measures of jaw motion for chewing are more sensitive to ALS than measures of jaw motion for speech. The sentence task distinguished between the two ALS subgroups, while no difference was detected between the two ALS subgroups during the chewing task. One reasons for this might be related to the fact that only one consistency was trialed during chewing. For example, it has been well documented that food consistency significantly influences jaw displacement (see Wilson et al., 2009 for review). Perhaps significant contrasts may have been detected had the chewers been further “taxed” with a more challenging, yet safe, consistencies. Further work investigating the role of chewing in the assessment of bulbar decline in ALS is warranted.
Task differences in measuring bulbar dysfunction
A central finding in this investigation was the robust directional task differences observed in jaw performance for speech and chewing with bulbar disease severity in ALS. Jaw movement speed for speech decreased significantly with disease involvement, while movement speed for chewing increased (see Figure 4). The distinct and contrasting trends in speed observed without significant changes in articulatory working space point to task-specific control strategies for these two behaviours.
The human neuromotor system is uniquely adept at maintaining task-specific proficiency (e.g. intelligible speech) by implementing specific biomechanic strategies to accommodate for underlying subsystem changes (Hughes & Abbs, 1976). During speech production, articulatory precision is of great importance and jaw position is highly specified to maintain speech production accuracy (Tremblay, Shiller, & Ostry, 2003). During the speaking task, the individuals with ALS may have slowed jaw movement to maintain jaw positioning requirements and resultant acoustic accuracy. In contrast, perhaps the greater jaw speed observed during chewing in individuals with ALS was indicative of increased force demands for this task. Arguably, occlusal force, rather than spatial position, is the specified parameter for chewing and, with disease progression, individuals may increase jaw velocity to maintain adequate force for bolus breakdown. Additional experimental work addressing the task demands and biomechanics of jaw motion for chewing and speech in individuals with ALS is warranted.
The role of jaw dependence
Another explanation for the current findings may be related to the nature of bulbar decline in ALS. While tongue function is generally most affected in individuals with ALS, jaw function remains relatively preserved (DePaul & Brooks, 1993; Langmore & Lehman, 1994). When jaw performance is altered to compensate for decreases in tongue function the phenomenon has been referred to as “jaw dependence” (Hirose et al., 1982). Recent work by Shellikeri and colleagues (2016) documented both tongue and jaw motion simultaneously during a speech task (i.e. BBP) and reported a decrease in the size and maximum speed of lingual movements with a concurrent increase in maximum jaw speed with disease progression.
While the relationship between tongue and jaw motion for chewing in ALS is currently unknown, the role of the tongue for bolus manipulation during chewing is arguably highly significant. During the chewing process, the tongue works to form a cohesive bolus as well as lateralize food (either unilaterally or bilaterally) for additional breakdown in preparation for the swallow. The distinct task differences observed in the current investigation lends support for future work investigating the role of the tongue during chewing in ALS and the potential compensatory strategies employed to maintain adequate bolus breakdown and propulsion.
Limitations and Future Directions
This project was an initial effort to identify candidate measures of bulbar decline. Our current sample size did not allow for longitudinal modeling; however, given the intriguing results, we will pursue work assessing the trend of change in each dependent variable as a function of time/speaking rate as additional data are collected. Further, given the task specific difference in speed trends observed, additional work is underway to better understand the physiologic mechanisms behind speech and masticatory changes in ALS. Our work on masticatory performance in ALS will also aim to identify correlates of change with standard functional assessments.
Conclusion
The findings from the current investigation suggest that 1) both sentence and chewing tasks were effective in distinguishing between the subgroups of individuals with ALS and neurologically intact controls, 2) jaw maximum speed was a more sensitive marker for bulbar dysfunction than articulatory working space, 3) the sentence task distinguished between the two ALS subgroups suggesting its future role as a candidate marker of bulbar disease onset and severity, and 4) distinct jaw movement differences exist between sentence production and chewing in individuals with bulbar disease of different severity. More specifically, movement speed for speech appears to be significantly reduced with severity, while movement speed for chewing increases with severity. This work sheds light on task-specific differences in the disease effects in ALS.
Acknowledgments
We would like to thank the participants and their families for their efforts. This work was supported by the National Institute on Deafness and Other Communication Disorders under Grant R01DC009890; under Grant R01DC0135470; and under Grant 3R01DC013547-04S1.
Footnotes
Declaration of interest
The authors report no declarations of interest.
References
- Allison KM, Yunusova Y, Campbell TF, Wang J, Berry JD, & Green JR (2017). The diagnostic utility of patient-report and speech-language pathologists’ ratings for detecting the early onset of bulbar symptoms due to ALS. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 1–9. 10.1080/21678421.2017.1303515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein NA (1967). The co-ordination and regulation of movements: Conclusions towards the Study of Motor Co-ordination. Biodynamics of Locomotion. 10.1097/00005072-196804000-00011 [DOI] [Google Scholar]
- Beukelman DR, Fager S, Ball L, & Dietz A (2007). AAC for adults with acquired neurological conditions: A review. Augmentative and Alternative Communication, 23(3), 230–242. 10.1080/07434610701553668 [DOI] [PubMed] [Google Scholar]
- Beukelman DR, Yorkston KM, Hakel M, & Dorsey M (2007). Sentence Intelligibility Test [software]. Lincoln, NE: Madonna Rehabilitation Hospital. [Google Scholar]
- Beukelman D, Fager S, & Nordness A (2011). Communication support for people with ALS. Neurology Research International. 10.1155/2011/714693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RH, & Al-Chalabi A (2017). Amyotrophic Lateral Sclerosis. New England Journal of Medicine, 377(2), 162–172. 10.1056/NEJMra1603471 [DOI] [PubMed] [Google Scholar]
- Bunton K (2008). Speech versus nonspeech: Different tasks, different neural organization. Seminars in Speech and Language. 10.1055/s-0028-1103390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, & Nakanishi A (1999). The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. Journal of the Neurological Sciences, 169(1–2), 13–21. 10.1016/S0022-510X(99)00210-5 [DOI] [PubMed] [Google Scholar]
- DePaul R, & Brooks BR (1993). Multiple orofacial indices in amyotrophic lateral sclerosis. Journal of Speech and Hearing Research, 36(6), 1158–67. 10.1044/jshr.3606.1158 [DOI] [PubMed] [Google Scholar]
- Green JR, & Wilson EM (2006). Spontaneous facial motility in infancy: A 3D kinematic analysis. Developmental Psychobiology, 48(1), 16–28. 10.1002/dev.20112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JR, Wilson EM, Wang Y-T, & Moore CA (2007). Estimating mandibular motion based on chin surface targets during speech. Journal of Speech, Language, and Hearing Research : JSLHR, 50(4), 928–939. 10.1044/1092-4388(2007/066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JR, Yunusova Y, Kuruvilla MS, Wang J, Pattee GL, Synhorst L, Berry JD (2013a). Bulbar and speech motor assessment in ALS: Challenges and future directions. Amyotrophic Lateral Sclerosis & Frontotemporal Degeneration, 14(7–8), 494–500. 10.3109/21678421.2013.817585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JR, Wang J, & Wilson DL (2013b). SMASH: A tool for articulatory data processing and analysis. In in Proceedings of the 14th Annual Conference of the International Speech Communication Association, Interspeech (pp. 1331–1335). Lyon, France. [Google Scholar]
- Hecht M, Hillemacher T, Gräsel E, Tigges S, Winterholler M, Heuss D, Hilz MJ, Neundörfer B (2002). Subjective experience and coping in ALS. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders, 3(4), 225–31. [DOI] [PubMed] [Google Scholar]
- Hirose H, Kiritani S, & Sawashima M (1982). Patterns of dysarthric movement in patients with amyotrophic lateral sclerosis and pseudobulbar palsy. Folia Phoniatrica, 34(2), 106–12. [DOI] [PubMed] [Google Scholar]
- Hughes OM, & Abbs JH (1976). Labial-mandibular coordination in the production of speech: Implications for the operation of motor equivalence. Phonetica, 33(3), 199–221. 10.1159/000259722 [DOI] [PubMed] [Google Scholar]
- Kent RD (2015). Nonspeech Oral Movements and Oral Motor Disorders: A Narrative Review. American Journal of Speech-Language Pathology, 24, 763–789. 10.1044/2015_AJSLP-14-0179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmore SE, & Lehman ME (1994). Physiologic Deficits in the Orofacial System Underlying Dysarthria in Amyotrophic Lateral Sclerosis. Journal of Speech and Hearing Research, 37(February 1994), 28–37. 10.1044/jshr.3701.28 [DOI] [PubMed] [Google Scholar]
- Mefferd AS, Green JR, & Pattee G (2012). A Novel Fixed-Target Task to Determine Articulatory Speed Constraints in Persons with Amyotrophic Lateral Sclerosis. J Commun Disord, 45(1), 35–45. 10.1016/j.jcomdis.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nip ISB, Wilson EM, & Kearney L (2018). Spatial Characteristics of Jaw Movements During Chewing in Children with Cerebral Palsy: A Pilot Study. Dysphagia, 33(1):33–40. 10.1007/s00455-017-9830-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong P, Yunusova Y, & Green JR (2015a). Speech intelligibility decline in individuals with fast and slow rates of ALS progression. Proceedings of the Annual Conference of the International Speech Communication Association, Interspeech, 2967–2971. [Google Scholar]
- Rong P, Yunusova Y, Wang J, & Green JR (2015b). Predicting early bulbar decline in amyotrophic lateral sclerosis: A speech subsystem approach. Behavioural Neurology, 15, 1–11. 10.1155/2015/183027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong P, Yunusova Y, Wang J, Zinman L, Pattee GL Berry JD, Perry B, & Green JR (2016). Predicting Speech Intelligibility Decline in Amyotrophic Lateral Sclerosis Based on the Deterioration of Individual Speech Subsystems. PloS One, 5;11(5):e0154971 10.1371/journal.pone.0154971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellikeri S, Green JR, Kulkarni M, Rong P, Martino R, Zinman L, & Yunusova Y (2016). Speech Movement Measures as Markers of Bulbar Disease in Amyotrophic Lateral Sclerosis. Journal of Speech, Language, and Hearing Research,, 59(5), 887–899. 10.1044/2016_JSLHR-S-15-0238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomik B, & Guiloff RJ (2010). Dysarthria in amyotrophic lateral sclerosis: A review. Amyotrophic Lateral Sclerosis, 11(1–2), 4–15. 10.3109/17482960802379004 [DOI] [PubMed] [Google Scholar]
- Tremblay S, Shiller DM, & Ostry DJ (2003). Somatosensory basis of speech production. Nature, 423(6942), 866–869. 10.1038/nature01710 [DOI] [PubMed] [Google Scholar]
- Turvey MT (1990). Coordination. American Psychologist, 45(8), 938–953. 10.1037//0003-066X.45.8.938 [DOI] [PubMed] [Google Scholar]
- van Es MA, Hardiman O, Chio A, Al-Chalabi A, Pasterkamp RJ, Veldink JH, & van den Berg LH (2017). Amyotrophic lateral sclerosis. The Lancet, 6736(17), 1–15. 10.1016/S0140-6736(17)31287-4 [DOI] [PubMed] [Google Scholar]
- Weismer G (2006). Philosophy of research in motor speech disorders. Clinical Linguistics & Phonetics, 20(5), 315–349. 10.1080/02699200400024806 [DOI] [PubMed] [Google Scholar]
- Weismer G, Yunusova Y, & Bunton K (2012). Measures to evaluate the effects of DBS on speech production. Journal of Neurolinguistics, 25(2), 74–94. 10.1016/j.jneuroling.2011.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijesekera LC, & Leigh PN (2009). Amyotrophic lateral sclerosis. Orphanet Journal of Rare Diseases, 4(1), 3 10.1186/1750-1172-4-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EM, & Green JR (2009). The development of jaw motion for mastication. Early Human Development, 85(5), 303–311. 10.1016/j.earlhumdev.2008.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EM, Green JR, & Weismer G (2012). A Kinematic Description of the Temporal Characteristics of Jaw Motion for Early Chewing: Preliminary Findings. Journal of Speech, Language, and Hearing Research. 10.1044/1092-4388(2011/10-0236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorkston K, Strand E, Miller R, Hillel A, & Smith K (1993). Speech deterioration in amyotrophic lateral sclerosis: implications for the timing of intervention. Journal of Medical Speech-Language Pathology, 1, 35–46. [Google Scholar]
- Yunusova Y, Weismer G, Westbury JR, & Lindstrom MJ (2008). Articulatory movements during vowels in speakers with dysarthria and healthy controls. Journal of Speech, Language, and Hearing Research, 51(3), 596–611. 10.1044/1092-4388(2008/043) [DOI] [PubMed] [Google Scholar]
- Yunusova Y, Green JR, Lindstrom MJ, Ball LJ, Pattee GL, & Zinman L (2010). Kinematics of disease progression in bulbar ALS. Journal of Communication Disorders, 43(1), 6–20. 10.1016/j.jcomdis.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunusova Y, Green J, Lindstrom M, Pattee G, & Zinman L (2013). Speech in ALS: Longitudinal Changes in Lips and Jaw Movements and Vowel Acoustics. Journal of Medical Speech-Language Pathology, 21(1), 1–13. [PMC free article] [PubMed] [Google Scholar]


